Abstract
To investigate the immunological state in amyloidosis, mice were twice intraperitoneally injected (2-week interval) with casein emulsified in complete Freund's adjuvant. Two weeks after the treatment, amyloid deposits were detected in the spleen and other organs of these mice. The number of lymphocytes yielded by the liver and spleen increased significantly. The most affected lymphocyte subset was found to be B cells, namely, the total number of B cells increased and unusual B220low B cells were newly generated in the liver and spleen. In other words, not only normal B220high B cells but also unusual B220low B cells were detected in these organs of mice with amyloidosis. In parallel with this phenomenon, autoantibodies against denatured DNA were detected in sera. Since such autoantibodies are known to accompany the functional activation of NKT cells, NKT cell-deficient mice were used for the induction of amyloidosis. Such mice showed less formation of amyloidosis and lower levels of autoantibodies in sera. Athymic nude mice were NKT cell-deficient but NK1·1−TCRint cells were present. These athymic mice showed an intermediate induction of amyloidosis. The cytokine profile seen in mice with amyloidosis was the Th0 type, showing simultaneous production of IL-4 and IFNγ. These results suggest that the generation of B220low B cells and the production of autoantibodies in aid of primordial T cells may be major immunological mechanisms in amyloidosis mice.
Keywords: amyloidosis, B cells, B220low B cells, autoantibodies, NKT cells
INTRODUCTION
Amyloidosis is often accompanied by certain autoimmune diseases (e.g. rheumatoid arthritis) in humans [1–5]. It is speculated that some autoimmune states induce or accelerate the deposition of amyloid proteins into tissues. However, there is a possibility that amyloid deposition itself induces or accelerates autoimmune diseases by modulating the immunological state in the body. Indeed, experimental amyloidosis (e.g. casein-induced amyloidosis) in mice has been reported to induce an autoimmune state [6]. For example, mice with experimental amyloidosis showed the activation of B cells and the production of certain autoantibodies. A similar phenomenon has been reported in a human case presenting Sjögren's syndrome [7].
In light of these findings, we further investigated what kinds of B cells were activated and then produced autoantibodies and what kinds of T cells supported such functions of B cells. These questions are raised from the fact that autoantibody-producing B cells (i.e. B-1 cells) require the help of primordial T cells rather than that of conventional T cells [8–10]. Conventional T cells are generated through the mainstream of T-cell differentiation in the thymus. On the other hand, primordial T cells, including natural killer T cells (i.e. NKT cells) and NK1·1− intermediate TCR cells (i.e. NK1·1−TCRint cells), are generated by an alternative intrathymic pathway [11–13] and by the extrathymic pathway in the liver [14,15], respectively.
We therefore used several types of NKT cell-deficient mice as well as normal mice to characterize immunological states in casein-induced amyloidosis. It was found that the activation of unique B cells (i.e. B220low B cells) was always accompanied by the onset of amyloidosis and that this phenomenon was induced in aid of primordial T cells.
MATERIALS AND METHODS
Mice
C57BL/6 (B6), CD1d(–/–) [16,17] and Jα281(–/–) mice [18], originally obtained from Jackson Laboratory, Inc. (Tokyo, Japan) were maintained in the animal facility of Niigata University under specific pathogen-free conditions. CD1d(–/–) and Jα281(–/–) mice were of B6 background. They were used at 7–12 weeks of age.
Induction of amyloidosis
Amyloidosis can be induced in mice with two intraperitoneal injections, with a 2-week interval, of a casein emulsified in complete Freund's adjuvant (CFA) (with a 2-week interval) [6]. In this procedure, amyloidosis was noted in mice two weeks after the second injection.
CFA was the product of Difco Laboratories (Detroit, MI, USA). Hammerstein casein was obtained from Nutritional Biochemical Corp. (Cleveland, OH, USA). Emulsions of the adjuvant with or without casein were prepared as follows: equal volumes of CFA and phosphate buffered saline (PBS) were added to a weighed amount of casein (8 mg/ml) and emulsified at 4°C under sterile conditions. Under aseptic conditions, 0·3 ml of the emulsion was injected intraperitoneally twice.
Cell preparations
Hepatic mononuclear cells (MNC) were isolated by an improved method previously described [19]. Briefly, mice anaesthetized with ether were sacrificed by exsanguination by means of cardiac puncture. To obtain MNC, the liver was removed, pressed through 200-gauge stainless steel mesh, and suspended in PBS (0·1 m, pH 7·2). After being washed once with PBS, MNC were isolated from hepatocytes and hepatocyte nuclei were obtained by the Percoll (35% Percoll containing 100 U/ml heparin) gradient centrifugation method. The MNC collected from the interface were then suspended in MEM medium supplemented with 2% fetal calf serum. The preparations of hepatic MNC contained less than 4% Kupffer cells. Splenocytes were obtained by pressing the spleen through 200-gauge steel mesh. Bone marrow cells were obtained by flushing bilateral femurs with the medium. Spleen cell suspensions and bone marrow cell suspensions were depleted of erythrocytes with 0·83% ammonium chloride-Tris buffer (0·17 m, pH 7·6).
Immunofluorescence tests
The surface phenotype of cells was identified using mAbs in conjunction with two-colour immunofluorescence tests [20]. FITC- or PE-conjugated anti-CD3 (145–2C11), anti-NK1·1 (PK136), B220 (RA3–6B2), anti-IgM, and anti-IgG mAbs were obtained from PharMingen Co. (San Diego, CA, USA). Biotin-conjugated reagents were developed with PE-conjugated streptavidin (Becton-Dickinson, Mountain View, CA). To prevent nonspecific binding of mAbs, CD32/16 (24G2) was added before staining with labelled mAbs. The fluorescence-positive cells were analysed with FACScan using Lysis II software (Becton-Dickinson).
Identification of autoantibodies
To detect anti-DNA antibodies, the ELISA method was applied [21]. Materials were coated with denatured DNA derived from salmon. The activity of such anti-DNA antibodies was divided into IgM and IgG fractions in sera by using isotype-specific anti-µ chain mAb and anti-γ chain mAb as secondary antibodies (PharMingen). The titre was determined by using a positive control of sera (100 U/ml) from MRL-lpr/lpr mice after the onset of autoimmune disease (at the age of 25 weeks). We also examined the titre of anti-hepatocyte antibodies by the ELISA method. Instead of denatured salmon DNA, materials were coated with denatured B6 hepatic tissue. The excess tissue was washed out by PBS.
Autoantibodies were also detected by using a Hep2 cell line in conjunction with an immunofluorescence test [21]. Sera obtained from the various mice were used after a dilution 1/20. FITC-conjugated anti-mouse Ig (PharMingen) was used as a secondary antibody.
ELISA assay for the detection of IL-4, IL-10 and IFNγ
Pooled sera were used to detect the concentrations of IL-4, IL-10 and IFNγ by ELISA assay using Opt EIA mouse IL-4, IL-10 and IFNγ sets (PharMingen).
Statistical analysis
Statistical differences were analysed by Student's t-test.
RESULTS
Induction of experimental amyloidosis
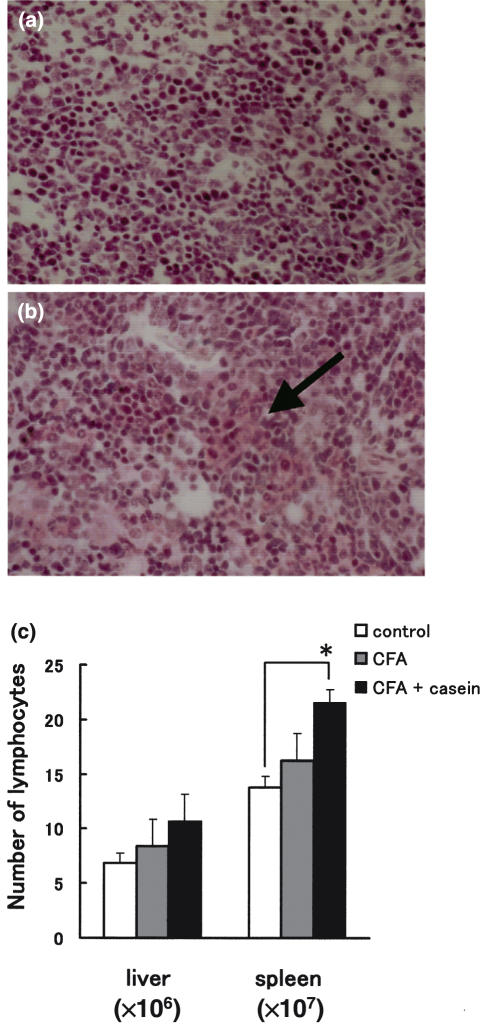
Mice were sacrificed at 4 weeks after an initial treatment and histology of the spleen was examined by Congo-red staining (Fig. 1a,b). In comparison with normal tissue (Fig. 1a), the spleen isolated from treated mice showed swelling of tissues (indicated by an arrow) which were positive to Congo-red (i.e. the extracelluar matrix became red) (Fig. 1b).
Fig. 1.
Induction of experimental amyloidosis (a) Histology of the spleen of normal mice, (b) Histology of the spleen of mice injected with CFA and casein, (c) Number of lymphocytes yielded by the liver and spleen. Mice were injected twice with either CFA alone or CFA + casein (2-week interval). Two weeks after the final injection, the liver tissues were isolated. The mean and one SD in the number of cells were produced from 4 mice in each experiment.
In addition to the development of amyloidosis, the number of lymphocytes yielded by the liver and spleen increased (Fig. 1c). This was significant in the spleen (P < 0·05) when mice were treated with both CFA and casein.
Characterization of lymphocyte subsets which expanded in the liver and spleen
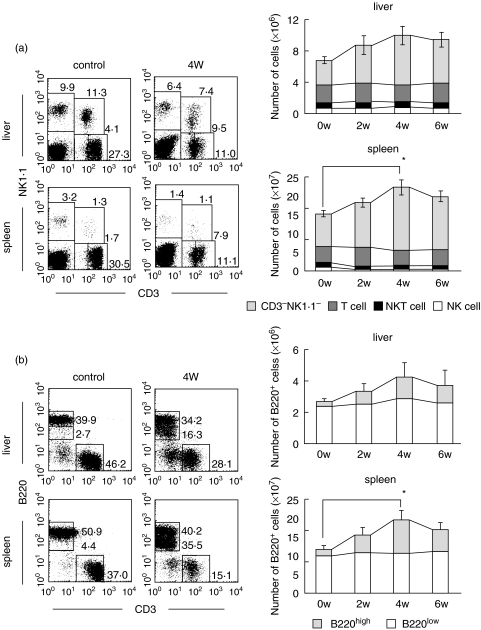
Two-colour staining for CD3 and NK1·1 was conducted in the liver and spleen of control and amyloidosis mice (Fig. 2a). This staining simultaneously identified NK cells (CD3−NK1·1+), NKT cells (CD3intNK1·1+), and conventional T cells (CD3highNK1·1−) [15]. All these lymphocyte subsets were found to remain unchanged or to rather decrease in both the liver and spleen of amyloidosis mice. In other words, the proportion of CD3−NK1·1− cells (mainly B cells) seemed to increase in the liver and spleen. The absolute numbers of each lymphocyte subset were calculated by repeated experiments (n = 4). It was demonstrated that the number of CD3−NK1·1− cells increased in both the liver and spleen of amyloidosis mice (Fig. 2a, right column).
Fig. 2.
Phenotypic characterization of lymphocytes in the liver and spleen of control and amyloidosis mice. (a) Two-colour staining for CD3 and NK1·1, and time-kinetics in the variation of lymphocyte subsets. (b) Two-colour staining for CD3 and B220, and time-kinetics in the variation of lymphocyte subsets. Representative results of three experiments are depicted in the case of immunofluorescence tests. The mean and one SD in the number of lymphocyte subsets were produced from 3 mice.
To identify what kind of lymphocytes expanded, two-colour staining for CD3 and B220 was then conducted (Fig. 2b). A unique population of possible B cells was identified as CD3−B220low cells in both the liver and spleen. Conventional B cells with CD3−B220high phenotype were also identified in control and amyloidosis mice. This result was confirmed by repeated experiments (n = 4) in which the absolute number of B220high and B220low cells was calculated.
Association of NKT cells and autoantibody production with the onset of amyloidosis
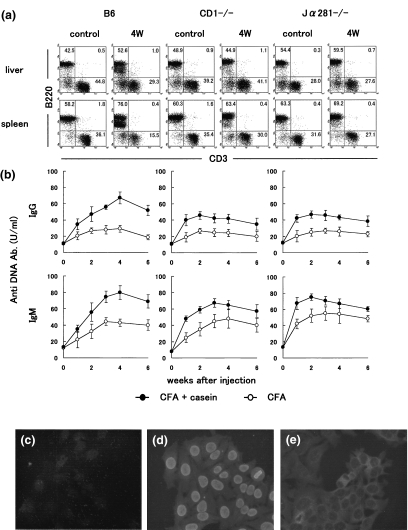
In parallel with a study of B6 mice, we conducted amyloidosis experiments in NKT-cell deficient mice, including CD1d(–/–) and Jα281(–/–) mice (Fig. 3a). It was found that the induction of amyloidosis was less prominent in these NKT-cell deficient mice (data not shown). Interestingly, B220low B cells did not appear in these mice.
Fig. 3.
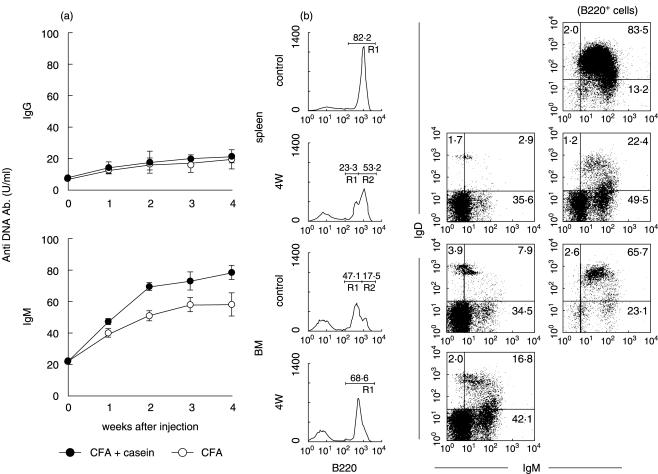
Experimental amyloidosis in NKT cell-deficient mice. (a) Two-colour staining for CD3 and B220. (b) Serum levels of the titre against anti-DNA antibody. • CFA + casein; ○ CFA (c) Immunofluorescence test of normal sera against Hep2 cells. (d) Sera from lpr mice. (e) Sera from amyloidosis mice. Numbers in the figure represent the percentages of fluorescence-positive cells in corresponding areas. The mean and one SD in serum levels of the titre against anti-DNA antibody were produced from 4 mice. Immunofluorescence test of sera was conducted using fixed Hep2 cells.
Since it is known that NKT cells sometimes activate B-1 cells which produce autoantibodies [8–10], one such autoantibody (i.e. anti-DNA antibody) was estimated in sera of control (CFA alone) and amyloidosis mice (CFA + casein) by the ELISA method (Fig. 3b). High titres of anti-DNA autoantibody (both IgG and IgM types) in sera were detected in B6 mice with amyloidosis. Although such titres in sera also increased in NKT-cell deficient mice, the magnitude was lower in these mice than in B6 mice.
Sera were obtained from control (Fig. 3c), MRL-lpr/lpr (Fig. 3d) and amyloidosis (Fig. 3e) mice. After a 1 : 20 dilution of sera, the reactivity against Hep2 cells was compared. Interestingly, sera of lpr mice reacted with the nucleus but that of amyloidosis mice reacted mainly with the cytoplasm.
Characterization of B220low B cells appeared in mice with amyloidosis
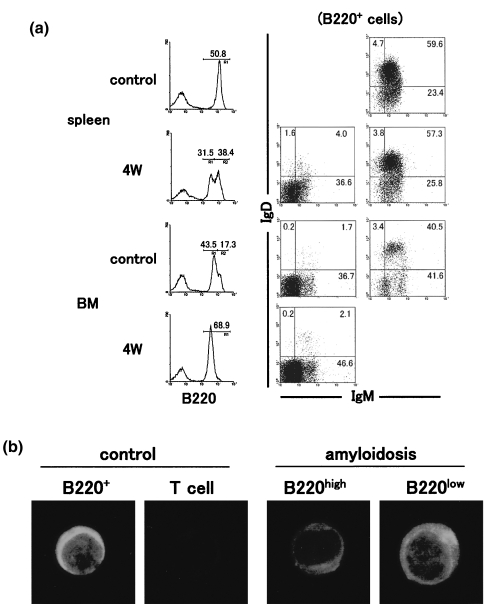
To further characterize B cells which newly appeared in mice with amyloidosis, three-colour staining for B220, IgM and IgD was conducted (Fig. 4a). The staining pattern of B220 was very interesting. The appearance of B220low B cells was confirmed in the spleen, whereas two peaks of B220 staining seen in the bone marrow of control mice became a single peak, namely, only the B220low peak. Gated analysis showed that B220low B cells were mainly IgM−D−. On the other hand, B220high B cells in both control and amyloidosis mice were a mixture of IgM+D+ and IgM+D−. B220low B cells in the bone marrow were also mainly IgM−D−.
Fig. 4.
Characterization of B220low B cells in the spleen and bone marrow of amyloidosis mice. (a) Three-colour staining for B220, IgM and IgD. (b) Immunofluorescence test of lymphocyte subsets for IgM. After three-colour staining for B220, IgM and IgD, gated analysis was conducted. Numbers in the figure indicate the fluorescence-positive cells in corresponding areas. Lymphocyte subsets were purified from the spleen in amyloidosis mice by a cell sorter and the fixed subsets were stained with FITC-conjugated anti-µ mAb.
An immunofluorescence test of purified B (and T) cells was then conducted in control and amyloidosis mice (Fig. 4b) and showed that, all B220+ B cells in control mice and B220high B cells were small and had cytoplasmic IgM+. In contrast, B220low B cells in amyloidosis mice were large and had cytoplasmic IgM+.
Appearance of B220low B cells even in athymic nude mice after the induction of amyloidosis
Usually, antibody production requires the help of conventional T cells while autoantibody production needs the help of extrathymic T cells. Athymic nude mice carry extrathymic T cells but lack conventional T cells [22]. Using these athymic mice, the induction of amyloidosis was found to be successful (data not shown). Interestingly, the IgM type of anti-DNA antibody was detected in sera of athymic mice injected with CFA + casein (Fig. 5a). The appearance of B220low cells was also detected in the spleen and bone marrow of these mice (Fig. 5b). Similar to the case of euthymic mice, B220low B cells in both the spleen and bone marrow were mainly IgM−D−, although they contained low levels of IgM+D+ and IgM+D−.
Fig. 5.
Induction of amyloidosis in athymic nude mice. (a) Serum levels of the titre against anti-DNA antibody. (b) Three-colour staining for B220, IgM and IgD in the spleen and bone marrow. The mean and one SD in serum levels of the titre against anti-DNA antibody were produced from 4 mice. After three-colour staining for B220, IgM and IgD, gated analysis was conducted. Numbers in the figure indicate the fluorescence-positive cells in corresponding areas.
Cytokine profiles in mice with amyloidosis
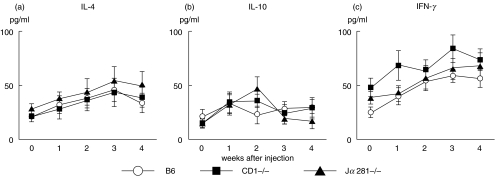
Serum levels of IL-4, IL-10 and IFNγ were measured by ELISA assay in mice with amyloidosis (Fig. 6). In these experiments, B6, CD1d(–/–) and Jα281 mice were injected with CFA + casein and sera were obtained at the indicated points of time. The level of both IL-4 and IFNγ increased, showing a peak at 3 weeks after the injection in all tested mice. However, the level of IL-10 remained almost unchanged. These results showed that the administration of CFA + casein induced a Th0 type of cytokine profile but that this response was not restricted to B6 mice which showed the onset of amyloidosis.
Fig. 6.
Serum levels of (a) IL-4, (b) IL-10 and (c) IFNγ in various mice after the injection of CFA and casein. Serum levels of cytokines were determined by ELISA assay. The mean and one SD were produced by 4 experiments at the indicated points of time.
DISCUSSION
In the present study, we demonstrated that B220low B cells were newly generated in the liver and spleen of mice with experimental amyloidosis. Since the majority of B220low B cells lacked the expression of IgM and IgD on the cell surface, these B cells might be an immature type of B cell lineage. In parallel with this phenomenon, the titre of anti-DNA antibody became positive in sera. We have previously reported that the production of autoantibodies is often accompanied by the activation of primordial T cells such as NKT cells [8–10]. The present results also suggest that NKT cells are associated with the production of autoantibodies in mice with experimental amyloidosis. Thus, NKT cell-deficient mice showed neither the onset of amyloidosis nor the appearance of B220low B cells. Taken together, these findings indicate that the onset of amyloidosis itself was able to induce an autoimmune state, accompanied by the activation of B220low B cells in the presence of NKT cells.
B220low B cells were primarily absent and only B220high cells were present in the liver and spleen of normal mice, whereas B220low B cells as well as B220high B cells were present in the bone marrow (Fig. 4a). The majority of B220low B cells were IgM−D− on the surface, but the majority of B220high B cells were IgM+D+. Therefore, the immature type of B cells were estimated to be generated in the liver and spleen in amyloidosis mice. Since B220low B cells became abundant even in the bone marrow of amyloidosis mice, the activation of B cell-differentiation is considered to have occurred at various sites of these mice. At present, it is still unknown whether the B220low B cells which appeared in the periphery of amyloidosis mice were derived from the bone marrow or were generated in situ in the periphery.
Both IgM and IgD types of anti-denatured DNA antibody were detected in sera of amyloidosis mice. These sera also had reactivity against the cytoplasm in perinuclear areas as shown by immunofluorescence tests using fixed cells. It is widely known that amyloidosis accompanies certain autoimmune diseases [1–5]. The present results also suggest that the deposition of amyloid proteins itself has the ability to induce autoimmune states. It is conceivable that both conditions of autoimmune diseases and amyloidosis may interact with each other.
It is well known that some autoantibody-producing B cells express CD5 antigen on the cell surface and are termed as B-1a cells, especially in autoimmune prone NZB/W F1 mice [23–25]. However, B220low cells appeared in all tested organs (including the peritoneal cavity) of B6 mice with experimental amyloidosis lacked the expression of CD5 (data not shown). There was no phenotypic difference among B cells in the spleen, liver and peritoneal cavity.
For a long time, it has been considered that autoreactive forbidden clones are generated by failure of their negative selection through the mainstream of T-cell differentiation (TCRhigh cells) in the thymus. However, in the autoimmune state the autoreactive clones are always present among primordial T cells (i.e. TCRint cells) which are generated extrathymically [26–28]. At this time, thymic atrophy is somewhat present, showing the arrest in the mainstream of T-cell differentiation [29,30]. In recent studies, the activation of primordial T cells (e.g. NKT cells) and the production of autoantibodies simultaneously were revealed to appear under various autoimmune conditions [8–10]. In this regard, we examined whether NKT cells are required to produce autoantibodies in amyloidosis mice. The onset of amyloidosis and the appearance of B220low B cells was less prominent in NKT cell-deficient mice. NKT cells might be important for the differentiation of autoantibody-producing B cells (i.e. B-1 cells).
In the case of NKT cells, mature cells or their precursors are generated by an alternative intrathymic pathway and they home to the liver thereafter [11–13]. Due to this situation, athymic nude mice lack NKT cells. However, these athymic mice carry another type of primordial T cells (i.e. NK1·1−TCRint cells) and these cells expand as a function of age, possibly as a compensatory effect [22]. In this study, athymic nude mice were found to produce autoantibodies, which resulted in amyloidosis. It is speculated that the activation of B220low B cells is also helped by NK1·1−TCRint cells as well as by NKT cells. In any case, the activation of B220low B cells and the production of autoantibodies may occur in aid of primordial T cells.
We, as well as others, have reported that the activation of primordial T cells results in production of their cytokines, showing Th0 [31,32] or Th2 type [33,34]. Since both IL-4 and IFNγ were detected in sera of amyloidosis mice, this pattern belonged to the Th0 type. However, this pattern was not restricted to the onset of amyloidosis. Irrespective of the onset of amyloidosis, repeated injections of CFA and casein induced the Th0 type-cytokine profile even in CD1d(–/–) and Jα281(–/–) mice.
Finally, it is concluded that the onset of amyloidosis itself induced the autoimmune state, showing the generation of B220low B cells in the periphery. Primordial T cells (i.e. NKT cells or NK1·1−TCRint cells) were found to be intimately associated with this phenomenon.
Acknowledgments
The authors wish to thank Yuko Kaneko for preparation of the manuscript. This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture, Japan.
REFERENCES
- 1.Piirainen HI, Helve AT, Tornroth T, Pettersson TE. Amyloidosis in mixed connective tissue disease. Scand J Rheumatol. 1989;18:165–8. doi: 10.3109/03009748909095415. [DOI] [PubMed] [Google Scholar]
- 2.Webb S, Segura F, Cervantes F, Darnell A, Soriano E, Ribas-Mundo M, Garcia-San Miguel J. Systemic lupus erythematosus and amyloidosis. Arthritis Rheum. 1979;22:554–6. doi: 10.1002/art.1780220518. [DOI] [PubMed] [Google Scholar]
- 3.Lender M, Wolf E. Incidence of amyloidosis in rheumatoid arthritis. Scand J Rheumatol. 1972;1:109–12. doi: 10.3109/03009747209103007. [DOI] [PubMed] [Google Scholar]
- 4.Laiho K, Hannula S, Savolainen A, Kautiainen H, Kauppi M. Cervical spine in patients with juvenile chronic arthritis and amyloidosis. Clin Exp Rheumatol. 2001;19:345–8. [PubMed] [Google Scholar]
- 5.Kaipiainen-Seppanen O, Myllykangas-Luosujarvi R, Lampainen E, Ikaheimo R. Intensive treatment of rheumatoid arthritis reduces need for dialysis due to secondary amyloidosis. Scand J Rheumatol. 2000;29:232–5. doi: 10.1080/030097400750041370. [DOI] [PubMed] [Google Scholar]
- 6.Hardt F, Wanstrup J, Elling P, Ranlov P. Occurrence of autoimmune phenomena during the development of casein-induced amyloidosis in C3H and NZB/BL mice. Acta Path Microbiol Scand. 1972;233:178–82. [PubMed] [Google Scholar]
- 7.Gogel HK, Searles RP, Volpicelli NA, Cornwell GG., III Primary amyloidosis presenting as Sjögren's syndrome. Arch Intern Med. 1983;143:2325–6. [PubMed] [Google Scholar]
- 8.Naito T, Kawamura H, Kawamura T, et al. Simultaneous activation of natural killer T cells and autoantibody production in mice injected with denatured syngeneic liver tissue. Clin Exp Immunol. 2002;129:397–404. doi: 10.1046/j.1365-2249.2002.01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morshed SRM, Mannoor MK, Halder RC, Kawamura H, Bannai M, Sekikawa H, Watanabe H, Abo T. Tissue-specific expansion of NKT and CD5+B cells at the onset of autoimmune diseases in (NZB×NZW) F1 mice. Eur J Immunol. 2002;32:2551–61. doi: 10.1002/1521-4141(200209)32:9<2551::AID-IMMU2551>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 10.Mannoor MK, Halder RC, Morshed SRM, et al. Essential role of extrathymic T cells in protection against malaria. J Immunol. 2002;169:301–6. doi: 10.4049/jimmunol.169.1.301. [DOI] [PubMed] [Google Scholar]
- 11.Hammond K, Cain W, van Driel I, Godfrey D. Three day neonatal thymectomy selectively depletes NK1·1+T cells. Int Immunol. 1998;10:1491–9. doi: 10.1093/intimm/10.10.1491. [DOI] [PubMed] [Google Scholar]
- 12.Tilloy F, Di Santo JP, Bendelac A, Lantz O. Thymic dependence of invariant Vα14+ natural killer-T cell development. Eur J Immunol. 1999;29:3313–8. doi: 10.1002/(SICI)1521-4141(199910)29:10<3313::AID-IMMU3313>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Coles MC, Raulet DH. NK1·1+T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4+CD8+ cells. J Immunol. 2000;164:2412–8. doi: 10.4049/jimmunol.164.5.2412. [DOI] [PubMed] [Google Scholar]
- 14.Sato K, Ohtsuka K, Hasegawa K, Yamagiwa S, Watanabe H, Asakura H, Abo T. Evidence for extrathymic generation of intermediate TCR cells in the liver revealed in thymectomized, irradiated mice subjected to bone marrow transplantation. J Exp Med. 1995;182:759–67. doi: 10.1084/jem.182.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe H, Miyaji C, Kawachi Y, Iiai T, Ohtsuka K, Iwanaga T, Takahashi-Iwanaga H, Abo T. Relationships between intermediate TCR cells and NK1·1+T cells in various immune organs. NK1·1+T cells are present within a population of intermediate TCR cells. J Immunol. 1995;155:2972–83. [PubMed] [Google Scholar]
- 16.Mendiratta SK, Martin WD, Hong S, Boesteanu A, Joyce S, Kaer LV. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6:469–77. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 17.Smiley ST, Kaplan MH, Grusby JJ. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science. 1997;275:977–9. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- 18.Cui J, Shin T, Kawano T, et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–6. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 19.Tsukahara A, Seki S, Iiai T, et al. Mouse liver T cells: Their change with aging and in comparison with peripheral T cells. Hepatology. 1997;26:301–9. doi: 10.1002/hep.510260208. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe H, Miyaji C, Seki S, Abo T. c-kit+ stem cells and thymocyte precursors in the livers of adult mice. J Exp Med. 1996;184:687–93. doi: 10.1084/jem.184.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyakawa R, Miyaji C, Watanabe H, Yokoyama H, Tsukada C, Asakura H, Abo T. Unconventional NK1·1− intermediate TCR cells as major T lymphocytes expanding in chronic graft-versus-host disease. Eur J Immunol. 2002;32:2521–31. doi: 10.1002/1521-4141(200209)32:9<2521::AID-IMMU2521>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 22.Halder RC, Kawamura T, Bannai M, Watanabe H, Kawamura H, Mannoor MDK, Morshed SRM, Abo T. Intensive generation of NK1·1− extrathymic T cells in the liver by injection of bone marrow cells isolated from mice with a mutation of polymorphic MHC antigens. Immunology. 2001;102:450–9. doi: 10.1046/j.1365-2567.2001.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oteki T, Abo T, Kusumi A, Sasaki T, Shibata S, Seki S, Kumagai K. Age-associated increase of CD5+ B cells in the liver of autoimmune (NZB×NZW) F1 mice. Microbiol Immunol. 1993;37:221–8. doi: 10.1111/j.1348-0421.1993.tb03203.x. [DOI] [PubMed] [Google Scholar]
- 24.Hardy RR, Hayakawa K. Development and physiology of Ly-1B and its human homolog, Leu-1B. Immunol Rev. 1986;93:53–79. doi: 10.1111/j.1600-065x.1986.tb01502.x. [DOI] [PubMed] [Google Scholar]
- 25.Hayakawa K, Hardy RR, Parks DR, Herzenberg LA. The ‘Ly-1B’ cell subpopulation in normal, immunodefective, and autoimmune mice. J Exp Med. 1983;157:202–18. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawachi Y, Watanabe H, Moroda T, Haga M, Iiai T, Hatakeyama K, Abo T. Self-reactive T cell clones in a restricted population of IL-2 receptor β+ cells expressing intermediate levels of the T cell receptor in the liver and other immune organs. Eur J Immunol. 1995;25:2272–8. doi: 10.1002/eji.1830250824. [DOI] [PubMed] [Google Scholar]
- 27.Moroda T, Iiai T, Kawachi Y, Kawamura T, Hatakeyama K, Abo T. Restricted appearance of self-reactive clones into intermediate T cell receptor cells in neonatally thymectomized mice with autoimmune disease. Eur J Immunol. 1996;26:3084–91. doi: 10.1002/eji.1830261239. [DOI] [PubMed] [Google Scholar]
- 28.Moroda T, Kawachi Y, Iiai T, et al. Self-reactive forbidden clones are confined to pathways of intermediate T cell receptor cell differentiation even under immunosuppressive conditions. Immunology. 1997;91:88–94. doi: 10.1046/j.1365-2567.1997.00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maruyama S, Tsukahara A, Suzuki S, Tada T, Minagawa M, Watanabe H, Hatakeyama K, Abo T. Quick recovery in the generation of self-reactive CD4low NKT cells by an alternative intrathymic pathway when restored from acute thymic atrophy. Clin Exp Immunol. 1999;117:587–95. doi: 10.1046/j.1365-2249.1999.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimizu T, Kawamura T, Miyaji C, et al. Resistance of extrathymic T cells to stress and a role of endogenous glucocorticoids in stress-associated immunosuppression. Scand J Immunol. 2000;51:285–92. doi: 10.1046/j.1365-3083.2000.00695.x. [DOI] [PubMed] [Google Scholar]
- 31.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–51. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 32.Mannoor MK, Weerasinghe A, Halder RC, Morshed SRM, Ariyashinghe A, Watanabe H, Sekikawa H, Abo T. Resistance to malarial infection is achieved by the cooperation of NK1·1+ and NK1·1− subsets of intermediate TCR cells which are constituents of the innate immunity. Cell Immunol. 2001;211:96–104. doi: 10.1006/cimm.2001.1833. [DOI] [PubMed] [Google Scholar]
- 33.Emoto M, Emoto Y, Kaufmann SH. IL-4 producing CD4+ TCRabint liver lymphocytes. Influence of thymus, b2-microglobulin and NK1·1 expression. Int Immunol. 1995;7:1729–39. doi: 10.1093/intimm/7.11.1729. [DOI] [PubMed] [Google Scholar]
- 34.Yoshimoto T, Bendelac A, Watson C, Hu-Li J, Paul WE. Role of NK1·1+T cells in a Th2 response and in immunoglobulin E production. Science. 1995;270:1845–7. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]