Abstract
The fusion protein of the respiratory syncytial virus (RSV) binds to the pattern recognition receptors, TLR4 and CD14, and initiates innate immunity response to the virus. The aim of the study was to investigate the expression of TLR4 on peripheral blood lymphocytes and monocytes in peripheral blood of infants in both acute and convalescent phase of RSV bronchiolitis (n = 26). In addition, TNF-α expression in lipopolysaccharide-stimulated monocytes was also assessed. The results showed TLR4 to be expressed predominantly by monocytes in both sick infants and controls. During the acute phase of infection monocytes up-regulated TLR4 in eight infants, which returned to the levels recorded in controls 4–6 weeks from infection. There was no difference in the percentage of TNF-α secreting monocytes. Of the clinical parameters tested, minimal oxygen saturation was found to correlate negatively with this expression in the group of infants with increased TLR4. Additional studies are under way to correlate this finding with the outcome of the immune response to RSV.
Keywords: bronchiolitis, infants, RSV, toll-like receptor 4
INTRODUCTION
Respiratory syncytial virus (RSV) is one of the most important causes of lower respiratory tract disease in infants and young children worldwide. Ample data show that some RSV glycoproteins such as fusion (F) and attachment (G) glycoproteins are important for the induction of neutralizing antibodies and long-term immunity [1]. In addition to the virus effect on acquired immunity, recent data indicate that different RSV proteins might alter early innate immune response as well [2]. It has been shown that G glycoprotein alters pulmonary trafficking of natural killer (NK) cells and polymorphonuclear cells, and influences the expression of cytokines and chemokines such as macrophage inflammatory protein (MIP)-1α, MIP-1β, MIP-2 and others [3,4]. In RSV immunopathology, monocytes might have a prominent role by direct interaction with T cells and production of cytokines, such as tumour necrosis factor (TNF), interleukin (IL)-1, IL-6, IL-8, IL-10, platelet-activating factor (PAF) and prostaglandin E2[5–9]. Human monocytes exposed to RSV express less intercellular adhesion molecule 1 (ICAM-1) and its ligand, lymphocyte function-associated antigen 1 (LFA-1) than do human monocytes exposed to influenza virus [8]. The effect of RSV on monocytes is suggested to dampen the immune response due to the reduction of lymphocyte maturation, decreased expression of adhesion molecules and reduced IL-12 and interferon-γ (IFN-γ) secretion [10]. Hence, the reaction to RSV infection is less robust, and skewed to the helper cell type 2 (Th2) direction of the immune pathway [11,12].
In addition to these findings, Kurt-Jones et al. showed the pattern recognition receptors TLR4 (toll-like receptor 4) and CD14 to participate in the antiviral response to RSV [13]. They showed that RSV F glycoprotein and to a lesser extent G glycoprotein failed to induce proinflammatory cytokine secretion in either CD14- or TLR4-deficient mice. TLR4 is a recently identified receptor that belongs to a family of proteins structurally similar to Drosophila Toll proteins [14]. In addition to its role in RSV pathogenesis, this molecule has been identified previously as a pattern recognition molecule for lipopolysaccharides isolated from the wall of Gram-negative bacteria, hsp60, fibronectin, and in a mouse system it also bound to plant-derived taxol [15]. The absence of TLR4 in a mouse model showed that, upon the challenge with RSV, TLR4-deficient mice had impaired NK and CD14+ pulmonary trafficking, deficient NK cell activity, impaired IL-12 expression and decreased virus clearance in comparison to TLR4-expressing mice [16].
Here we investigated the expression of TLR4 in 28 acutely ill patients with RSV bronchiolitis. Expression of TLR4 in 17 children was determined in the convalescence phase, at weeks 4–6 of the disease onset. In addition, the levels of TNF-α-secreting monocytes following lipopolysaccharide (LPS) stimulation were determined by intracellular cytokine staining in five children.
PATIENTS AND METHODS
Patients and controls
Infants (nine girls and 17 boys) aged 1–12 months (mean 3·4 months) admitted to University Children's Hospital or Dr Fran Mihaljević University Hospital for Infectious Diseases with verified RSV infection were included in the study during the 2001–02 winter epidemic. All enrolled infants had bronchiolitis defined as wheezing, hypoxia with O2 saturation <95% and hyperinflation but infiltrate-free chest radiograph if taken. Controls were recruited among gender- and age-matched infants without evident allergic, immune, infectious or haematological disorders. The study was approved by the Ethics Committees of the two participating hospitals. Parents gave their informed consent for collection of blood specimens. Because of the small amount of blood that could be obtained, not all tests were performed in every infant.
Clinical and laboratory findings
Symptoms (wheezing, MOSmin oxygen saturation, MRRmax respiratory rate, fever) and findings were determined according to standard values for these parameters (MOS <95%, MRR <50). Routine white blood count (WBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), chest X-ray and bacteriological analyses of nasopharyngeal swabs were determined.
Viral diagnosis
Clinical specimen (nasopharyngeal secretion) was taken at an early stage of infection (1–3 days of the onset of infection). RSV-infection was verified by rapid detection (DFA, direct fluorescent antibody, Light Diagnostics, Temecula, USA) or/and virus isolation (in cell culture: HEp-2, HeLa, MCR-5) [17].
Immunological tests
Samples of heparinized peripheral blood were taken up to day 10 (mean 7·2 days) of the onset of acute illness and in convalescent phase, weeks 4–6 of the disease onset.
Monoclonal antibodies for surface analysis
The following murine antibodies to human lymphocyte surface antigens were used: anti-CD4 (peridinin chlorophyll protein (PerCP)-conjugated), anti-CD8 (FITC-conjugated), anti-CD3 (allophycocyanin (APC)-conjugated), anti-CD19 (FITC-conjugated), anti-CD14 (FITC-conjugated), anti-CD33 (PE-conjugated) (all from Becton Dickinson, Heidelberg, Germany) and anti-TLR4 (PE-conjugated; eBioscience, San Diego, CA, USA). In each experiment, FITC-, PE-, PerCP- and APC-conjugated isotype controls for determination of non-specific binding were included.
Flow cytometry analysis
Fifty µl of heparinized blood were incubated in 12 × 75 mm polystyrene round-bottomed tubes (Becton Dickinson, Heidelberg, Germany) with 5 µl of murine antihuman monoclonal antibodies to CD3, CD4, CD8, CD19 and CD14 for 15 min in the dark at room temperature (RT) (Table 1).
Table 1.
Antibody combinations used in flow cytometry analysis
| FITC | PE | PerCP | APC | |
|---|---|---|---|---|
| Tube 1 | Anti-CD8 | Anti-TLR4 | Anti-CD4 | Anti-CD3 |
| Tube 2 | Anti-CD19 | Anti-TLR4 | – | – |
| Tube 3 | Anti-CD14 | Anti-TLR4 | – | – |
| Tube 4 | Isotype control | Isotype control | Isotype control | Isotype control |
| MoAb IgG1 | MoAb IgG1 | MoAb IgG1 | MoAb IgG1 |
FITC, fluorescein isothiocyanate; PE, phycoerythrin; PerCP, peridinin chlorophyll protein; APC, allophycocyanin; MoAb, monoclonal antibody.
Red blood cells (RBC) were lysed by adding 2 ml of 10% FACS Lysing Solution (Becton Dickinson, San Jose, USA) for 10 min at RT in the dark. After washing, the cells were resuspended in 0·5 ml of the staining buffer.
Correlated analysis of forward- and right-angle scatter was used to establish a lymphocyte or monocyte gate. A minimum of 20 000 events for four-colour and 5000 events for two-colour immunofluorescence analysis in lymphocyte gate or 3000 events in monocyte gate was analysed by a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, USA). The non-specific staining, assessed by an isotype control, was adjusted to less than 1%. The collected data were analysed using CELLQuest software and presented as percentage.
Analysis of TNF-α expression in CD14+ monocytes by intracellular cytokine staining
Two samples of heparinized blood (300 µl) were diluted 1 : 1 in RPMI-1640 medium (Institute of Immunology, Zagreb, Croatia) and cultured in sterile 12 × 75 mm polystyrene round-bottomed tubes (Becton Dickinson). First sample was cultured in the presence of an inhibitor of protein secretion, brefeldin A (BFA, 10 µg/ml, Sigma, Poole, Dorset, UK) only, and the second in the presence of 100 ng/ml of lipopolysaccharide (LPS, Sigma) and BFA for 4 h at 37°C in 5% CO2.
Samples of diluted blood (100 µl) were then transferred into new tubes and stained first with 5 µl anti-CD14 MoAb for 15 min in the dark at RT and washed. RBC were lysed with 2 ml of 10% FACS Lysing Solution (Becton Dickinson, San Jose, USA) for 10 min at RT in the dark. After washing, the cells were fixed for 20 min or left overnight at 4°C with 4% paraformaldehyde in PBS. Cells were then permeabilized with 0·1% saponin in PBS for 10 min prior to the addition of APC-conjugated anti-TNF-α antibody or isotypic control (0·5 µg/106 cells) (PharMingen) and then incubated for additional 30 min at 4°C. Cells were then washed in PBS containing 0·1% saponin to wash out unbound antibody, resuspended in PBS and analysed immediately on a flow cytometer. Results are expressed as a percentage of TNF-α expressing CD14+ monocytes among all monocytes. In preliminary experiments, peripheral blood mononuclear cells were also stained simultaneously with FITC-conjugated anti-CD14 MoAb and PE-conjugated CD33, followed by subsequent staining of intracellular APC-conjugated anti-TNF-α.
Statistical analysis
Expression of TLR4 on different lymphocyte subsets and monocytes, TNF-α secretion and clinical parameters (days of wheezing, days of O2 supplementation, minimal O2 saturation and maximal respiratory rate) were analysed. Wilcoxon's test for paired data and a Mann–Whitney U-test for unpaired data were applied. Correlation was evaluated using Spearman's rank correlation coefficient (rs). The level of significance was set at P < 0·05.
RESULTS
Expression of TLR4
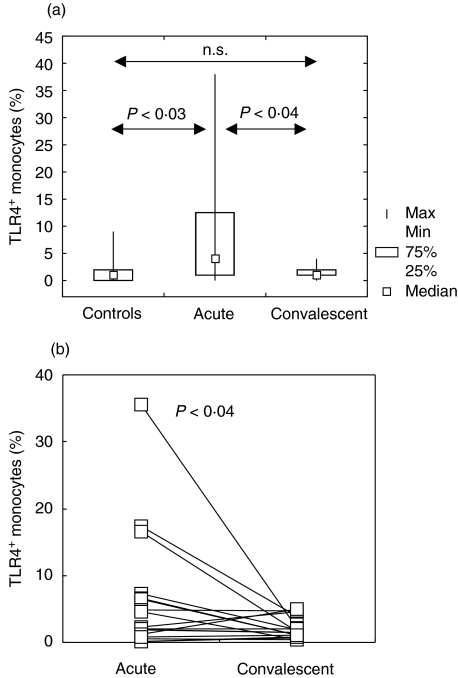
Flow cytometric analysis of TLR4 expression showed this molecule to be virtually absent on the surface of either CD3 (total T lymphocytes), CD3+ CD4+ (helper T lymphocytes), CD3+ CD8+ (cytotoxic T lymphocytes), CD19+ (B lymphocytes) cells or granulocytes in peripheral blood of both control subjects and infants with RSV bronchiolitis (Fig. 1a-e). The cells that expressed TLR4 in tested infants were CD14+ monocytes (Fig. 1f). TLR4 expression on CD14+ monocytes was significantly higher in acutely ill infants (P < 0·04) than in age- and gender-matched controls (Fig. 2a). In 17 children, samples were analysed for TLR expression at 4–6 weeks of the onset of symptoms. The percentage of TLR on CD14+ monocytes decreased significantly (P < 0·04) and was similar to the percentage recorded in control subjects (Fig. 2b).
Fig. 1.
A representative dot plot of TLR4 expression on CD3+ T lymphocytes (a), CD3+ CD4+ T lymphocytes (b), CD3+ CD8+ T lymphocytes (c), CD19+ B lymphocytes (d), granulocytes (e) and CD14+ monocytes (f). Gates were set around lymphocytes (a–d), granulocytes (e) or monocytes (f) according to the forward- and side-scatter values on a flow cytometer. The horizontal and vertical lines were set with an irrelevant isotype-matched fluorescent conjugate. The percentage of positive cells is indicated. Results obtained from a RSV infected infant are shown.
Fig. 2.
Expression of TLR4 on peripheral blood monocytes. (a) Comparison of TLR4-expressing CD14+ monocytes in acute (n = 26) and convalescent (n = 17) phases of RSV infection, and in control infants (n = 10). Box indicates 25th and 75th percentile, central point median and whiskers indicate minimum and maximum data values. Probabilities (P) of rejecting null hypothesis for computed Conover's inequalities are indicated by dotted lines are showed. n.s. = not significant. (b) Down-regulation of the percentage of TLR4-expressing CD14+ monocytes in 17 infants at weeks 4–6 of disease onset. The values obtained in a single infant in acute and convalescent phase of disease are linked with a line. Each symbol represents one tested infant.
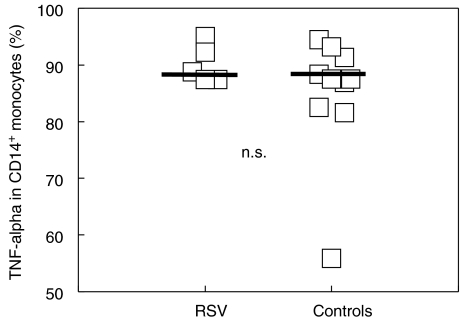
Expression of TNF-α
In five acutely ill infants and 10 controls, LPS-induced TNF-α was determined by intracellular cytokine staining (Fig. 3). Preliminary experiments showed that both CD14 and CD33 were appropriate markers of peripheral blood monocytes as no differences were observed between these molecules (data not shown). Expression of TNF-α in unstimulated cells was always less than 1% in all children tested (data not shown). There was no significant difference in the percentage of TNF-α secreting monocytes, and no correlation between the levels of TLR4 and TNF-α expression was found.
Fig. 3.
Analysis of TNF-α expression in CD14+ monocytes by intracellular cytokine staining in acutely infected RSV infants (n = 5) and controls (n = 10). Blood samples were cultured with brefeldin A (10 µg/ml) or LPS (100 ng/ml) and brefeldin A for 4 h. LPS-induced TNF-α was determined by intracellular cytokine staining as described in Methods. Expression of TNF-α in unstimulated cells was always less than 1% in all tested children (data not shown). Each symbol represents one tested infant. n.s. = not significant.
Relation between immunological and clinical parameters
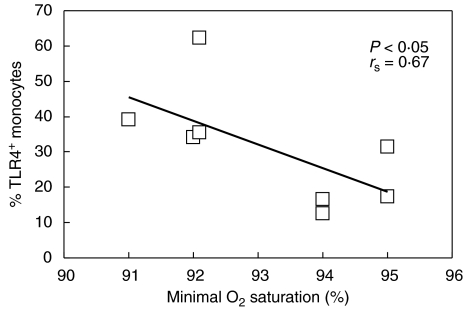
Analysis of the data obtained from all RSV-infected infants yielded no significant correlation between either the percentage of TLR4 or TNF-α levels and clinical parameters examined (days of wheezing, days of O2 supplementation, minimal O2 saturation and maximal respiratory rate). However, as illustrated in Fig. 2, two groups of infants could be distinguished clearly: those with substantial, transitory increase in TLR4 expression (n = 8) and the remainder of the RSV-infected children exhibiting only slight changes in TLR expression (n = 18). Additional analysis showed that in the former, the substantial increase in TLR4 (increased TLR4 expression in this group was higher than the maximal value obtained in healthy infants) was accompanied by a statistically significant decrease in minimal O2 saturation (rs = 0·67, P < 0·05), while no correlation was found in infants with moderate changes in TLR4 expression (Fig. 4).
Fig. 4.
Negative correlation between minimal oxygen saturation and percentage of TLR4-expressing monocytes in eight children with increased TLR4 expression during acute phase of RSV-infection.
DISCUSSION
The main finding of this study was that in a subgroup of infants, RSV infection induced TLR4 expression on monocytes obtained from peripheral blood in the acute phase of RSV bronchiolitis. In the infants showing a substantial increase in TLR4 expression, the minimal oxygen saturation correlated negatively with this expression, indicating that TLR4 might be related to the severity of illness in RSV-infected infants. This was the first study to assess the expression of TLR4 in RSV-infected humans. We failed to demonstrate TLR4 expression on either of the lymphocyte subsets investigated. In control infants, low expression of TLR4 was also observed on peripheral blood monocytes only. TLR expression has been observed on a variety of cells other than monocytes and several stimuli have been identified so far which modulate the expression of TLR4. TLR4 expression is increased on intestinal epithelial cells during inflammation, and it can also be up-regulated by LPS on corneal epithelial cells and in the presence of recombinant (r)IFN-γ on human monocytes [18–20]. Stimuli such as Porphyromonas gingivalis-derived LPS and rIL-4 down-regulate TLR4 expression [20,21]. Irrespective of these findings, it still has to be determined whether the dynamic regulation of TLR4 expression observed is connected with responsiveness to TLR4.
Routine bacterial analysis of nasopharyngeal swabs was performed in all children enrolled in the study, and additional bacterial analyses such as urine analysis to minimize the possible effect of bacterial co-infection on TLR4 expression were performed in some study children as necessary. Although the possibility of other infections (bacterial or others) influencing the study results could not be absolutely ruled out, we did not demonstrate any association between TLR4 expression and presence of bacterial infection in study infants. In addition, the level of C-reactive protein was within the normal range in all study children.
We aimed to investigate whether the up-regulation of TLR4 on monocytes in acutelly ill infants was linked to the impaired capacity of these monocytes to secrete proinflammatory cytokines. Using human peripheral blood monocytes, Kurt-Jones et al. have shown that both LPS and RSV F protein induce significant secretion of proinflammatory cytokines such as IL-6, TNF-α, IL-1β and IL-8 [13]. In the present study, we investigated the expression of TNF-α in peripheral blood monocytes following in vitro stimulation with LPS, another ligand for TLR4. We showed that, despite an increased percentage of TLR4, the acutely ill RSV-infected infants had a similar percentage of TNF-α-expressing monocytes as that measured in controls. Because LPS binds to other ligands on the monocyte cell surface [15], the possibility cannot be ruled out that LPS stimulation was too crude to detect the possible differences in TNF-α expression. Additional experiments using recombinant RSV F protein might help address this issue more specifically.
It is of interest that overexpression of TLR4 did not confer LPS responsiveness on human embryonic kidney 293 cells, suggesting that an additional molecule, recently identified as MD-2, is required for TLR4-mediated LPS signalling [22]. MD-2 is associated with the extracellular domain of TLR4 and expression of MD-2 imparts LPS responsiveness to cells expressing TLR4 alone. Additional studies are required to elucidate the possible role of MD-2 during the innate immune system response to RSV.
Two major surface viral glycoproteins, F and G, have been reported to induce distinct types of immune response: Th1 and Th2, respectively [23]. An interesting observation that rIFN-γ can induce TLR4 expression on human monocytes might indicate that in those individuals in whom RSV F protein activates innate immune system through TLR4-directed pathway, consequent IFN-γ secretion by RSV-specific Th1 cells up-regulates TLR4 on peripheral blood monocytes. Once the patient had recovered and the virus had been cleared from the body, cytokine secretion was also dampened resulting in TLR4 down-regulation. Experiments are under way to test whether this could be the underlying mechanism for the regulation of TLR4 in RSV patients.
All our patients had a clinical picture of bronchiolitis, giving us no opportunity to investigate whether the level of TLR4 expression correlated with disease severity. However, the substantial increase in TLR4 expression correlated negatively with minimal oxygen saturation, indicating that TLR4 expression might be linked to the severity of illness in RSV-infected infants. We have shown previously that the level of IFN-γ secreted by both CD4+ and CD8+ T cells during acute RSV illness correlates with disease severity [24]. The absence of TLR4 was associated with reduced expression of IL-12, a major Th1 skewing cytokine. Inadequate activation of CD14+ cells might contribute to the low IL-12, a phenomenon already observed in patients with severe bronchiolitis [25]. It has to be determined whether the substantial increase of TLR4 observed in eight infants during the acute phase of infection contributed to exaggerated inflammatory airway response and consequent decrease in O2 saturation.
In addition to the Th1/Th2 imbalance observed in RSV infection, another surrogate marker might be monitored to assess how the modulation of TLR4 expression and activation of a certain type of innate immune response influence the immune players that participate in the later, acquired immune response to RSV. Namely, we have shown previously that in some infants acute RSV illness is accompanied with an increase in CD23-bearing B cells and RSV-specific IgE response [26]. Additional studies are under way to show whether the infants who demonstrate Th2-skewed immune reponse and possibly an increased risk of allergic disease fail to up-regulate TLR4 during acute RSV infection. TLR4 mutations have been associated with endotoxin hyporesponsiveness in humans and represent a possible link to asthma [27]. RSV infection early in childhood has been postulated as a risk for subsequent allergic disease later in life [28,29]. Additional follow-up studies are necessary to show whether our finding of RSV infection failing to induce TLR4 in some children could be a sensitive predictor of abberant response to environmental antigens (allergens).
Acknowledgments
We thank Dr Ante Sabioncello for the valuable help with statistical analysis. Grant support was received from the Croatian Ministry of Science and Technology (0021001 to S.R.) and The Wellcome Trust, UK (to A.G.).
REFERENCES
- 1.Connors M, Collins PL, Firestone CY, Murphy BR. Respiratory syncytial virus (RSV) F, G, M2 (22K) and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J Virol. 1991;65:1634–7. doi: 10.1128/jvi.65.3.1634-1637.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garofalo RP, Habearle H. Epithelial regulation of innate immunity to respiratory syncytial virus. Am J Respir Cell Mol Biol. 2000;23:581–5. doi: 10.1165/ajrcmb.23.5.f204. [DOI] [PubMed] [Google Scholar]
- 3.Tripp RA, Jones L, Anderson LJ. Respiratory syncytial virus G and/or SH glycoproteins modify CC and CXC chemokine mRNA expression in the BALB/c mouse. J Virol. 2000;74:6227–9. doi: 10.1128/jvi.74.13.6227-6229.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tripp RA, Moore D, Jones L, Sullender W, Winter J, Anderson LJ. Respiratory syncytial virus G and/or SH protein alters Th1 cytokines, natural killer cells, and neutrophils responding to pulmonary infection in BALB/c mice. J Virol. 1999;73:7099–107. doi: 10.1128/jvi.73.9.7099-7107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panuska JR, Merolla R, Rebert NA, et al. Respiratory syncytial virus induces IL-10 by human alveolar macrophages. Suppression of early cytokine production and implications for incomplete immunity. J Clin Invest. 1995;96:2445–53. doi: 10.1172/JCI118302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker S, Quay J, Soukup J. Cytokine (tumor necrosis factor, IL-6, and IL-8) production by respiratory syncytial virus-infected human alveolar macrophages. J Immunol. 1991;147:4307–12. [PubMed] [Google Scholar]
- 7.Villani A, Cirino NM, Baldi E, Kester M, McFadden ER, Jr, Panuska JR. Respiratory syncytial virus infection of human mononuclear phagocytes stimulates synthesis of platelet-activating factor. J Biol Chem. 1991;15:5472–9. [PubMed] [Google Scholar]
- 8.Salkind AR, Nichols JE, Roberts NR., Jr Suppressed expression of ICAM-1 and LFA-1 and abrogation of leukocyte collaboration after exposure of human mononuclear leucocytes to respiratory syncytial virus in vitro: comparison with exposure to influenza virus. J Clin Invest. 1991;88:505–11. doi: 10.1172/JCI115332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panuska JR, Midulla F, Cirino NM, et al. Virus-induced alterations in macrophage production of tumor necrosis factor and prostaglandin E2. Am J Physiol. 1990;259:L396–402. doi: 10.1152/ajplung.1990.259.6.L396. [DOI] [PubMed] [Google Scholar]
- 10.Kimpen JLL. Respiratory syncytial virus and asthma. The role of monocytes. Am J Respir Crit Care Med. 2001;163:S7–S9. doi: 10.1164/ajrccm.163.supplement_1.2011110. [DOI] [PubMed] [Google Scholar]
- 11.Sung RY, Hui SH, Wong CK, Lam CW, Yin J. A comparison of cytokine responses in respiratory syncytial virus and influenza A infections in infants. Eur J Pediatr. 2001;160:117–22. doi: 10.1007/s004310000676. [DOI] [PubMed] [Google Scholar]
- 12.Gentile DA, Skoner DP. Effect of respiratory syncytial virus infection during early infancy on the ontogeny of cytokine immune responses. Allergy Asthma Proc. 2002;23:399–405. [PubMed] [Google Scholar]
- 13.Kurt-Jones EA, Popova L, Kwinn L, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 14.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 15.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 16.Haynes LM, Moore DD, Kurt-Jones EA, Finberg RW, Anderson LJ, Tripp RA. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J Virol. 2001;75:10730–7. doi: 10.1128/JVI.75.22.10730-10737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mlinarić-Galinović G, Varda-Brkić D. Nosocomial respiratory syncytial virus infections in children's wards. Diagn Microbiol Infect Dis. 2000;37:237–46. doi: 10.1016/s0732-8893(00)00154-1. [DOI] [PubMed] [Google Scholar]
- 18.Hausmann M, Kiessling S, Mestermann S, et al. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- 19.Song PI, Abraham TA, Park Y, et al. The expression of functional LPS receptor proteins CD14 and toll-like receptor 4 in human corneal cells. Invest Ophthalmol Vis Sci. 2001;42:2867–77. [PubMed] [Google Scholar]
- 20.Mita Y, Dobashi K, Endou K, et al. Toll-like receptor 4 surface expression on human monocytes and B cells is modulated by IL-2 and IL-4. Immunol Lett. 2002;81:71–5. doi: 10.1016/s0165-2478(01)00328-5. [DOI] [PubMed] [Google Scholar]
- 21.Wang PL, Oido-Mori M, Fujii T, et al. Heterogeneous expression of toll-like receptor 4 and downregulation of toll-like receptor 4 expression on human gingival fibroblasts by Porphyromonas gingivalis lipopolysaccharide. Biochem Biophys Res Commun. 2001;288:863–7. doi: 10.1006/bbrc.2001.5842. [DOI] [PubMed] [Google Scholar]
- 22.Nagai Y, Akashi S, Nagafuku M, et al. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–72. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 23.Hancock GE, Speelman DJ, Heers K, Bortell E, Smith J, Cosco C. Generation of atypical pulmonary inflammatory responses in BALB/c mice after immunization with the native attachment (G) glycoprotein of respiratory syncytial virus. J Virol. 1996;70:7783–91. doi: 10.1128/jvi.70.11.7783-7791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bendelja K, Gagro A, Bace A, et al. Predominant type-2 response in infants with respiratory syncytial virus (RSV) infection demonstrated by cytokine flow cytometry. Clin Exp Immunol. 2000;121:332–8. doi: 10.1046/j.1365-2249.2000.01297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bont L, Kavelaars A, Heijnen CJ, van Vught AJ, Kimpen JL. Monocyte interleukin-12 production is inversely related to duration of respiratory failure in respiratory syncytial virus bronchiolitis. J Infect Dis. 2000;181:1772–5. doi: 10.1086/315433. [DOI] [PubMed] [Google Scholar]
- 26.Rabatic S, Gagro A, Lokar-Kolbas R, et al. Increase in CD23+ B cells in infants with bronchiolitis is accompanied by appearance of IgE and IgG4 antibodies specific for respiratory syncytial virus. J Infect Dis. 1997;175:32–7. doi: 10.1093/infdis/175.1.32. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz DA. TLR4 and LPS hyporesponsiveness in humans. Int J Hyg Environ Health. 2002;205:221–7. doi: 10.1078/1438-4639-00117. [DOI] [PubMed] [Google Scholar]
- 28.Sigurs N, Biarnason R, Sigurbergsson F, Kielman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161:1501–7. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 29.Hancock GE, Scheuer CA, Sierzega R, et al. Adaptive immune responses of patients with asthma to the attachment (G) glycoprotein of respiratory syncytial virus. J Infect Dis. 2001;184:1589–93. doi: 10.1086/324583. [DOI] [PubMed] [Google Scholar]