Abstract
T-cell-mediated immunoregulation is one of the main mechanisms implicated in induction and maintenance of transplantation tolerance. In this regard, deletion or modulation of xeno/alloantigen-specific T cells, as well as blocking of their interactions with other cell populations, are currently being pursued for tolerance induction in humans as well as nonhuman primates. In order to investigate whether cytotoxic T-lymphocyte antigen-4 (CTLA-4) may represent a suitable target for a T cell depletion approach in nonhuman primate models, we analysed CTLA-4 expression in peripheral blood mononuclear cells (PBMCs) from nonhuman primates and the potential role of two anti-CTLA-4 saporin-conjugated immunotoxins. The analysis was performed in PBMCs from 8 cynomolgus monkeys from Philippines and from Mauritius both at protein level by flow cytometry and at transcriptional level by RT-PCR. In addition, the apoptotic role of the immunotoxins was investigated. The results showed that CTLA-4 was expressed at variable levels depending on the origin of the cynomolgus monkeys and the resting or activated cell condition. CTLA-4 was not expressed on resting Mauritius PBMCs and showed a lower up-regulation upon PMA/PHA activation compared to the Philippines PBMCs that expressed CTLA-4 also before activation. Two CTLA-4 RNA transcripts (672 and 550 bp) were detected with levels variations after cell stimulation. Two anti-CTLA-4 immunotoxins induced in vitro apoptosis of activated PBMCs from both sources of cynomolgus monkeys. This is the first report that documents CTLA-4 expression both at protein and transcriptional level by nonhuman primate PBMCs and provides novel perspectives of xeno/allograft rejection immunotherapy based on CTLA-4 targeting.
Keywords: CTLA-4, cynomolgus monkeys, tolerance, immunotoxins
INTRODUCTION
Porcine xenografts transplanted into unmodified or pharmacologically immunosuppressed primates [1] generally undergo hyperacute rejection (HAR) primarily triggered by the binding of preexisting (natural) antibodies (Abs) and subsequent activation of the complement cascade [2]. Anti-xenograft natural antibodies are primarily directed against the Galα1–3Gal (Gal) carbohydrate determinants on the vascular endothelial cells of the donor organ and their levels vary both between and within a same species depending on age, diet and exposure to microorganisms and parasites [3]. Anti-Gal Abs often account for almost 100% of circulating natural anti-pig Abs in nonhuman primates [3] and for more than 80% in humans [4]. Consequently, several approaches have been undertaken to obtain selective elimination or inhibition of the anti-Gal Abs [5,6]. In this view attention has been directed towards a specific suppression or elimination of the B cell subsets involved in anti-Gal antibody production. The possibility of destroying these B cell subsets by an α-Gal oligosaccharide or anti-idiotypic antibody conjugated to a toxin has been explored, together with other B cell and plasma cell immunotoxins [7]. Alternatively, some have recently produced alpha 1,3-galactosyltransferase-deficient pigs [8].
Other types of anti-xenograft antibodies contributing to the development of xenograft rejection are directed to a large variety of xenopeptides (designated as anti-non-Gal) [9,10] and are usually absent in pretransplantation sera. However, both anti-Gal and anti-non Gal elicited anti-xenograft antibodies are T-cell dependent as shown by the complete inhibition of antibody response by administration of anti-CD40L antibodies that prevents T-cell/B–cell interaction [11]. Therefore, immunosuppression targeted toward helper T cells may affect B cells and effectively prevent production of elicited anti-xenograft antibodies.
In this study we performed in vitro experiments aimed at investigating whether the cytotoxic T lymphocyte associated protein-4 (CTLA-4, CD152) might be a suitable target molecule for a T cell depletion and/or inhibition approach in nonhuman primates expected to receive a porcine xenograft.
In contrast to CD28, which delivers positive signals, CTLA-4 molecule has been widely described in human T cells as a costimulatory molecule which negatively regulates T cell activation [12,13]. CTLA-4 engagement by its ligands CD80 (B7·1) and CD86 (B7·2) expressed on antigen presenting cells (APCs), results in impaired IL-2, IFN-γ, IL-4 cytokines production and cell cycle arrest [13]. In contrast to CD28 that is constitutively expressed on resting T cells, CTLA-4 is mainly expressed on activated human T cells with maximal expression after 48- to 72-h from the activation hit [12].
We previously described the generation of human recombinant anti-human CTLA-4 scFv monoclonal antibodies (mAbs) [14] and their use as therapeutic reagents in transplantation either as unconjugated antibodies [15] or as antibodies conjugated to saporin [16], a type-1 ribosome-inactivating protein (RIP) [17]. Because of the high degree of homology (greater than 95%) between human CTLA-4 and its nonhuman primate analogue [18], anti-human CTLA-4 scFv mAbs were used to analyse the CTLA-4 expression pattern on resting or activated peripheral blood mononuclear cells (PBMCs) from two sources of cynomolgus monkeys with different immunological backgrounds [19].
The analysis was performed by flow cytometry with two anti-CTLA-4 scFv mAbs and by reverse transcriptase-polymerase chain reaction (RT-PCR) with the CTLA-4 full-length coding sequence specific primers. In addition, we investigated the ability of two immunotoxins, consisting of an anti-CTLA-4 scFv mAb or an anti-T lymphocyte globulin preparation (ATG) conjugated with saporin RIP, to induce in vitro apoptosis of non human-primate PBMCs.
MATERIALS AND METHODS
Monoclonal antibodies and immunotoxins
Recombinant human anti-human CTLA-4 scFv mAbs, namely scFvs. no. 40 and no. 83, were obtained by selecting the Nissim scFv phage library, as described previously [14]. They were conjugated to fluorescein isothiocyanate (FITC) and utilized for direct immunofluorescence followed by flow cytometry analysis. The FITC-conjugated anti-bovine serum albumin (BSA) scFv no. 26 was used as negative control.
The anti-T lymphocyte globulin preparation obtained from rabbits (ATG, Fresenius, Bad-Homburg, Germany) has been already characterized [20] and contains an anti-CTLA-4 polyclonal component, as well as other anti-lymphocyte polyclonal antibodies. This reagent has been utilized as a positive control, since it was found positive both on resting and activated cynomolgus lymphocytes (results not shown).
The scFv no. 83 mAb and the ATG anti-T lymphocyte globulin preparation were chemically linked to the type-1 single-chain RIP saporin-S6 and the resulting immunotoxins were tested for reactivity with activated T cells and for toxicity with haematopoietic precursors according to standard protocols [16].
Source of nonhuman primate blood samples
All blood samples were obtained from healthy cynomolgus monkeys (Macaca fascicularis) from the Philippines and from Mauritius. The study was conducted in primates stabled in facilities licensed by the Italian Home Office, in accordance with the Italian Animals Act (Law no. 116 of the 27/1/1992). Animals used were 4- to 6-years-old females weighing from 2·9 to 4·7 kg (Philippines monkeys; n = 3) and 4- to 5-years-old males weighing from 4·9 to 7·4 kg (Mauritius monkeys; n = 5). All the animals in the study were purpose-bred and housed in similar experimental conditions in the same primate facility for more than 1 year. No treatment was administered to these animals at the time of this study. In addition, no overt infection developed in any of these animals neither during the observation period preceding the study nor during the subsequent follow up phase.
Haemolytic anti-pig antibody assay (APA)
Haemolytic antibodies to porcine red blood cells (PBRC) were detected using a standard assay as described previously [21]. Briefly, test serum samples and a human standard serum were heat-inactivated at 56°C for 30 min. Samples were serially diluted with complement fixation diluent (CFD, Oxoid Ltd, Basingstoke, UK) in a V-bottomed 96-well plate. Sera were incubated with 1% porcine erytrocytes (washed in CFD) and incubated for 1 h at 37°C. Plates were then washed twice with CFD buffer and PRBC were incubated with baby rabbit complement (Serotec, Oxford, UK) at 37°C for 1 h on an orbital incubator. After centrifugation 100 µl of supernatant was transferred from each well to flat-bottom 96-well plate for the measurement of absorbance at 420 nm. The absorption of each sample was then plotted as a function of dilution, and the area under the curve (AUC) was calculated. Data were expressed relative to the standard human serum to which the value of 1000 arbitrary units (AU) was assigned.
Anti-Gal antibody assay
An enzyme-linked immunosorbent assay (ELISA) was performed with slight modification from a previously described method [22]. Briefly, 96-well microtiter plates were coated overnight at 4°C with 50 µl/well of Gal-α-1,3-Gal-β-1,4-GlcNAc linked to human serum albumin (Dextra Laboratories, Reading, UK) or HSA (Sigma, Poole, UK) as background control, in 0·1 m bicarbonate buffer, pH 9·0 at 5 µg/ml. The plates were then blocked for 1 h with 0·5% Tween 20 in PBS. Test sera and standard human serum were serially diluted twofold from 1 : 5 to 1 : 320 in a separate plate and 50 µl aliquots were transferred to the assay plates and incubated for 1 h at room temperature. Separate test plates were prepared to measure IgM antibodies. Plates were then washed seven times with 0·1% Tween 20 in PBS. Horseradish peroxidase-labelled goat anti-human IgM (µ-chain specific, Sigma) were then added at 1 : 200 dilution. The plates were incubated for 1 h at room temperature before being washed seven times. The colour reaction was developed with orthophenylene diamine hydrochloride in phosphate citrate buffer (Sigma) and subsequently quenced with 1 m sulphuric acid. Sigmoidal dose–response curves were processed as described for the APA assay and the AUC was calculated. Data were expressed relative to the human standard serum to which the arbitrary value of 1000 AU was assigned.
Cells and culture conditions
Peripheral blood mononuclear cells (PBMCs) were isolated from 5 to 7 ml of heparinized whole blood derived from cynomolgus monkeys by density gradient centrifugation over Ficoll/Biocoll (Biochrom KG, Berlin, Germany). PBMCs were freshly tested or used for activation by culturing them in RPMI 1640 (Biochrom KG) supplemented with 10% FCS (Biochrom KG), antibiotics, 2 mm l-glutamine in the presence of phorbol ester (PMA) (Sigma) at 5 ng/ml and/or phytohemagglutinin (PHA) (Life Technologies, Milano, Italy) at a final concentration of 2 µg/ml for 48 h at 37°C. Activation of human PBMCs from healthy adult human volunteers (with prior informed consent) was performed by the same procedure and used as positive control for CTLA-4 expression.
Immunofluorescence and flow cytometry
A direct immunofluorescence staining was performed for analysing surface expression of CTLA-4 in resting and activated PBMCs either from monkeys or humans. Briefly, a pellet of 4 × 105 cells, was incubated for 30 min at room temperature (RT) with different FITC-anti-CTLA-4 scFvs. (no. 40, no. 83), or with the FITC-anti-BSA (no. 26) negative control. T cell specific staining was performed with phycoerythrin (PE)-conjugated anti-CD2 mAb, cross-reacting with CD2 + cynomolgus lymphocytes (Beckman-Coulter, Miami FL). The fluorescence intensity was measured by flow cytometry (EPICS XL, Beckman-Coulter); at least 15 000 cells/sample were counted. Positive control samples were run with an indirect immunofluorescence, utilizing ATG and FITC-anti-rabbit Ig (Dako, Copenhagen, DK).
RNA preparation and cDNA synthesis
Total cellular RNA and cDNA were prepared as previously described [23].
RT-PCR
PCRs were carried out in 50 µl volume, using 1/10 of the reverse transcriptase (RT) mixture (500 ng RNA). Specific amplification of CTLA-4 full lenght transcript was performed on each cDNA sample using the set of primers previously described for human CTLA-4 coding sequence [24].
The human CTLA-4 primer sequence used showed 100% homology with at least two different cynomolgus monkeys (Macaca nemestrina and M. mulatta) CTLA-4 deposited coding sequence (accession numbers AF344854 and AF344846 respectively).
PCR reactions were run with the following cycle profile: denaturation at 94°C for 1 min, annealing temperature at 60°C for 1 min, elongation at 72°C for 1 min and a total of 35 cycles. The amplification reactions were initially hot started (94°C for 3 min) and terminally extended at 72°C for 5 min. The quality of RNA retro-transcription was checked by β-actin gene amplification using primers specific both in human and macacus as previously described [25]. Each PCR reaction was setting up in order to maintain the reaction-efficiency in logarithmic phase of amplification. To this scope the PCR conditions of β-actin gene amplification were identical to the CTLA-4 gene reaction conditions but the number of cycles was reduced to 25. Absence of contaminant DNA was controlled by concomitant amplification of the PCR mixture without template (H2O). The obtained PCR products were analysed by electrophoresis on a 2% agarose gel. The identity and specificity of CTLA-4 PCR products were confirmed after direct sequencing analysis in both directions using an ABI-PRISM 377 Perkin-Elmer DNA Sequencer.
Apoptosis
In a series of eight independent experiments, PBMCs derived from the two groups of monkeys were incubated with increasing concentrations of no. 83-saporin or ATG-saporin immunotoxins, or a mix of saporin and no. 83 scFv or ATG, in complete RPMI 1640 medium for 72 h. Viable and dead cells were evaluated by a double staining via FITC-Annexin V and 7-aminoactinomycin D (7-AAD) (kit from Beckman-Coulter), following manufacturer's instructions. Analysis was performed by flow cytometry, calculating the percentage of apoptotic (annexin V+/7-ADD-) cells.
RESULTS
CTLA-4 protein expression in cynomolgus monkey lymphoid cells by flow cytometry
We have recently shown that CTLA-4, expressed on the surface of activated human cells of the lymphoid lineage, represents a target molecule suitable for apoptosis induction by immunotoxins [16,23]. To investigate whether this finding could be extended to nonhuman primate lymphoid cells, we analysed the CTLA-4 expression levels in PBMCs from two sources of cynomolgus monkeys, respectively, from the Philippines (N100, N116, N140) and from Mauritius (N915, N917, N181, N850, N857) (Table 1).
Table 1.
Cynomolgus monkeys used in this study
| Cynomolgus monkeys (source) | Age (years) | Sex | Weight (kg) | Haemolytic Ig APA* (AUC) | Anti-Gal IgM* (AUC) |
|---|---|---|---|---|---|
| Philippines | |||||
| N100 | 4 | Female | 4·55 | 1021 ± 140 | 525 ± 150 |
| N116 | 4 | Female | 2·90 | 1615 ± 105 | 1303 ± 336 |
| N140 | 6 | Female | 4·71 | 553 ± 90 | 368 ± 250 |
| Mauritius | |||||
| N915 | 4 | Male | 7·11 | 1405 ± 37 | 1343 ± 253 |
| N917 | 4 | Male | 5·22 | 1390 ± 48 | 1373 ± 113 |
| N181 | 5 | Male | 4·96 | 1334 ± 314 | 701 ± 117 |
| N850 | 5 | Male | 7·49 | 1696 ± 38 | 3296 ± 65 |
| N857 | 5 | Male | 6·26 | 1640 ± 139 | 2132 ± 111 |
APA, anti-pig antibody assay. AUC, area under the curve (see Materials and methods). Results are the mean of three different experiments.
PBMCs were analysed by immunofluorescence staining with two human anti-human CTLA-4 FITC-scFv no. 40 and no. 83 mAbs whose CTLA-4 specificity was previously well defined by enzymatic assay (ELISA), Western blot and immunofluorescence [14,23]. These scFv mAbs, cross-reacting with monkey CTLA-4, were tested with monkey PBMCs either in resting conditions or after activation with PMA/PHA, agents known to up-regulate the expression of human CTLA-4 molecule [26].
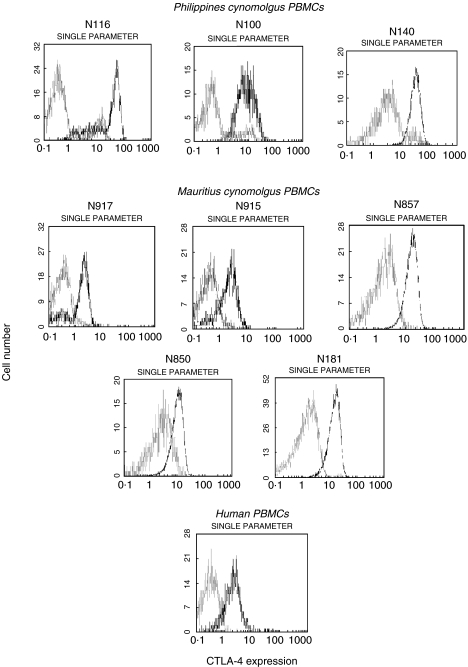
CTLA-4 was not detected on resting PBMCs from the Mauritius monkeys, mimicking the expression profile observed with human PBMCs, whereas it was consitutively expressed on 10%-17% of resting PBMCs from the Philippines monkeys (Fig. 1).
Fig. 1.
Flow cytometric profiles of surface CTLA-4 expression in freshly isolated cynomolgus monkeys and human PBMCs. Cells were tested before (□) and after (▪) activation for 48 h as described in ‘Materials and Methods’. Resting and activated PBMCs were stained with FITC-conjugated anti-CTLA-4 scFv no. 83 and analysed by flow cytometry. Results are expressed as fluorescence intensity with mean fluorescence intensity (MFI) values of 21·8, 16·3, 11·4, 2·36, 2·74, 8·8, 5·7, 7·4, 2·93 for N116, N100, N140, N917, N915, N857, N850, N181 and human resting PBMC samples, respectively. The FITC-conjugated anti-BSA scFv no. 26 was used as negative control for anti-CTLA-4 scFv no. 83. Data are representative of two similar experiments.
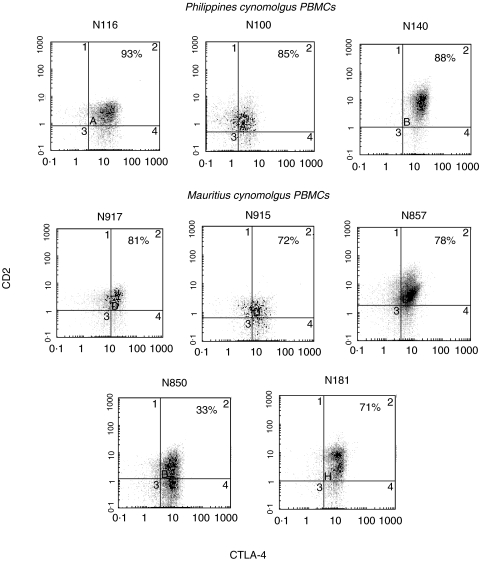
Upon activation with PMA/PHA, PBMCs from both groups of monkeys were induced to up-regulate surface CTLA-4 although with different degrees of intensity. The Mauritius group showed lower up-regulation compared to the Philippines group. The CTLA-4 expressing-cells in the monkeys PMA/PHA activated PBMCs were likely to belong to the T cell populations as suggested by the double positive staining with FITC-scFv no. 83 and PE-anti-CD2 mAb shown in Fig. 2.
Fig. 2.
Double staining of PMA/PHA activated PBMCs from cynomolgus monkeys with FITC-conjugated anti-CTLA-4 scFv no. 83 (x-axis) and PE-conjugated anti-CD2 mAb (y-axis) by flow cytometry. Appropriate gating for lymphocyte populations has been performed by forward scattering vs. surface scattering on lymphoid cells. Data are representative of two similar experiments.
Similar flow cytometric profiles were obtained with the FITC-anti-CTLA-4 scFv no. 40. As observed in humans [26] after activation with PMA/PHA, CTLA-4 was expressed at similar levels on CD4- and on CD8-positive T lymphocytes (data not shown).
Furthermore, as demonstrated for PMA/PHA activated human T-lymphocytes. ATG was positive on monkey activated PBMCs [20]. In contrast, the anti-BSA scFv no. 26 consistently resulted in negative staining (data not shown).
CTLA-4 transcripts expression in cynomolgus monkey lymphoid cells by RT-PCR
Expression of CTLA-4 specific transcripts in cynomolgus monkey PBMCs was investigated by RT-PCR using the same set of primers specific for human CTLA-4 full length coding sequence [24].
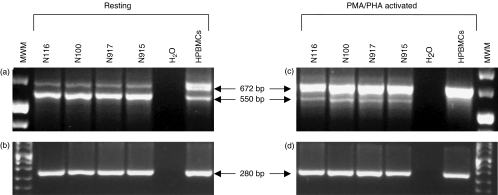
In resting PBMCs derived from two representative cynomolgus monkeys from each group, two RNA transcript variants of 672 and 550 bp were similarly detected which corresponded, respectively, to the membrane CTLA-4 transcript and to the spliced CTLA-4-deleted (delTM) transcript also detectable in human resting PBMCs [23,24] although in different proportions (Fig. 3a). In fact, in cynomolgus PBMCs the predominant variant corresponded to the deleted 550 bp band, whereas in human PBMCs it corresponded to the full length 672 bp variant, as previously reported [23].
Fig. 3.
Total RNA from two representative Philippines (N116, N100) and Mauritius (N917, N915) cynomolgus PBMCs and from human PBMCs (HPBMCs) was reverse transcribed and PCR-amplified with primers specific for human CTLA-4 full length coding sequence (a,c), that show 100% homology to the macacus CTLA-4 coding sequence deposited in GenBank. As internal control, β-actin gene amplification was carried out (b,d). The molecular weight marker used was Boehringer marker VIII.
Stimulation of cynomolgus PBMCs with PMA/PHA resulted in a significant decrease of the CTLA-4delTM band whereas the membrane CTLA-4 band increased considerably as shown in Fig. 3c. In activated human PBMCs the only detectable band corresponded to the full length variant transcript whereas the delTM variant could not be observed.
Therefore, CTLA-4 RNA levels upon activation were proportional to the levels of CTLA-4 protein expression on the surface of PBMCs from cynomolgus monkeys.
Effect of anti-CTLA4 immunotoxins on the induction of apoptosis of cynomolgus monkey lymphoid cells
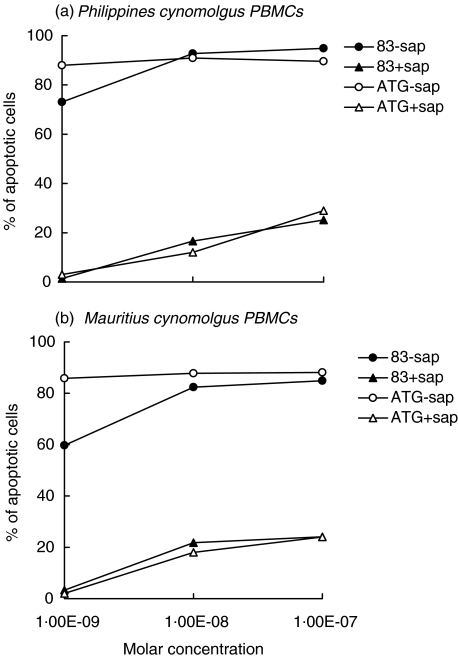
We evaluated the apoptosis of PMA/PHA activated PBMCs from both groups of cynomolgus monkeys induced by two immunotoxins. These were obtained by chemical conjugation of the anti-CTLA-4 scFv no. 83 or the ATG anti-T lymphocyte globulin preparation to the saporin type-1 RIP. Both immunotoxins at a concentration of 10−8m caused apoptosis of >90% of activated PBMCs from the Philippines group and of >80% of activated PBMCs from the Mauritius group whereas they did not affect unstimulated PBMCs (data not shown), as evaluated by FITC-annexin V and 7-AAD staining (Fig. 4). In contrast, saporin mixed with scFv no. 83 or ATG was weakly toxic at concentrations of 10−8 and 10−7m. Since anti-CTLA-4 immunotoxins have been previously shown [16] to be only slightly toxic for bone marrow precursors at a concentration of 10−8m, these results suggest their possible in vivo application in xenotransplant models.
Fig. 4.
Percentage of apoptotic PMA/PHA activated PBMCs from cynomolgus monkeys treated for 72 h with increasing doses of two immunotoxins (• 83-sap and ○ ATG-sap), or a mixture of scFv or ATG and saporin (▴ no. 83+sap, Δ ATG+sap). PBMCs from the (a) Philippines and from (b) Mauritius were stained with FITC-Annexin V and 7-AAD. Results are expressed as the mean value of apoptotic cells in individual monkeys from both groups in triplicate experiments. SD never exceeded 10%.
As we previously detected anti-CTLA-4 antibodies in ATG globulin preparations [20], the apoptotic effect exerted by the polyclonal ATG-immunotoxin on monkey PBMCs may be due to the binding to CTLA-4 in addition to other lymphocyte molecules recognized by the polyclonal ATG.
Natural anti-pig antibodies
The analysis of the natural anti-pig antibody repertoire in the sera of the two cynomolgus monkeys groups, by determination of APA and anti-Gal IgM antibodies, is reported in Table 1. These experiments indicate that in most cases the APA and anti-Gal antibody titres were greater than those observed in a pooled human control sample to which the value of 1000 AU was assigned. In addition, APA levels and anti-Gal IgM antibody titres were considerably lower in the animals from the Philippines compared to the animals from Mauritius.
DISCUSSION
Because of their relevance to clinical transplantation, nonhuman primate models are critical for organ transplantation studies. Several strategies have been designed in the past years to induce tolerance in nonhuman primates including approaches aimed at reducing the T-cell-dependent B-cell responses. In this context, reagents able to provide T cell depletion from blood and lymph nodes, by targeting the TCR-CD3 complex, were useful in prolonging graft survival. This was convincingly demonstrated by Armstrong and colleagues who were able to significantly prolong renal allograft acceptance using a toxin-conjugated anti-CD3 mAb, given to recipient monkeys before transplantation without any additional immunosuppressive therapy [27].
Furthermore, the discovery that costimulation is essential for T-cell activation prompted considerable attention to costimulatory blockade as a means of manipulating T-cell-mediated immune responses in vivo[28].
Among the costimulatory signals that have been identified the CD28/CTLA-4-CD80/CD86 pathways and the CD40-CD40 ligand (CD154) pathway have been determined to be of pivotal importance for T cell proliferation and cytokine release. Several tolerance-inducing regimens have been designed to evaluate the effect of interrupting these critical pathways in nonhuman primate species. In particular, administration of the CTLA-4-Ig fusion protein to rhesus monkey kidney allotransplant recipients blockades the CD28/CTLA−4 interaction with their CD80 or CD86 counter receptors by acting as a high affinity antagonist of such ligands on APCs. This treatment resulted in a significantly prolonged graft survival [29]. Similar results were obtained using the anti-CD154 (CD40L) humanized mAbs or the combination of these reagents [30].
There is much information on the expression of CTLA-4 molecule in human and murine cells of the T lymphoid lineage whereas no data are available on its expression in nonhuman primate lymphoid cells. Therefore, in this study we have analysed the expression pattern of the CTLA-4 costimulatory molecule on peripheral blood lymphocytes from cynomolgus monkeys with the aim of investigating whether targeting this molecule could represent a tool to obtain selective depletion of activated T cells. Clearly this analysis would have important implications in view of possible subsequent transplant studies in nonhuman primates.
We first performed in vitro studies at a protein level by immunofluorescence staining and flow cytometry using previously described human recombinant anti-human CTLA-4 scFvs. [14,23], that are crossreactive with nonhuman primate CTLA-4. The analysis, carried out in vitro on both resting and activated PBMCs from two groups of cynomolgus monkeys, indicated that CTLA-4 was differently expressed at the cell surface of PBMCs depending on the source of cynomolgus monkeys. CTLA-4 was constitutively expressed by a small proportion of resting PBMCs from Philippines monkeys and was markedly increased upon activation. On the contrary, CTLA-4 was not expressed by resting PBMCs from Mauritius monkeys and was up-regulated following stimulation, although at lower levels. The difference in the CTLA-4 expression profile observed between the 2 groups of primates studied could be related to the different immunological background of the monkeys analysed. This is also suggested by the higher titres of APA and anti-Gal IgM antibodies in the Mauritius group compared to the Philippines group. In this context, it is of interest that differences in the natural repertoire of the humoral immune response similar to those observed in this series had previously been reported by others using cynomolgus monkeys from the same 2 sources [19]. In addition, it should be noted that the expression pattern detected in the Mauritius group paralleled that found in human PBMCs both in intensity and frequency. However, although this is a remote possibility, at this stage we cannot exclude that the different CTLA-4 expression pattern observed between Philippines and Mauritius groups may be related to the different sex of the animals available for the study. Indeed, even if no gender-related differences in CTLA-4 expression have previously been reported, sex-dependent differences in both humoral and cellular arm of the immune response have already been documented [31,32].
To determine whether the different levels of CTLA-4 protein expression between the two groups of monkeys in resting condition and following activation was transcriptionally regulated, a semiquantitative RT-PCR was carried out. This analysis showed two transcript variant bands, thus confirming previous studies in human PBMCs [23], but revealed differentially expressed levels compared to humans. In cynomolgus monkeys PBMCs the CTLA-4 deleted transcript variant was more abundant than the CTLA-4 membrane variant whereas in human PBMCs there was an opposite pattern of expression.
Interestingly, the observed CTLA-4 transcriptional profile while confirming a conserved mechanism of CTLA-4 activation between humans and macaques, at the same time emphasizes the role that CTLA-4 del-TM spliced form may play in resting lymphocytes of both primate groups. However, following activation with PMA/PHA, the deleted variant decreased in both groups of primates and completely disappeared in human PBMCs as previously reported [23,24], whereas the membrane variant increased leading to enhanced protein expression.
Efforts have been made in the development of new therapeutic strategies that specifically target molecules involved in the rejection process. Therefore, we evaluated in vitro the possibility of using anti-CTLA-4 specific immunotoxins for apoptosis induction of nonhuman primate PBMCs. In particular, two immunotoxins containing anti-CTLA-4 ligands (anti-CTLA-4 scFv or ATG preparation) and the type-1 RIP saporin, were able to kill CTLA-4 expressing monkey cells. It was already reported that RIP-containing immunotoxins are able to induce apoptosis in target cells, with cytotoxic mechanisms comprehending protein synthesis inhibition and possibly direct damages to DNA or other type of RNA [17]. Our results show that both immunotoxins used are highly efficient in inducing apoptosis of monkey cells supporting the future use of CTLA-4 as a new target molecule for in vivo tolerance inducing immunotherapeutic strategies in nonhuman transplant models. This approach could be applicable even in the case of the hypothetical existence of CD4+ CD25+ CTLA-4+ regulatory T cells [33] in macaques. In this case collection of these regulatory T cells prior to treatment followed by their reinfusion (possibly, after in vitro expansion), at the end of the immunotoxin treatment, to restore the subset could be envisaged. Alternatively, since lymphocytes are constantly regenerating cells, spontaneous repopulation of these cells after immunotoxin treatment could occur.
Taken together the results of the present study show that there are variable levels of CTLA-4 expression in nonhuman primates and this can be demonstrated either at protein or transcriptional level with a reasonable level of correlation. Notwithstanding the variability observed between animals from different sources, our data suggest that in vivo treatment with anti-CTLA-4 immunotoxins should efficiently reduce the incidence of allo and xenograft rejection in cynomolgus monkeys from either sources by killing activated T cells. Moreover treatment with a CTLA-4-toxin conjugate could lead to the specific elimination of allo/xeno-reactive T cells preventing a possible subsequent recognition by the same T cells. This is substantially different from the effect mediated by the use of CTLA-4-Ig. Indeed, although effective during its administration, CTLA-4-Ig only causes a defective recognition and does not lead to elimination of the allo/xeno-reactive T-cells. Finally, our results also suggest that an ATG-saporin conjugate could be used as a therapeutic option in case a more pronounced T-lymphocyte depletion is needed.
In conclusion, this study demonstrates that strategies aimed at targeting CTLA-4 molecules expressed by T cells using immunotoxins such as those presented here, may represent an effective tool to extend the survival of allo- and xeno-grafts transplanted into primates.
Acknowledgments
This study was supported in part by funds of Progetto Strategico Oncologia N.74 from Ministero Istruzione Università e Ricerca, Roma, Italy, by funds of Ministero della Salute Ricerca Finalizzata 2000 and by the Veneto Region.
REFERENCES
- 1.Lambrigts D, Sachs DH, Cooper DK. Discordant organ xenotransplantation in primates: world experience and current status. Transplantation. 1998;66:547–61. doi: 10.1097/00007890-199809150-00001. [DOI] [PubMed] [Google Scholar]
- 2.Platt JL, Vercellotti GM, Dalmasso AP, et al. Transplantation of discordant xenografts: a review of progress. Immunol Today. 1990;11:450–6. doi: 10.1016/0167-5699(90)90174-8. [DOI] [PubMed] [Google Scholar]
- 3.Teranishi K, Manez R, Awwad M, Cooper DK. Anti-Gal alpha 1–3Gal IgM and IgG antibody levels in sera of human and old world non-human primates. Xenotransplantation. 2002;9:148–54. doi: 10.1034/j.1399-3089.2002.1o058.x. [DOI] [PubMed] [Google Scholar]
- 4.Sandrin MS, Vaughan HA, Dabkowski PL, McKenzie IF. Anti-pig IgM antibodies in human serum react predominantly with Gal (alpha 1–3) Gal epitopes. Proc Natl Acad Sci USA. 1993;90:11391–5. doi: 10.1073/pnas.90.23.11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alwayn IP, Basker M, Buhler L, Cooper DK. The problem of anti-pig antibodies in pig-to-primate xenografting: current and novel methods of depletion and/or suppression of production of anti-pig antibodies. Xenotransplantation. 1999;6:157–68. doi: 10.1034/j.1399-3089.1999.00030.x. [DOI] [PubMed] [Google Scholar]
- 6.Galili U, Matta KL. Inhibition of anti-Gal IgG binding to porcine endothelial cells by synthetic oligosaccharides. Transplantation. 1996;62:256–62. doi: 10.1097/00007890-199607270-00018. [DOI] [PubMed] [Google Scholar]
- 7.Alwayn IP, Xu Y, Basker M, et al. Effects of specific anti-B and/or anti-plasma cell immunotherapy on antibody production in baboons: depletion of CD20- and CD22-positive B cells does not result in significantly decreased production of anti-alphaGal antibody. Xenotransplantation. 2001;8:157–71. doi: 10.1034/j.1399-3089.2001.008003157.x. [DOI] [PubMed] [Google Scholar]
- 8.Phelps CJ, Koike C, Vaught TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–4. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindeborg E, Kumagai-Braesch M, Tibell A, Moller E. Continued production of xenoimmune antibodies 6–8 years after clinical transplantation of fetal pig islet-like cell-clusters. Xenotransplantation. 2001;8:273–83. doi: 10.1034/j.1399-3089.2001.00124.x. [DOI] [PubMed] [Google Scholar]
- 10.Richards AC, Davies HF, McLaughlin ML, et al. Serum anti-pig antibodies as potential indicators of acute humoral xenograft rejection in pig-to-cynomolgus monkey kidney transplantation. Transplantation. 2002;73:881–9. doi: 10.1097/00007890-200203270-00009. [DOI] [PubMed] [Google Scholar]
- 11.Buhler L, Awwad M, Basker M, et al. High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced anti-pig humoral response. Transplantation. 2000;69:2296–304. doi: 10.1097/00007890-200006150-00013. [DOI] [PubMed] [Google Scholar]
- 12.Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–13. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 13.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–65. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pistillo MP, Tazzari PL, Ellis JH, Ferrara GB. Molecular characterization and applications of recombinant scFv antibodies to CD152 costimulatory molecule. Tissue Antigens. 2000;55:229–38. doi: 10.1034/j.1399-0039.2000.550306.x. [DOI] [PubMed] [Google Scholar]
- 15.Pistillo MP, Tazzari PL, Stirpe F, et al. Anti-CTLA-4 human scFv antibodies could prevent T cell activation in transplantation. Transplant Proc. 2001;33:285–7. doi: 10.1016/s0041-1345(00)02012-1. [DOI] [PubMed] [Google Scholar]
- 16.Tazzari PL, Polito L, Bolognesi A, et al. Immunotoxins containing recombinant anti-CTLA-4 single-chain fragment variable antibodies and saporin: in vitro results and in vivo effects in an acute rejection model. J Immunol. 2001;167:4222–9. doi: 10.4049/jimmunol.167.8.4222. [DOI] [PubMed] [Google Scholar]
- 17.Barbieri L, Bolognesi A, Valbonesi P, Polito L, Olivieri F, Stirpe F. Polynucleotide adenosine glycosidase activity of immunotoxins containing ribosome-inactivating proteins. J Drug Target. 2000;8:281–8. doi: 10.3109/10611860008997906. [DOI] [PubMed] [Google Scholar]
- 18.Villinger F, Bostik P, Mayne A, et al. Cloning, sequencing, and homology analysis of nonhuman primate Fas/Fas-ligand and co-stimulatory molecules. Immunogenetics. 2001;53:315–28. doi: 10.1007/s002510100322. [DOI] [PubMed] [Google Scholar]
- 19.Holmes BJ, Richards AC, Awwad M, et al. Anti-pig antibody levels in naive baboons and cynomolgus monkeys. Xenotransplantation. 2002;9:135–47. doi: 10.1034/j.1399-3089.2002.1o056.x. [DOI] [PubMed] [Google Scholar]
- 20.Pistillo MP, Tazzari PL, Bonifazi F, et al. Detection of a novel specificity (CTLA-4) in ATG/TMG globulins and sera from ATG-treated leukemic patients. Transplantation. 2002;73:1295–302. doi: 10.1097/00007890-200204270-00019. [DOI] [PubMed] [Google Scholar]
- 21.Cozzi E, Bhatti F, Schmoeckel M, et al. Long-term survival of nonhuman primates receiving life-supporting transgenic porcine kidney xenografts. Transplantation. 2000;70:15–21. [PubMed] [Google Scholar]
- 22.Taylor CJ, Tang KG, Smith SI, White DJ, Davies HF. HLA-specific antibodies in highly sensitized patients can cause a positive crossmatch against pig lymphocytes. Transplantation. 1998;65:1634–41. doi: 10.1097/00007890-199806270-00016. [DOI] [PubMed] [Google Scholar]
- 23.Pistillo MP, Tazzari PL, Palmisano GL, et al. CTLA-4 is not restricted to the lymphoid cell lineage and can function as a target molecule for apoptosis induction of leukemic cells. Blood. 2003;101:202–9. doi: 10.1182/blood-2002-06-1668. [DOI] [PubMed] [Google Scholar]
- 24.Magistrelli G, Jeannin P, Herbault N, et al. A soluble form of CTLA-4 generated by alternative splicing is expressed by nonstimulated human T cells. Eur J Immunol. 1999;29:3596–602. doi: 10.1002/(SICI)1521-4141(199911)29:11<3596::AID-IMMU3596>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 25.Sachdeva G, Patil V, Katkam RR, et al. Expression profiles of endometrial leukemia inhibitory factor, trasforming growth factor β2 (TGFβ2), and TGFβ2 receptor in infertile bonnet monkeys. Biol Reprod. 2001;65:1–8. doi: 10.1095/biolreprod65.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Freeman GJ, Lombard DB, Gimmi CD, et al. CTLA-4 and CD28 mRNA are coexpressed in most T cells after activation. Expression of CTLA-4 and CD28 mRNA does not correlate with the pattern of lymphokine production. J Immunol. 1992;149:3795–801. [PubMed] [Google Scholar]
- 27.Armstrong N, Buckley P, Oberley T, et al. Analysis of primate renal allografts after T-cell depletion with anti-CD3-CRM9. Transplantation. 1998;66:5–13. doi: 10.1097/00007890-199807150-00002. [DOI] [PubMed] [Google Scholar]
- 28.Wekerle T, Kurtz J, Bigenzahn S, Takeuchi Y, Sykes M. Mechanisms of transplant tolerance induction using costimulatory blockade. Rev Curr Opin Immunol. 2002;14:592–600. doi: 10.1016/s0952-7915(02)00378-3. [DOI] [PubMed] [Google Scholar]
- 29.Kirk AD, Harlan DM, Armstrong NN, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci USA. 1997;94:8789–94. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirk AD, Burkly LC, Batty DS, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5:686–93. doi: 10.1038/9536. [DOI] [PubMed] [Google Scholar]
- 31.Remou F, Rogerie F, Gallissot MC, et al. Sex-dependent neutralizing humoral response to Schistosoma mansoni 28GST antigen in infected human populations. J Infect Dis. 2000;181:1855–9. doi: 10.1086/315454. [DOI] [PubMed] [Google Scholar]
- 32.Vine MF, Stein L, Weigle K. Gender differences in response to the multitest CMI skin test in the general population. Ann Allergy Asthma Immunol. 2000;84:445–50. doi: 10.1016/S1081-1206(10)62279-X. [DOI] [PubMed] [Google Scholar]
- 33.Levings MK, Sangregorio R, Sartirana C, et al. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor beta, but not interleukin 10, and are distinct from type 1 T regulatory cells. J Exp Med. 2002;196:1335–46. doi: 10.1084/jem.20021139. [DOI] [PMC free article] [PubMed] [Google Scholar]