Abstract
A rise in interleukin (IL) 4-dependent immunoglobulin E (IgE) is a hallmark of the mercuric chloride (HgCl2)-induced Th2-mediated autoimmune syndrome in the Brown Norway (BN) rat, and one of the mediators in allergic asthma in human. Oxidative stress, a potential factor related to the pathogenesis of allergy and asthma, has been shown to up-regulate IL-4 in mast cells and predispose to degranulation in vitro. However, it remains unknown whether oxidative/antioxidative imbalance plays a role in this Th2-driven model of autoimmunity in the rat. Here we show that administration of the non-sulphydryl-containing antioxidant desferrioxamine i.p. and s.c. to BN rats reduces HgCl2-enhanced IL-4 gene expression and inhibits HgCl2-induced Th2-mediated autoimmunity. Desferrioxamine treatment suppresses significantly IgE production and lymphoproliferation, and reduces tissue injury in the form of caecal vasculitis in the HgCl2-induced autoimmune syndrome. These results support a role for oxidative stress in the pathogenesis of the HgCl2-induced Th2-dominated autoimmune syndrome. This finding might have implications for understanding the mechanisms involved in Th2 cell responses as seen in allergy and asthma and thereby aid the development of new therapeutic strategies for these diseases.
Keywords: animal model, antioxidant, interleukin 4, IgE, vasculitis
INTRODUCTION
Immunoglobulin E (IgE) is a key antibody that mediates atopic or allergic conditions such as asthma, seasonal allergy and urticaria. IgE binds to receptors on the surface of effector cells, such as mast cells and basophils, and when cross-linked causes them to release bioactive mediators. In many patients, the total serum IgE level correlates roughly with disease severity. Local IgE synthesis in allergic rhinitis and asthma has been demonstrated recently [1–3].
IgE synthesis by B lymphocyte is dependent upon at least two types of signals. The first signal is delivered through cytokines produced primarily from T helper 2 (Th2) cells such as interleukin (IL)-4 and IL-13, of which IL-4 is the most important cytokine required for isotype switching to IgE and for the development of a Th2 cell response [4]. The second signal is provided by interaction of the CD40 antigen expressed on the B cell surface with its ligand (CD154) expressed on activated T cells [5]. Elevated concentrations of IgE in allergic individuals may result from the preferential activation of Th2 cells. For managing the allergic response, different immunotheraputic strategies have been developed to inhibit IgE production including anti-IgE monoclonal antibodies, IL-4 antibodies, IL-4 receptor antibodies, soluble IL-4 receptors and interferon (IFN)-γ[6].
We have been investigating an animal model of autoimmunity that is dominated by a Th2 cell type response. Administration of chemical compounds such as mercuric chloride (HgCl2), gold salts or d-penicillamine to the Brown Norway (BN) rat induces an autoimmune syndrome characterized by the production of autoantibodies [7], a marked increase in total serum IgE concentration, generalized lymphoproliferation [8] and tissue injury in the form of a necrotizing vasculitis and inflammatory polyarthritis [9]. The induction of a necrotizing vasculitis and antineutrophil cytoplasm antibody (ANCA) of the antimyeloperoxidase (MPO) type in the rats is analogous to human systemic vasculitis [7,10]; in particular, the elevated IgE concentration provides similarities to the Churg–Strauss syndrome [11]. The syndrome in the rat has been shown to be T cell-dependent [12]. The rise in serum IgE implicates Th2 cells, as isotype switching to IgE is dependent on IL-4, a major cytokine product of Th2 cells [13].
Although the Th2-dominated autoimmune syndrome peaks about 2 weeks after the administration of HgCl2 in the BN rat, significant vasculitis with elevated levels of IL-4 mRNA occurs in the caecum within 24–48 h of an injection of HgCl2, a time at which there is no significant T cell infiltrate [14]. Our work has demonstrated that mast cells play a role in this early phase of vasculitis [15].
We have also found that the compounds that cause Th2 cell-driven autoimmunity in vivo generate reactive oxygen species (ROS) and enhance IL-4 expression in mast cells in vitro, that oxidative stress by H2O2 mimics the effect of HgCl2 in enhancing IL-4 expression, and that antioxidants (sulphydryl and non-sulphydryl-containing) diminish the HgCl2-induced enhancement of IL-4 [16]. These data suggest a mechanism of IL-4 transcriptional regulation by oxidative stress. We therefore propose the hypothesis that up-regulation of IL-4 and sensitization for mediator release in mast cells, caused by production of intracellular ROS, contributes to HgCl2-induced autoimmunity. Our recent data have shown that administration of antioxidants to BN rats inhibits HgCl2-induced early vasculitis in vivo, indicating that oxidative stress plays a role in the pathogenesis of HgCl2-induced mast cell-mediated early vasculitis [14]. However, it is known that T cells are not involved in this early phase of vasculitis [15]. An important separate question, therefore, is whether the later Th2 cell-dependent aspects of the syndrome would also be susceptible to antioxidant regulation, either because they are induced by the earlier mast cell production of IL-4, and/or because Th2 cell activation can be modulated directly by antioxidant treatment.
The aim of this study was to extend and test further our hypothesis by determining whether the administration of the non-sulphydryl-containing antioxidant desferrioxamine to BN rats could inhibit the later HgCl2-induced Th2-dominated autoimmune response, and to examine whether modulation of oxidative/antioxidative balance influences IL-4 expression in vivo.
MATERIALS AND METHODS
Animals
Male BN rats (250–400 g) were bred and maintained in the Biological Research Facilities of St George's Hospital Medical School (London, UK) or obtained from Harlan-Olac (Bicester, Oxon, UK), and used in aged-matched groups. All procedures were conducted in accordance with the Home Office Animal (Scientific Procedures) Act of 1986.
Experimental protocols
BN rats received HgCl2 (Sigma, Poole, UK) s.c. at a dose of 1 mg/kg on 1–3 alternate days starting on day 0. Desferrioxamine (Ciba, Horsham, UK) was dissolved in distilled water and was injected i.p. or i.p. and s.c. at a dose of 400 mg/kg each injection (when given i.p. and s.c., 800 mg/kg in total) for a total of five doses given on consecutive days from day 0. Blood samples were taken from tail vessels at various time-points, and serum was stored at −20°C for measurement of IgE concentration. Animals were killed and scored for macroscopic evidence of vasculitis [17] at day 14 after the start of HgCl2 by an experienced observer (D. B. G. O.) blinded to treatment group. Biopsies were taken from caecal tissues.
In another set of experiments, the rats were killed on day 4 after two doses of HgCl2, with or without desferrioxamine, and spleen cells were prepared and stored in guanidinium thiocyanate solution at −80°C for extraction of RNA.
IgE enzyme-linked immunosorbent assay (ELISA)
Total IgE was measured by ELISA [9]. Briefly, 96-well plates (Dynex Technologies, Billingshurst, UK) were coated with monoclonal antirat IgE heavy chain (Serotec, Oxford, UK), diluted to 5 µg/ml in carbonate buffer. After blocking unoccupied binding sites with 5% skimmed milk in phosphate buffered saline (PBS), known concentrations of rat IgE κ myeloma protein (Serotec) or serum samples were added in duplicate to coated wells and singly to anti-IgE free wells. Binding was detected with alkaline phosphatase-conjugated antirat κ and λ light chain antibodies followed by p-nitrophenyl phosphate substrate (Sigma). The optical density (OD) at 405 nm was read using a Dynatech multiplate reader (Dynex Technologies). A standard curve from the OD of the known concentrations of IgE on each plate was used to calculate the IgE concentration from mean OD of the unknown test samples.
Reverse transcription-polymerase chain reaction (RT-PCR)
RNA was prepared by a method adapted from Chomczynski and Sacci [18]. cDNA was synthesized from about 5 µg of total RNA using a cDNA cycle kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's recommendations. For PCR, cDNA was made up with 25 pmol of each PCR primer, 200 µm concentration of each deoxynucleoside triphosphate (Amersham Pharmacia Biotech, Little Chalfont, UK), 1× reaction buffer and 5 U Taq polymerase (Appligene-Oncor, Ilkirch, France) for β-actin amplification or 7·5 U ampliTaq Gold (PE Applied Biosystems, Foster City, CA, USA) with 1 mm MgCl2 for IL-4 amplification. The forward and reverse primers are as follows: β-actin 5′-ATGCCATC CTGCGTCTGGACCTGGC-3′ and 5′-AGCATTTGCGGTGC ACGATGGAGGG-3′; IL-4 5′-TGATGGGTCTCAGCCCCCA CCTTGC-3′ and 5′-CTTTCAGTGTTGTGAGCGTGGACTC-3′; IFN-γ 5′-ATGAGTGCTACACGCCGCGTCTTGG-3′ and 5′-GAGTTCATTGACAGCTTTGTGCTGG-3′. The amount of starting material (cDNA) and the number of PCR cycles were selected by pilot experiments and samples of the PCR reactions were taken at multiple points throughout the amplification process for appropriate quantification. PCR was performed with 20–40 cycles of 94°C (1 min), 60°C (2 min) and 72°C (3 min). Pre-PCR incubation at 95°C for 10 min was performed for amplification of IL-4 cDNA.
The PCR products were separated in 1·5% agarose gels containing ethidium bromide and photographed. Densitometric analysis of the bands was carried out using the Gel Imager Program (Alpha Innotech Co., San Leandro, CA, USA). Results are expressed as the ratio of the intensity of the band for IL-4 or IFN-γ to the intensity of the band for β-actin. The specificity of the PCR products was checked further by DNA sequencing using the ABI Prism Dye Terminator Cycle Sequencing Ready Reaction Kit and ABI 373 A DNA sequencer (Applied Biosystems).
Histology
Directed biopsies from rat caecal mucosa were fixed in buffered formalin, and paraffin-embedded sections were stained with haematoxylin and eosin. Scores were assigned by an observer unaware of treatment group (S. D. J. H.) according to the presence and severity of vasculitis (0, normal; 1, grade I; 2, grade II; 3, grade III; 4 grade IV) [19].
Statistics
For comparisons between groups over time, repeated-measures analysis of variance (anova) was used on log-transformed data for IgE concentrations. Pearson's r was calculated to evaluate the relationship between IgE production and spleen weight. All other comparisons were made by the non-parametric Mann–Whitney U-test.
RESULTS
Desferrioxamine suppresses IgE production in HgCl2-induced autoimmunity
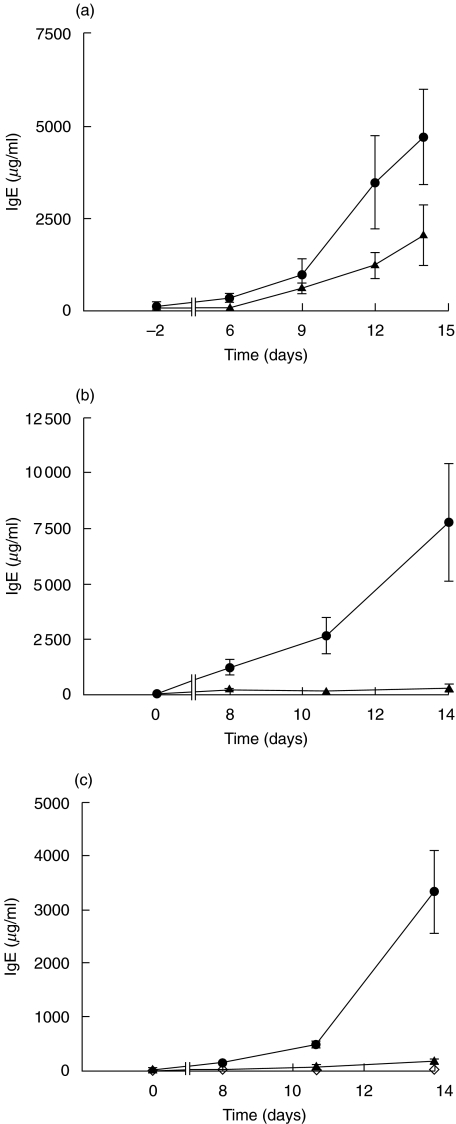
To determine whether the non-sulphydryl-containing antioxidant desferrioxamine inhibits the elevation of IgE caused by HgCl2 treatment, groups of rats were given different doses of HgCl2 with or without desferrioxamine, or desferrioxamine only. The serum total IgE was measured. Administration of a single dose of HgCl2 to BN rats resulted in a marked elevation of serum IgE concentration (Fig. 1a). The level of IgE started to rise after day 6, and then rose significantly after day 9 (P = 0·0003). Injection of desferrioxamine (i.p.) for 5 days decreased the elevated concentration of IgE stimulated by HgCl2, although these differences did not achieve statistical significance (P = 0·077, repeated-measures anova). BN rats treated with two or three doses of HgCl2 (Fig. 1b,c, respectively) again showed increased IgE concentrations, but in this case the elevated levels of IgE were abolished by the administration of an increased dose of desferrioxamine (i.p. and s.c.) (P = 0·001, P < 0·001, respectively). No significant changes in IgE concentration were observed in rats given five doses of desferrioxamine (i.p. and s.c.) alone (Fig. 1c).
Fig. 1.
Suppression of IgE production by desferrioxamine in HgCl2-induced autoimmunity. Serum IgE concentration (mean ± s.e.) is shown for groups of BN rats treated with (•) HgCl2+ saline (▴) HgCl2+ desferrioxamine, or (◊) saline + desferrioxamine. Rats were injected with (a) one dose of HgCl2 with (n = 6) or without desferrioxamine (n = 6) (i.p.) or (b) two doses of HgCl2 with (n = 5) or without desferrioxamine (n = 5) (i.p. and s.c.) or (c) three doses of HgCl2 with (n = 10) or without desferrioxamine (n = 12) (i.p. and s.c.), or saline with desferrioxamine (n = 6) (i.p. and s.c.).
Desferrioxamine inhibits lymphoproliferation in HgCl2-treated BN rats
The spleen enlarges considerably following HgCl2 treatment, with a marked increase in B cells and germinal centre formation [19]. Both spleen weight and the spleen index (spleen weight/rat weight) were recorded to quantify lymphoproliferation. Results in Table 1 show that treatment of the rats with three doses of HgCl2 significantly increase spleen weight and its index (P = 0·0007, P = 0·0001, respectively). Animals given both HgCl2 and desferrioxamine showed a decrease in spleen weight and spleen index (Table 1). There was a significant difference, either in spleen weight or spleen index, between the HgCl2+ saline and the HgCl2+ desferrioxamine groups (P-values ranged between 0·009 and lower than 0·0001, Mann–Whitney U-test, two-tailed). The current data indicate that desferrioxamine inhibits lymphoproliferation in BN rats treated by HgCl2. There was a significant correlation in individual rats between final IgE concentration and spleen weight (r = 0·804, P < 0·0001, n = 28).
Table 1.
Effect of desferrioxamine on spleen weight and spleen index in HgCl2-induced autoimmunityA
| HgCl2 treatment | HgCl2+ saline | HgCl2+ desf. | Saline + saline |
|---|---|---|---|
| Spleen weight (g, mean ± s.d.) | |||
| 2 doses | 0·916 ± 0·078 (n = 5) | 0·634 ± 0·069 (n = 5)B | n.d. |
| 3 doses | 0·935 ± 0·296 (n = 12) | 0·640 ± 0·133 (n = 10)C | 0·563 + 0·045 (n = 6)D |
| Spleen weight/rat weight (mg/g, mean ± s.d.) | |||
| 2 doses | 2·738 ± 0·099 | 2·010 ± 0·200E | n.d. |
| 3 doses | 2·988 ± 0·479 | 2·030 ± 0·188F | 1·667 ± 0·075G |
Statistics represent two-tailed Mann–Whitney U-test.
P = 0·009 versus HgCl2+ saline.
P < 0·0001 versus HgCl2+ saline.
P = 0·0007 versus HgCl2+ saline.
P = 0·009 versus HgCl2+ saline.
P < 0·0001 versus HgCl2+ saline.
P = 0·0001 versus HgCl2+ saline.
Desferrioxamine influences T cell-dependent HgCl2-induced caecal vasculitis in BN rats
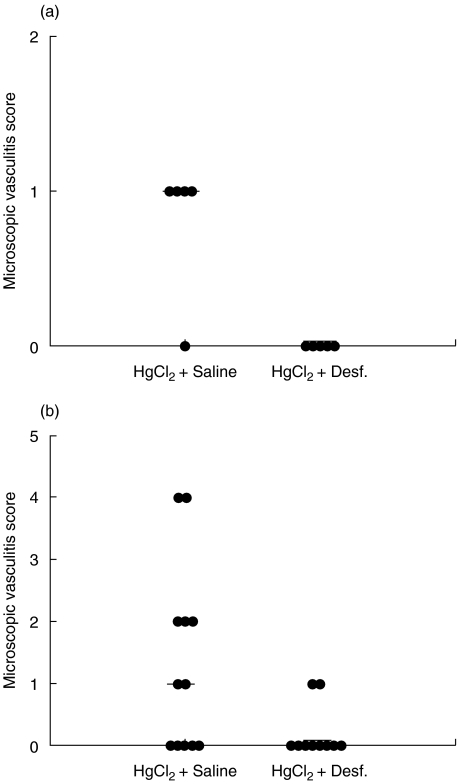
BN rats treated with HgCl2 develop a mucosal vasculitis primarily affecting the caecum. The late vasculitis (in contrast to early mast cell-mediated vasculitis, known to be inhibited by desferrioxamine [14]) is T cell-dependent [15]. To assess whether desferrioxamine treatment could reduce the late phase of tissue injury in HgCl2-induced autoimmunity, both microscopic and macroscopic caecal vasculitis at day 14 after start of HgCl2 were examined. Microscopic caecal vasculitis scores were recorded by examination of biopsies either from affected areas of the caecum or from random biopsies in animals with no macroscopic evidence of vasculitis. In BN rats treated with two doses of HgCl2, four of five rats developed microscopic vasculitis (Fig. 2a). By contrast, none of the animals treated with two doses of HgCl2 and desferrioxamine showed microscopic vasculitis. There was a statistically significant difference between the groups (P = 0·014, Mann–Whitney U-test, two-tailed). Similarly, the microscopic vasculitis scores in rats treated with three doses of HgCl2 and desferrioxamine were lower than those of rats given HgCl2 and saline (Fig. 2b) (P = 0·0373).
Fig. 2.
Suppression of caecal vasculitis by desferrioxamine in HgCl2-induced autoimmunity. Microscopic caecal vasculitis scores for individual rats and median scores are shown for (a) BN rats treated with two doses of HgCl2 with or without desferrioxamine or (b) BN rats treated with three doses of HgCl2 with or without desferrioxamine.
In agreement with the above results, two of five rats receiving two injections of HgCl2 developed macroscopic caecal vasculitis (scored as 2 and 5, respectively), while none of five rats given two injections of HgCl2 and desferrioxamine had macroscopic vasculitis. Treatment of the rats with desferrioxamine decreased the incidence and total macroscopic vasculitis scores induced by three doses of HgCl2: median score HgCl2+ saline = 0·5; HgCl2+ desferrioxamine = 0; P = 0·039 (Mann–Whitney U-test, one-tailed). Taken together, these results indicate that the antioxidant desferrioxamine can reduce HgCl2-induced late vasculitis in BN rats in vivo.
Desferrioxamine modulates splenic cytokine gene expression in HgCl2-induced autoimmunity
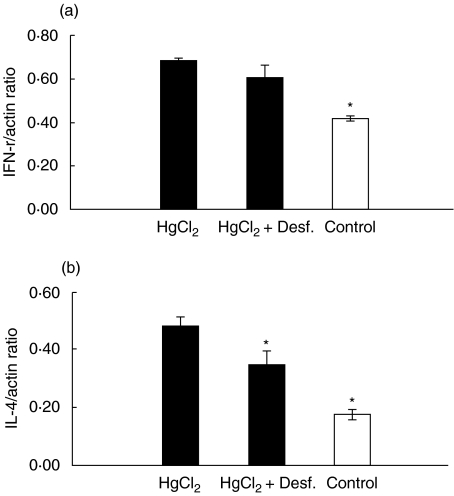
It is known that there are reciprocal regulatory interaction between Th1 and Th2 cells, with IL-4 inhibiting Th1 cells and IFN-γinhibiting Th2 cells [20]. However, recent work has revealed that IL-4 secreted by CD4+ T cells can play a role in the development of a Th1 immune response against an infectious pathogen [21]. RT-PCR analysis for IFN-γ and IL-4 mRNA were performed. The data shown in Fig. 3 are pooled from two identical independent experiments. As reported by others [22], administration of HgCl2 to BN rats leads to up-regulation of splenic IFN-γ expression (Fig. 3a); desferrioxamine treatment causes a non-significant inhibition of the up-regulation of IFN-γ mRNA by HgCl2 (Fig. 3a). A significant increase of IL-4 gene expression in splenocytes was noted on day 4 following two injections of HgCl2 (Fig. 3b). In rats treated additionally with desferrioxamine, the increase in the amount of IL-4 mRNA transcript was decreased (P = 0·0315, Mann–Whitney U-test, two-tailed).
Fig. 3.
Modulation of splenic cytokine mRNA expression in HgCl2-induced autoimmunity. BN rats were treated with two doses of HgCl2 in the presence or absence of desferrioxamine for 4 days and then spleen cells were prepared for mRNA and RT-PCR. Data were pooled from two identical independent experiments with a total of six animals in each group, and expressed as the ratio of the intensity of the band for IFN-γ (a) or IL-4 (b) to the intensity of the band for β-action. *P < 0·05 compared with HgCl2 group (rats treated with HgCl2 only).
DISCUSSION
The data presented in this study demonstrate that the antioxidant desferrioxamine can inhibit lymphoproliferation, IgE production and late caecal vasculitis in HgCl2-induced autoimmunity in BN rats. We have also shown that desferrioxamine decreases HgCl2-enhanced IL-4 gene expression in splenocytes in vivo, although this conclusion should be interpreted with some caution in view of the fact that we only measured IL-4 mRNA with a semiquantitative method. This study therefore provides evidence to support our hypothesis that oxidative stress plays a role in the up-regulation of IL-4 in vivo and contributes to the HgCl2-induced Th2-driven autoimmune syndrome. The importance of this observation is that it extends our work on the in vivo regulation of mast cells to Th2 cells; whether this is due to a direct effect of antioxidants on Th2 cells, or an indirect effect via the inhibition of mast cell driven activation, remains to be determined.
One study found that only sulphydryl-containing antioxidants decreased IL-4 production and IgE synthesis [23]. However, desferrioxamine is an iron chelator which inhibits iron-catalysed hydroxyl radical formation via the Fenton reaction [24]. We have shown previously that desferrioxamine, which has affinity for trivalent cations, does not chelate Hg++[25]. Desferrioxamine has been shown to improve cardiac endothelial dysfunction caused by ROS production [26], and to restore dilation of the coronary microcirculation and a normal matching between myocardial metabolic demand and coronary blood flow in type II diabetic patients [27]. Our previous studies demonstrated that desferrioxamine decreased HgCl2-induced up-regulation of IL-4 mRNA expression in mast cells in vitro and inhibited HgCl2-induced early vasculitis in this animal model [14,16]. The rise in serum IgE is a hallmark of the HgCl2-induced autoimmunity in the experimental model. After even a single injection of HgCl2, elevated serum IgE levels were seen in all rats with a 10–1000-fold increase (data not shown). The data showing that desferrioxamine treatment results in a significant decrease in HgCl2-enhanced serum IgE suggests an inhibitory effect on the production of IgE in vivo, putatively via its antioxidative effect.
IL-4 is a critical cytokine required for development of Th2 responses and isotype switching to IgE. A recent study on knock-out mice has revealed that IL-4 alone can induce Th2 responses, even in the combined absence of IL-5, IL-9 and IL-13 [4]. It has been shown that IL-4 reduces the expression of inhibitory receptors on B cells, abolishes B cell suppression by CD22 and CD32 and enhances B cell activation [28]. We have found previously that oxidative stress mimicked the effect of HgCl2 on IL-4 expression by mast cells, while antioxidants inhibited the HgCl2-enhanced IL-4 expression in mast cells and spleen cells in vitro[16]. In addition, antioxidants can also inhibit HgCl2-induced early vasculitis in BN rats, with a trend to a decrease in HgCl2-enhanced IL-4 expression in mast cells in vivo[14]. Our present data have shown that desferrioxamine suppresses HgCl2-induced Th2-mediated autoimmunity with a decrease in HgCl2-induced IL-4 expression in spleen cells in vivo, consistent with the hypothesis that the antioxidant desferrioxamine modulates HgCl2-induced Th2 cell responses via inhibition of IL-4 production in vivo.
We have found previously a more significant increase of IL-4 mRNA expression in the spleen of HgCl2-treated BN rats [29]. However, this was not as obvious in the present study, and decreased only moderately by the addition of desferrioxamine. This could be explained by our experimental procedures. To minimize use of animals, a single time-point (4 days after administration of HgCl2) was chosen in the present experiments based on published time-course data [29]. Methods to detect human and mouse IL-4 are well established, whereas there has been a lack of sensitive assays for the measurement of rat IL-4 protein. We have been unable to detect IL-4 protein levels in vivo by intracellular staining/flow cytometry and IL-4 ELISA (BD Biosciences) due to high background staining and low sensitivity, respectively.
Although IL-4 is likely to be a major cytokine modulating the HgCl2-induced Th2-dominated autoimmunity, the initial cellular source of IL-4 required for instigation of Th2 cell development remains to be determined. We have reported previously that an antioxidant inhibits HgCl2-enhanced IL-4 expression moderately in the mast cells in the caecum 2 days after injection of HgCl2, a time at which there is no obvious lymphocyte infiltration or IL-4+ T cells present in the tissues [14]. A T cell infiltrate was evident in the caecum and lung 5 days after injection of HgCl2[19]. Mast cells can release prestored IL-4 within minutes of stimulation and synthesize more hours after the stimulation [30], whereas IL-4 gene expression in T cells is controlled more strictly than in mast cells [31]. It is possible that mast cells could be activated and provide an initial source of IL-4 which could induce or expand T cells for an effective Th2 response.
In conclusion, our results demonstrate that the antioxidant desferrioxamine can inhibit HgCl2-induced Th2-mediated autoimmunity in BN rats, suggesting that oxidative stress plays a role in the pathogenesis of the HgCl2-induced Th2-driven autoimmune syndrome. This finding might have implications for understanding the processes involved in Th2 autoimmune responses such as allergy and asthma, and thereby facilitate the development of new therapeutic strategies for these diseases.
Acknowledgments
This work was supported by grants from the Wellcome Trust UK.
REFERENCES
- 1.Burrows B, Martinez FD, Halonen M, et al. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–7. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 2.Kleinjan A, Vinke JG, Severijnen LW, et al. Local production and detection of (specific) IgE in nasal B-cells and plasma cells of allergic rhinitis patients. Eur Respir J. 2000;3:491–7. doi: 10.1034/j.1399-3003.2000.15.11.x. [DOI] [PubMed] [Google Scholar]
- 3.Ying S, Humbert M, Meng, et al. Local transcription of e germline gene transcripts and RNA for the e heavy chain of IgE in the bronchial mucosa in atopic and nonatopic asthma. J Allergy Clin Immunol. 2001;107:686–92. doi: 10.1067/mai.2001.114339. [DOI] [PubMed] [Google Scholar]
- 4.Fallon PG, Jolin HE, Smith P, et al. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity. 2002;17:7–17. doi: 10.1016/s1074-7613(02)00332-1. [DOI] [PubMed] [Google Scholar]
- 5.Ferlin WG, Severinson E, Strom L, et al. CD40 signaling induces interleukin-4-independent IgE switching in vivo. Eur J Immunol. 1996;26:2911–5. doi: 10.1002/eji.1830261216. [DOI] [PubMed] [Google Scholar]
- 6.Faty JV. Reducing IgE levels as a strategy for the treatment of asthma. Clin Exp Allergy. 2000;30(Suppl. 1):16–21. doi: 10.1046/j.1365-2222.2000.00091.x. [DOI] [PubMed] [Google Scholar]
- 7.Esnault VLM, Mathieson PW, Thiru S, et al. Autoantibodies to myeloperoxidase in Brown Norway rats treated with mercuric chloride. Lab Invest. 1992;67:114–20. [PubMed] [Google Scholar]
- 8.Goldman M, Druet P, Gleichmann E. Th2 cells in systemic autoimmunity: insights from allogeneic diseases and chemically-induced autoimmunity. Immunol Today. 1991;12:223–7. doi: 10.1016/0167-5699(91)90034-Q. [DOI] [PubMed] [Google Scholar]
- 9.Kiely PDW, Thiru S, Oliveira DBG. Inflammatory polyarthritis induced by mercuric chloride in the Brown Norway rat. Lab Invest. 1995;73:284–93. [PubMed] [Google Scholar]
- 10.Jennette JC, Falk RJ, Milling DM. Pathogenesis of vasculitis. Semin Neurol. 1994;14:291–9. doi: 10.1055/s-2008-1041088. [DOI] [PubMed] [Google Scholar]
- 11.Cohen Tervaert JW, Limburg PC, Elema JD, et al. Detection of auto-antibodies against myeloid lysosomal enzymes: a useful adjunct to classification of patients with biopsy-proven necrotizing arteritis. Am J Med. 1991;91:59–66. doi: 10.1016/0002-9343(91)90074-8. [DOI] [PubMed] [Google Scholar]
- 12.Pelletier L, Pasquier R, Rossert J, et al. Autoreactive T cells in mercury-induced autoimmunity: ability to induced the autoimmune disease. J Immunol. 1988;140:2335–41. [PubMed] [Google Scholar]
- 13.Finkelman FD, Katona IM, Urban JF, et al. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988;141:2335–41. [PubMed] [Google Scholar]
- 14.Wu Z, Turner DR, Oliveira DBG. Antioxidants inhibit mercuric chloride-induced early vasculitis. Int Immunol. 2002;14:267–73. doi: 10.1093/intimm/14.3.267. [DOI] [PubMed] [Google Scholar]
- 15.Kiely PDW, Pecht I, Oliveira DBG. Mercuric chloride-induced vasculitis in the Brown Norway rat: αβ T cell dependent and independent phases − role of the mast cell. J Immunol. 1997;159:5100–6. [PubMed] [Google Scholar]
- 16.Wu Z, Turner DR, Oliveira DBG. Interleukin (IL)-4 gene expression upregulated by mercury in rat mast cells: a role of oxidant stress in IL-4 transcription. Int Immunol. 2001;13:297–304. doi: 10.1093/intimm/13.3.297. [DOI] [PubMed] [Google Scholar]
- 17.Qasim FJ, Mathieson PW, Thiru S, et al. Cyclosporin A exacerbates mercuric chloride-induced vasculitis in the Brown Norway rat. Laboratory Invest. 1995;72:183–90. [PubMed] [Google Scholar]
- 18.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 19.Qasim FJ, Mathieson PW, Thiru S, et al. Time course and characterisation of mercuric chloride induced autoimmunity in the Brown Norway rat. J Autoimmun. 1995;8:195–208. doi: 10.1006/jaut.1995.0015. [DOI] [PubMed] [Google Scholar]
- 20.Morel PA, Oriss TB. Crossregulation between Th1 and Th2 cells. Crit Rev Immmunol. 1998;18:275–303. doi: 10.1615/critrevimmunol.v18.i4.10. [DOI] [PubMed] [Google Scholar]
- 21.Carvalho LH, Sano G, Hafalla JCR, et al. IL-4 secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat Med. 2002;8:166–70. doi: 10.1038/nm0202-166. [DOI] [PubMed] [Google Scholar]
- 22.Gillespie KM, Saoudi A, Kuhn J, et al. Th1/Th2 cytokine gene expression after mercuric chloride in susceptible and resistant rat strains. Eur J Immunol. 1996;26:2388–92. doi: 10.1002/eji.1830261018. [DOI] [PubMed] [Google Scholar]
- 23.Jeannin P, Delneste Y, Lecoanet-Henchoz S, et al. Thiols decrease human interleukin (IL) 4 production and IL-4-induced immunoglobulin synthesis. J Exp Med. 1995;182:1785–92. doi: 10.1084/jem.182.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klebanoff SJ. The iron–H2O2–iodide cytotoxic system. J Exp Med. 1982;156:1262–7. doi: 10.1084/jem.156.4.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfreys K, Oliveira DBG. Alterations in intracellular reactive oxygen species generation and redox potential modulate mast cell function. Eur J Immunol. 1997;27:297–306. doi: 10.1002/eji.1830270143. [DOI] [PubMed] [Google Scholar]
- 26.MacCarthy PA, Grieve DJ, Li J, et al. Impaired endothelial regulation of ventricular relaxation in cardiac hypertropy. Circulation. 2001;104:2967–74. doi: 10.1161/hc4901.100382. [DOI] [PubMed] [Google Scholar]
- 27.Nitenberg A, Ledoux S, Valensi P, et al. Coronary microvascular adaptation to myocardial metabolic demand can be restored by inhibition of iron-catalyzed formation of oxygen free radicals in type 2 diabetic patients. Diabetes. 2002;51:813–8. doi: 10.2337/diabetes.51.3.813. [DOI] [PubMed] [Google Scholar]
- 28.Rudge EU, Cutler AJ, Pritchard NR, et al. Interleukin 4 reduces expression of inhibitory receptors on B cells and abolishes CD22 and FcrRII-mediated B cell suppression. J Exp Med. 2002;195:1079–85. doi: 10.1084/jem.20011435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillespie KM, Qasim LM, Tibbatts LM, et al. Interleukin-4 gene expression in mercury-induced autoimmunity. Scand J Immunol. 1995;41:268–72. doi: 10.1111/j.1365-3083.1995.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 30.Bradding P, Feather IH, Howarth PH, et al. Interleukin 4 is localized to and released by human mast cells. J Exp Med. 1992;176:1381–6. doi: 10.1084/jem.176.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bird JJ, Brown DR, Mullen AC, et al. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229–37. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]