Abstract
CD4+ T cells are essential for the immune response against cancer. Vaccination against cancer will likely only be effective at preventing growth of micrometastatic disease while adoptive T cell therapy will be better suited for eradication of bulky pre-existing disease (Knutson et al. Expert Opin Biol Ther 2002; 2:55–66). Problems with the use of adoptive T cell therapy include lack of CD4+ T cell help, low frequency of antigen-specific T cells, and lack of effective ex vivo expansion techniques. In this study, we focused on improving ex vivo expansion of CD4+ T helper cells. The effects of IL-12, along with IL-2, on the ex vivo generation of HER-2/neu antigen-specific T cells were examined. Patients were immunized with a peptide-based vaccine that contained a helper epitope, p776–790, derived from the intracellular domain of HER-2/neu. While T cell immunity to p776–790, assessed by proliferation assays, could be readily measured in short-term cultures, cell line generation by multiple in vitro stimulation with peptide and IL-2 as the only added cytokine resulted in loss of antigen-specific proliferation. The inclusion of IL-12, along with IL-2, restored antigen-specific proliferation in a dose-dependent fashion. The resulting p776–790-specific T cells responded readily to antigen by proliferating and producing type I cytokines (IFN-γ and TNF-α). The increased proliferative response of the cultures was due in part to an increase in the number of HER-2/neu-specific T cells. These results suggest that IL-12 is an important cytokine for ex vivo recovery and maintenance of antigen-specific CD4+ T lymphocytes that would otherwise be lost by using IL-2 alone in combination with antigen. Furthermore, these results have important implications for ex vivo expansion of CD4+ T cell for use in anti-tumour adoptive immunotherapy.
Keywords: IL-12, T lymphocyte, cytokine, tumour antigen, peptide
INTRODUCTION
CD4+ T helper cells are central to the development of adaptive immune responses for the protection against infection and possibly malignancy by activating antigen-specific effector cells (CTL and B cells) and recruiting cells of the innate immune system such as macrophages and mast cells [1–4]. Two predominant CD4+ T helper cell subtypes exist, Th1 and Th2, each with a unique role in the type of the global immune response elicited. Th1 cells, characterized by secretion of IFN-γ and TNF-α, are primarily responsible for activating and regulating the development and persistence of cell-mediated (i.e. CTL) immunity [1]. In contrast, Th2 cells are responsible for the development of a broad therapeutic humoral response. Because of the central role of Th1 cells in cell-mediated immunity, there has been recent interest in developing adoptive T cell therapy for the treatment of malignancy using CD4+ T helper cells. However, the ex vivo expansion of human tumour antigen-specific CD4+ T helper cells has not been well defined and the elucidation of improved culture conditions for the ex vivo expansion of CD4+ T cells is an important issue [5]. Currently used ex vivo culture conditions are often inadequate for expansion of CD4+ T cells.
The manipulation of the cytokines in the culture medium is one technique that is rigorously being explored for specifically expanding certain T cell subsets. The most common cytokine to be added to T cell cultures is IL-2. However, antigen specificity is often lost when using IL-2 alone for the generation of tumour antigen-specific CD4+ T helper cells, particularly when antigen-specific T cell precursors are low [5–7]. Another cytokine that has been extensively characterized over the past decade and which could be useful for the ex vivo expansion of human tumour-antigen-specific T cells for adoptive T cell therapy is IL-12. IL-12 is a cytokine that is uniquely different from other commonly used cytokines, such as IL-7, in that it only acts on activated T cells [8]. While the effects of IL-12 are well characterized on the ex vivo expansion of antigen-specific CD8+ T cells [9,10] less is known about the role of IL-12 during the ex vivo expansion of CD4+ T cells, particularly T cells specific for tumour antigens.
In the present study, the in vitro effects of IL-12, along with IL-2, on CD4+ T cell line growth, phenotype, and antigen-specific function were evaluated. HER-2/neu peptide, p776–790 [11] was used as a model to study the role of IL-12 during in vitro cell growth of antigen-specific CD4+ T cells.
MATERIALS AND METHODS
Materials
All peptides were >95% purity and manufactured by United Biochemical Inc. (Seattle, WA, USA). HER-2/neu peptides used were p42–56 (p42), HLDMLRHLYQGCQVV and p776–790 (p776), GVGSPYVSRLLGICL [12]. Nitrocellulose-backed plates were purchased from Millipore Corp (Bedford, MA, USA). Anti-IFN-γ monoclonal antibodies were purchased from Mabtech (Nacka, Sweden). IL-12 (batch # 4D18I002, 1·7 × 107 U/mg) was a generous gift from the Genetics Institute (Cambridge, MA, USA). Anti-CD8, anti-CD4, anti-αβ-TCR, anti-CD3, anti-TNF-α antibodies and purified recombinant human TNF-α were from Pharmingen (San Diego, CA, USA), streptavidin-alkaline phosphatase and alkaline-phosphatase colour detection reagents from Bio-Rad Laboratories (Hercules, CA, USA), human AB+ serum from Valley Biomedical, Incorporated (Winchester, VA, USA), and Immulon IV ELISA plates from Dynex Technologies (Chantilly, VA, USA). TMB peroxidase substrate was obtained from Kirkegaard and Perry Laboratories (Gaithersburg, MD, USA) and streptavidin-horseradish peroxidase was obtained from Amersham Pharmacia Biotech (Uppsala, Sweden).
Source and preparation of PBMC
Breast cancer patients participating in a Phase I trial of HER-2/neu peptide vaccines were leukapheresed at the completion of the trial after informed consent. PBMC derived from those leukaphereses were used in these studies [12]. PBMC were isolated by density gradient centrifugation as previously described [12]. Cells were aliquoted and cryopreserved in liquid nitrogen in freezing media (90% fetal bovine serum and 10% dimethylsulfoxide) at a cell density of 25–50 × 106 cells/ml. PBMC from HER-2/neu immunized patients were used because PBMC from naïve cancer patients or normal healthy volunteers would require multiple labourious in vitro stimulations as we have previously reported [13].
In vitro stimulation (IVS) of PBMC with peptide
In all experiments, except as indicated, PBMC were stimulated in vitro for 12 days in the presence of p776 peptide. For each experiment, 40–80 × 106 cryopreserved cells were rapidly thawed, washed 2 times in 10 mls of RPMI-1640 containing streptomycin, penicillin, and 10% human AB serum (complete medium), and resuspended at 1·0–2·0 × 106 cells/ml in the same medium and incubated in a humidified incubator at 37°C at 5% CO2. Peptide was added on day 0 to a concentration of 10 µg/ml. Ten U/ml of IL-2 and 0·01–10 ng/ml of IL-12 were added on days 4 and 8 and the cells were further incubated for 4 days. Viable cell yield was assessed by standard Trypan Blue exclusion.
T-lymphocyte proliferation
Thymidine incorporation was measured as previously described [14]. The generation of immune responses by peptide vaccination were detected from PBMC samples and presented as the mean of 24 well replicates, each well containing 2·5 × 105 cells. The detection of proliferation following IVS is presented as the mean SI of 3–6 replicate wells each containing 7·5 × 104 cells. Autologous irradiated (3300 rad) PBMC were added as antigen presenting cells at a 1 : 1 ratio with cultured T cells. Peptides were added into each well at concentrations indicated in each figure. No cytokines (i.e. IL-2 and IL-12) were added during proliferation assessment. The stimulation index (SI) was defined as the ratio of the mean of the experimental wells and the mean of control (no antigen) wells. SI's of greater than 2 were considered a positive proliferation response as previously described [12].
Cytokine secretion
Following IVS, cells were restimulated with 10 µg/ml peptide and irradiated, autologous PBMC at 2·5 × 105/well for 40 h. No cytokines (i.e. IL-2 and IL-12) were added during restimulation. TNF-α content of the media was measured by ELISA. Immulon IV ELISA plates were coated with 50 µl of mouse anti-TNF-α monoclonal antibody at 2·5 µg/ml in carbonate buffer (15 mm Na2CO3, 35 mm NaH/CO3, pH 8·0). Wells were blocked 2 h at room temperature with 200 µl of PBS containing 1% BSA and 0·5% Tween-20. The plates were washed 4 times using PBS containing 0·1% Tween-20 (PBST). Cell culture supernatants were added at 100 µl/well and the plates were further incubated overnight at 4°C. The plates were washed 4 times with PBST. One hundred microliters PBST containing 10% goat serum and 0·5 µg/ml biotinylated anti-TNF-α antibody were added to wells and the plates were further incubated for 2 h at room temperature followed by washing 4 times with PBST. The plates were subsequently incubated with 100 µl/well of PBS containing 1 : 3000 streptavidin-horseradish peroxidase, washed, and developed with 100 µl/well TMB peroxidase substrate. The developing reaction was terminated with 1 N hydrochloric acid and the OD was measured at 450 nm optical wavelength using a Molecular Devices (Sunnyvale, CA, USA) kinetic microplate reader. Purified TNF-α was analysed in parallel and the OD values of the samples were used to establish a standard curve (ng/ml).
Enzyme-linked immunosorbent spot (ELIspot)
An ELIspot assay (3-day format) was used to determine frequencies of peptide-specific T lymphocytes as previously described with only minor modifications (i.e. substituting MHC class I peptide with MHC class II peptide antigen) [14]. On day 1, T cells (3–6 replicates per condition) were plated into 96-well, anti-IFN-γ-coated nitrocellulose plates in 100 µl media. The cells were stimulated with 100 µl of media containing a 1 : 1 ratio of autologous, irradiated (3000 rads) PBMC with or without antigen (10 µg/ml). The cells were further incubated for 20 h at 37°C and detection of bound IFN-γ was performed as previously described [14]. Data is presented as the mean peptide-specific T cell frequency/million cells (± sem) calculated from 6 (patient 1276) or 3 replicates (patient 6622).
Flow cytometry
Flow cytometry of human T cells was done as previously described [14].
RESULTS
Generation of immunity to HER-2/neu helper peptide, p776–790, in patients with HER-2/neu-overexpressing tumours
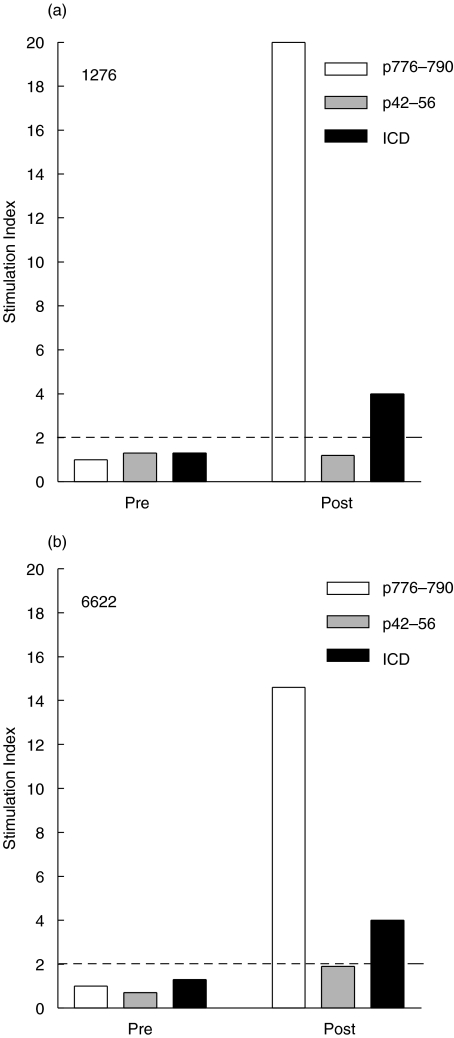
Patients 1276 and 6622 were vaccinated with a HER-2/neu peptide-based vaccine consisting of helper peptides including p776 [15]. The patients had no detectable preexisting immunologic response to the p776 or the HER-2/neu ICD. However, after the immunizations were complete, patients had detectable p776-specific proliferation responses (Fig. 1). Both patients also developed ICD-specific protein proliferation responses. The patients did not develop responses to another HER-2/neu-derived peptide, p42, which was not contained in the vaccine preparation. PBMC derived from each patient donor were used to assess the effects of IL-12 on antigen-specific T cell line expansion and phenotype using p776 as a model antigen.
Fig. 1.
Generation of immunity to HER-2/neu helper peptide, p776–790, and HER-2/neu protein, ICD, in patients with HER-2/neu-overexpressing cancers. Patients were vaccinated with HER-2/neu peptides once a month for 6 months. Peptide-specific immunity was assessed prior to (Pre) and after vaccination (Post). T cell proliferation was assessed by 3H-thymidine incorporation assays and stimulation index was calculated from 24-well replicates. □ p776–790 (immunizing peptide) response,  p42–56 (irrelevant, nonimmunizing, HER-2/neu peptide) response, ▪ ICD response (protein). S.I.s greater than 2 (-----) are indicative of a positive proliferation response to antigens as described in the Materials and methods. All S.I.s greater than 2 were statistically significant (P < 0·05) than control (no antigen) wells as assessed by unpaired 2-tailed student's T-test.
p42–56 (irrelevant, nonimmunizing, HER-2/neu peptide) response, ▪ ICD response (protein). S.I.s greater than 2 (-----) are indicative of a positive proliferation response to antigens as described in the Materials and methods. All S.I.s greater than 2 were statistically significant (P < 0·05) than control (no antigen) wells as assessed by unpaired 2-tailed student's T-test.
The addition of IL-12 to IL-2 increases antigen-specific proliferation after IVS with HER-2/neu helper peptide
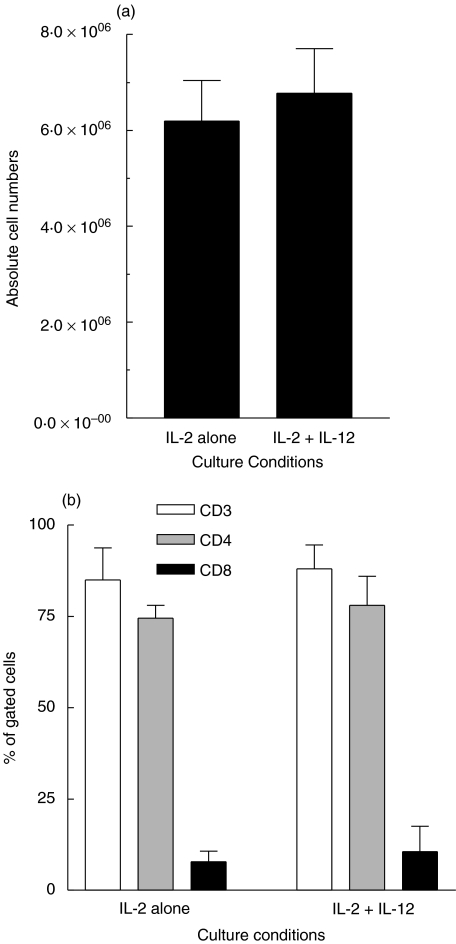
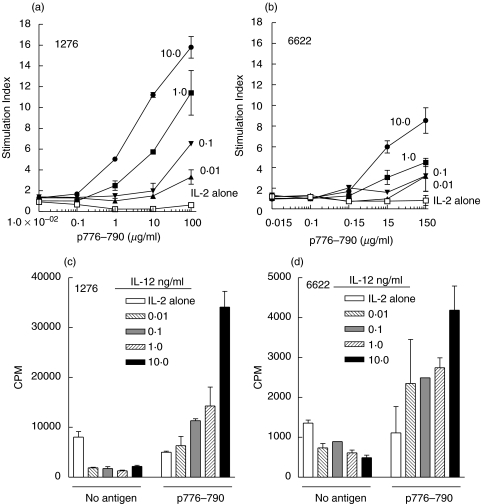
The addition of IL-12 to p776 antigen and IL-2 did not change the overall recovery of viable cells following IVS (Fig. 2a). Furthermore, as expected, the cells were predominantly (>74%) CD3+/CD4+ when incubated in the presence of p776 and either IL-2 alone or IL-2 and IL-12 (10 ng/ml) (Fig. 2b). The phenotype was not significantly altered by the addition of IL-12. In similar experiments, but without MHC class II peptide, we also observed that CD3, CD4, and CD8 T cells were unaltered by the inclusion of IL-12 (data not shown). Proliferation was assessed following IVS of patient PBMC with p776, IL-2 and varying (0, 0·01, 0·1, 1, and 10 ng/ml) concentrations of IL-12. As shown in Fig. 3, those cells expanded in culture in the presence of IL-2 alone, i.e. in the absence of IL-12, did not demonstrate a proliferative response to p776. That is, proliferation observed in the original samples before IVS was not preserved when using IL-2 alone. The addition of IL-12, during IVS, resulted in augmentation of antigen-specific proliferation in a dose-dependent manner. As an example, the SI of T cells taken through IVS in 10 ng/ml IL-12 was 16 in PBMC from patient 1276 (Fig. 3a) and 9 in PBMC from patient 6622 (Fig. 3b). The proliferative responses were specific for the p776, as there was no proliferative response to the irrelevant HER-2/neu peptide, p42 (data not shown). The inclusion of IL-12 along with IL-2 during IVS substantially reduced nonspecific cellular proliferation (Fig. 3c,d). The reduction in nonspecific cellular proliferation was similar at all concentrations of IL-12 used and was not dose-dependent within the range of IL-12 concentrations used. Results shown from 2 patients are representative of a minimum of 3 independent experiments from each patient. The observed effects were also observed in PBMC from at least 3 different patient donors.
Fig. 2.
Cultures established with IL-12, IL-2 and HER-2/neu helper peptide at predominantly CD4. (a) shows the absolute viable cell number recovery from cell cultures carried through IVS with p776–790 (10 µg/ml) and either IL-2 or IL-2 and IL-12 (10 ng/ml). Cells were initially cultured using 20 × 106 cells per condition. The data are the mean (± sem) calculated from 6 independent determinations. (b) shows the percentage of cells in the cultures after IVS that stained positive for CD3, CD4, or CD8. The cells were incubated with p776–790 (10 µg/ml) and either IL-2 alone or with IL-2 and IL-12 (10 ng/ml). Each bar is representative (mean ± sem) of 2 independent determinations from patients 1276 and 6622.
Fig. 3.
The addition of IL-12 to IL-2 increases antigen-specific clonal proliferation after IVS with HER-2/neu helper peptide. (a, b) proliferation responses to various concentrations of p776–790 (0–150 µg/ml) of cultures carried through 1 IVS in the presence of 10 µg/ml p776–790, 10 U/ml IL-2 and varying concentrations of IL-12 (ng/ml, filled symbols). (a) shows results with PBMC derived from patient 1276 and (b) from patient 6622. The concentration of IL-12 used during IVS is shown next to the corresponding lines. In (a, b) in some cases error bars are low enough for the error bars to be obscured by the symbols.(c) patient 1276 and (d) patient 6622, data is presented as the counts per minute (CPM) for proliferation responses to media alone (No antigen) or p776–790 following IVS with 10 µg/ml p776–790, 10 U/ml IL-2, and varying concentrations of IL-12 from the same patients as indicated in graph. All values are the mean (± sem) of duplicate determinations. In (d) the value for the 0·1 ng/ml was from only a single determination.
The addition of IL-12 to IL-2 increases the number of peptide antigen-specific CD4+ T cells after IVS with HER-2/neu helper peptide
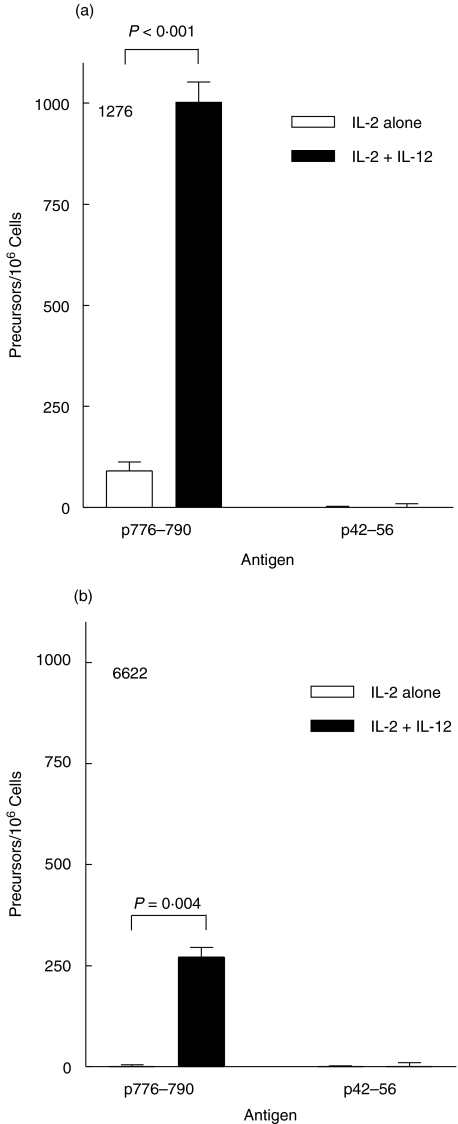
The T cell frequency was evaluated following IVS of patient PBMC with p776 and IL-2 in the absence or presence of IL-12 (Fig. 4). Following IVS with peptide and IL-2 alone, the p776-specific and p42-specific T cell frequencies in PBMC from patient 1276 were 91 ± 22/million cells and 0/million cells, respectively (Fig. 4a). The addition of IL-12 during IVS resulted in an increase of p776-specific T cells to 1003 ± 50/million cells. In the absence of IL-12 the T cell frequencies to p776 and p42 in PBMC from patient 6622 were not detectable following IVS with p776 and IL-2 alone (Fig. 4b). The inclusion of IL-12 resulted in increased p776-specific T cells to 272 ± 24/million cells. T cells specific for p42 were not elevated in PBMC from either patient as a result of inclusion of IL-12 during IVS (Fig. 4a,b). T cell lines derived with IL-2 and IL-12 were 86% and 89% CD4+ for patients 1276 and 6622, respectively (data not shown). Results shown are representative data of at least 3 experiments in 3 different patients yielding similar data.
Fig. 4.
The addition of IL-12 to IL-2 increases the numbers of peptide-specific CD4+ T cells after IVS with HER-2/neu helper peptide. PBMC from patient 1276 (a) and 6622 (b) were cultured with 10 µg/ml p776–790 peptide in the presence of 10 U/ml IL-2 alone (□) or 10 U/ml IL-2 and 10 ng/ml IL-12 (▪). Peptide-specific T cells were measured by IFN-γ ELIspot analysis following restimulation of the cultured cells with no antigen, p776–790, or the irrelevant HER-2/neu peptide, p42–56. Data is presented as the mean T cell frequency/million cells (± sem) calculated from 6 (a) or 3 replicates (b).
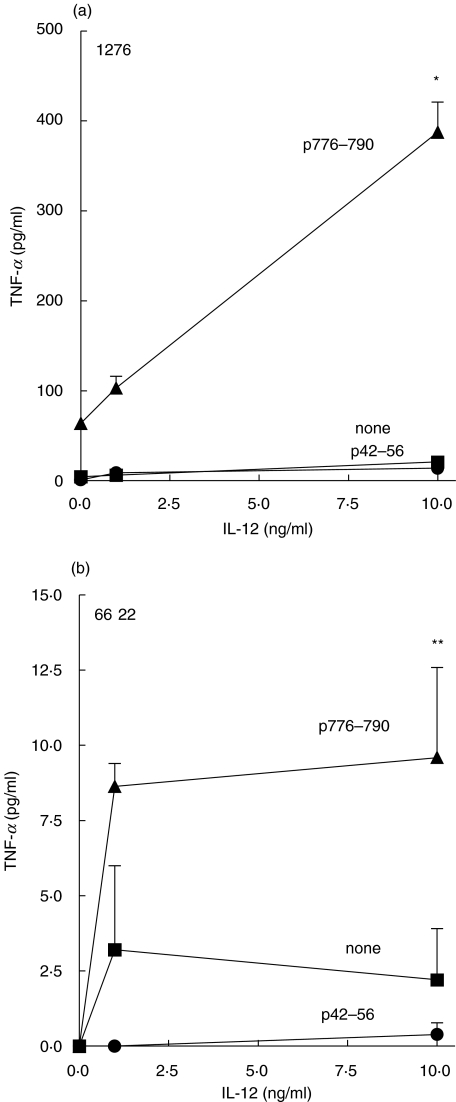
The addition of IL-12 to IL-2 increases antigen-specific TNF-α release by T cells after IVS with HER-2/neu helper peptide
Figure 4 demonstrates that IL-12 in culture can enhance tumour antigen-derived peptide-induced TNF-α release compared to cultures incubated in IL-2 alone. For patient 1276, the TNF-α yield from T cell lines established in p776 and IL-2 alone was 63 ± 7 pg/ml and increased to 390 ± 33 pg/ml by the inclusion of IL-12 (Fig. 5a). For patient 6622, the TNF-α yield from T cell lines established in p776 and IL-2 alone was not detectable and was increased to 9·5 ± 3 pg/ml for cells incubated by the inclusion of IL-12 (Fig. 5b). Increased TNF-α release to the irrelevant HER-2/neu peptide, p42–15, was not observed in PBMC in either patient (Fig. 5c,d). Results shown are representative of 3 experiments yielding similar results.
Fig. 5.
The addition of IL-12 to IL-2 increases peptide-specific TNF-α release by T cells after IVS with HER-2/neu helper peptide. PBMC from patient donors 1276 (panel A) and 6622 (panel B) were cultured with 10 µg/ml p776–790, 10 U/ml IL-2, and varying concentrations of IL-12 (0, 1, and 10 ng/ml). The cells were subsequently tested for TNF-α release against no antigen (none, squares), 10 µg/ml p776–790 (triangles) or 10 µg/ml p42–56 (circles). Each determination is the average (± sem) for triplicate determination. *P = 0·004; **P = 0·04, compared to TNF-α release from cultures carried through IVS with IL-2 alone and subsequently restimulated with 10 µg/ml p776–790. The absence of an error indicates an standard error of the mean of less than 1%.
DISCUSSION
In general, active immunization of cancer patients with tumour antigen-specific vaccine does not result in regression or cure of existing malignancy. Most likely, the greatest utility of cancer vaccines will be in the prevention of cancer recurrence or even the initiation of disease. Infusion of competent antigen-specific T cells (i.e. adoptive T cell therapy) however, may result in eradication of cancer [16]. Successful ex vivo expansion of tumour-specific T cells for therapeutic use in humans has been limited by the lack of defined tumour antigens which would allow expansion of antigen-specific T cells, the understanding of the in vitro expansion requirement of T cells which would allow the generation of maximal numbers while retaining optimal antigen-specific function, and the ability to develop methods for ex vivo culture which could expand polyclonal T cell lines, both CD4+ and CD8+. Recent studies in adoptive T cell therapy of malignant melanoma have demonstrated the potential utility of this approach. Dudley and colleagues evaluated the therapeutic efficacy of infusion of T cells expanded in the presence of tumour cell lysate [17]. In that study infusion of polyclonal cell lines resulted in approximately a 50% objective response rate. While the mechanism of tumour killing in this study was unclear, a reasonable hypothesis is that the cell lines had a strong CD4+ T cell component which resulted in direct CD4+ T cell killing of tumours, augmentation of endogenous or transferred CD8+ tumour-specific T cell immunity or both. In fact, in a similar parallel study by the same group, infusion of pure CD8+ T cell populations under the same conditions had little impact on tumour cell growth, suggesting that CD4+ T helper cells played an active role in anti-tumour immunity [18].
It is well known that the CD4+ T cell is critical in controlling the activation and persistence of the immune response against viral infections [1,19]. Recent evidence also suggests that CD4+ T cells are essential for anti-tumour immunity and for function and survival of tumour-specific CD8+ T cells [20]. In murine tumour models, lymphocyte depletion studies reveal that both CD8+ and CD4+ T cells are required for therapeutic efficacy [21]. In fact, CD4+ T cell-depleted mice showed faster tumour development than CD8+-depleted mice suggesting a dominant role for CD4+ T cells [22]. The importance of CD4+T cells in the evolution of the tumour-specific immune response is underscored by recent studies demonstrating that infusion of tumour antigen-specific CD4+ T cells can initiate a de novo tumour antigen-specific CTL response [23]. CD4+ T cells can also modify the immune microenvironment by producing cytokines that attract DC, macrophages, and eosinophils to promote an inflammatory environment [3]. In recent times, there have been increased efforts to identify tumour antigens and, in particular, tumour antigen MHC class II epitopes to generate a strong Th1 immune response. The generation of Th1 CD4+ T cell immunity may be particularly important for effective anti-tumour immunity as suggested by recent murine studies in which it was demonstrated that the Th1 phenotype is absolutely necessary for therapeutic efficacy of adoptive T cell therapy [24]. Additionally, Th1 CD4+ T cells are important for broadening the epitope recognition (i.e. epitope spreading) of the anti-tumour T cell repertoire and preventing the outgrowth of antigen-negative variants [25].
HER-2/neu is a tumour antigen overexpressed by a wide variety of adenocarcinomas including breast and ovarian cancers. Active immunization of cancer patients with HER-2/neu helper peptide vaccines can boost antigen-specific T cell frequencies substantially in vivo [26]. We hypothesized that active immunization of cancer patients followed by ex vivo expansion of T cells may offer the clinical potential of increasing cancer-specific T cells to numbers greater than what could be achieved by vaccination alone. We evaluated ex vivo expansion of T cells specific for HER-2/neu helper epitopes as a model using IL-2 and IL-12 to augment T cell number and function. IL-12 has been well defined for ex vivo expansion of viral-specific CD8+ T cells. For example, the cytolytic activity of HIV-specific T lymphocytes is greatly enhanced by including IL-12 along with IL-2 during ex vivo expansion of T cells with HIV-derived antigens [27]. Less is known about a role for ex vivo expansion of CD4+ T cell lines using IL-12 and specifically the generation of Th1 T cell lines reactive against human tumour antigens. Experiments, in the present study, demonstrated that inclusion of IL-12 along with IL-2 during the ex vivo expansion of human antigen-specific CD4+ T cells resulted in a significant increase in the blastogenic response to a subsequent rechallenge with antigen, the number of antigen-specific T cells, and augmented function of Th1 phenotype lymphocytes. These findings, however, are in contrast to the observed effects of IL-12 in vivo where profound suppression of the CD4 T helper cell population has been observed [28,29]. The reason for this difference is unclear but likely is the effect of environmental differences such as the actions of IL-12 on other tissue types that interact with immune effectors. For example, other tissues respond to IL-12 such as hepatocytes which could lead to altered haematopoetic cells dynamics [30]. Whatever the mechanism, the suppressive effects of IL-12 in vivo also extend to the CD8 T cells, monocytes and neutrophils indicating a lack of specificity. Thus the in vitro effects of IL-12 on CD4 T cells may be markedly different than the effects in vivo.
The results presented here demonstrate that IL-12, when added to cell cultures during IVS with IL-2 and tumour antigen helper peptides promotes increased recovery of CD4+ T cells specific for HER-2/neu helper peptide. T cell lines incubated with antigen, IL-2 and IL-12 show augmented function and secretion of Th1 type cytokines. The resulting cultures, depending on the levels of antigen-specific T cell precursors, could be used directly for infusion or could be manipulated by further ex vivo expansion. Although it is not currently known how many tumour-antigen specific CD4 T cells would be needed to eradicate tumour, previous studies in murine models have shown that infusion of tumour-specific CD4 T cells at doses as low as 3 × 105 CD4 T cells is effective at inhibiting tumour growth and eliciting an endogenous CD8 T cell response [23]. A number of other factors including tumour aggressiveness and disease burden would also greatly influence the amount of CD4 T helper cells required. Our findings could potentially be useful for translating adoptive T cell therapy to the clinic where increasing both numbers and function of polyclonal tumour antigen-specific Th1 CD4+ T cells may be important in mediating an anti-tumour response.
Acknowledgments
This work was supported for KLK by a fellowship from the Department of Defense Breast Cancer Research Program (DAMD 17-00-1-0492) and for MLD by NCI grants R01CA85374 and K24CA85218.
REFERENCES
- 1.Kalams SA, Walker BD. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J Exp Med. 1998;188:2199–204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen PA, Peng L, Plautz GE, Kim JA, Weng DE, Shu S. CD4+ T cells in adoptive immunotherapy and the indirect mechanism of tumor rejection. Crit Rev Immunol. 2000;20:17–56. [PubMed] [Google Scholar]
- 3.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–68. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ossendorp F, Toes RE, Offringa R, van der Burg SH, Melief CJ. Importance of CD4(+) T helper cell responses in tumor immunity. Immunol Lett. 2000;74:75–9. doi: 10.1016/s0165-2478(00)00252-2. [DOI] [PubMed] [Google Scholar]
- 5.Knutson KL, Disis ML. Expansion of HER-2/neu specific T cells ex vivo following immunization with a HER-2/neu peptide-based vaccine. Clin Breast Ca. 2001;2:73–9. doi: 10.3816/CBC.2001.n.014. [DOI] [PubMed] [Google Scholar]
- 6.Pawelec G, Schneider EM, Wernet P. Acquisition of suppressive activity and natural killer-like cytotoxicity by human alloproliferative ‘helper’ T cell clones. J Immunol. 1986;136:402–11. [PubMed] [Google Scholar]
- 7.Shortman K, Wilson A, Scollay R. Loss of specificity in cytolytic T lymphocyte clones obtained by limit dilution culture of Ly-2+ T cells. J Immunol. 1984;132:584–93. [PubMed] [Google Scholar]
- 8.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–27. [PubMed] [Google Scholar]
- 9.Lee K, Overwijk WW, O'Toole M, Swiniarski H, Restifo NP, Dorner AJ, Wolf SF, Sturmhoefel K. Dose-dependent and schedule-dependent effects of interleukin-12 on antigen-specific CD8 responses. J Interferon Cytokine Res. 2000;20:589–96. doi: 10.1089/10799900050044787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McFarland EJ, Harding PA, MaWhinney S, Schooley RT, Kuritzkes DR. In vitro effects of IL-12 on HIV-1-specific CTL lines from HIV-1- infected children. 1998:513–9. 161. [PubMed] [Google Scholar]
- 11.Tuttle TM, Anderson BW, Thompson WE, et al. Proliferative and cytokine responses to class II HER-2/neu-associated peptides in breast cancer patients. Clin Cancer Res. 1998;4:2015–24. [PubMed] [Google Scholar]
- 12.Disis ML, Gooley TA, Rinn K, Davis D, Piepkorn M, Cheever MA, Knutson KL, Schiffman K. Generation of T-cell immunity to the her-2/neu protein after active immunization with HER-2/neu Peptide-based vaccines. J Clin Oncol. 2002;20:2624–32. doi: 10.1200/JCO.2002.06.171. [DOI] [PubMed] [Google Scholar]
- 13.Disis ML, Smith JW, Murphy AE, Chen W, Cheever MA. In vitro generation of human cytotoxic T cells specific for peptides derived from the HER-2/neu proto-oncogene protein. Ca Res. 1994;54:1071–6. [PubMed] [Google Scholar]
- 14.Knutson KL, Disis ML. Clonal diversity of the T-cell population responding to a dominant HLA- A2 epitope of HER-2/neu after active immunization in an ovarian cancer patient. Hum Immunol. 2002;63:547–57. doi: 10.1016/s0198-8859(02)00401-9. [DOI] [PubMed] [Google Scholar]
- 15.Disis ML, Grabstein KH, Sleath PR, Ma C. Generation of immunity to the HER-2/neu oncogenic protein in patients with breast and ovarian cancer using a peptide-based vaccine. Clin Cancer Res. 1999;5:1289–97. [PubMed] [Google Scholar]
- 16.Cheever MA, Chen W. Therapy with cultured T cells: principles revisited. Immunol Rev. 1997;157:177–94. doi: 10.1111/j.1600-065x.1997.tb00982.x. [DOI] [PubMed] [Google Scholar]
- 17.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudley ME, Wunderlich JR, Yang JC, et al. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother. 2002;25:243–51. doi: 10.1097/01.CJI.0000016820.36510.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, Riddell SR. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–44. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–4. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 21.Hu HM, Winter H, Urba WJ, Fox BA. Divergent roles for CD4+ T cells in the priming and effector/memory phases of adoptive immunotherapy. Jimmunol. 2000;165:4246–53. doi: 10.4049/jimmunol.165.8.4246. [DOI] [PubMed] [Google Scholar]
- 22.Reilly RT, Gottlieb MB, Ercolini AM, et al. HER-2/neu is a tumor rejection target in tolerized HER-2/neu transgenic mice. Cancer Res. 2000;60:3569–76. [PubMed] [Google Scholar]
- 23.Surman DR, Dudley ME, Overwijk WW, Restifo NP. Cutting edge. CD4+ T cell control of CD8+ T cell reactivity to a model tumor antigen. J Immunol. 2000;164:562–5. doi: 10.4049/jimmunol.164.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winter H, Hu HM, Poehlein CH, et al. Tumour-induced polarization of tumour vaccine-draining lymph node T cells to a type 1 cytokine profile predicts inherent strong immunogenicity of the tumour and correlates with therapeutic efficacy in adoptive transfer studies. Immunology. 2003;108:409–19. doi: 10.1046/j.1365-2567.2003.01596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pilon SA, Kelly C, Wei WZ. Broadening of epitope recognition during immune rejection of ErbB-2-positive tumor prevents growth of ErbB-2-negative tumor. J Immunol. 2003;170:1202–8. doi: 10.4049/jimmunol.170.3.1202. [DOI] [PubMed] [Google Scholar]
- 26.Knutson KL, Schiffman K, Disis ML. Immunization with a HER-2/neu helper peptide vaccine generates HER-2/neu CD8 T-cell immunity in cancer patients. J Clin Invest. 2001;107:477–84. doi: 10.1172/JCI11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson CC, Olson WC, Tuting T, Rinaldo CR, Lotze MT, Storkus WJ. HIV-1-specific CTL responses primed in vitro by blood-derived dendritic cells and Th1-biasing cytokines. 1999:3070–8. 162. [PubMed] [Google Scholar]
- 28.Portielje JE, Lamers CH, Kruit WH, Sparreboom A, Bolhuis RL, Stoter G, Huber C, Gratama JW. Repeated administrations of interleukin (IL)-12 are associated with persistently elevated plasma levels of IL-10 and declining IFN-gamma, tumor necrosis factor-alpha, IL-6, and IL-8 responses. Clin Cancer Res. 2003;9:76–83. [PubMed] [Google Scholar]
- 29.Robertson MJ, Cameron C, Atkins MB, Gordon MS, Lotze MT, Sherman ML, Ritz J. Immunological effects of interleukin 12 administered by bolus intravenous injection to patients with cancer. Clin Cancer Res. 1999;5:9–16. [PubMed] [Google Scholar]
- 30.Park JW, Gruys ME, McCormick K, et al. Primary hepatocytes from mice treated with IL-2/IL-12 produce T cell chemoattractant activity that is dependent on monokine induced by IFN-gamma (Mig) and chemokine responsive to gamma-2 (Crg-2) J Immunol. 2001;166:3763–70. doi: 10.4049/jimmunol.166.6.3763. [DOI] [PubMed] [Google Scholar]