Abstract
Psoriasis is a common autoimmune skin disease characterized by T cell-mediated hyperproliferation of keratinocytes. The disease has a strong but complex genetic background with a concordance of approximately 60% in monozygotic twins, and recent linkage and high resolution association studies indicate that HLA-Cw*0602 is itself a major susceptibility allele for psoriasis. Patients carrying this allele have been shown to have different clinical features and earlier age of disease onset, and patients homozygous for this allele have about 2·5 times higher disease risk than heterozygotes. Published data indicate that CD8+ T cells may play a major effector role in psoriasis. Epidermal infiltration of predominantly oligoclonal CD8+ T cells, and probably also of CD4+ T cells in the dermis, is a striking feature of chronic psoriasis lesions, indicating that these cells are responding to specific antigens. We argue that CD4+ T cells are essential for initiating and maintaining the pathogenic process of psoriasis but that cross-primed CD8+ T cells are the main effector cells responding to antigens in the HLA-Cw*0602 binding pocket of keratinocytes. It is further proposed that CD8+ T cells are involved in the control of the Th1 polarization, which is observed in psoriasis lesions, through a complex interplay between CD4+, CD8+ T cells and cross-presenting dendritic cells. It is also suggested that spontaneous remissions or fluctuations in disease activity may be determined by a balance within the lesions between effector and suppressor CD4+ and CD8+ T cells.
Keywords: CD8+ T cells, cross-presentation, HLA-Cw*0602, psoriasis, Th 1 regulation
INTRODUCTION
Psoriasis is a common inflammatory skin disease characterized by T cell-mediated hyperproliferation of keratinocytes. The disease has certain distinct but overlapping clinical phenotypes including chronic plaque lesions (psoriasis vulgaris), acute and usually self-limiting guttate type eruptions, seborrhoeic psoriasis, pustular lesions, and at least 10% of these patients develop arthritis [1]. The disease has a strong but complex genetic background with a concordance of approximately 60% in monozygotic twins [2]. Psoriasis vulgaris is genetically not a homogenous disease, and distinct clinical subphenotypes of the disease seem to be dependent on different genetic components [Karason et al. unpublished observation]. Interestingly, psoriasis is the only chronic inflammatory disease that has a strong association with HLA-C, and about two-thirds of the patients carry the HLA-Cw*0602 allele compared to 10–15% in the population at large. Recent linkage and high resolution association studies strongly indicate that HLA-Cw*0602 is itself a major susceptibility risk allele for psoriasis [3]. Patients carrying this allele have been shown to have distinct clinical features including an earlier age of disease onset [4,5]. Furthermore, individuals who are homozygous for the HLA-Cw*0602 allele have about 2·5 times higher disease risk than heterozygotes [6]. Although chronic plaque psoriasis is likely to be somewhat heterogeneous with respect to genetic and pathogenic components, available evidence indicates a final common pathogenic pathway involving specific antigen recognition by T cells that results in stimulation of keratinocyte proliferation [7]. The predominance of CD8+ T cells in the epidermis of psoriatic lesions is a striking feature in psoriasis, while CD4+ T cells are predominant in the upper dermis [8,9].
In normal skin the ratio of proliferating to nonproliferating keratinocytes, is around 60%[10] whereas in psoriasis it is almost 100%, and the mean cell cycle time is reduced from 311 to 36 h in psoriatic lesions [11]. It has been suggested that this keratinocyte hyperproliferation is not restricted to the basal cell compartment containing the stem cells, but may also involve suprabasal cells [12]. Besides the increased proliferation of keratinocytes observed in psoriasis there are distinct changes in the expression of various keratins, mainly involving the type I keratins, K16 and K17, that are not expressed in normal epidermis except for K17 which is present at a low level in normal hair follicles [13].
INVOLVEMENT OF β-HAEMOLYTIC STREPTOCOCCI
Although many environmental factors have been implicated, throat infection with β-haemolytic streptococci is the only well defined external trigger that has convincingly been associated with initiation and acute exacerbation of psoriasis [14,15,16], especially in patients who carry the Cw*0602 allele [4,17]. Streptococcal superantigens have been shown to induce cutaneous lymphocyte associated antigen (CLA) expression by T cells [18,19], thereby facilitating T cell migration into the skin. Although streptococci have traditionally been viewed as extracellular pathogens they are able to invade several eukaryotic cell types including squamous epithelium and macrophages [20,21], and this has been associated with persistent streptococcal carriage and recurrent infections [22]. Protective immunity against streptococci is therefore likely to be dependent on both CD4+ and CD8+ T cells. It is of interest, in this context, that both CD4+ and CD8+ T cells isolated from psoriatic skin lesions have been shown to be respond to crude streptococcal antigens [23,24].
In psoriasis, the importance of effector/memory T cells is now generally recognized [25], but it remains to be elucidated where the effector T cells are activated. It has been observed that CLA + T cells, especially the CD8 + subpopulation, resides within the tonsils in higher frequency than in blood or abdominal lymph nodes and selective homing of CLA + T cells into the tonsils is unlikely as endothelial expression of E-selectin is very low in the tonsils [Thorleifsdottir et al. unpublished observation]. Furthermore, high frequency of clinical remission of psoriasis has been reported after tonsillectomy [26,27]. It is therefore tempting to argue that the priming of the effector and memory T cells that cause psoriasis, especially the guttate variant, may at least to some extent take place in the tonsils, which interestingly is the only mucosal lymphoid organ lined with stratified epithelium [28].
PSORIASIS IS A T CELL-MEDIATED DISEASE
Although a case report in 1979 suggested that cyclosporin A could clear psoriasis [29], this disease was generally considered to be a primary disorder of keratinocytes in the early eighties. A direct role of T cells in the pathogenesis of psoriasis was first suggested in 1983 [30], and it was independently demonstrated that the eruption of psoriatic skin lesions coincided with epidermal influx of dendritic cells (DCs) and T cells [31] and that resolution of psoriatic lesions during phototherapy was preceded by depletion of T cells, especially from the epidermis [32]. The efficacy of cyclosporin A in psoriasis was subsequently confirmed in two independent studies [33,34] and trials with anti-CD4 monoclonal antibodies [35] and an interleukin-2-toxin conjugate [36] further supported that psoriasis is a T cell-mediated disease. These T cell-specific treatments resulted in the normalization of the keratinocyte proliferation and epidermal thickening. The key role of T cells in psoriasis was conclusively demonstrated in 1996 when psoriatic lesions were induced by injecting autologous T cells into uninvolved psoriatic skin transplanted to SCID mice [37]. It was further shown in this model that psoriatic lesions could be induced by injecting purified CD4+ T cells into uninvolved psoriatic skin but no changes were seen when purified CD8+ T cells were injected [38]. Although CD4+ T cells therefore seem to be essential for initiating psoriatic lesions, CD8+ T cells may also have an important role in the pathogenic process as uninvolved psoriatic epidermis contains an increased number of CD8+ T cells that may be able to proliferate locally with the help of IL-7 and IL-15 [39,40], cytokines that are produced by keratinocytes [41].
THE CYTOKINE NETWORK IN PSORIASIS
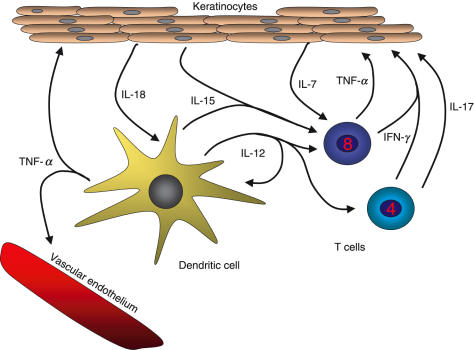
The Th1 cytokines IL-12 [42], IFN-γ and TNF-α are predominant in psoriatic lesions [43]. IFN-γ is produced both by CD4+ and CD8+ T cells, and it may be a central cytokine in psoriasis as lesions can be induced by intradermal injection of IFN-γ [44]. DCs and keratinocytes can also produce large amounts of other cytokines and chemokines. Thus, IFN-γ and TNF-α can induce keratinocytes to produce IL-6, IL-7, IL-8, IL-12, IL-15 IL-18, TNF-α and a multitude of other cytokines and growth factors [41]. IL-18 acts on DCs synergistically with IL-12 [45], which is also produced by the DCs, thereby greatly increasing the IFN-γ production. IL-17, which is produced by activated CD4+ T cells, synergizes with IFN-γ to enhance the production of pro-inflammatory cytokines by human keratinocytes [46], including IL-6 and IL-8, thereby increasing the influx of T cells to the skin. In this way the cytokine network in psoriasis can become a self-sustaining process (Fig. 1).
Fig. 1.
The cytokine network in psoriasis in mainly characterized by Th1 cytokines such as IFN-γ, IL-12 and TNF-α. Major cytokine producers in the lesions are DCs, CD4+ and CD8+ T cells and keratinocytes. IFN-γ and TNF-α induce keratinocytes to produce IL-6, IL-7, IL-8, IL-12, IL-15, IL-18 and TNF-α besides multitude of other cytokines, chemokines and growth factors. IL-18 acts on DCs synergistically with IL-12, to increase the production of IFN-γ. IL-7 and IL-15 are important for the proliferation and homeostatic maintenance of the CD8+ T cells. IL-17 which is produced by activated CD4+ T cells, synergizes with IFN-γ to elicit further production of pro-inflammatory cytokines by the keratinocytes. In this way the cytokine network in psoriasis can become a self-sustaining process.
Autocrine TGF-α [47], IL-20 [48], IFN-γ [49] and other cytokines [49], cause keratinocyte hyperproliferation, and IFN-γ and IL-15 increase the apoptotic resistance of the keratinocytes [50,51]. With Th1 cytokines, such as IL-23 and IL-27, being recently identified, the cytokine network in psoriasis will become increasingly complex. The exact role of TNF-α in the pathomechanism of psoriasis is still unclear, but anti-TNF-α therapy is highly effective in psoriasis [52] indicating that this cytokine has, together with IFN-γ, a central role in the pathogenesis. Chemokines, produced by keratinocytes and inflammatory cells, are an integral part of the immune-mediated cascade in psoriasis, mainly in the recruitment, compartmentalization, adhesion and trafficking of leucocytes in the disease process [53]. The interplay between these and other cytokines and growth factors generated in psoriasis lesions can explain most of the clinical features of psoriasis, such as the hyperproliferation of keratinocytes, increased neovascularization and inflammation. Determining which cytokines and chemokines play a central role in the disease process, is an ongoing challenge which may provide interesting therapeutic targets in the future.
IS PSORIASIS AN AUTOIMMUNE DISEASE?
Acute guttate psoriasis is often self-limiting and reflects an abnormal immune reaction to streptococcal throat infection. Chronic plaque psoriasis, however, behaves like most autoimmune disease, being characterized by a chronic but fluctuating HLA-linked inflammatory process. In contrast to other autoimmune diseases that are mostly linked to certain class II HLA alleles, psoriasis is the only known chronic inflammatory disease that has the strongest association with an HLA-C allele. Thus, over 60% of psoriasis patients carry the HLA-Cw6 allele, and the predominance of oligoclonal CD8+ T cells in lesional epidermis [54] suggests that the pathogenic process is driven by autoantigen(s), that may be presented by HLA-Cw6 in those patients who carry this allele. The CD4+ T cells in stable lesions are also likely to be oligoclonal [55], further supporting the notion that chronic psoriasis is an antigen driven disease.
It has been reported that patients with chronic plaque psoriasis can experience an exacerbation after streptococcal throat infection [56], and we have previously postulated that psoriasis is mediated by T cells that cross-react with epitopes which are common to streptococcal M proteins and those keratins that are up-regulated in psoriatic lesions [57]. Streptococcal M proteins have extensive amino acid sequence homologuey to type I keratins [58–60], including keratins 14, 16 and 17. Interestingly, these keratins are usually only expressed at low levels or not at all in normal skin but are up-regulated during inflammation or trauma [61]. T cell responses against certain amino acid sequences that streptococcal M proteins share with these keratins have been shown to be increased in patients with active psoriasis but absent during disease remissions [59,60]. Furthermore, CLA + CD8+ T cells in HLA-Cw*0602 positive psoriasis patients with an active disease showed IFN-γ responses against the homologueous sequences present both in keratins and M proteins while nonpsoriatic HLA-Cw*0602 positive controls only responded to peptides from the M protein [Johnston et al. unpublished observation]. This indicates that patients with active psoriasis have a recirculating subset of skin homing CD8+ T cells that react specifically to keratin peptides.
DO CD8+ T CELLS HAVE AN IMPORTANT ROLE IN THE PATHOGENESIS OF CHRONIC PSORIASIS?
As previously stated, psoriasis is characterized by hyperproliferation of keratinocytes. It should be reiterated in this context that at least 80% of the T cells in chronic lesional epidermis are of the CD8 phenotype, and while most of the epidermal CD8+ T cells associate closely with keratinocytes, the relatively few epidermal CD4+ T cells preferentially adhere to dendritic cells [57,62]. Furthermore, an increase in CD8+ T cells has been observed in the epidermis of uninvolved skin of psoriasis patients, and eruption of psoriatic lesions is associated with epidermal influx and activation of CD4+ T cells [31,63]. Thus, although psoriatic lesions could only be induced with purified CD4+ T cells in the SCID model [38], the striking predominance of CD8+ T cells that was observed when the grafts had become lesional [38] indicated that resident CD8+ T cells had proliferated during the pathogenic process. It is interesting in this context that IL-7 and IL-15 stimulate the proliferation of CD8+ but not CD4+ T cells [40], and that both these cytokines are abundantly produced by keratinocytes [41]. Furthermore, oligoclonality has predominantly been observed for the CD8+ T cell population in lesional epidermis as judged both by the V beta repertoire [64] and sequence length of CDR3 [65]. This indicates that the CD8+ T cells are responding to specific antigen(s) in the psoriatic lesion with the help of CD4+ T cells that are probably also antigen specific [55]. It is therefore unlikely that nonspecific NK activity of CD8+ T cells has a major pathogenic role [65]. Taken together these observations suggest that while the CD4+ T cells may be necessary for providing critical inductive and helper signals, the CD8+ T cells are likely to be the principal effector agents in the pathogenesis of psoriasis. Thus, interaction of CD4+ T cells with antigen-presenting cells, both in the dermis and epidermis, could create the microenvironment required for the activation and local proliferation of CD8+ T cells in the epidermal compartment of psoriatic lesions.
Why then is apoptosis of keratinocytes not a prominent feature of psoriasis? Keratinocytes from psoriatic skin have an increased capacity to resist the induction of apoptosis compared with keratinocytes from normal skin [66], and this effect may be due to some of the inflammatory cytokines found in psoriatic skin, such as IFN-γ and IL-15 [50,51]. Furthermore, soluble or surface expressed HLA-G in the epidermis may also have a role in preventing apoptosis [67].
WHY CAN TREATMENT WITH ULTRAVIOLET LIGHT (UVB) INDUCE RELATIVELY LONG-TERM REMISSION OF PSORIASIS?
Unlike treatment with cyclosporin A or methotrexate where disease exacerbation usually occurs within a few days after discontinuation, it is not uncommon for psoriasis patients to go into remission for several months after treatment with UVB irradiation. It has been demonstrated that T cells are about 10-times more sensitive than keratinocytes to UVB induced apoptosis [68]. The extended remission achieved by UVB treatment may therefore reflect apoptosis of psoriasis specific epidermal CD8+ T cells that are preferentially exposed to the UVB irradiation. Although UVB irradiation also induces apoptosis of epidermal dendritic cells (DCs) [69], it has been shown that the epidermis is replenished by blood borne DCs within two weeks after UVB exposure [70]. The long remission commonly achieved by UVB treatment in psoriatic patients is therefore more likely to be due to depletion of specific epidermal CD8+ T cells than dendritic cells.
HIV INFECTION AND CD8+ T CELLS IN PSORIASIS
The relative importance of the CD8+ T cell subset in the pathogenesis of chronic psoriasis can explain why psoriasis can worsen dramatically in HIV infected individuals with relative few circulating CD4+ T cells [71], and improves with effective anti-retroviral therapy [72]. It should be pointed out in this context that some of the CD4+ T cells that infiltrate psoriasis lesions might be CD4+ CD25 + regulatory T cells although no data have so far been reported in support of that. Interestingly, it has recently been shown that the regulatory CD4+ CD25+ T cells restrict memory CD8+ T cell responses as depletion of the CD4+ CD25 + regulatory cells is associated with at least a 10-fold expansion of the CD8+ T cell population [73], and an increase in the activity of the CD8+ T cell population has been observed in HIV infected individuals [74]. Thus, the exacerbation observed in HIV positive psoriasis patients might, at least in part, be due to depletion of the regulatory CD4+ T cells and a subsequent enhancement in the activity of CD8+ T cells. Predictably, if CD4+ T cells can both stimulate and restrain the CD8+ T cell responses, a near depletion of the CD4+ T cell population, as happens in full-blown AIDS, will lead to remission of psoriasis [75,76].
HOW HLA-CW6 MAY PLAY A DIRECT ROLE IN THE PATHOGENESIS OF PSORIASIS
The HLA-class I molecules, HLA-A, HLA-B and HLA-C, are ubiquitously expressed whereas HLA-class II genes are normally only expressed on antigen-presenting cells (APCs). Although HLA-C molecules have less allele diversity and are expressed at much lower levels than HLA-A and -B molecules [77], the nature of endogenous peptides presented by HLA-C molecules both with respect to anchor motives and peptide diversity, is comparable to that of HLA-A and -B molecules [77]. HLA-C molecules have also been shown to be involved in cell-mediated cytotoxicity and to be active as alloantigens [78–80].
HLA-Cw*0602 is strongly linked as ancestral haplotypes to three HLA-B alleles, B57, B37 and B13, and these haplotypes are all associated with increased risk of developing psoriasis [81, Gudjonsson et al. unpublished observation]. The fact that not all patients with psoriasis carry HLA-Cw*0602, does not exclude HLA-Cw*0602 as a causative gene. It is evident from other diseases such as Crohn's disease, that there can be different genetic causes for a disease that has been defined as a homogenous clinical entity [82,83]. Furthermore, it should be noted that only 10% of HLA-Cw*0602 carriers get psoriasis, and only 20–30% of HLA-Cw*0602 homozygotes [6]. This is much lower than the 60–70% concordance rate observed in identical twins [2], and strongly indicates that other genetic factors interact with HLA-Cw*0602 in causing psoriasis. HLA-Cw*0602 is, however, an attractive candidate gene because it encodes a protein that can participate in immune reactions by presenting peptide epitopes to CD8+ T cells. It is easy to envisage that psoriasis may be a heterogeneous disease, both in terms of genetic predisposition [Karason et al. unpublished observation] and pathogenic mechanisms, but HLA class I allele(s) that can present psoriasis specific antigens in HLA-Cw6 negative patients remain to be identified and could be heterogeneous.
MAINTENANCE OF THE TH1 CYTOKINE NETWORK AND REGULATORY FUNCTION OF THE CD8+ T CELLS
Beside the Th1 cytokine network in psoriasis, there is evidence for another mechanism that operates at the T cell – DC level which can promote a Th1 polarization. It has recently been reported that the recognition of HLA-class I presented epitopes by antigen-specific CD8+ T cells results in a TNF-α and IFN-γ-dependent increase in the activation level of DCs, and also induces the maturation of type-1 polarized DCs capable of producing high levels of IL-12p70 upon a subsequent CD40 ligation [84] (Fig. 2). This polarization was strongly inhibited by the addition of either anti-TNF-α or soluble IFN-γ receptors, and was greatly enhanced by direct contact between the CD8+ T cells and the DCs. Interestingly, the exposure of DCs to only IFN-γ in the absence of any maturation stimulation, or TNF-α, did not induce the type-1 polarized DC phenotype, but paradoxically reduced the ability of DCs to produce IL-12 [84]. Thus, CD8+ T cells and TNF-α may be of critical importance for the maintenance of the Th1 cytokine network and explain the marked efficacy of anti-TNF-α treatment in recalcitrant psoriasis [52] as well as the observed increase in immature-dendritic cells in lesional biopsies from psoriasis patients treated with CTLA4-Ig [85].
Fig. 2.
It is proposed that CD8+ T cells together with TNF-α and IFN-γ increase the activation level and maturation of DCs (imDC to mDC). This results in the induction of type-1 polarized mature DCs capable of producing high levels of IL-12p70 upon a subsequent CD40 ligation. Thus, CD8+ T cells may be essential for the maintainance of the Th1 environment, and this tight control of the Th1 pattern in psoriasis can explain why a Th2 skin disease, such as atopic eczema, very rarely occurs in patients with psoriasis.
IS CROSS–PRESENTATION AN IMPORTANT MECHANISM FOR THE ACTIVATION OF CD8+ T CELLS IN THE PATHOGENESIS OF CHRONIC PSORIASIS?
The process of cross-presentation, involving the uptake by antigen-presenting cells (APCs) of antigens from adjacent cells, may be a key mechanism whereby CD8+ T cells are involved in the pathogenesis of chronic psoriasis. Cross-presentation was first described in relation to induction of T cell cytotoxicity to minor histocompatibility antigens [86] and has now been shown to occur both in the context of HLA-class I and HLA-class II molecules [87–89]. Dendritic cells are particularly efficient at cross-presenting antigens and are the only cells that can cross-prime CD8+ T cells [90]. It should be noted, however, that cross-priming also has been shown to be dependent on CD4+ T cells [91]. It has been reported that dendritic cells can take up and cross-present antigens not only from apoptotic cells [92] but also from nearby live cells [93,94] (Fig. 2). Cross-presentation is dependent on a relatively large amount of antigen presented on the surface of the DCs. Cells that express sufficient antigen to be recognized directly by an effector CD8+ T cell, may therefore not contain enough antigen for effective cross-presentation by DCs [87,95]. It is therefore more likely that HLA-Cw*0602 homozygotes, having twice as many HLA-Cw*0602 molecules on their surface compared to heterozygotes, can reach the activation threshold for cross-priming CD8+ T cells to peptides that can be presented by this HLA-C allele. This may, at least in part, explain why HLA-Cw*0602 homozygous individuals are about 2·5 fold more likely to develop psoriasis than HLA-Cw*0602 heterozygotes [6].
It is of interest in this context that the keratins containing the putative psoriasis autoepitope(s) are only present in small amount or not at all in normal skin, but are up-regulated during inflammation or trauma [96,97], and this increase might be sufficient for effective cross-presentation by epidermal DCs. It is known that HLA-Cw*0602 is able to present amino-acid sequences present both in streptococcal M-proteins and type I keratins [Johnston et al. unpublished observation], a type of molecular mimicry that is believed to operate in several other autoimmune diseases [98,99].
Thus, the CD4+ T cells that are closely adherent to the dendritic epidermal cells in guttate psoriasis may be able to recognize cross-reactive streptococcal (and keratin) epitopes on the surface of the DCs presented by HLA-class II molecules and thereby activate the DCs through ligation with CD40. The activated DCs may then in turn be able to activate CD8+ T cells that recognize antigens presented by the DCs HLA-class I molecules [100]. CD4+ T cells have been shown to be essential for inducing cross-priming of CD8+ T cells [91], and for maintaining CD8+ T cell effector function [101]. After being activated in this way by DCs it is envisaged that the CD8+ T cells can recognize the autoantigen(s) in the binding pocket of HLA-Cw*0602 on the keratinocytes themselves (Fig. 2).
It is possible that DCs carrying cross-reactive streptococcal antigens can migrate from the tonsils into the skin (Fig. 3), as bacterial peptidoglycan has been detected within dendritic cells in nonlymphoid tissues, including brain lesions of multiple sclerosis patients [102]. In psoriasis such DCs may then mature further, as described above, through interaction with CD4+ T cells in the skin, and subsequently induce CD8 effector responses against the putative psoriasis epitope presented by class I HLA molecules on keratinocytes (Fig. 2). The responses induced by DCs carrying bacterial antigen would be expected to be relatively short lived (e.g. guttate psoriasis) but the development of persistent plaques would probably require a successful cross-presentation of keratinocyte antigens in the absence of effective regulatory mechanisms. The cross-primed effector CD8+ T cell should then induce a further maturation and Th1 polarization of the dendritic cells, which subsequently migrate to the lymph nodes to recruit more effector CD4 and CD8+ T cells whereby the self-sustaining cycle of chronic plaque psoriasis is established (Fig. 3).
Fig. 3.
Schematic presentation of the processes suggested to be involved in the generation of guttate psoriasis and chronic plaque psoriasis. In guttate psoriasis, or exacerbation of chronic plaque psoriasis after streptococcal throat infection, it is envisaged that streptococcal specific T cells, and possibly DCs carrying streptococcal antigens migrate from the tonsils into the skin where the specific T cells recognize streptococcal antigens or cross-reactive antigens derived from keratinocytes with a resulting eruption of guttate psoriasis. If effective cross-presentation does not occur, perhaps due to sufficient infiltration and activation of CD8 + suppressor T cells, the disease process is self-limited, while effective cross-presentation results in the recruitment of cross-reactive T cells that recirculate between lymph nodes and the skin lesions. This scenario predicts that oligoclonal T cells would be more pronounced in chronic plaques than in guttate lesions.
As previously mentioned the recognition of the putative psoriasis epitope in the binding pocket of HLA-Cw*0602 on keratinocytes does not result in keratinocyte apoptosis, but promotes instead the release of various pro-inflammatory cytokines with resulting hyperproliferation of keratinocytes, neutrophil influx and vascular neogenesis, features that are characteristic for psoriatic lesions.
POSSIBLE REGULATORY MECHANISMS IN PSORIASIS
As stated previously most guttate psoriasis lesions resolve spontaneously, and chronic plaque psoriasis often has a fluctuating course that is independent of treatment. Early studies showed that eruption of guttate lesions coincided with epidermal influx and activation of CD4+ T cells while their resolution was associated with decreasing numbers of CD4+ T cells and increase in the numbers of activated CD8+ T cells [31]. This suggests that somewhat different immune mechanisms may operate in acute guttate and persistent plaque psoriasis. It is relevant in this context that in addition to the CD4+ CD25+ regulatory T cells, three types of CD8 + suppressor T cells are currently being studied in humans [reviewed in 103]. It is therefore possible that the majority of the CD8+ T cells observed in resolving guttate lesions are suppressor T cells that can switch the pathogenic process off before effective cross-presentation of autoantigen(s) is achieved. It can also be envisaged that a balance could exist in all types of psoriatic lesions between effector and suppressor CD4+ and CD8+ T cells, and that changes in this balance may be involved in fluctuations of disease activity and spontaneous remissions of chronic lesions. Furthermore, as the great majority of patients with guttate psoriasis carry the HLA-Cw6 allele, and patients with chronic psoriasis who carry this allele have a more fluctuating disease course than those patients who are Cw6 negative [4], it is possible that these putative regulatory mechanisms are more active in psoriasis patients who carry the genetic constellation associated with the HLA-Cw6 allele. It should not be difficult to test this possibility for a disease like psoriasis in which longitudinal tissue samples can easily be obtained.
CONCLUSIONS
To conclude, it has been demonstrated that psoriasis is an immunological disease and recent data indicate that HLA-Cw*0602 may play an important pathogenic role in the majority of the psoriasis patients. CD4+ T cells are necessary for inducing and maintaining the disease, whereas it is argued that cross-primed antigen-specific CD8+ T cells recognizing autoantigen(s) are the main effector cells. It is also envisaged that CD8+ T cells are involved in the control of the Th1 polarization that is observed in psoriasis lesions, and that fluctuations in the severity of psoriasis, and even the spontaneous remissions that are common in guttate psoriasis, can be explained by changes in the balance between CD4+ and CD8 + effector and regulatory cell subsets.
REFERENCES
- 1.Espinoza LR, Cuellar ML, Silveira LH. Psoriatic arthritis. Curr Opin Rheumatol. 1992;4:470–8. [PubMed] [Google Scholar]
- 2.Brandrup F, Holm N, Grunnet N, et al. Psoriasis in monozygotic twins: variations in expression in individuals with identical constitution. Acta Derma Venereol. 1982;62:229–36. [PubMed] [Google Scholar]
- 3.Veal CD, Capon F, Allen MH, et al. Family-based analysis using a dense single-nucleotide polymorphism-based map defines genetic variation at PSORS1, the major psoriasis-susceptibility locus. Am J Hum Genet. 2002;71:554–64. doi: 10.1086/342289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gudjonsson JE, Karason A, Antonsdottir AA, et al. HLA-Cw6-Positive and HLA-Cw6-Negative Patients with Psoriasis Vulgaris have Distinct Clinical Features. J Invest Dermatol. 2002;118:362–5. doi: 10.1046/j.0022-202x.2001.01656.x. [DOI] [PubMed] [Google Scholar]
- 5.Enerback C, Martinsson T, Inerot A, et al. Evidence that HLA-Cw6 determines early onset of psoriasis, obtained using sequence-specific primers (PCR-SSP) Acta Derm Venereol. 1997;77:273–6. doi: 10.2340/0001555577273276. [DOI] [PubMed] [Google Scholar]
- 6.Gudjonsson JE, Karason A, Antonsdottir A, et al. Psoriasis patients who are homozygous for the HLA-Cw*0602 allele have a 2.5 fold increased risk of developing psoriasis compared with Cw6 heterozygotes. Br J Dermatol. 2003;148:233–5. doi: 10.1046/j.1365-2133.2003.05115.x. [DOI] [PubMed] [Google Scholar]
- 7.Valdimarsson H, Baker BS, Jonsdottir I, et al. Psoriasis: a disease of abnormal proliferation induced by T lymphocytes. Immunol Today. 1986;7:256–9. doi: 10.1016/0167-5699(86)90005-8. [DOI] [PubMed] [Google Scholar]
- 8.Austin LM, Coven TR, Bhardwaj N, et al. Intraepidermal lymphocytes in psoriatic lesions are activated GMP-17 (TIA-1) +CD8+CD3+ CTLs as determined by phenotypic analysis. J Cutan Pathol. 1998;25:79–88. doi: 10.1111/j.1600-0560.1998.tb01694.x. [DOI] [PubMed] [Google Scholar]
- 9.Paukkonen K, Naukkarinen A, Horsmanheimo M. The development of manifest psoriatic lesions is linked with the invasion of CD8+ T cells and CD11c + macrophages into the epidermis. Arch Dermatol Res. 1992;284:375–9. doi: 10.1007/BF00372065. [DOI] [PubMed] [Google Scholar]
- 10.Gelfant S. On the existence of non-cycling germinative cells in human epidermis in vivo and cell cycle aspects of psoriasis. Cell Tissue Kinet. 1982;15:393–7. doi: 10.1111/j.1365-2184.1982.tb01056.x. [DOI] [PubMed] [Google Scholar]
- 11.Weinstein GD, McCullough JL, Ross PA. Cell kinetic basis for pathophysiology of psoriasis. J Invest Dermatol. 1985;85:579–83. doi: 10.1111/1523-1747.ep12283594. [DOI] [PubMed] [Google Scholar]
- 12.Leigh IM, Pulford KA, Ramaekers FC, Lane EB. Psoriasis. maintenance of an intact monolayer basal cell differentiation compartment in spite of hyperproliferaiton. Br J Dermatol. 1985;113:53–64. doi: 10.1111/j.1365-2133.1985.tb02044.x. [DOI] [PubMed] [Google Scholar]
- 13.Leigh IM, Navsaria H, Purkis PE, et al. Keratins (K16 and K17) as markers of keratinocyte hyperproliferation in psoriasis in vivo and in vitro. Br J Dermatol. 1995;133:501–11. doi: 10.1111/j.1365-2133.1995.tb02696.x. [DOI] [PubMed] [Google Scholar]
- 14.Fry L. Psoriasis. Br J Dermatol. 1988;119:445–61. doi: 10.1111/j.1365-2133.1988.tb03248.x. [DOI] [PubMed] [Google Scholar]
- 15.Telfer NR, Chalmers RJ, Whale K, Colman G. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1994;128:39–42. [PubMed] [Google Scholar]
- 16.Gudjonsson JE, Thorarinsson AM, Sigurgeirsson B, et al. Streptococcal throat infections and exacerbation of chronic plaque psoriasis: a propestive study. Br J Dermatol. 2003;149:530–4. doi: 10.1046/j.1365-2133.2003.05552.x. [DOI] [PubMed] [Google Scholar]
- 17.Mallon E, Bunce M, Savoie H, et al. HLA-C and guttate psoriasis. Br J Dermatol. 2000;143:1177–82. doi: 10.1046/j.1365-2133.2000.03885.x. [DOI] [PubMed] [Google Scholar]
- 18.Leung DY, Gately YM, Trumbla A, et al. Bacterial superantigens induce T cell expression of the skin-selective homing receptor, the cutaneous lymphocyte-associatied antigen, via stimulation of interleukin 12 production. J Exp Med. 1995;181:747–53. doi: 10.1084/jem.181.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sigmundsdottir H, Gudjonsson JE, Valdimarsson H. Interleukin-12 alone can not enhance the expression of the cutaneous lymphocyte antigen (CLA) by superantigen stimulated T lymphocytes. Clin Exp Immunol. 2003;132:430–5. doi: 10.1046/j.1365-2249.2003.02169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaPenta D, Rubens C, Chi E, Cleary PP. Group A streptococci efficiently invade human respiratory epithelial cells. Proc Natl Acad Scie USA. 1994;91:12115–9. doi: 10.1073/pnas.91.25.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dombek PE, Cue D, Sedgewick J, et al. High-frequency intracellular invasion of epithelial cells by serotype M1 group A streptococci: M1 protein-mediated invasion and cytoskeletal rearrangements. Mol Microbiol. 1999;31:859–70. doi: 10.1046/j.1365-2958.1999.01223.x. [DOI] [PubMed] [Google Scholar]
- 22.Osterlund A, Popa R, Nikkila T, et al. Intracellular reservoir of Streptococcus pyogenes in vivo: a possible explanation for recurrent pharyngotonsillitis. Laryngoscope. 1997;107:640–7. doi: 10.1097/00005537-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Ovigne JM, Baker BS, Davison SC, et al. Epidermal CD8+ T cells reactive with group A streptococcal antigens in chronic plaque psoriasis. Exp Dermatol. 2002;11:357–64. doi: 10.1034/j.1600-0625.2002.110410.x. [DOI] [PubMed] [Google Scholar]
- 24.Brown DW, Baker BS, Ovigne JM, et al. Skin CD4+ T cells produce interferon-gamma in vitro in response to streptococcal antigens in chronic plaque psoriasis. J Invest Dermatol. 2000;114:576–80. doi: 10.1046/j.1523-1747.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- 25.Ellis CN, Krueger GG. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. N Engl J Med. 2001;345:248–55. doi: 10.1056/NEJM200107263450403. [DOI] [PubMed] [Google Scholar]
- 26.McMillin BD, Maddern BR, Graham WR. A role for tonsillectomy in the treatment of psoriasis? Ear Nose Throat J. 1999;78:155–8. [PubMed] [Google Scholar]
- 27.Takahara M, Bandoh N, Imada M, et al. Efficacy of tonsillectomy on psoriasis and tonsil histology. Nippon Jibiinkoka Gakkai Kaiho. 2001;104:1065–70. doi: 10.3950/jibiinkoka.104.1065. [DOI] [PubMed] [Google Scholar]
- 28.Perry M, Whyte A. Immunology of the tonsils. Immunol Today. 1998;19:414–21. doi: 10.1016/s0167-5699(98)01307-3. [DOI] [PubMed] [Google Scholar]
- 29.Mueller W, Hermann B. Cyclosporin A for psoriasis. N Engl J Med. 1979;301:555. doi: 10.1056/NEJM197909063011016. [DOI] [PubMed] [Google Scholar]
- 30.Bos JD, Hulsebosch HJ, Krieg SR, et al. Immunocompetent cells in psoriasis. In site immunophenotyping by monoclonal antibodies. Arch Dermatol Res. 1983;275:181–9. doi: 10.1007/BF00510050. [DOI] [PubMed] [Google Scholar]
- 31.Baker BS, Swain AF, Fry L, Valdimarsson H. Epidermal T lymphocytes and HLA-DR expression in psoriasis. Br J Dermatol. 1984;110:555–64. doi: 10.1111/j.1365-2133.1984.tb04678.x. [DOI] [PubMed] [Google Scholar]
- 32.Baker BS, Swain AF, Griffiths CE, et al. Epidermal T lymphocytes and dendritic cells in chronic plaque psoriasis: the effects of PUVA treatment. Clin Exp Immunol. 1985;61:536–4. [PMC free article] [PubMed] [Google Scholar]
- 33.Ellis CN, Gorsulowsky DC, Hamilton TA, et al. Cyclosporine improves psoriasis in a double-blind study. J Am Med Assoc. 1986;256:3110–6. [PubMed] [Google Scholar]
- 34.Griffiths CE, Powles AV, Leonard JN, et al. Clearance of psoriasis with low dose cyclosporin. Br Med J. 1986;293:731–2. doi: 10.1136/bmj.293.6549.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morel P, Revillard JP, Nicolas JF, et al. Anti-CD4 monoclonal antibody therapy in severe psoriasis. J Autoimmun. 1992;5:465–77. doi: 10.1016/0896-8411(92)90006-c. [DOI] [PubMed] [Google Scholar]
- 36.Gottlieb SL, Gilleaudeau P, Johnson R, et al. Response of psoriasis to a lymphocyte-selective toxin (DAB389IL-2) suggests a primary immune, but not keratinocyte, pathogenic basis. Nat Med. 1995;1:442–7. doi: 10.1038/nm0595-442. [DOI] [PubMed] [Google Scholar]
- 37.Wrone-Smith T, Nickoloff BJ. Dermal injection of immunocytes induces psoriasis. J Clin Invest. 1996;98:1878–87. doi: 10.1172/JCI118989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nickoloff BJ, Wrone-Smith T. Injection of pre-psoriatic skin with CD4+ T cells induces psoriasis. Am J Pathol. 1999;155:145–58. doi: 10.1016/S0002-9440(10)65109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kieper WC, Tan JT, Bondi-Boyd B, et al. Overexpression of interleukin (IL) -7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J Exp Med. 2002;195:1533–9. doi: 10.1084/jem.20020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan JT, Ernst B, Kieper WC, et al. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–32. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gröne A. Keratinocytes and cytokines. Vet Immunol Immunopathol. 2002;88:1–12. doi: 10.1016/s0165-2427(02)00136-8. [DOI] [PubMed] [Google Scholar]
- 42.Yawalkar N, Karlen S, Hunger R, et al. Expression of interleukin-12 is increased in psoriatic skin. J Invest Dermatol. 1998;111:1053–7. doi: 10.1046/j.1523-1747.1998.00446.x. [DOI] [PubMed] [Google Scholar]
- 43.Gearing AJ, Fincham NJ, Bird CR, et al. Cytokines in skin lesions of psoriasis. Cytokine. 1990;2:68–75. doi: 10.1016/1043-4666(90)90045-u. [DOI] [PubMed] [Google Scholar]
- 44.Fierlbeck G, Rassner G, Muller C. Psoriasis induced at the injection site of recombinant interferon gamma. Results of immunohistologic investigations. Arch Dermatol. 1990;126:351–5. [PubMed] [Google Scholar]
- 45.Tominaga K, Yoshimoto T, Torigoe K, et al. IL-12 synergizes with IL-18 or IL-1beta for IFN-gamma production from human T cells. Int Immunol. 2000;12:151–60. doi: 10.1093/intimm/12.2.151. [DOI] [PubMed] [Google Scholar]
- 46.Teunissen MB, Koomen CW, de Waal Malefyt R, et al. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645–9. doi: 10.1046/j.1523-1747.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- 47.Turbitt MI, Akhurst RJ, White SI, MacKie RM. Localization of elevated transforming growth factor-alpha in psoriatic epidermis. J Invest Dermatol. 1990;95:229–32. doi: 10.1111/1523-1747.ep12478077. [DOI] [PubMed] [Google Scholar]
- 48.Blumberg H, Conklin D, Xu W, et al. Interleukin 20. Discovery, receptor identification, and role in epidermal function. Cell. 2001;104:9–19. doi: 10.1016/s0092-8674(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 49.Prinz JC, Gross B, Vollmer S, et al. T cell clones from psoriasis skin lesions can promote keratinocyte proliferation in vitro via secreted products. Eur J Immunol. 1994;24:593–8. doi: 10.1002/eji.1830240315. [DOI] [PubMed] [Google Scholar]
- 50.Quin JZ, Chaturvedi V, Denning MF, et al. Role of NF-kappaB in the apoptotic-resistant phenotype of keratinocytes. J Biol Chem. 1999;274:37957–64. doi: 10.1074/jbc.274.53.37957. [DOI] [PubMed] [Google Scholar]
- 51.Ruckert R, Asadullah K, Seifert M, et al. Inhibition of keratinocyte apoptosis by IL-15: a new parameter in the pathogenesis of psoriasis? J Immunol. 2000;165:2240–50. doi: 10.4049/jimmunol.165.4.2240. [DOI] [PubMed] [Google Scholar]
- 52.Schopf RE, Aust H, Knop J. Treatment of psoriasis with the chimeric monoclonal antibody against tumor necrosis factor alpha, infliximab. J Am Acad Dermatol. 2002;46:886–91. doi: 10.1067/mjd.2002.120472. [DOI] [PubMed] [Google Scholar]
- 53.Schon MP, Ruzicka T. Psoriasis: the plot thickens…. Nat Immunol. 2001;2:91. doi: 10.1038/84293. [DOI] [PubMed] [Google Scholar]
- 54.Lin WJ, Norris DA, Achziger M, et al. Oligoclonal expansion of intraepidermal T cells in psoriasis skin lesions. J Invest Dermatol. 2001;117:1546–53. doi: 10.1046/j.0022-202x.2001.01548.x. [DOI] [PubMed] [Google Scholar]
- 55.Vollmer S, Menssen A, Prinz JC. Dominant lesional T cell receptor rearrangements persist in relapsing psoriasis but are absent from nonlesional skin: Evidence for a stable antigen-specific pathogenic T cell response in psoriasis vulgaris. J Invest Dermatol. 2001;117:1296–301. doi: 10.1046/j.0022-202x.2001.01494.x. [DOI] [PubMed] [Google Scholar]
- 56.Wardrop P, Weller R, Marais J, Kavanagh G. Tonsillitis and chronic psoriasis. Clin Otolaryngol. 1998;23:67–8. doi: 10.1046/j.1365-2273.1998.00084.x. [DOI] [PubMed] [Google Scholar]
- 57.Valdimarsson H, Baker BS, Jonsdottir I, et al. Psoriasis: a T cell-mediated autoimmune disease induced by streptococcal superantigens? Immunol Today. 1995;16:145–9. doi: 10.1016/0167-5699(95)80132-4. [DOI] [PubMed] [Google Scholar]
- 58.McFadden J, Valdimarsson H, Fry L. Cross-reactivity between streptococcal M surface antigen and human skin. Br J Dermatol. 1991;125:443–7. doi: 10.1111/j.1365-2133.1991.tb14769.x. [DOI] [PubMed] [Google Scholar]
- 59.Gudmundsdottir AS, Sigmundsdottir H, Sigurgeirsson B, et al. Is an epitope on keratin 17 a major target for autoreactive T lymphocytes in psoriasis? Clin Exp Immunol. 1999;117:580–6. doi: 10.1046/j.1365-2249.1999.01013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sigmundsdottir H, Sigurgeirsson B, Troye-Blomberg M, et al. Circulating T cells of patients with active psoriasis respond to streptococcal M-peptides sharing sequences with human epidermal keratins. Scand J Immunol. 1997;45:688–97. doi: 10.1046/j.1365-3083.1997.d01-438.x. [DOI] [PubMed] [Google Scholar]
- 61.McGowan K, Coulombe PA. The wound repair-associated keratins 6, 16, and 17. Insights into the role of intermediate filaments in specifying keratinocyte cytoarchitecture. Subcell Biochem. 1998;31:173–204. [PubMed] [Google Scholar]
- 62.Fry L. An atlas of psoriasis. New Jersey: The Parthenon Publications Group; 1992. [Google Scholar]
- 63.Onuma S. Immunohistochemical studies of infiltrating cells in early and chronic lesions of psoriasis. J Dermatol. 1994;21:223–32. doi: 10.1111/j.1346-8138.1994.tb01727.x. [DOI] [PubMed] [Google Scholar]
- 64.Chang JC, Smith LR, Froning KJ, et al. CD8+ T cells in psoriatic lesions preferentially use T cell receptor V beta 3 and/or V beta 13.1 genes. Proc Natl Acad Sci USA. 1994;91:9282–6. doi: 10.1073/pnas.91.20.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nickoloff BJ. The Immunologic and genetic basis of psoriasis. Arch Dermatol. 1999;135:1104–10. doi: 10.1001/archderm.135.9.1104. [DOI] [PubMed] [Google Scholar]
- 66.Wrone-Smith T, Mitra RS, Thompson CB, et al. Keratinocytes derived from psoriatic plaques are resistant to apoptosis compared with normal skin. Am J Pathol. 1997;151:1321–9. [PMC free article] [PubMed] [Google Scholar]
- 67.Aractingi S, Briand N, Le Danff C, et al. HLA-G and NK receptor are expressed in psoriatic skin: a possibly pathway for regulating infiltrating T cells? Am J Pathol. 2001;159:71–7. doi: 10.1016/S0002-9440(10)61675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krueger JG, Wolfe JT, Nabeya RT, et al. Successful ultraviolet B treatment of psoriasis is accompanied by a reversal of keratinocyte pathology and by selective depletion of intraepidermal T cells. J Exp Med. 1995;182:2057–68. doi: 10.1084/jem.182.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rattis FM, Concha M, Dalbiez-Gauthier C, et al. Effects of ultraviolet B radiation on human Langerhans cells: functional alteration of CD86 upregulation and induction of apoptotic cell death. J Invest Dermatol. 1998;111:373–9. doi: 10.1046/j.1523-1747.1998.00320.x. [DOI] [PubMed] [Google Scholar]
- 70.Merad M, Manz MG, Karsunky H, et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;4:1135–41. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Myskowski PL, Ahkami R. Dermatologic complications of HIV infection. Med Clin North Am. 1996;80:1415–35. doi: 10.1016/s0025-7125(05)70496-x. [DOI] [PubMed] [Google Scholar]
- 72.Fischer T, Schworer H, Vente C, et al. Clinical improvement of HIV-associated psoriasis parallels a reduction of HIV viral load induced by effective antiretroviral therapy. AIDS. 1999;13:628–9. doi: 10.1097/00002030-199904010-00018. [DOI] [PubMed] [Google Scholar]
- 73.Kursar M, Bonhagen K, Fensterle J, et al. Regulatory CD4+CD25+T cells restrict memory CD8+ T cell responses. J Exp Med. 2002;196:1585–92. doi: 10.1084/jem.20011347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ribeiro RM, Mohri H, Ho DD, Perelson AS. In vivo dynamics of T cell activation, proliferation, and death in HIV-1 infection: why are CD4+ but not CD8+ T cells depleted? Proc Natl Acad Sci USA. 2002;99:15572–7. doi: 10.1073/pnas.242358099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Obuch ML, Maurer TA, Becker B, Berger TG. Psoriasis and human immunodeficiency virus infection. J Am Acad Dermatol. 1992;27:667–73. doi: 10.1016/0190-9622(92)70234-7. [DOI] [PubMed] [Google Scholar]
- 76.Colebunders R, Blot K, Mertens V, Dockx P. Psoriasis regression in terminal AIDS. Lancet. 1992;339:1110. doi: 10.1016/0140-6736(92)90701-4. [DOI] [PubMed] [Google Scholar]
- 77.Falk CS, Schendel DJHLA-C. revisited. Ten years of change. Immunol Res. 1997;16:203–14. doi: 10.1007/BF02786363. [DOI] [PubMed] [Google Scholar]
- 78.Malissen B, Kristensen T, Goridis C, et al. Clones of human cytotoxic T lymphocytes derived from an allosensitized individual: HLA specificity and cell surface markers. Scand J Immunol. 1981;14:213–24. doi: 10.1111/j.1365-3083.1981.tb00558.x. [DOI] [PubMed] [Google Scholar]
- 79.Schendel DJ, Reinhardt C, Nelson PJ, et al. Cytotoxic T lymphocytes show HLA-C-restricted recognition of EBV-bearing cells and allorecognition of HLA class I molecules presenting self-peptides. J Immunol. 1992;149:2406–14. [PubMed] [Google Scholar]
- 80.van der Bruggen P, Szikora JP, Boel P, et al. Autologous cytolytic T lymphocyte recognize a MAGE-1 nonapeptide on melanomas expressing HLA-Cw*1601. Eur J Immunol. 1994;24:2134–40. doi: 10.1002/eji.1830240930. [DOI] [PubMed] [Google Scholar]
- 81.Jenisch S, Henseler T, Nair RP, et al. Linkage analysis of human leukocyte antigen (HLA) markers in familial psoriasis: a strong disequilibrium effects provide evidence for a major determinant in the HLA-B/-C region. Am J Hum Genet. 1998;63:191–9. doi: 10.1086/301899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 83.Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–6. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 84.Maillard RB, Egawa S, Cai Q, et al. Complementary dendritic cell-activating function of CD8+ and CD4+ T cells: helper role of CD8+ T cells in the development of T helper type 1 responses. J Exp Med. 2002;195:473–83. doi: 10.1084/jem.20011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abrarms JR, Kelley SL, Hayes E, et al. Blockade of T lymphocyte costimulatin with cytotoxic T lymphocyte-associated antigen 4-immunoglobulin (CTLA4Ig) reverses the cellular pathology of psoriatic plaques, including the activation of keratinocytes, dendritic cells, and endothelial cells, and endothelial cells. J Exp Med. 2000;192:681–94. doi: 10.1084/jem.192.5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976;143:1283–8. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 88.Kurts C, Heath WR, Carbone FR, et al. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J Exp Med. 1996;184:923–30. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Skoberne M, Schenk S, Hof H, Geginat G. Cross-presentation of Listeria monocytogenes-derived CD4 T cell epitopes. J Immunol. 2002;169:1410–8. doi: 10.4049/jimmunol.169.3.1410. [DOI] [PubMed] [Google Scholar]
- 90.den Haan JM, Bevan MJ. Antigen presentation to CD8+ T cells: cross-priming in infectious diseases. Curr Opin Immunol. 2001;13:437–41. doi: 10.1016/s0952-7915(00)00238-7. [DOI] [PubMed] [Google Scholar]
- 91.Bennett SR, Carbone FR, Karamalis F, et al. Help for cytotoxic-T cell responses is mediated by CD40 signalling. Nature. 1998;393:478–80. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 92.Larsson M, Fonteneau JF, Somersan S, et al. Efficiency of cross presentation of vaccinia virus-derived antigens by human dendritic cells. Eur J Immunol. 2001;31:3432–42. doi: 10.1002/1521-4141(200112)31:12<3432::aid-immu3432>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 93.Harshyne LA, Watkins SC, Gambotto A, Barratt-Boyes SM. Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J Immunol. 2001;166:3717–23. doi: 10.4049/jimmunol.166.6.3717. [DOI] [PubMed] [Google Scholar]
- 94.Ramirez MC, Sigal LJ. Macrophages and dendritic cells use the cytosolic pathway to rapidly cross-present antigen from live, vaccinia-infected cells. J Immunol. 2002;169:6733–42. doi: 10.4049/jimmunol.169.12.6733. [DOI] [PubMed] [Google Scholar]
- 95.Carbone FR, Kurts C, Bennett SR, et al. Cross-presentation. a general mechanism for CTL immunity and tolerance. Immunol Today. 1998;19:368–73. doi: 10.1016/s0167-5699(98)01301-2. [DOI] [PubMed] [Google Scholar]
- 96.de Mare S, van Erp PE, Ramaekers FC, van de Kerkhof PC. Flow cytometric quantification of human epidermal cells expressing keratin 16 in vivo after standardized trauma. Arch Dermatol Res. 1990;282:126–30. doi: 10.1007/BF00493471. [DOI] [PubMed] [Google Scholar]
- 97.Wei L, Debets R, Hegmans JJ, et al. IL-1 beta and IFN-gamma induce the phenotype of psoriasis in the transwell skin organ culture system. IFN-gamma upregulates the expression of keratin 17 and keratinocyte transglutaminase via endogenous IL-1 production. J Pathol. 1999;187:358–64. doi: 10.1002/(SICI)1096-9896(199902)187:3<358::AID-PATH253>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 98.Roep BO. Autoreactive T cells in endocrine/organ-specific autoimmunity: why has progress been so slow? Springer Semin Immunopathol. 2002;24:261–71. doi: 10.1007/s00281-002-0109-8. [DOI] [PubMed] [Google Scholar]
- 99.Wucherpfennig KW. Mechanisms for the induction of autoimmunity by infectious agents. J Clin Invest. 2001;108:1097–104. doi: 10.1172/JCI14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ronchetti A, Iezzi G, Crosti MC, et al. Role of antigen-presenting cells in cross-priming of cytotoxic T lymphocytes by apoptotic cells. J Leukoc Biol. 1999;66:247–51. doi: 10.1002/jlb.66.2.247. [DOI] [PubMed] [Google Scholar]
- 101.Serbina NV, Lazarevic V, Flynn JL. CD4 (+) T cells are required for the development of cytotoxic CD8 (+) T cells during Mycobacterium tuberculosis infection. J Immunol. 2001;167:6991–7000. doi: 10.4049/jimmunol.167.12.6991. [DOI] [PubMed] [Google Scholar]
- 102.Schrijver IA, van Meurs M, Melief MJ, et al. Bacterial peptidoglycan and immune reactivity in the central nervous system in multiple sclerosis. Brain. 2001;124:1544–54. doi: 10.1093/brain/124.8.1544. [DOI] [PubMed] [Google Scholar]
- 103.Filaci G, Suciu-Foca N. CD8+ T suppressor cells are back to the game: are they players in autoimmunity. Autoimmunity Rev. 2002;1:279–83. doi: 10.1016/s1568-9972(02)00065-4. [DOI] [PubMed] [Google Scholar]