Abstract
Complement activation plays a relevant role in the development of tissue damage under inflammatory conditions, and clinical and experimental observations emphasize its contribution to inflammatory vasculitides. Statins have recently been shown to reduce cardiovascular morbidity independently of plasma cholesterol lowering and in vitro studies support a direct anti-inflammatory action of these drugs. The aim of this study was to verify the in vivo effect of fluvastatin on complement-mediated acute peritoneal inflammation. The effect of oral treatment with fluvastatin was investigated in normo-cholesterolaemic rats that received intraperitoneal injection of either yeast-activated rat serum (Y-act RS) or lipopolysaccharide to induce peritoneal inflammation monitored by the number of PMN recruited in peritoneal fluid washes. In addition, vascular adherence and extravasation of leucocytes were evaluated by direct videomicroscopy examination on mesentery postcapillary venules topically exposed to Y-act RS. The number of PMN in the peritoneal washes of rats treated with fluvastatin was 38% lower than that of untreated animals (P < 0·05) 12 h after LPS injection, and was even lower (56%) in rats treated with Y-act RS already 8 h after injection (P < 0·02). Firm adhesion to endothelium and extravasation of leucocytes evaluated under direct videomicroscopy observation were significantly inhibited in fluvastatin treated rats (77% and 72%, respectively; P < 0·01), 120 min after treatment with Y-act RS. Our results demonstrate that fluvastatin inhibits in vivo complement-dependent acute peritoneal inflammation and suggest a role for statins in preventing the inflammatory flares usually associated with complement activation in chronic diseases, such as SLE or rheumatoid arthritis.
Keywords: fluvastatin, complement, leucocytes, rat, videomicroscopy
INTRODUCTION
The complement (C) system is an important component of innate immunity that actively contributes to host protection against bacterial infections and promotes clearance of immune complexes [1]. More recent data have disclosed an additional role of C in the removal of apoptotic cells through the recognition units of the classical and lectin pathways [2]. The critical importance of this system in host defence is supported by the finding of an in-creased susceptibility to bacterial infections and to immune-complex-mediated diseases observed in patients with inherited complement deficiencies [3].
C activation plays also a key role in the development of inflammation, and it may therefore contribute to tissue alterations in the course of several clinical disorders. Any undesidered effect of C activation that may be harmful to self cells and tissues is usually neutralized by regulators and inhibitors acting both in the fluid phase and on the cell surface [4]. However, unrestricted C activation that overcomes the neutralizing control of the C regulators may often occur in various pathological conditions that are both acute, such as sepsis, multiple trauma, burns or large ischaemia, or chronic, such as autoimmune diseases, and result in tissue damage [5]. Other chronic degenerative diseases, including atherosclerosis, Alzheimer disease, or primary glomerulopathies, are also characterized by the recurrent acute inflammatory phases contributed by C activation [6].
The C system can induce the inflammatory process through the action of different activation products that are mainly derived from C3 and C5 [7]. Evidence collected over the last few years indicate that the terminal C complex (TCC) is also an important proinflammatory product of C activation [8] and may promote adhesion of leucocytes to endothelial cells (EC) and their extravascular migration [9]. Tissue deposition of this complex component was shown to be related with the degree of inflammation in the synovial tissue from patients with rheumatoid arthritis [10,11]. Moreover, the serum levels of TCC appear to be a sensitive marker of disease activity in SLE patients [12], and the complex is easily detectable in the cerebrospinal fluid of patients suffering from primary degenerative neurological syndromes or neuro-SLE [13].
Given the important contribution of the C activation products to inflammation and tissue damage, it is not surprising that different therapeutic strategies have been devised to neutralize these biologically active products including competing peptides and blocking antibodies [14]. C5 is an ideal target because the activation of this component results in the release of the chemotactic fragment C5a and of C5b that initiates the assembly of the cytolytically destructive and proinflammatory complex C5b-9. Humanized scFv against C5 has proved to be beneficial in patients undergoing cardiopulmonary bypass [15] and more recently our group has produced a human scFv directed to the cleavage site of C5 that inhibits the development of antigen-induced arthritis [16]. Based on the available in vitro and in vivo data showing that statins, a family of 3-hydroxy methyl glutaryl Coenzyme A (HMGCoA)-reductase inhibitors, exert anti-inflammatory effects, we decided to evaluate the ability of these drugs to control C-induced inflammation that occurs both in immune and in nonimmune pathological conditions. In vitro, statins have been shown to inhibit the production of chemokines [17,18], the expression of adhesion molecules on endothelial cells [19,20] and the transendothelial migration and chemotaxis of PMN [21]. Also, statins are able to interact within a binding site on β2 integrin (Leucocyte Function Antigen-1) cell adhesion molecule [22] and to inhibit IFN-γ-induced expression of MHC class II on human endothelial cells and macrophages [23]. In vivo, prolonged treatment of hypercholesterolaemic rats with statins reduces adhesion of leucocytes to vascular endothelium triggered by leukotriene (LTB4) or PAF [24]. In addition, statins have been found to be effective in inhibiting adhesion and extravasation of leucocytes stimulated by thrombin [25,26], LPS [18] or in the presence of nitric oxide synthase inhibitors [25] even after short-term administration to normocholesterolaemic rats.
We now present data showing that statins prevent migration of leucocytes into the peritoneal cavity of rats induced by C activation products and provide evidence indicating that this drug inhibits in particular adhesion and transendothelial migration of PMN.
MATERIALS AND METHODS
Drugs and reagents
Fluvastatin (FLU) was provided by Novartis Pharma (Basel, Switzerland). Lipopolysaccharide (LPS) from E. coli O55:B5, recombinant human C5a, and Acridine Orange were purchased from Sigma-Aldrich (Milan, Italy). Thiobutabarbital sodium (Inactin) was obtained from BYK (Konstanz, Germany).
Rat sera
Sera collected from at least 5 normal rats were pooled, divided in small aliquots and kept at −80°C to be used as source of rat C6 deficient (C6def) serum, from a rat colony established at the Department of Nephrology, University of Leiden, the Netherlands, was kindly provided by Prof M. Daha [27].
Preparation of yeast-activated rat serum
Yeast cell suspension and yeast-activated rat serum (Y-act RS) were prepared according to Harrison and Lachmann [28]. Briefly, rat serum (1 ml) was incubated with a 10% yeast cell suspension for 45 min at 37°C to activate the C system through the alternative pathway followed by centrifugation at 2000× g for 15 min and filtration through a Millipore filter (0·45 µm pore size) (Millipore Corp., Bedford, MA, USA) to remove the yeast cells. Under these conditions, the C activity of Y-act RS became undetectable as tested by haemolytic assay on IgM-sensitized sheep red blood cells.
Induction of acute peritoneal inflammation
Male Wistar rats weighing 250–280 g were used for all experiments. The animals were kept under a standardized diet with free food and water intake over the entire study. Two groups of 4 rats were used to assess the effect induced by intraperitoneal (ip) administration of Y-act RS (250 µl) prepared from either C sufficient or C6 deficient rats. An additional 12 rats were used to investigate the inflammatory effect of increasing doses of recombinant human C5a ranging between 10−6m and 10−10m, where 10−6m corresponds to a C5a concentration of 8·6 µg/ml. Control groups of 4 rats received an ip injection of sterile saline. The rats were anaesthetized by intraperitoneal (i.p) injection of 80 mg/kg thiobutabarbital sodium, and half an hour later received an ip injection of the reagents under evaluation to a final volume of 0·5 ml. The peritoneal cavities were then filled with 40 ml of sterile saline injected via an infusion set with a 21G needle. At selected time points (basal, T = 0, and 3, 6, 12 h after injection), the abdomen was gently massaged to favour detachment of adherent peritoneal cells and 5 ml aliquots of peritoneal fluid were collected into a sterile vial containing 3 mm EDTA. Total and differential cell counts were then performed using a haemocytometer, and the number of PMN were also assessed measuring their myeloperoxidase activity [29].
Peritoneal inflammation in FLU-treated rats
A total number of 16 animals divided into two groups of 8 rats received FLU dissolved in a solution of 98% ethanol and water (1 : 10 = vol : vol) by gavage at the dose of either 5 or 10 mg/kg of body weight over a period of 15 days. At the end of treatment, peritoneal inflammation was induced either by Y-act RS (250 µl) or LPS (1 mg/kg body weight). An additional group of 8 animals received the same volume of water and ethanol solution (vehicle-treated group) and served as a control.
Intravital microscopy
Two groups of rats were treated with 5 mg/kg body weight FLU (8 animals) or vehicle (8 animals) as indicated above. Before intravital microscopy analysis, all rats received an i.p. injection of LPS (0·25 mg/kg body weight) and, four hours later, were anaesthetized with sodium thiobarbital (100 mg/kg). Details on the surgical procedure, including insertion of intravascular catheters to monitor blood pressure and to inject the fluorescent leucocyte marker, acridine orange (AO), were reported previously [9]. AO was diluted in sterile saline and then slowly infused over the first 3 h of the experimental procedure at the concentration of 0·025 mg/kg/min and at a rate of 0·5 ml/ h.
After a midline incision of the rat abdomen, a loop of ileal mesentery was exteriorized and carefully draped over a transparent pedestal. The exposed tissue was superfused throughout the study with sterile buffered saline and placed on an adjustable stage of an upright microscope (mod. BX50WI, Olympus Optical Co., Tokyo, Japan). The AO-labelled leucocytes were made visible by epifluorescent transillumination. Images were recorded by CCD camera connected through a PCI interface-board (SensiCam PCO, Kelheim, Germany) to a computer device.
Y-act RS (50 µl) was topically applied to the mesentery over a 10-min period after baseline evaluation and image sequences were recorded intermittently up to 180 min. A control group of 8 untreated rats received only a topical application of 50 µl saline after priming with the same dose of LPS. Segments of 3–5 unbranched postcapillary venules (25–40 µm diameter and 200 µm long) were selected for analysis. Venular diameter and centre-line red blood cell velocity were off-line evaluated using both a video caliper (Image Research, Ontario, Canada) and a customized frame by frame analogical image analysis (Analytica Lite, Milan, Italy). Red blood cell velocity (VRBC) and venular diameter (D) were used to calculate venular wall shear rate (g) by employing the formula g = 8(Vmean/D), where Vmean = VRBC/1·6. Intravascular circulation of leucocytes in postcapillary venules and their extravascular emigration were analysed off-line during playback of the digital file sequences. Labeled leucocytes were classified as rolling (if they moved more slowly than red blood cells thus becoming visible), or adherent (if they remained stationary for more than 30 s). The rolling flux was expressed as the number of leucocytes that were seen moving past a reference point per minute and adherence was measured by counting the number of adherent leucocytes per 200 µm of venule length. Leucocyte emigration out of the vessel was evaluated by counting the cells that had extravasated up to 100 µm away from the wall in parallel with 200 µm vessel segments.
Statistical analysis
Results were expressed as mean ± SD. Data were compared by anova using posthoc analysis for paired multiple comparisons with Fisher's corrected t-test. A P-value ≤0·05 was considered statistically significant.
RESULTS
C activation induces inflammation in the peritoneal cavity
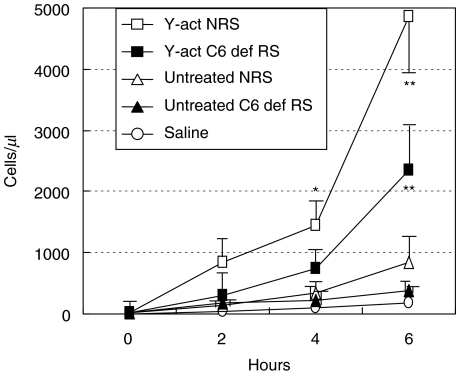
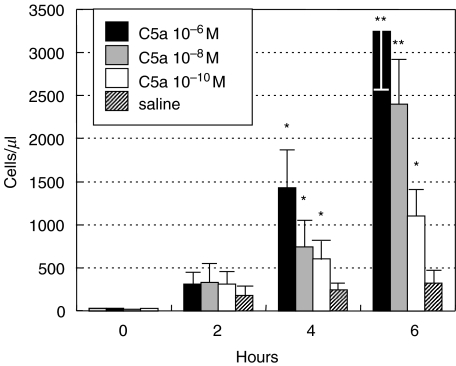
To test the pro-inflammatory effect of C activation products, C was activated in the rat serum by yeast cells through the alternative pathway by incubating for 45 min at 37°C one ml of rat serum with the yeast cell suspension that caused C depletion as measured by haemolytic assay. In the initial experiments different volumes of Y-act RS were tested for their ability to induce inflammation and 250 µl were selected as the lowest volume that was able to induce a substantial leucocyte migration into the peritoneal cavity. As shown in Fig. 1, the number of PMN in the peritoneal cavity started to increase from 1000 cells/µl at 2 h postinjection, doubled by 4 h and reached a value of approximately 5000 cells/µl. It is important to note that treatment with either C6 deficient or normal unactivated rat sera showed to induce a slight increase of inflammatory cells which did not exceeded a number of 850/µl after 6 h. Occasional leucocytes were found in the control rats treated with sterile saline. Essentially similar results were obtained by using yeast treated heat inactivated serum or yeast treated saline (data not shown). To evaluate the contribution of C5a and of the terminal C complex (TCC) to PMN migration, the animals received an ip injection of Y-act RS prepared either with C sufficient or with C6 deficient rat sera. As shown in Fig. 1. Y-act RS obtained from C6 deficient rat serum elicited a PMN response that was less than half of that induced by Y-act RS prepared with C sufficient serum suggesting that TCC contributed with C5a to promote PMN intraperitoneal migration. Experiments performed on rats receiving an ip injection of recombinant human C5a revealed a dose dependent migration of PMN in numbers comparable with those induced by yeast activated C6 deficient serum (Fig. 2).
Fig. 1.
Acute peritoneal inflammation induced by yeast activated rat serum. Five groups of 4 rats received an ip injection of one of the following reagents: yeast-activated normal rat serum (Y-act NRS, □), yeast-activated C6 deficient rat serum (Y-act C6def RS, ▪), untreated normal (NRS, Δ) or C6 def rat sera (C6def RS, ▴), and sterile saline. The PMN number was counted in the peritoneal washings obtained at different time intervals after injection. Data are expressed as mean ± SD; *P < 0·05, and **P < 0·01 compared with the other groups at the same time of observation.
Fig. 2.
Acute peritoneal inflammation induced by C5a. Four groups of 4 rats received an ip injection of increasing concentrations of C5a or sterile saline and the PMN number was counted in the peritoneal washings obtained at different time intervals after injection. Data are expressed as mean ± SD; *P < 0·05, or **P < 0·01 compared with saline-treated animals (used as controls) at the same time of observation.
Statin inhibits the pro-inflammatory effect of C activation
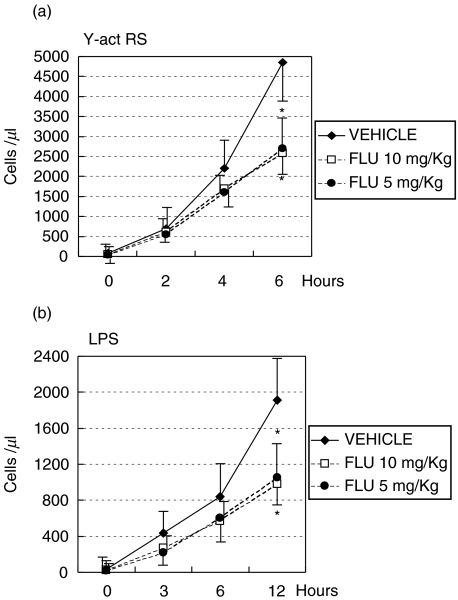
Having established the optimal conditions for the induction of C-dependent acute peritonitis, we decided to investigate the effect of a prolonged treatment of rats with FLU on the peritoneal inflammation induced by Y-act RS. To this end, FLU was administered by gavage to two groups of rats at doses of 10 mg and 5 mg/kg body weight, respectively, and the numbers of PMN migrating into the peritoneal cavity at different time intervals following the injection of Y-act RS were compared with those observed in vehicle treated rats. As expected, the number of peritoneal PMN in the control group rose progressively with time to a mean value of 4800 cell/µl 6 h after injection of Y-act RS. By contrast, the rats treated with 10 mg/kg fluvastatin showed a reduced number of PMN compared to the control rats at 4 h postinjection. The difference at this time was not significant, but reached the level of significance 6 h after injection of Y-act RS (Fig. 3a). FLU proved to be effective in inhibiting intraperitoneal migration of PMN also at the dose of 5 mg/kg. To compare the effect of Y-act RS with that of another pro-inflammatory substance, we decided to test LPS, a bacterial product that is able to activate C through the alternative pathway. Unlike Y-act RS, LPS was marginally effective and promoted migration of only 800 cell/µl at 6 h, that doubled at 12 h reaching a number that was in any case much lower than that mobilized by Y-act RS (Fig. 3b). FLU exhibited an inhibitory activity also on the migration of PMN induced by LPS, despite the relatively low number of mobilized PMN.
Fig. 3.
C-mediated acute peritoneal inflammation induced in FLU-treated rats. Six groups of 4 rats were daily treated with either FLU at two different doses of 10 mg/kg (□) or 5 mg/kg (•) body weight, or with vehicle (water plus ethanol alone, ⋄). At the end of treatment each rat received an ip injection of (a) yeast-activated normal rat serum (Y-act RS) or (b) LPS (1 mg/kg). The PMN number was counted in the peritoneal washings obtained at different time intervals after injection. Data are expressed as mean ±SD; *P < 0·05, when compared with the results obtained in the vehicle-treated rats at the same time of observation.
Statin inhibits adhesion of leucocytes to vascular endothelium and their extravascular emigration from the mesenteric venules
The finding that fluvastatin inhibited the intraperitoneal migration of leucocytes stimulated by Y-act RS injection, prompted us to elucidate the mechanism of action of this statin using intravital microscopy to follow the migration of PMN in vivo. Videomicroscopy experiments allowed us to directly assess the pro-inflammatory effects of Y-act RS applied to the abluminal side of mesenteric microvessels of animals that had been pretreated with an ip injection of LPS. Unfortunately, local treatment with the volume of Y-act RS (250 µl) used for i.p. injection caused haemodynamic disturbances in the exposed vessels. We were forced to reduce the volume of Y-act RS to 50 µl, that failed to induce inflammation unless the animals were pretreated with LPS (0·25 mg/kg body weight) for 4 h. As shown in our previous experiments, LPS injected ip had no significant effect on leucocyte extravasation at 4–8 h. Under these conditions, blood pressure levels did not change significantly nor overt modification of red blood cells velocity was detected during intravital microscopy examination. The values of venular shear rate observed at the start of the videomicroscopy analysis in the control (670 ± 84 s−1) or in FLU-treated animals (642 ± 94 s−1) were comparable. Control rats primed with LPS and treated with saline did not show any significant change in the rolling flux or vascular adhesion and extravasation throughout the whole period of observation.
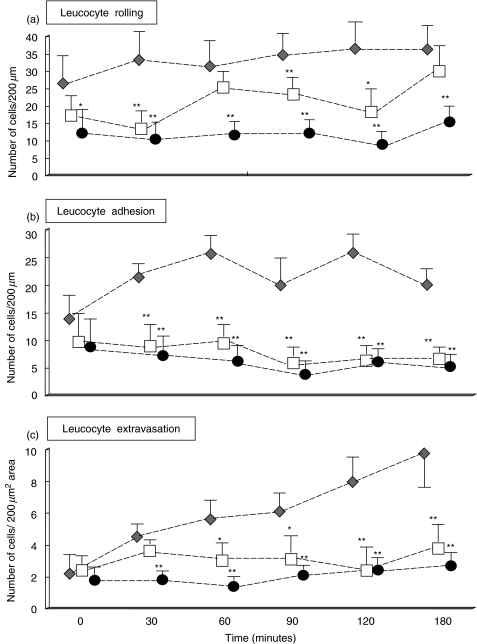
The data of leucocyte mobilization obtained after application of Y-act RS to the mesenteric venules are presented in Fig. 4. The rolling flux and the number of adherent leucocytes were slightly reduced in FLU treated animals primed with LPS prior to stimulation with Y-act RS when compared to the values of vehicle treated animals, though not significantly.
Fig. 4.
Intravital videomicroscopy evaluation of C-stimulated leucocyte trafficking in FLU-treated rats. Two groups of 8 rats were treated for 15 days with either 5 mg/kg body weight FLU (□) or vehicle (⋄). On the day of the experiment, all rats received an ip injection of LPS (0·25 mg/kg) followed 4 h later by topical application of yeast-activated normal rat serum (50 µl). An additional group of 8 untreated rats received only a topical application of saline 4 h after the ip treatment with LPS (0·25 mg/kg), and served as controls (•). Data of the rolling flux, adhesion and extravasation of leucocytes are expressed as mean ± SD; *P < 0·05, or **P < 0·01, compared with the vehicle-treated animals at each considered time.
Exposure to Y-act RS led to a slight increase in the values of rolling flux which was partially inhibited by fluvastatin (Fig. 4a).
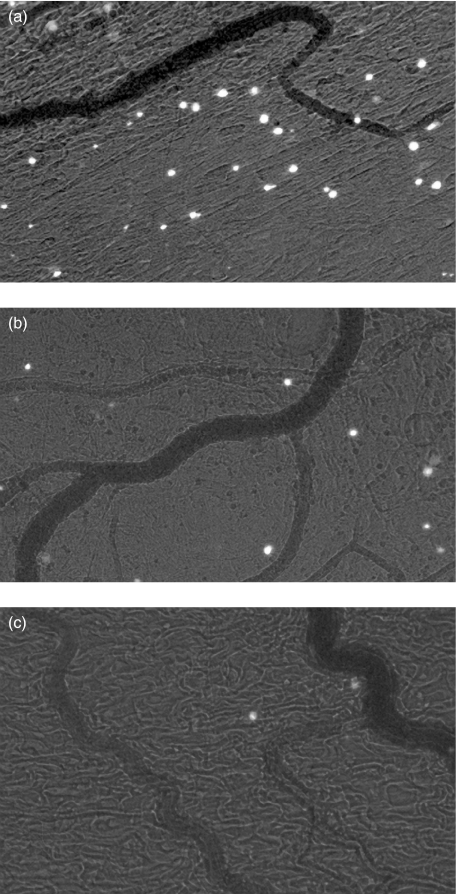
This statin had a more clear effect on leucocyte adherence. Stable arrest of fluorescinated leucocytes on endothelial cells was observed in vehicle treated animals after Y-act RS superfusion with peak levels of 25 cells/200 µm vessel lenght at 60 min (Fig. 4b). Treatment with FLU caused a significant decrease in the number of adherent leucocytes that started 30 min after application of Y-act RS and persisted for the entire period of observation up to 3 h. A similar inhibition pattern of leucocytes extravasation was noticed in rats treated with FLU. Fifty percentage less cells migrated out of the vessels in rats receiving FLU as compared to the control group, 1 h after exposure to Y-act RS (P < 0·05), and the difference became more marked at the end of the observation (3·8 cells/200 µm2 in flu-treated rats vs. 9·2/200 µm2 of controls, P < 0·01) (Figs 4c and 5)
Fig. 5.
Videomicrograph images showing the degree of extravasation of fluorescinated leucocytes following exposure to yeast-activated rat serum in the mesentery of rats treated with either (a) vehicle or (b) FLU. (c) Untreated rats exposed to sterile saline served as controls. Magnification, ×10.
Effect of FLU treatment on the levels of plasma lipids
We next decided to ascertain whether the ability of FLU to affect leucocyte migration was associated with its known pharmacological effect on the cholesterol level. To this end, the concentration of total plasma cholesterol and of triglycerides was measured at various time intervals from the start of treatment with FLU (fluvastatin) and compared with the values obtained from control animals. As shown in Table 1, treatment of rats with this drug had no effect on the plasma levels of both total cholesterol and triglycerides and did not induce any change in the C reactive protein value.
Table 1.
Values of plasma lipid levels and of serum C reactive protein concentrations in the examined groups of animals. Data are expressed as mean ± SD, and concern a selected group of rats whose blood was drawn either before (Basal values, n = 10 animals) and after treatment with Fluvastatin (10 mg/kg body weight for 15 days, n = 5 animals) or Vehicle (water plus ethanol alone, n = 5 animals)
| Examined parameter | Basal values | After fluvastatin treatment | After vehicle treatment |
|---|---|---|---|
| Total cholesterol(mg/dl) | 78·0 ± 8·0 | 74·0 ± 11·0 | 76·0 ± 6·0 |
| Triglycerides (mg/dl) | 115·0 ± 8·0 | 108·0 ± 14·0 | 105·0 ± 11·0 |
| C reactive protein(mg/dl) | 1·22 ± 0·16 | 1·08 ± 0·20 | 1·12 ± 0·25 |
DISCUSSION
A large body of evidence collected over the years indicates that the C system contributes to the induction and the maintenance of the inflammatory process in several clinical conditions not necessarily immune-mediated. In the present investigation we have established a model system of C-mediated acute inflammation induced in rats by ip injection of Y-act RS and show that the inflammatory reaction can be inhibited by prolonged oral treatment with FLU.
Our aim was to use a C-mediated experimental approach that mimics to a large extent the situation encountered in vivo in the course of bacterial and fungal infections. To this end, we decided to follow a well established procedure to activate C through the alternative pathway using homologous serum treated with yeast cells as a source of pro-inflammatory stimuli [30,31]. The evidence that C plays a critical role in this model is provided by our failure to promote the inflammatory reaction using serum that had been heat inactivated prior to treatment with yeast cells.
Several biologically active products are released as a result of incubation of rat serum with yeast cells, including C3a, C5a and TCC. However, C3a is unlikely to contribute in a significant manner to the accumulation of PMN in the peritoneal cavity under our experimental conditions since DiScipio et al. [32] clearly demonstrated that the C3 peptide failed to induce stable adherence of neutrophils to the endothelium of postcapillary venules and leucocyte extravasation in the rabbit mesentery superfused with this C activation product.
C5a seems to play a more direct role in this inflammatory process as suggested by the substantial number of PMN found in the peritoneal washes of rats 6 h after injection of C5a. These data are compatible with the known ability of C5a to up-regulate the expression of adhesion molecules on endothelium [33] and are in line with previous findings showing that C5a stimulates adhesion of PMN to the endothelium and their egression across the wall of mesenteric venules in rabbits and rats [9,32]. C5a also proved to be effective in promoting pulmonary sequestration of PMN when administered into the airway or into the pulmonary artery [34] further supporting the critical role of this chemotactic peptide as an important pro-inflammatory agent in vivo. However, Y-act RS also contains elevated levels of TCC, which are shown in the present study to significantly contribute to the accumulation of PMN in the peritoneal cavity of rats receiving Y-act RS. The evidence supporting this conclusion is provided by the finding that yeast cell-activated C6 deficient serum mobilized a number of PMN comparable with that found in rats receiving purified C5a, but definitely lower than the total number recruited by Y-act RS. These results were not surprising since we had previously shown that the cytolytically inactive TCC is still able to interact with EC up-regulating the expression of adhesion molecules [8] and stimulates the transendothelial migration of PMN both in vitro and in vivo[9].
FLU administered by gavage at the dose of either 10 or 5 mg/kg significantly reduced the number of PMN that migrated into the peritoneal cavity following injection of Y-act RS. The inhibitory effect of FLU was also seen in LPS-treated rats, though the extravasation of PMN in the latter group of animals was delayed to about 12 h, as observed also by Ajuebor et al. [35]. Romano et al.[18] reported essentially similar results using other statins, lovastatin and simvastatin, to inhibit the extravascular efflux of leucocytes stimulated by LPS in a mouse air-pouch model. The fact that FLU and other statins inhibit recruitment of leucocytes by different pro-inflammatory stimuli suggests that these drugs may control common mechanisms involved in the development of the inflammatory process. Endothelial cells represent a potential target of the inhibitory effects of FLU, as indicated by our previous finding that this drug down-regulates the expression of E-selectin and ICAM-1 by human cultured ECs (HUVEC) stimulated with antiphospholipid antibodies, cytokines or LPS [19]. Other statin analogue, such as pravastatin and cerivastatin, were shown to be effective in inhibiting the chemotaxis of PMN and their transmigration across HUVEC monolayers in response to cytokines [21,36], and simvastatin and lovastatin were able to reduce the release of MCP-1 by stimulated cultured peripheral blood mononuclear cells and ECs [18].
All three steps of leucocyte interaction with the endothelium, including rolling, adhesion and transendothelium migration, were inhibited by treatment with FLU, though to a different extent. This was confirmed using direct intravital videomicroscopy to analyse the fate of leucocytes circulating in the mesenteric vessels superfused with Y-act RS in rats primed with LPS. Our aim was to apply onto the rat mesentery the same volume of Y-act RS (250 µl) that induced a significant sequestration of PMN when injected into the peritoneal cavity. However, exposure of mesentery to high volumes of Y-act RS caused alterations of the arteriolar diameters and modifications of vascular flow confirming previous observations reported by other groups that zymosan particles infused intravascularly in rats induced systemic haemodynamic modifications [37,38]. This problem was circumvented by applying 50 µl of Y-act RS, but this small volume of activated rat serum had a markedly reduced activity in mobilizing circulating leucocytes and required priming of rats with LPS to be effective. It's worth emphasizing that the inflammatory reaction elicited by the low dose of LPS after 4 h was insufficient to induce any significant increase of leucocyte margination.
The beneficial effect of FLU as an agent capable of inhibiting leucocyte adherence and extravasation induced by PAF and LTB4 has been investigated by Kimura et al. [24] in hypercholesterolaemic rats that manifest a markedly increased leucocyte– endothelial cell interaction. The anti-inflammatory activity of FLU observed in our study appears to be independent from its ability to lower the cholesterol level since the rats used in our study were normocholesterolaemic and the level of cholesterol remained unchanged after chronic treatment with FLU. Similar results were obtained by Pruefer et al. [25] and Stalker et al. [26] in normocholesterolaemic rats treated with other statins and exposed to different pro-inflammatory stimuli.
It is well established that cholesterol is not the only product of the ‘mevalonate pathway’ to be selectively inhibited by statins. Evidence has been collected in favour of a role played by mevalonate metabolites in inducing several regulatory effects on cell physiology. These effects include post-trascriptional isoprenylation of membrane-associated proteins, which influence signal transduction, cell differentiation and proliferation, endo- and exocitosis, and cytoskeleton dynamics [39]. Statins have been shown to directly induce apoptosis of different leucocyte cells, and modulate migration of neutrophils owing to some potential direct effects also exerted on mesenchymal cells [36]. As suggested by ex vivo models of atherosclerosis [17,40], statins also up-regulate NO synthase activity, which in turn limit ECs dysfunction resulting in reduced chemokine production and monocytes infiltration into the areas of early hyperlipidaemic vascular lesions. Experimental data support the view that statins can inhibit in vitro the activation of endothelial cells by exerting detectable antioxidant effects [41]. Statins have also been shown to exert an anti-inflammatory effect by interfering with MHC class II expression [23], and by inhibiting stable adhesion between leucocytes and endothelial cells as a result of its binding to an allosteric site of LFA-1 [22].
In conclusion, the results of the present study show that FLU is effective in inhibiting the local recruitment of PMN triggered by the activation of C components and suggest that statins may be proposed as an additional therapeutic tool to control C–mediated inflammatory damage of different tissues under a variety of pathological conditions.
Acknowledgments
This work was supported by grants provided by the Italian Ministero dell’Istruzione, dell’Università e della Ricerca (60% and Cofin) and European concerted action (contract QLG1-CT-2001–01039).
REFERENCES
- 1.Müller-Eberhard HJ. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321–47. doi: 10.1146/annurev.bi.57.070188.001541. [DOI] [PubMed] [Google Scholar]
- 2.Rus HG, Niculescu FI, Shin ML. Role of the C5b-9 complement complex in cell cycle and apoptosis. Immunol Rev. 2001;180:49–55. doi: 10.1034/j.1600-065x.2001.1800104.x. [DOI] [PubMed] [Google Scholar]
- 3.Morgan BP, Walport MJ. Complement deficiency and disease. Immunol Today. 1991;12:301–6. doi: 10.1016/0167-5699(91)90003-C. [DOI] [PubMed] [Google Scholar]
- 4.Liszewski MK, Farries TC, Lublin DM, Rooney IA, Atkinson JP. Control of the complement system. Adv Immunol. 1996;61:201–83. doi: 10.1016/s0065-2776(08)60868-8. [DOI] [PubMed] [Google Scholar]
- 5.Kirschfink M. Targeting complement in therapy. Immunol Rev. 2001;180:177–89. doi: 10.1034/j.1600-065x.2001.1800116.x. [DOI] [PubMed] [Google Scholar]
- 6.Tedesco F, Fischetti F, Pausa M, Dobrina A, Sim RB, Daha MR. Complement–endothelial cell interactions: pathophysiological implications. Mol Immunol. 1999;36:261–8. doi: 10.1016/s0161-5890(99)90054-8. [DOI] [PubMed] [Google Scholar]
- 7.Hugli TE. Biochemistry and biology of anaphylatoxins. Complement. 1986;3:111–27. doi: 10.1159/000467889. [DOI] [PubMed] [Google Scholar]
- 8.Tedesco F, Pausa M, Nardon E, Introna M, Mantovani A, Dobrina A. The cytolytically inactive terminal complement complex activates endothelial cells to express adhesion molecules and tissue factor procoagulant activity. J Exp Med. 1997;185:1619–27. doi: 10.1084/jem.185.9.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobrina A, Pausa M, Fischetti F, et al. Cytolytically inactive terminal complement complex causes transendothelial migration of polymorphonuclear leukocytes in vitro and in vivo. Blood. 2002;99:185–92. doi: 10.1182/blood.v99.1.185. [DOI] [PubMed] [Google Scholar]
- 10.Neumann E, Barnum SR, Tarner IH, et al. Local production of complement proteins in rheumatoid arthritis synovium. Arthritis Rheum. 2002;46:934–45. doi: 10.1002/art.10183. [DOI] [PubMed] [Google Scholar]
- 11.Oleesky DA, Daniels RH, Williams BD, Amos N, Morgan BP. Terminal complement complexes and C1/C1 inhibitor complexes in rheumatoid arthritis and other arthritis conditions. Clin Exp Immunol. 1991;84:250–5. [PMC free article] [PubMed] [Google Scholar]
- 12.Gawryl MS, Chudwin DS, Langlois PF, Lint TF. The terminal complement complex, C5b-9, a marker of disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 1988;31:188–95. doi: 10.1002/art.1780310206. [DOI] [PubMed] [Google Scholar]
- 13.Sanders ME, Alexander EL, Koski CL, Frank MM, Joiner KA. Detection of activated terminal complement (C5b−9) in cerebrospinal fluid from patients with central nervous system involvement of primary Sjogren's syndrome or systemic lupus erythematosus. J Immunol. 1987;138:2095–9. [PubMed] [Google Scholar]
- 14.Sahu A, Morikis D, Lambris JD. Complement inhibitors targeting C3, C4, and C5. In: Lambris JD, Holers VM, editors. Therapeutic Interventions in the Complement System. Totowa NJ: Humana Press Inc.; 2000. pp. 75–112. [Google Scholar]
- 15.Fitch JC, Rollins S, Matis L, et al. Pharmacology and biological efficacy of a recombinant, humanized, single-chain antibody C5 complement inhibitor in patients undergoing coronary artery bypass graft surgery with cardiopulmonary bypass. Circulation. 1999;100:2499–506. doi: 10.1161/01.cir.100.25.2499. [DOI] [PubMed] [Google Scholar]
- 16.Marzari R, Sblattero D, Macor P, et al. The cleavage site of C5 from man and animals as a common target for neutralizing human monoclonal antibodies: in vitro and in vivo studies. Eur J Immunol. 2002;32:2773–82. doi: 10.1002/1521-4141(2002010)32:10<2773::AID-IMMU2773>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 17.Ni W, Egashira K, Kataoka C, Kitamoto S, Koyanagi M, Inoue S, Takeshita A. Antiinflammatory and antiarteriosclerotic actions of HMG-CoA reductase inhibitors in a rat model of chonic inhibition of nitric oxide synthesis. Circ Res. 2001;89:415–21. doi: 10.1161/hh1701.096614. [DOI] [PubMed] [Google Scholar]
- 18.Romano M, Diomede L, Sironi M, et al. Inhibition of monocyte chemotactic Protein-1 synthesis by statins. Laboratory Invest. 2000;80:1095–100. doi: 10.1038/labinvest.3780115. [DOI] [PubMed] [Google Scholar]
- 19.Meroni PL, Raschi E, Testoni C, et al. Statins prevent endothelial cell activation induced by antiphospholipid (anti-β2-glycoprotein I) antibodies. Effect on the proadhesive and proinflammatory phenotype. Arthritis Rheum. 2001;44:2870–8. doi: 10.1002/1529-0131(200112)44:12<2870::aid-art475>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 20.Meroni PL, Luzzana C, Ventura D. Anti-inflammatory and immunomodulating properties of statins. An additional tool for the therapeutic approach of systemic autoimmune diseases? Clin Rev Allergy Immunol. 2002;23:263–77. doi: 10.1385/CRIAI:23:3:263. [DOI] [PubMed] [Google Scholar]
- 21.Dunzendorfer S, Rothbucher D, Schratzberger P, Reinish N, Kähel CM, Wiedermann CJ. Mevalonate-dependent inhibition of transendothelial migration and chemotaxis of human peripheral blood neutrophils by pravastatin. Circ Res. 1997;81:963–9. doi: 10.1161/01.res.81.6.963. [DOI] [PubMed] [Google Scholar]
- 22.Weitz-Schmidt G, Welzenbach K, Brinkmann V, et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7:687–92. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 23.Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6:1399–402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 24.Kimura M, Kurose I, Russel J, Granger N. Effects of fluvastatin on leukocyte-endothelial cell adhesion in hypercholesterolemic rats. Arterioscler Thromb Vasc Biol. 1997;17:1521–6. doi: 10.1161/01.atv.17.8.1521. [DOI] [PubMed] [Google Scholar]
- 25.Pruefer D, Scalia R, Lefer AM. Simvastatin inhibits leukocyte–endothelial cell interactions and protects against inflammatory processes in normocholesterolemic rats. Arterioscler Thromb Vasc Biol. 1999;19:2894–900. doi: 10.1161/01.atv.19.12.2894. [DOI] [PubMed] [Google Scholar]
- 26.Stalker T, Lefer AM, Scalia R. A new HMG-CoA reductase inhibitor, rosuvastatin, exerts anti-inflammatory effects on the microvascular endothelium: the role of mevalonic acid. Br J Pharmacol. 2001;133:406–12. doi: 10.1038/sj.bjp.0704070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leenaerts PL, Stad RK, Hall BM, Van Damme BJ, Vanrenterghem Y, Daha MR. Hereditary C6 deficiency in a strain of PVG/c rats. Clin Exp Immunol. 1994;97:478–82. doi: 10.1111/j.1365-2249.1994.tb06113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison RA, Lachmann PJ. Complement technology. In: Weir DM, Herzenberg LA, Blackwell C, Herzenberg LA, editors. Handbook of Experimental Immunology. Oxford: Blackwell; 1986. pp. 31–49. [Google Scholar]
- 29.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–50. [PubMed] [Google Scholar]
- 30.Forrest MJ, Jose PJ, Williams TJ. Kinetics of the generation and action of chemical mediators in zymosan-induced inflammation in the rabbit peritoneal cavity. Br J Pharmacol. 1986;89:719–30. doi: 10.1111/j.1476-5381.1986.tb11176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Issekutz AC, Movat KW, Movat HZ. Enhanced vascular permeability and haemorrhage-inducing activity of zymosan-activated plasma. Clin Exp Immunol. 1980;41:505–11. [PMC free article] [PubMed] [Google Scholar]
- 32.DiScipio RG, Daffern PJ, Jagels MA, Broide DH, Sriramarao PJ. A comparison of C3a and C5a-mediated stable adhesion of rolling eosinophils in postcapillary venules and transendothelial migration in vitro and in vivo. J Immunol. 1999;162:1127–36. [PubMed] [Google Scholar]
- 33.Ember JA, Jagels MA, Hugli TE. Characterization of complement anaphylatoxins and their biological responses. In: Volanakis JE, Frank MM, editors. The Human Complement System. New York: Marcel Dekker; 1998. pp. 241–84. [Google Scholar]
- 34.Lien DC, Henson PM, Capen RL, et al. Neutrophyl kinetics in the pulmonary microcirculation during acute inflammation. Laboratory Invest. 1991;65:145–59. [PubMed] [Google Scholar]
- 35.Ajuebor MN, Das AM, Viràg L, Flower RJ, Szabò C, Perretti M. Role of resident macrophages and mast cells in chemokine production and neutrophil migration in acute inflammation: evidence for an inhibitory loop involving endogenous IL-10. J Immunol. 1999;162:1685–91. [PubMed] [Google Scholar]
- 36.Kaneider NC, Reinisch CM, Dunzendorfer S, Maierhofer C, Djanani A, Wiedermann CJ. Induction of apoptosis and inhibition of migration of inflammatory and vascular wall cells by cerivastatin. Atherosclerosis. 2001;158:23–33. doi: 10.1016/s0021-9150(00)00764-4. [DOI] [PubMed] [Google Scholar]
- 37.Lübbe AS, Garrison RN, Harris PD. Endothelium-dependent microvascular responses to activated complement. J Surg Res. 1994;57:654–60. doi: 10.1006/jsre.1994.1196. [DOI] [PubMed] [Google Scholar]
- 38.Ishikawa S, Tsukada H, Bhattacharya J. Soluble complex of complement increases hydraulic conductivity in single microvessels of rat lung. J Clin Invest. 1993;91:103–9. doi: 10.1172/JCI116157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bellosta S, Bernini F, Ferri N, et al. Direct vascular effects of HMG-CoA reductase inhibitors. Atherosclerosis. 1998;137:S101–S109. doi: 10.1016/s0021-9150(97)00319-5. [DOI] [PubMed] [Google Scholar]
- 40.Sumi D, Hayashi T, Thakur NK, Jayachandran M, Asai Y, Kano H, Matsui H, Iguchi A. A HMG-CoA reductase inhibitor possesses a potent anti-atherosclerotic effect other than serum lipid lowering effects. The relevance of endothelial nitric oxide synthase and superoxide anion scavenging action. Atherosclerosis. 2001;155:347–57. doi: 10.1016/s0021-9150(00)00597-9. [DOI] [PubMed] [Google Scholar]
- 41.Nakashima A, Ohtawa M, Iwasaki K, Wada M, Kuroda N, Nakashima K. Inhibitory effects of fluvastatin and its metabolites on the formation of several reactive oxygen species. Life Sci. 2001;69:1381–9. doi: 10.1016/s0024-3205(01)01223-1. [DOI] [PubMed] [Google Scholar]