Abstract
The aetiology of chronic fatigue syndrome (CFS) is not known. However, it has been suggested that CFS may be associated with underlying immune activation resulting in a Th2-type response. We measured intracellular production of interferon (IFN)-γ and interleukin (IL)-2; type 1 cytokines), IL-4 (type 2) and IL-10 (regulatory) by both polyclonally stimulated and non-stimulated CD4 and CD8 lymphocytes from patients with CFS and control subjects by flow cytometry. After polyclonal activation we found evidence of a significant bias towards Th2- and Tc2-type immune responses in CFS compared to controls. In contrast, levels of IFN-γ, IL-2 and IL-10-producing cells were similar in both study groups. Non-stimulated cultures revealed significantly higher levels of T cells producing IFN-γ or IL-4 in CFS patients. Concluding, we show evidence for an effector memory cell bias towards type 2 responsiveness in patients with CFS, as well as ongoing type 0 immune activation in unstimulated cultures of peripheral blood cells.
Keywords: chronic fatigue syndrome, cytokines, immune activation, Th1/Th2 cytokines
INTRODUCTION
Chronic fatigue syndrome (CFS) is an illness characterized by severe disabling fatigue lasting for at least 6 months and made worse by minimal physical or mental exertion, for which there is no adequate medical explanation. There are no key features or typical symptoms, but a sore throat, depression and myalgia may all be present [1–3]. Factors causing the condition remain unclear, although there is some consensus that psychological and social factors influence outcome. Altered hypothalamus–pituitary–adrenal gland (HPA) axis activity has been identified as a feature, as has the frequent association of CFS with viral infections, suggesting that under certain circumstances virus infection is a prelude to the development of CFS [4–8].
In the light of observations in relation to viruses, a number of studies have sought evidence of abnormalities in immune function, but in general there has been no consistent pattern observed, and abnormalities rarely correlate with clinical status [9–13] even though immunological changes have been observed and reported frequently. Most recently, the Th1/Th2 paradigm of immune-mediated disease has been invoked and explored in relation to CFS [14–16]. One of the main supporting arguments for this proposal is the frequent reporting of a reduction in natural killer (NK) cell activity in CFS [10,17,18]. NK cells play a key role in the generation of Th1 type antiviral responses, and loss of their activity could result in a Th2 bias and persistent viral activation and chronic infection. More direct evidence of potential Th2 bias has been reported recently [15] in a study in which an increased level of interleukin (IL)-4 mRNA was associated with a CFS-like syndrome in Gulf War veterans.
Cytokines are the main parameters measured in assessing Th1/Th2 balance and information on cytokine levels and the nature of cellular production could provide important insights into the immune hypothesis in relation to CFS. To date few studies have examined Th1/Th2 balance directly in this disorder. Studies that have been performed have employed enzyme-linked immunosorbent assay (ELISA) or bioassay measurement of cytokines in supernatants from peripheral blood mononuclear cell culture [19] or from serum samples. However, these approaches are not able to provide a clear picture of Th1/Th2 balance. First, the relative contribution of individual cell subsets to cytokine production, and whether they are lymphocytes or macrophages, cannot be determined. Subtle changes in lymphocyte polarization could be masked by cytokine production by non-lymphoid cells. In addition, the presence of soluble natural inhibitors of cytokine function in serum is usually not determined simultaneously, and therefore the functional balance of the immune system remains obscured. Finally, cytokines are known to be sensitive to degradation in serum and are present typically only at the limits of detection of conventional assays [20]. In contrast with these approaches, in recent years direct visualization of cytokine production, using intracellular staining and flow cytometry, has become a powerful technique for examining the immune response, being both sensitive and cell-specific [21–23]. This method therefore allows direct enumeration of polarized, cytokine-producing effector cells. Additionally the use of a whole-blood system avoids potential subtle effects on lymphocyte function arising from gradient separations [22,24–26], and is also reported to yield greater cytokine production when compared with purified peripheral blood mononuclear cell (PBMC) preparations [27]. The choice of phorbol esters and ionomycin as stimulants that act independently of accessory cells is based on earlier work [23], showing their superiority in terms of their ability to induce intracellular cytokine secretion to lectins such as phytohaemagglutinin [21].
With these technical issues in mind, in the present study we examined the frequency of type 1 (interferon (IFN)-γ and IL-2-producing), type 2 (IL-4) and regulatory (IL-10) CD4 and CD8 T cells by direct visualization of intracellular cytokine production in CFS patients and healthy blood donors. Our results demonstrate an expansion of type 2 effector memory cells in CFS patients.
MATERIALS AND METHODS
Patients
Blood samples in this study were drawn from 35 patients (13 males and 22 females) from our clinic (Table 1), which receives both secondary and tertiary referrals. In order to recruit patients fulfilling criteria for CFS, our published experience is that it is necessary to screen approximately three patients for every two recruited [28]. The clinic is a well-established assessment and treatment centre for CFS. It is the only such centre in South London, and has published results previously on many aspects of the illness. Its patients are known to be very typical of CFS patients seen in specialist care in the United Kingdom, United States and Australia [29] but, like all specialist clinics, are not typical of patients seen in primary care [30].
Table 1.
Demographic data on patients with chronic fatigue syndrome (CFS)
| Minimum | Maximum | Mean | s.d. | |
|---|---|---|---|---|
| Age (years) | 19 | 62 | 39·0 | 12·4 |
| Duration of CFS1 (years) | 1·5 | 16·0 | 5·1 | 4·1 |
| Weight (kg) | 44·1 | 95·0 | 63·3 | 13·3 |
| Height (cm) | 152·0 | 183·0 | 169·8 | 8·1 |
| Total WSAS2 disability score (max = 40) | 5·0 | 38·0 | 26·2 | 8·9 |
| Total GHQ3 score (depression/anxiety; max = 36) | 3·0 | 29·0 | 16·8 | 7·7 |
| Total fatigue score (max = 33) | 17·0 | 33·0 | 26·4 | 4·5 |
| Physical fatigue score (max = 21) | 9·0 | 21·0 | 17·3 | 3·2 |
| Mental fatigue score (max = 12) | 4·0 | 12·0 | 9·1 | 2·4 |
CFS: chronic fatigue syndrome;
WSAS: Work and Social Adjustment Scale;
GHQ: General Health Questionnaire. s.d.: standard deviation.
All patients attending the service complete routinely a well-established group of questionnaires, which we have reported on previous occasions. For the purpose of this study, the questionnaires that we used were: (1) Chalder Fatigue Scale: a questionnaire measuring subjective physical and mental fatigue and fatigability [31]; (2) General Health Questionnaire (GHQ): a scale measuring psychological distress [32]; and (3) Work and Social Adjustment: a simple visual analysis scale measuring self-reported functioning in five domains (work, social, domestic, leisure, relationship) [33].
Consecutive referrals were assessed fully using a semistructured interview [34], and those fulfilling the Center for Diseases Control (CDC) diagnostic criteria for CFS [1] were asked to provide blood samples. In keeping with our normal procedures all patients are also assessed using a semistructured psychiatric interview and checklist, designed to ensure that patients fulfilled the criteria for the CFS case definition, and to assess the presence of psychiatric comorbidity [34]. Thus, patients suffering from a serious psychiatric disorder such as psychosis, eating disorder or substance dependence were excluded. The presence of other, non-exclusionary psychiatric conditions such as depression or anxiety disorders was also assessed. Similarly, organic conditions that might explain fatigue were ruled out by ensuring there were no abnormalities on physical examination and relevant investigation, with a minimum of urinalysis, full blood count, urea and electrolytes, liver function tests, calcium and phosphate, thyroid function tests, morning cortisol and antigliadin antibodies. Medication use over the previous 2 months was assessed by patient history. Patients not meeting the CDC criteria or with concomitant physical illness were excluded from the study.
As a control group, we obtained blood from 28 healthy hospital/laboratory professionals matching for sex and age.
Cell culture and intracellular cytokine staining
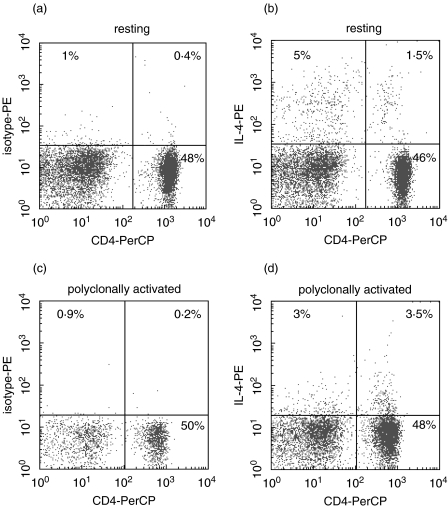
Flow cytometry was used to measure intracellular cytokine production by CD4 and CD8 T lymphocytes. Heparinized venous whole blood was taken from all subjects at approximately the same time of day (in the morning) to avoid any effects of diurnal variation. Blood was mixed with tissue culture medium (TCM; RPMI-1640, 10% fetal calf serum (FCS), 2 mm l-glutamine, 100 IU/ml penicillin, 100 µg/ml streptomycin, Life Technologies, Paisley, Scotland, UK) in the proportion 1 : 3·5 TCM. In preliminary studies the stimulation and protein secretion blockade conditions were optimized. The following stimuli were tested: M phorbol 12-myristate 13-acetate (PMA)/ionomycin, anti-CD3/anti-CD28 and phytohaemagglutinin, each at varying concentrations. In addition we optimized protein secretion blockade, selecting from monensin alone, brefeldin A alone and monensin in combination with brefeldin A. Finally, we optimized the duration of polyclonal stimulation from 6, 16 and 24 h. Optimal conditions were selected as those providing the best overall performance for detection of CD4 cells positive for all cytokines, with the minimum of cell toxicity. Under the selected conditions, 1-ml aliquots of blood suspension were supplemented either with the polyclonal activators PMA (5 ng/ml final concentration) and ionomycin (745 ng/ml; both Sigma Chemical Co, Poole, UK) or left unstimulated. To improve the sensitivity of intracellular cytokine detection, two protein secretion inhibitors with different mechanisms of action (brefeldin A at 5 µg/ml and monensin at 2·08 mg/ml; both Sigma) were added to all cultures and incubated at 37°C for 16 h in 5% CO2. Under these conditions, cellular viability was ≥92% as assessed by trypan blue exclusion and flow cytometric staining with 7-amino actinomycin-D. In addition, down-regulation of CD4 after polyclonal stimulation under these conditions was minimal and non-significant. A representative dot-plot, showing intact CD4 staining and IL-4 positive cells, is shown in Fig. 1.
Fig. 1.
Representative flow cytometric plot of cells (lymphocyte gate from forward- and side-scatter plot) stained for CD4 (x-axis) and either isotype control antibody (a, c) or anti-IL-4 (b, d) after 16 h culture in the absence (upper panels, ‘resting’) or presence (lower panels, ‘polyclonally activated’) of PMA/ionomycin. It is apparent that there is minimal down-regulation of CD4 under these stimulation conditions, and that the percentage of CD4+ cells remains unchanged after stimulation.
Cells were harvested by gentle agitation with warm medium, washed and stained with PerCP and fluorescein isothiocyanate (FITC)-conjugated antihuman CD4 and CD8 monoclonal antibodies (MoAb) (Becton Dickinson, San Jose, CA, USA), respectively, then washed twice with phosphate-buffered saline (PBS) containing 5% FCS and 0·01% sodium azide and intracellular cytokine staining carried out according to the manufacturer's recommendations (Fix & Perm Permeabilization Kit, Caltag Laboratory, Burlingame, USA). In brief, the cells were fixed with Reagent A and incubated for 15 min at room temperature in the dark. After washing twice, permeabilizing Reagent B was added along with appropriate PE-conjugated anticytokine (IL-2, IFN-γ, IL-4, IL-10) MoAbs or fluorochrome and isotype-matched control MoAbs (all Becton Dickinson) and incubated in the dark at 4°C for 30 min. Finally, the stained cells were washed and suspended in 200 µl of phosphate buffered saline (PBS) containing 0·01% sodium azide for flow cytometry analysis, which was always carried out within 2 h of the end of the staining procedure.
Flow cytometry analysis
Stained cells were analysed on a FACSCalibur cytometer using CELLQuest software (Becton Dickinson, San Jose, CA, USA) and cytokine-positive cells expressed as percentage of positive total lymphocytes. Lymphocytes were gated on the basis of forward- and side-scatter properties and fluorescent channel dot-plot quadrant statistics set on the basis of corresponding isotype-matched control MoAbs to determine the frequencies of CD4 and CD8 T cells producing IL-2, IFN-γ, IL-4 and IL-10. Contamination by CD14+ monocytes in the gated CD4 population was routinely assessed as <1%.
Serum levels of total and allergen specific IgE
Serum IgE levels were measured by latex enhanced laser nephelometry using specific antisera and a Behring Laser Nephelometer II as recommended by the manufacturers. Allergen specific IgE against a panel of 15 common allergens was measured by Phadebas radioallergosorbent test (RAST; Pharmacia & Upjohn, Uppsala, Sweden).
Statistical analysis
We determined that all the cytokine data were non-normally distributed on testing, therefore the non-parametric Mann–Whitney U-test was used to assess differences between the CFS patients and healthy control subjects. All correlation analyses were carried out using Spearman's rank correlation test.
RESULTS
Types 1 and 2 T cell balance in chronic fatigue syndrome
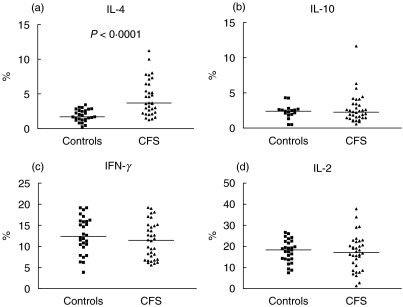
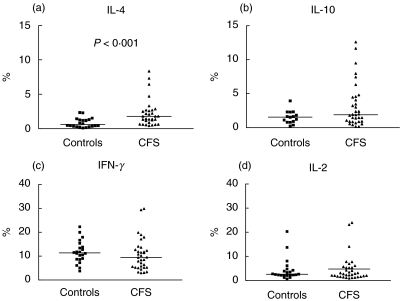
We found evidence of a bias towards Th2- and Tc2-type immune responses in CFS. The frequency of CD4 and CD8 T cells producing IL-4 following polyclonal stimulation was significantly higher in CFS patients compared with healthy control subjects (P < 0·0001 for CD4 and P < 0·001 for CD8 T cells; Figs 2 and 3). Because there was a positive correlation between IL-4+ CD4 and IL-4+ CD8 T cell numbers (r = 0·54, P = 0·0016) it can be concluded that in general those CFS patients with elevated levels of IL-4-secreting Th2 cells tended to also have high levels of Tc2 cells. In contrast, mean levels of IFN-γ, IL-2 and IL-10-producing cells were similar in both study groups. The lack of increase in IFN-γ-producing CD4 and CD8 T cells suggests that the expansion in IL-4 producing cells we observed is attributable to greater numbers of type 2 rather than type 0 cells that secrete both IFN-γ and IL-4 (Figs 2 and 3). These differences were not attributable to differences in percentages of CD4+ and CD8+ lymphocytes, which were similar in the two groups.
Fig. 2.
Percentage levels of CD4 T cells producing (a) IL-4, (b) IL-10, (c) IFN-γ and (d) IL-2 after polyclonal activation in patients with CFS and control subjects. There is a significant difference in levels of IL-4-producing CD4 T cells between the groups (P < 0·0001). Horizontal bars represent medians.
Fig. 3.
Percentage levels of CD8 T cells producing (a) IL-4, (b) IL-10, (c) IFN-γ and (d) IL-2 after polyclonal activation in patients with CFS and control subjects. There is a significant difference in levels of IL-4-producing CD8 T cells between the groups (P < 0·0001). Horizontal bars represent medians.
Analysis of the mean fluorescence intensity of positive staining for each of the cytokines tested showed that levels were similar in the two study groups, indicating that the differences we observe reflect changes in numbers of cytokine-producing cells, but not the quantity secreted.
Evidence of ongoing immune activation
In parallel with the analysis of cytokine-producing polyclonally stimulated CD4 and CD8 T cells, which represent polarized cells, we also measured intracellular cytokine production by CD4 and CD8 cells that had been cultured for 16 h in the absence of stimulation. Cells producing cytokines under these conditions could represent circulating cells that were activated in vivo just before the time of blood sampling.
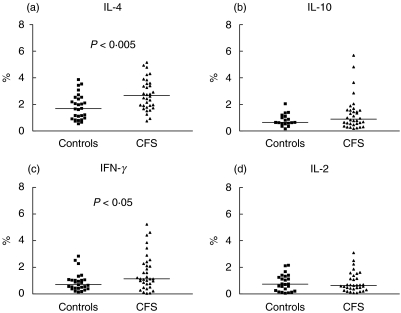
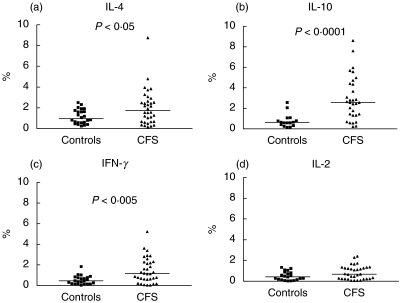
Typically, under such resting conditions, only small numbers of cytokine-producing cells are detected. Despite this, we found significantly higher levels of CD4 and CD8 T cells spontaneously producing IFN-γ or IL-4 in CFS patients compared with healthy controls (Figs 4 and 5). Levels of CD4 cells producing IFN-γ or IL-4 were correlated positively with each other among CFS patients (r = 0·58, P = 0·0004), suggesting that they may be produced by the same subset of cells, or that the stimuli driving their induction are similar or related. This type of response is typical of a type 0 response. IL-10 production by non-stimulated CD8+ lymphocytes was also significantly higher in CFS patients in comparison to the control group (Fig. 5; P < 0·0001).
Fig. 4.
Percentage levels of CD4 T cells producing (a) IL-4, (Bb IL-10, (c) IFN-γ and (d) IL-2 after overnight culture in the absence of activation stimuli (unstimulated/resting cells) in patients with CFS and control subjects. There is a significant difference in levels of IL-4 and IFN-γ -producing CD4 T cells between the groups (P < 0·005 and P < 0·05, respectively). Horizontal bars represent medians.
Fig. 5.
Percentage levels of CD8 T cells producing (a) IL-4, (Bb IL-10, (c) IFN-γ and (d) IL-2 after overnight culture in the absence of activation stimuli (unstimulated/resting cells) in patients with CFS and control subjects. There is a significant difference in levels of IL-4, IFN-γ and IL-10-producing CD8 T cells between the groups (P < 0·05 and P < 0·005, respectively). Horizontal bars represent medians.
Changes in levels of IL-4-producing CD4 and CD8 cells after PMA/ionomycin stimulation were less appreciable than for the other cytokines. In particular, in control subjects, mean levels of IL-4+ cells were similar in the unstimulated and stimulated cultures (mean ± SD; 1·8 ± 0·95 and 1·89 ± 0·85, respectively, for CD4 and 1·15 ± 0·68 and 0·87 ± 0·7, respectively, for CD8). In contrast, mean levels in CFS patients showed an increase after stimulation compared with unstimulated cultures (mean ± SD; 2·92 ± 1·54 and 4·47 ± 2·58 in the unstimulated and stimulated cultures, respectively, for CD4 (P = 0·005) and 2·03 ± 1·7 and 2·24 ± 1·97 for CD8 P = NS).
IgE levels and type 1 and type 2 cells
Because ‘allergy’ is a common subjective complaint in CFS [35,36], and is itself associated with type 2 T cell responses, we assessed the presence of objective markers of atopy and allergy by measuring serum levels of total and allergen-specific IgE. Levels of serum IgE were not statistically different between CFS patients and healthy controls (median levels in CFS patients, 76·7 IU/ml (range 0–493) and in control subjects 43·7 IU/ml (5–205); P > 0·05) and there was only one significant correlation observed in the CFS group between IgE levels and cytokine-positive cell numbers, namely with IL-4 produced by non-stimulated CD4 (r = 0·46; P = 0·022). The prevalence of positive RAST tests for allergen-specific IgE in CFS patients (11/33; 33%) was similar to that reported in the control subjects (12/28, 42%, P > 0·05).
Cytokine production and clinical status
We examined whether levels of cytokine-producing cells were related to clinical status. We found no correlation between non-stimulated or polyclonally stimulated IFN-γ+, IL-2+ or IL-4+ cell numbers and fatigue (total, physical and mental), GHQ score (for psychiatric symptoms), Work and Social Adjustment Scale (for disability), age, duration of illness and weight. For IL-10-producing cells we found two weak correlations, between polyclonally stimulated IL-10+ cells and age (r = 0·36, P = 0·038) and duration of illness (r = 0·36, P = 0·038). These results, however, may reflect the performance of multiple statistical analyses. There was no relationship between any of the immune parameters and age in the control subjects.
Of the CFS patients, 22 had been free from psychotropic drug use for more than 2 months at the time of blood sampling. There were no differences in immune parameters between these and those patients on psychotropic drug therapy. Similarly, 19 patients had been free from all medication for more than 2 months at the time of blood sampling, and there were no differences in immune parameters between these and those patients on medication. Ten patients had current comorbid psychiatric illness at the time of blood sampling (depression in eight cases, depression and panic disorder in one case and agoraphobia in one case) but did not differ in any of the immune parameters from those patients with pure CFS. Similarly, when patients were divided according to those with and without (n = 15) past or present psychiatric history, immune parameters were similar in the two groups.
DISCUSSION
The aim of this study was to examine the hypothesis that CFS is associated with a type 2 immune response, in which IL-4 production by T cells is predominant over IFN-γ. We found evidence for a bias towards type 2 responsiveness in patients with CFS, in whom there were increased numbers of IL-4-producing T cells compared with control subjects. We did not measure secretion of IL-4 and IFN-γ simultaneously from the same cell, and cannot therefore be certain that the increase in levels of IL-4-producing cells represents an expansion of pure Th2 and Tc2 cells. However, IL-4-producing cells were expanded as a proportion of both CD4 and CD8 T cells and levels of IL-4-producing cells correlated between the two T cell subsets but not with IFN-γ-producing cells. The use of polyclonal stimuli is designed to reveal the cytokine potential of effector memory lymphocytes, whereas naive T cells do not secrete these polarizing cytokines under these conditions [37,38]. We therefore interpret these data as indicating a consistent and coordinated bias towards type 2 responsiveness in CFS. Importantly, however, we were unable to identify any correlation between the degree of type 2 responsiveness and any clinical measurement of illness severity.
Numerous studies have been carried out to evaluate immune function in CFS patients, as studies on stress have shown a strong interrelation between the immune, central nervous and endocrine systems [39]. Differences in study design, diagnostic criteria, confounding physiological and pathological states, including depression and laboratory factors such as methodology and reagents, probably account for the fact that this remains a controversial area of research in which even systematic reviews fail to reveal an overall pattern [40]. However, some findings have greater consistency than others. For example, an indication of reduced Th1 activity leading towards Th2 bias has been reported previously by two separate studies [10,41], in both of which reduced IFN-γ production was reported. Immune activation has been observed frequently, and is reflected mainly in raised/lowered levels of various cytokines [14,15,42], reduced levels and activity of natural killer cells [10,18,43,44] and a change in the balance of numbers of memory and naive T cells [2,45]. Some studies in CFS have reported increased activation of CD8 T lymphocytes [46,47], a finding that could be interpreted as being consistent with the proposal that latent viruses have a role in CFS aetiology. The present study supports the view that CFS is associated with ongoing immune activation, as we found evidence of low-grade T cell activation in non-stimulated cultures of peripheral blood cells, which had increased levels of both type 1 and type 2 cytokines in CD4 and CD8 T cells (consistent with a type 0 pattern of responsiveness). We interpret these findings as indicating that such cells were activated at the time of blood sampling, although we cannot exclude the possibility that the cells were activated by the culture process itself.
A key question arising from our study is the nature of the factors that drive the immune response towards type 2 commitment in CFS, and following on from this, what can be offered in terms of therapy? It is well established that allergic symptoms are a result of dysregulation in Th1/Th2 immune balance towards a Th2 type of response [48,49]. Although there are studies claiming a higher prevalence of allergy and delayed type hypersensitivity [44,50–52] in CFS patients, this is a controversial area of research [50,53]. We considered it important to examine whether the Th2 bias we detected was associated with evidence of allergy, which would thus act as a confounder. In the present study we were able to measure total serum and allergen-specific IgE, neither of which were associated with high levels of IL-4 producing cells or with CFS. Future studies will be required to address any possible association using additional formal tests of allergic disease. Another mechanism through which an expansion in IL-4-producing cells could be mediated would be pre-existing chronic viral infections [54,55], which are known to be type 2-associated. Because we did not test for existence of any viral infections in our CFS patients we cannot exclude or include this hypothesis. Recent literature also indicates that Th2 type of responses may accompany autoimmune disorders. One study [56] has reported the association of CFS with a novel subtype of antinuclear autoantibody (ANA) that reacts with nuclear envelope (NE) antigens, but we were not able to reproduce these results [57]. A final factor that may be relevant is the status of the hypothalamo–pituitary–adrenal (HPA) axis, as glucocorticoids are well-known Th2 driving factors [58–60]. A number of CFS investigators [61–63] have suggested that abnormalities in the HPA axis play a crucial role in the pathogenesis of CFS. On balance, the evidence supports heightened negative feedback and increased glucocorticoid receptor function [64]. Specifically, the lymphocytes of adult CFS patients are more sensitive to glucocorticoids compared to controls [41,65,66]. Thus, even though there is little evidence for raised glucocorticoid levels in CFS, if anything there being a slight decrease in circulating cortisol in some patients [64], the increased sensitivity of lymphocytes to glucocorticoids might lead to increased Th2 polarity
Although we demonstrate a significant difference in immune responsiveness between CFS patients and healthy controls, levels of IL-4-producing CD4 and CD8 T cells, and other subsets that we measured, showed considerable overlap. Moreover, we were unable to identify clinical correlates with these immune changes. It is conceivable that such correlates exist but were not recorded for the current patient cohort. However, our data imply that the major defining symptom of CFS, namely fatigue, is neither due to nor responsible for type 2 immune responsiveness. It is possible that the type 2 immune biasing we observed is genetically determined, although no genes specifically associated with CFS have been identified, or that it is a component of the host susceptibility necessary for the development, but not chronicity or severity of the fatigue symptoms. The fact that we did not observe a correlation between our immunological findings and clinical parameters supports the proposal that such immune changes are not suitable for the development of an objective diagnostic test. The mechanisms and consequences of type 2 changes remain to be elucidated, and may require prolonged prospective studies on well-defined patient cohorts, as well as an examination of the natural history of such changes as patients improve clinically.
Acknowledgments
This study was supported by the Linbury Trust. Dorothy Blair was supported by The Psychiatry Research Trust and The Joint Research Committee of King's College Hospital.
REFERENCES
- 1.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome. a comprehensive approach to its definition and study. Ann Intern Med. 1994;121:953–9. doi: 10.7326/0003-4819-121-12-199412150-00009. International Chronic Fatigue Syndrome Study Group. [DOI] [PubMed] [Google Scholar]
- 2.Sharpe MC, Archard LC, Banatvala JE, et al. A report − chronic fatigue syndrome: guidelines for research. J Roy Soc Med. 1991;84:118–21. doi: 10.1177/014107689108400224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wessely S, Chalder T, Hirsch S, Wallace P, Wright D. The prevalence and morbidity of chronic fatigue and chronic fatigue syndrome: a prospective primary care study. Am J Public Health. 1997;87:1449–55. doi: 10.2105/ajph.87.9.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White PD, Thomas JM, Kangro HO, et al. Predictions and associations of fatigue syndromes and mood disorders that occur after infectious mononucleosis. Lancet. 2001;358:1946–54. doi: 10.1016/S0140-6736(01)06961-6. [DOI] [PubMed] [Google Scholar]
- 5.White PD, Thomas JM, Amess J, et al. Incidence, risk and prognosis of acute and chronic fatigue syndromes and psychiatric disorders after glandular fever. Br J Psychiatry. 1998;173:475–81. doi: 10.1192/bjp.173.6.475. [DOI] [PubMed] [Google Scholar]
- 6.Buchwald DS, Rea TD, Katon WJ, Russo JE, Ashley RL. Acute infectious mononucleosis: characteristics of patients who report failure to recover. Am J Med. 2000;109:531–7. doi: 10.1016/s0002-9343(00)00560-x. [DOI] [PubMed] [Google Scholar]
- 7.Hotopf M, Noah N, Wessely S. Chronic fatigue and minor psychiatric morbidity after viral meningitis: a controlled study. J Neurol Neurosurg Psychiatry. 1996;60:504–9. doi: 10.1136/jnnp.60.5.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Straus SE, Tosato G, Armstrong G, et al. Persisting illness and fatigue in adults with evidence of Epstein–Barr virus infection. Ann Intern Med. 1985;102:7–16. doi: 10.7326/0003-4819-102-1-7. [DOI] [PubMed] [Google Scholar]
- 9.Patarca-Montero R, Antoni M, Fletcher MA, Klimas NG. Cytokine and other immunologic markers in chronic fatigue syndrome and their relation to neuropsychological factors. Appl Neuropsychol. 2001;8:51–64. doi: 10.1207/S15324826AN0801_7. [DOI] [PubMed] [Google Scholar]
- 10.Klimas NG, Salvato FR, Morgan R, Fletcher MA. Immunologic abnormalities in chronic fatigue syndrome. J Clin Microbiol. 1990;28:1403–10. doi: 10.1128/jcm.28.6.1403-1410.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landay AL, Jessop C, Lennette ET, Levy JA. Chronic fatigue syndrome: clinical condition associated with immune activation. Lancet. 1991;338:707–12. doi: 10.1016/0140-6736(91)91440-6. [DOI] [PubMed] [Google Scholar]
- 12.Lloyd A, Hickie I, Wakefield D, Boughton C, Dwyer J. A double-blind, placebo-controlled trial of intravenous immunoglobulin therapy in patients with chronic fatigue syndrome. Am J Med. 1990;89:561–8. doi: 10.1016/0002-9343(90)90173-b. [DOI] [PubMed] [Google Scholar]
- 13.See DM, Tilles JG. alpha-Interferon treatment of patients with chronic fatigue syndrome. Immunol Invest. 1996;25:153–64. doi: 10.3109/08820139609059298. [DOI] [PubMed] [Google Scholar]
- 14.Swanink CM, Vercoulen JH, Galama JM, et al. Lymphocyte subsets, apoptosis, and cytokines in patients with chronic fatigue syndrome. J Infect Dis. 1996;173:460–3. doi: 10.1093/infdis/173.2.460. [DOI] [PubMed] [Google Scholar]
- 15.Hanson SJ, Gause W, Natelson B. Detection of immunologically significant factors for chronic fatigue syndrome using neural-network classifiers. Clin Diagn Lab Immunol. 2001;8:658–62. doi: 10.1128/CDLI.8.3.658-662.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rook GA, Zumla A. Gulf War syndrome: is it due to a systemic shift in cytokine balance towards a Th2 profile? Lancet. 1997;349:1831–3. doi: 10.1016/S0140-6736(97)01164-1. [DOI] [PubMed] [Google Scholar]
- 17.Tirelli V, Pinto A, Marotta G, et al. Clinical and immunologic study of 205 patients with chronic fatigue syndrome: a case series from Italy. Arch Intern Med. 1993;153:120. [PubMed] [Google Scholar]
- 18.Caligiuri M, Murray C, Buchwald D, et al. Phenotypic and functional deficiency of natural killer cells in patients with chronic fatigue syndrome. J Immunol. 1987;139:3306–13. [PubMed] [Google Scholar]
- 19.Thorpe R, Wadhwa M, Bird CR, Mire-Sluis AR. Detection and measurement of cytokines. Blood Rev. 1992;6:133–48. doi: 10.1016/0268-960x(92)90025-l. [DOI] [PubMed] [Google Scholar]
- 20.Bienvenu JAD, Monneret G, Gutowski MC, Fabien N. Cytokine assays in human sera and tissues. Toxicology. 1998;129:55–61. doi: 10.1016/s0300-483x(98)00063-8. [DOI] [PubMed] [Google Scholar]
- 21.North ME, Ivory K, Funauchi M, Webster AD, Lane AC, Farrant J. Intracellular cytokine production by human CD4+ and CD8+ T cells from normal and immunodeficient donors using directly conjugated anti-cytokine antibodies and three-colour flow cytometry. Clin Exp Immunol. 1996;105:517–22. doi: 10.1046/j.1365-2249.1996.d01-795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maino VC, Suni MA, Ruitenberg JJ. Rapid flow cytometric method for measuring lymphocyte subset activation. Cytometry. 1995;20:127–33. doi: 10.1002/cyto.990200205. [DOI] [PubMed] [Google Scholar]
- 23.Sander B, Andersson J, Andersson U. Assessment of cytokines by immunofluorescence and the paraformaldehyde–saponin procedure. Immunol Rev. 1991;119:65–93. doi: 10.1111/j.1600-065x.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 24.Kasahara K, Strieter RM, Chensue SW, Standiford TJ, Kunkel SL. Mononuclear cell adherence induces neutrophil chemotactic factor/interleukin-8 gene expression. J Leukoc Biol. 1991;50:287–95. doi: 10.1002/jlb.50.3.287. [DOI] [PubMed] [Google Scholar]
- 25.Nerad JL, Griffiths JK, Van der Meer JW, et al. Interleukin-1 beta (IL-1 beta), IL-1 receptor antagonist, and TNF alpha production in whole blood. J Leukoc Biol. 1992;52:687–92. doi: 10.1002/jlb.52.6.687. [DOI] [PubMed] [Google Scholar]
- 26.Kirchner H, Kleinicke C, Digel W. A whole-blood technique for testing production of human interferon by leukocytes. J Immunol Meth. 1982;48:213–9. doi: 10.1016/0022-1759(82)90195-8. [DOI] [PubMed] [Google Scholar]
- 27.Zangerle PF, De Groote D, Lopez M, et al. Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood. II. Application to rheumatoid arthritis and osteoarthritis. Cytokine. 1992;4:568–75. doi: 10.1016/1043-4666(92)90021-i. [DOI] [PubMed] [Google Scholar]
- 28.Deale A, Chalder T, Marks I, Wessely S. Cognitive behavior therapy for chronic fatigue syndrome: a randomized controlled trial. Am J Psychiatry. 1997;154:408–14. doi: 10.1176/ajp.154.3.408. [DOI] [PubMed] [Google Scholar]
- 29.Wilson A, Hickie I, Hadzi-Pavlovic D, et al. What is chronic fatigue syndrome? Heterogeneity within an international multicentre study. Aust NZ J Psychiatry. 2001;35:520–7. doi: 10.1046/j.1440-1614.2001.00888.x. [DOI] [PubMed] [Google Scholar]
- 30.Euba R, Chalder T, Deale A, Wessely S. A comparison of the characteristics of chronic fatigue syndrome in primary and tertiary care. Br J Psychiatry. 1996;168:121–6. doi: 10.1192/bjp.168.1.121. [DOI] [PubMed] [Google Scholar]
- 31.Chalder T, Berelowitz G, Pawlikowska T, et al. Development of a fatigue scale. J Psychosom Res. 1993;37:147–53. doi: 10.1016/0022-3999(93)90081-p. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg DP, Hillier VF. A scaled version of the General Health Questionnaire. Psychol Med. 1979;9:139–45. doi: 10.1017/s0033291700021644. [DOI] [PubMed] [Google Scholar]
- 33.Mundt JC, Marks IM, Shear MK, Greist JM. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry. 2002;180:461–4. doi: 10.1192/bjp.180.5.461. [DOI] [PubMed] [Google Scholar]
- 34.Sharpe M, Chalder T, Palmer I, Wessely S. Chronic fatigue syndrome. A practical guide to assessment and management. Gen Hosp Psychiatry. 1997;19:185–99. doi: 10.1016/s0163-8343(97)80315-5. [DOI] [PubMed] [Google Scholar]
- 35.Olson GB, Kanaan MN, Gersuk GM, Kelley LM, Jones JF. Correlation between allergy and persistent Epstein–Barr virus infections in chronic-active Epstein–Barr virus-infected patients. J Allergy Clin Immunol. 1986;78:308–14. doi: 10.1016/s0091-6749(86)80081-1. [DOI] [PubMed] [Google Scholar]
- 36.Straus SE, Dale JK, Wright R, Metcalfe DD. Allergy and the chronic fatigue syndrome. J Allergy Clin Immunol. 1988;81:791–5. doi: 10.1016/0091-6749(88)90933-5. [DOI] [PubMed] [Google Scholar]
- 37.Mascher B, Schlenke P, Seyfarth M. Expression and kinetics of cytokines determined by intracellular staining using flow cytometry. J Immunol Meth. 1999;223:115–21. doi: 10.1016/s0022-1759(98)00200-2. [DOI] [PubMed] [Google Scholar]
- 38.Carter LL, Zhang X, Dubey C, Rogers P, Tsui L, Swain SL. Regulation of T cell subsets from naive to memory. J Immunother. 1998;21:181–7. doi: 10.1097/00002371-199805000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Glaser R, Kiecolt-Glaser JK. Stress-associated immune modulation: relevance to viral infections and chronic fatigue syndrome. Am J Med. 1998;105:35S–42. doi: 10.1016/s0002-9343(98)00160-0. [DOI] [PubMed] [Google Scholar]
- 40.Lyall MPM, Wessely S. A. critical review of the immunology of chronic fatigue syndrome. J Psychosom Res. 2003. pp. 79–90. [DOI] [PubMed]
- 41.Visser J, Blauw B, Hinloopen B, et al. CD4 T lymphocytes from patients with chronic fatigue syndrome have decreased interferon-gamma production and increased sensitivity to dexamethasone. J Infect Dis. 1998;177:451–4. doi: 10.1086/517373. [DOI] [PubMed] [Google Scholar]
- 42.Patarca R. Cytokines and chronic fatigue syndrome. Ann NY Acad Sci. 2001;933:185–200. doi: 10.1111/j.1749-6632.2001.tb05824.x. [DOI] [PubMed] [Google Scholar]
- 43.Gupta S, Vayuvegula B. A comprehensive immunological analysis in chronic fatigue syndrome. Scand J Immunol. 1991;33:319–27. doi: 10.1111/j.1365-3083.1991.tb01777.x. [DOI] [PubMed] [Google Scholar]
- 44.Lloyd A, Hickie I, Hickie C, Dwyer J, Wakefield D. Cell-mediated immunity in patients with chronic fatigue syndrome, healthy control subjects and patients with major depression. Clin Exp Immunol. 1992;87:76–9. doi: 10.1111/j.1365-2249.1992.tb06416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Komaroff AL, Buchwald D. Symptoms and signs of chronic fatigue syndrome. Rev Infect Dis. 1991;13(Suppl. 1):S8–11. doi: 10.1093/clinids/13.supplement_1.s8. [DOI] [PubMed] [Google Scholar]
- 46.Borysiewicz LK, Haworth SJ, Cohen J, Mundin J, Rickinson A, Sissons JG. Epstein–Barr virus-specific immune defects in patients with persistent symptoms following infectious mononucleosis. Q J Med. 1986;58:111–21. [PubMed] [Google Scholar]
- 47.Gold D, Bowden R, Sixbey J, et al. Chronic fatigue. A prospective clinical and virologic study. JAMA. 1990;264:48–53. doi: 10.1001/jama.264.1.48. [DOI] [PubMed] [Google Scholar]
- 48.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–6. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 49.Singh VK, Mehrotra S, Agarwal SS. The paradigm of Th1 and Th2 cytokines: its relevance to autoimmunity and allergy. Immunol Res. 1999;20:147–61. doi: 10.1007/BF02786470. [DOI] [PubMed] [Google Scholar]
- 50.Steinberg P, Pheley A, Peterson PK. Influence of immediate hypersensitivity skin reactions on delayed reactions in patients with chronic fatigue syndrome. J Allergy Clin Immunol. 1996;98:1126–8. doi: 10.1016/s0091-6749(96)80204-1. [DOI] [PubMed] [Google Scholar]
- 51.Borish L, Schmaling K, DiClementi JD, Streib J, Negri J, Jones JF. Chronic fatigue syndrome: identification of distinct subgroups on the basis of allergy and psychologic variables. J Allergy Clin Immunol. 1998;102:222–30. doi: 10.1016/s0091-6749(98)70090-9. [DOI] [PubMed] [Google Scholar]
- 52.Conti F, Magrini L, Priori R, Valesini G, Bonini S. Eosinophil cationic protein serum levels and allergy in chronic fatigue syndrome. Allergy. 1996;51:124–7. [PubMed] [Google Scholar]
- 53.Mawle AC, Nisenbaum R, Dobbins JG, et al. Immune responses associated with chronic fatigue syndrome: a case-control study. J Infect Dis. 1997;175:136–41. doi: 10.1093/infdis/175.1.136. [DOI] [PubMed] [Google Scholar]
- 54.Fan XG, Liu WE, Li CZ, et al. Circulating Th1 and Th2 cytokines in patients with hepatitis C virus infection. Med Inflamm. 1998;7:295–7. doi: 10.1080/09629359890992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romagnani S, Maggi E, Del Prete G. An alternative view of the Th1/Th2 switch hypothesis in HIV infection. AIDS Res Hum Retroviruses. 1994;10:iii–ix. doi: 10.1089/aid.1994.10.iii. [DOI] [PubMed] [Google Scholar]
- 56.Konstantinov K, von Mikecz A, Buchwald D, Jones J, Gerace L, Tan EM. Autoantibodies to nuclear envelope antigens in chronic fatigue syndrome. J Clin Invest. 1996;98:1888–96. doi: 10.1172/JCI118990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skowera A, Stewart E, Davis ET, et al. Antinuclear autoantibodies (ANA) in Gulf War-related illness and chronic fatigue syndrome (CFS) patients. Clin Exp Immunol. 2002;129:354–8. doi: 10.1046/j.1365-2249.2002.01912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rook GA. Glucocorticoids and immune function. Baillières Best Pract Res Clin Endocrinol Metab. 1999;13:567–81. doi: 10.1053/beem.1999.0044. [DOI] [PubMed] [Google Scholar]
- 59.Wilckens T, De Rijk R. Glucocorticoids and immune function. unknown dimensions and new frontiers. Immunol Today. 1997;18:418–24. doi: 10.1016/s0167-5699(97)01111-0. [DOI] [PubMed] [Google Scholar]
- 60.Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–63. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- 61.Demitrack MA, Dale JK, Straus SE, et al. Evidence for impaired activation of the hypothalamic–pituitary–adrenal axis in patients with chronic fatigue syndrome. J Clin Endocrinol Metab. 1991;73:1224–34. doi: 10.1210/jcem-73-6-1224. [DOI] [PubMed] [Google Scholar]
- 62.Scott LV, Medbak S, Dinan TG. The low dose ACTH test in chronic fatigue syndrome and in health. Clin Endocrinol (Oxf) 1998;48:733–7. doi: 10.1046/j.1365-2265.1998.00418.x. [DOI] [PubMed] [Google Scholar]
- 63.Gaab J, Huster D, Peisen R, et al. Hypothalamic–pituitary–adrenal axis reactivity in chronic fatigue syndrome and health under psychological, physiological, and pharmacological stimulation. Psychosom Med. 2002;64:951–62. doi: 10.1097/01.psy.0000038937.67401.61. [DOI] [PubMed] [Google Scholar]
- 64.Cleare AJ. The neuroendocrinology of chronic fatigue syndrome. Endocr Rev. 2003;24:236–52. doi: 10.1210/er.2002-0014. [DOI] [PubMed] [Google Scholar]
- 65.Visser J, Graffelman W, Blauw B, et al. LPS-induced IL-10 production in whole blood cultures from chronic fatigue syndrome patients is increased but supersensitive to inhibition by dexamethasone. J Neuroimmunol. 2001;119:343–9. doi: 10.1016/s0165-5728(01)00400-3. [DOI] [PubMed] [Google Scholar]
- 66.Visser J, Lentjes E, Haspels I, et al. Increased sensitivity to glucocorticoids in peripheral blood mononuclear cells of chronic fatigue syndrome patients, without evidence for altered density or affinity of glucocorticoid receptors. J Invest Med. 2001;49:195–204. doi: 10.2310/6650.2001.34047. [DOI] [PubMed] [Google Scholar]