Abstract
Diets rich in soy phytoestrogens have many potential health benefits but isoflavones such as genistein may suppress cell mediated immune function. The effect of dietary phytoestrogens on the host response to infection has not been extensively examined. Mice were fed a diet containing soy phytoestrogens and infected with Mycobacterium avium to establish a chronic infection and inflammatory response. As phytoestrogens may act through classical oestrogen receptors (ER), mice deficient in ERα signalling and wild type mice were evaluated for a panel of Type 1-associated cytokines (IFNγ, IL-12 and IL-18) in the spleen. IFNγ production in the spleen was increased approximately 4-fold in ERα-deficient mice fed a casein-based diet over wild type mice fed a casein-based diet (P < 0·05), suggesting a role for ERα in suppressing IFNγ production. IL-18 levels in spleens of wild type mice were decreased compared to ERα-deficient mice on a casein diet. Splenic IL-12 and IL-18 levels were not affected in wild type and ERα-deficient mice on the phytoestrogen containing diets, with the exception that whole soy increased IL-12 levels in the tissues of ERα deficient mice. We conclude that ERα and dietary phytoestrogens can influence production of key regulatory cytokines in response to chronic bacterial infection.
Keywords: phytoestrogens, oestrogen receptor, interferon, interleukin, inflammation
INTRODUCTION
Dietary soy products have been proposed to be beneficial as an alternative therapy to oestrogen replacement therapy for oestrogenic stimulation of postmenopausal women. Soy products also have potent antioxidative activity and are a major component of the Asian diet. Other potential benefits of dietary consumption of soy phytoestrogens are decreased risk of developing breast and prostate cancer as noted in Asian populations [1,2]. Host immune responses to chronic infection can resemble immune responses to tumours in many ways, involving similar protective cell populations and shared cytokine signalling pathways. There is growing interest in the potential influence of diet, especially soy phytoestrogens, on the host immune function including aspects related to tumour initiation and growth in addition to other potential health benefits.
There have been few if any studies of the effects of soy foods and dietary hormones on modulation of cytokine responses during chronic inflammation. Dietary soy phytoestrogens may influence the differentiation, signalling and actions of numerous cells of the immune system as the receptor(s) have been identified on many cell types including lymphocytes and antigen presenting cells. This is further supported by the correlation between oestrogen levels and susceptibility to certain infectious agents in addition to the mounting evidence linking gender bias in cytokine responses [3]. Several studies have noted altered gender-specific Th1/Th2 immune responses attributable in part to signalling via oestrogen or oestrogen-like compounds. In general, females are considered more prone towards a Th2 response and males a Th1 response [4,5]. The action of phytoestrogens in the diet is likely complex with the potential to act either as agonists or antagonists on signalling through ER pathways. Additionally, higher concentrations can inhibit tyrosine kinases, constituents of many signalling pathways in immune cells [6]. Genistein has also been shown to modulate metabolism of E2 [7]. Collectively, signalling by dietary phytoestrogens through these pathways may influence the innate and adaptive immune responses to infection via alteration of cytokine responses.
Mouse models of infection have been of great benefit in elucidating immune regulatory mechanisms involved in inflammatory responses to a variety of intracellular and extracellular pathogens [8]. Infection models with intracellular pathogens which result in chronic infection allow evaluation of the role of dietary compounds such as phytoestrogens on the production of key potential regulatory type I cytokines such as IFNγ, IL-12 and IL-18. To determine whether dietary phytoestrogens, such as genistein, can alter the cytokine responses to chronic infection and inflammation, mice were fed diets containing either genistein or whole soy and then infected with the facultative intracellular pathogen, Mycobacterium avium. Bacterial growth and levels of the Th1-associated cytokines, interleukin-12 (IL-12), interleukin-18 (IL-18) and interferon-γ (IFNγ) were determined in liver and spleen tissue. Our studies demonstrate that IFNγ production following chronic bacterial infection is negatively impacted by a soy- or genistein-based diet. The effects of oestrogen and oestrogen-like compounds on IFNγ production was influenced by the presence of one of the key oestrogen receptors identified to date, ERα. Importantly, these studies establish a link for further investigation between ERα and regulation of IFNγ production.
MATERIALS AND METHODS
Animals, surgery and diets
C57BL/6 mice heterozygous for the disrupted ERα coding sequence (ER+/ER-) were maintained as a breeding colony at the University of Missouri-Columbia [9,10]. All experiments were conducted according to Animal Care and Use (ACUC) guidelines as recommended by the National Institutes of Health. Wild type (ER+/ER +) and homozygous ERαKO (ER–/ER–) animals used in these experiments were either littermates or age-matched whenever possible. Mice were maintained at constant temperature (21°C) and humidity (45%) on a 12 h light/12 h dark cycle. Animals were supplied with Purina 5053 laboratory mouse chow and water ad libitum. For ovariectomy, 30 wild type and 30 ERαKO animals were anaesthetized by inhalation of isoflurane, and bilateral flank incisions were made in the abdominal cavity. Each ovary was removed, the wound closed, and the animals allowed to recover for 7 days. After surgery, all animals were maintained on sterile feed and water until the end of the experiment. At 7 days postsurgery, animals were randomly assigned to groups of 5 animals each and fed one of the diets described in Results. The diets were prepared by dry extrusion pelleting at low temperature and sterilization by gamma irradiation. This experiment was repeated once.
Bacterial infection
M. avium strain 49601 was obtained from American Type Culture Collection (Rockville, MD, USA) and cultured on Middlebrook 7H11 agar. Smooth transparent colonies were selected and propagated in Middlebrook 7H9 broth enriched with oleic albumin dextrose catalase (OADC). Bacteria were grown to approximately 108 CFU/ml, which was determined by plating bacteria on Middlebrook 7H11 agar. Aliqouts were frozen at −70°C and used for infections. Mice were fed their respective diets for 3 weeks and then infected by intraperitoneal (IP) injection with 107 CFU of M. avium in saline. Infected animals were continued on their respective diets for 60 days under the standard conditions listed previously. At this time, animals were sacrificed by CO2 asphyxiation under aseptic conditions. Tissues were removed, weighed, and aliquots partitioned for bacteriology, histopathology and cytokine analysis as indicated. Samples for histopathology were fixed in formalin; samples for cytokine analysis were snap frozen in liquid N2 and stored at −70°C. Liver tissue for bacteriology was weighed, homogenized, and passed through a 70 µm mesh filter; the resulting suspension was serially diluted and plated on Middlebrook 7H11 Agar (Remel, Lenexa, KS, USA). Plates were incubated at 37°C for approximately 14 days at which time bacterial colonies were counted.
Genistein measurements
Serum from individual mice were pooled by genotype and diet regimen and 100 µl aliquots were combined with an equal volume of distilled water and then treated with 400 µl ethanol to precipitate protein. Samples were vortexed and centrifuged at 3000 r.p.m. for 5 min. A 300 µl aliquot of the supernatant was combined with 1·5 ml 0·1 m sodium acetate buffer (pH 5·1 containing 0·1% ascorbic acid and 0·01% EDTA) and 1000 units of beta-glucuronidase HP-2 (Sigma Chemical, St. Louis, MO, USA) and incubated overnight at 37°C. Samples were acidified with 300 µl 2 N HCl and applied to preconditioned Waters 3 ml C18 Sep-Pac-Vac disposable columns. Columns were washed with 2 ml methanol : 0·1% acetic acid (1 : 1 v/v) and vacuum dried for 30 min. Genistein was eluted with 4 ml methanol and the sample eluates taken to dryness. Samples were reconstituted in methanol:water (80 : 20 v/v) for HPLC analysis, which was done with a 4-channel ESA CoulArray Model 5600 HPLC detection system together with an ESA isocratic HPLC pump connected to a Thermo Separations Products Spectrasystem AS3500 autosampler. Coularray settings were 450, 650, 700, and 875 mV. The system was controlled and data acquired and processed using the CoulArray software on a Pentium-based computer. A Supelco Discovery HS F5 (15 × 4·6 mm, 5 µm) column with a mobile phase of 50 mm sodium acetate buffer (pH 4·8) : methanol : acetonitrile (48 : 26 : 26) was used at a flow rate of 1 ml/minute. Genistein was purchased from LC Laboratories (Woburn, MA, USA). Primary standards (500 p.p.m) were prepared in methanol. Working standards (10, 25, 50, 50 and 125 p.p.b) were prepared in methanol : water (80 : 20). Serum samples spiked with genistein (100 p.p.b) had recoveries of greater than 85%. The serum samples were pooled within a single experiment not between experiments and the data is presented as the mean of separate determinations from 2 different experiments. The SE represents one-half of the range.
Cytokine assays
The concentration of IFNγ, IL-12p70 and IL-18 in spleen homogenates was measured by an enzyme-linked immunosorbent assay (ELISA). Spleen tissue (approximately 40–60 mg) was homogenized in lysing buffer (150 mm NaCl, 15 mm Tris, 1 mm CaCl2, 1 mm MgCl2, 0·5% Triton-X100; 1 mg spleen/10 µl buffer) on ice, centrifuged at 3500 × g for 10 min and 100 µl of supernatant was assayed in triplicate by ELISA assay using commercially available kits. IFNγ and IL-12p70 was analysed using the DuoSet Elisa Development System (R & D Systems, Minneapolis, MN) according to manufacturer's instructions. IL-18 was analysed using the OptEIA Mouse IL-18 Set (BD Biosciences, San Diego, CA) according to manufacturer's instructions. Cytokine levels from each group were evaluated statistically using a multifactorial, 2-way anova with genotype and diet as factors (P < 0·05 was considered significant).
RESULTS
Circulating phytoestrogen levels achieved through dietary supplementation
Serum was collected by cardiac puncture at the time of sacrifice from M. avium infected mice that had been fed one of the diets listed in Table 1. Samples were pooled by genotype and diet regimen and phytoestrogen levels in the serum of the mice on diets were analysed by HPLC for genistein, one of the major phytoestrogens in soy (Table 2). Wild type and ERα-deficient mice on casein-based diet had no detectable genistein in their serum. Wild type and ERα-deficient mice fed a casein + genistein diet or wild type mice fed a whole soy diet had levels of genistein in their serum comparable to those of Japanese adults consuming a diet traditionally high in soy [11–13]. ERα-deficient mice on whole soy were not analysed for serum genistein.
Table 1.
Composition of experimental diets
| Ingredient | Supplier† | Casein‡ | Casein + Genistein‡ | Whole soy‡ |
|---|---|---|---|---|
| Casein* | ICN Biomedicals, Aurora OH | 200 | 200 | 0 |
| Soy protein§ | Solae, St. Louis, MO. | 0 | 0 | 200 |
| Corn starch | National Starch and Chemical Co, Bridgewater NJ | 397·5 | 397·2 | 396·5 |
| Dyetrose | Dyets, Inc., Bethlehem PA | 132 | 132 | 132 |
| Sucrose | Allen Foods, St Louis MO | 100 | 100 | 100 |
| Cellulose | Alphacell, ICN Biomedicals, Aurora OH | 50 | 50 | 50 |
| Safflower oil | ICN Biomedicals, Aurora OH | 20 | 20 | 20 |
| Corn oil | Dyets, Inc., Bethlehem PA | 50 | 50 | 50 |
| Salt mix | AIN 93G, ICN Biomedicals, Aurora OH | 35 | 35 | 35 |
| Vitamin mix | AIN 93VX, ICN Biomedicals, Aurora OH | 10 | 10 | 10 |
| Choline bitartrate | ICN Biomedicals, Aurora OH | 2·5 | 2·5 | 2·5 |
| DL-methionine | ICN Biomedicals, Aurora OH | 3 | 3 | 4 |
| Genistein | LC Laboratories, Woburn MA | 0 | 0·3 | 0 |
Diets prepared by RS MacDonald, University of Missouri and pelleted by low temperature, dry extrusion. Estimated energy content 4·84 kcal/g.
High nitrogen casein;
Soy Protein contained 3·03 mg total isoflavones/g which included 1·52 mg genistein-containing compounds/g. The whole soy diet provided 0·3 mg genistein-containing compounds/kg diet.
All Suppliers are USA.
All values are g/kg.
Table 2.
Serum genistein levels in mice on treatment diets. The measurements are the mean of determinations from 2 separate experiments. The SE represent one-half of the range
| Genistein (µmol/l) | |||
|---|---|---|---|
| Genotype | Diet | Average | SE |
| Wild Type | Casein | ND | |
| Wild Type | Casein + Genistein | 0·96 | 0·262 |
| Wild Type | Whole soy | 0·41 | 0·038 |
| ERαKO | Casein | ND | |
| ERαKO | Casein + Genistein | 0·41 | 0·160 |
| ERαKO | Whole soy | NM | |
ND, None detected; NM, Not measured.
M. avium infection
Bacterial levels in tissues following infection were analysed at 60 days following establishment of chronic infection and the accompanying focal inflammatory responses typical of mycobacterial infection (1–3). Spleen weights from infected wild type and ERα-deficient mice on each diet showed dramatically enlarged spleens when compared to uninfected controls (data not shown). There were no statistically significant differences (2 way anova, P >0·05) in spleen weights between any of the groups on the different diets. The majority of mice examined had typical histological manifestations of infection and resulting local inflammatory response, including multifocal granulomas and associated perivascular granulomatous regions in the spleen and liver (data not shown). Bacterial loads were evaluated in the livers of mice on the respective diets at 60 days post infection (Figs 2 and 3). There were no statistically significant differences in bacterial numbers in the livers of ERα-deficient mice on control diet versus wild type mice on control diet (Fig. 1a) or in ERα-deficient mice fed dietary soy products relative to wild-type mice (Figs 2a, 3a).
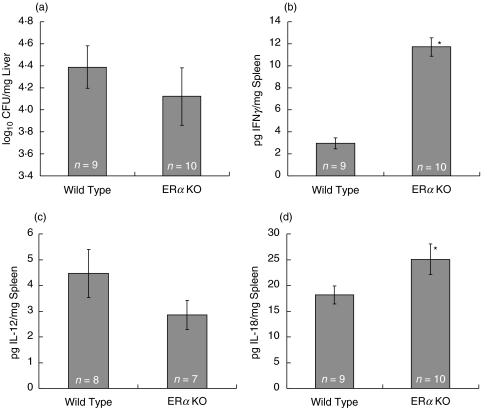
Fig. 1.
Mycobacterial counts and cytokine production in M. avium infected wild type and ERαKO mice fed a nonestrogenic casein diet. Ovariectomized wild type and ERαKO were fed a casein diet for three weeks and then infected with 1 × 107 CFU of M. avium for 60 days. Diets were continued during the entire infection period. Liver tissue was collected and bacterial load (a) determined by plating serial dilutions of macerated liver tissue. Spleen tissue was collected and frozen at −70°C. Aliquots were macerated and analysed for (b) IFNγ, (c) IL-12 and (d) IL-18 production by ELISA. Values represent the average of the values for individual mice in a treatment group, with error bars representing the standard error of the mean. The number of animals evaluated is listed in each column. Mean differences were evaluated statistically using a multifactorial, 2-way anova with genotype and diet as factors (*P < 0·05)
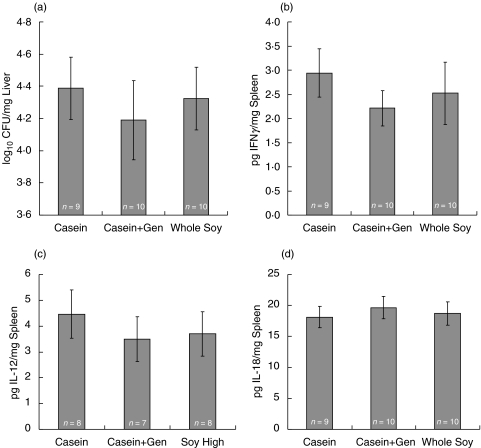
Fig. 3.
Mycobacterial counts and cytokine production in M. avium infected ERαKO mice fed either a casein-, casein + 300 mg/kg genistein-, or a whole soy-based diet. Ovariectomized ERαKO mice were fed one of the above diets for three weeks before infection with 1 × 107 CFU of M. avium. Diets were continued during the entire infection period (60d). Liver tissue was collected and bacterial load (a) determined. Spleens were analysed for (b) IFNγ, (c) IL-12 and (d) IL-18 production by ELISA. Values represent the average of the values for individual mice in a treatment group, with error bars representing the standard error of the mean. The number of animals evaluated is listed in each column. Mean differences were evaluated statistically using a multifactorial, 2-way anova with genotype and diet as factors (*P < 0·05)
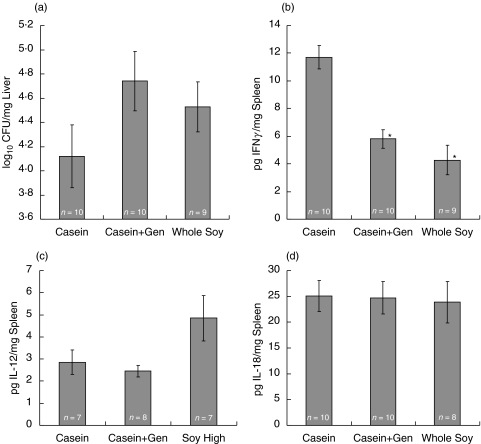
Fig. 2.
Mycobacterial counts and cytokine production in M. avium infected wild type mice fed a casein-, casein + 300 mg/kg genistein-, or a whole soy-based diet. Ovariectomized wild type mice were fed one of the above diets for three weeks before infection with 1 × 107 CFU of M. avium. Diets were continued during the entire infection period (60d). Liver tissue was collected and bacterial load determined by plating serial dilutions of macerated liver tissue (a). Spleen tissue was analysed for (b) IFNγ, (c) IL-12 and (d) IL-18 production by ELISA. Values represent the average of the values for individual mice in a treatment group, with error bars representing the standard error of the mean. The number of animals evaluated is listed in each column. Mean differences were evaluated statistically using a multifactorial, 2-way anova with genotype and diet as factors (*P < 0·05)
Loss of ERα signalling modulates IFNγ and IL-18 production in M. avium infected mice
Immunological control of mycobacterial infection requires IFNγ production by CD4 and CD8 T cells and NK cells [14–17]. Both mice and humans lacking IFNγ production or signalling are more susceptible to mycobacterial infection [18,19]. To determine the effects of dietary soy (or genistein) on cytokine production in response to chronic infection and inflammation, we measured IFNγ production in wild type and ERα-deficient mice infected with M. avium. IFNγ production in the spleen was increased approximately 4-fold in ERα-deficient mice fed a casein-based diet over wild type mice fed a casein-based diet (P < 0·05), suggesting a role for ERα in suppressing IFNγ production (Fig. 1b). Diminished IFNγ in spleens of wild type mice was not however, associated with higher bacterial loads in the liver of wild type mice compared to ERα-deficient mice on a casein diet (Fig. 1a). IL-12 and IL-18 contribute to IFNγ production in response to M. avium infection of resistant mice [20]. Splenic IL-18 levels were significantly increased in ERα-deficient versus wild type mice (Fig. 1d) (P < 0·05). There was no effect of diet and no significant interactions between genotype and diet. These data suggest IL-18 may in part be responsible for the increased IFNγ in the spleens of ERα-deficient mice following infection (Fig. 1d). IL-12 levels appeared to be inversely correlated with IFNγ in ERα-deficient versus wild type mice at this time point (Fig. 1c). IL-12 alternative pathways have been described in murine mycobacterial infection models.
Soy phytoestrogens modulate IFNγ production in M. avium infected mice
Estrogens have been shown to modulate IFNγ expression in various model systems [21–25]. We hypothesized that dietary phytoestrogens may also influence IFNγ production and the inflammatory response to mycobacterial infection. To test this, we infected wild type and ERα-deficient mice fed a casein, casein plus genistein, or whole soy diet with M. avium and measured IFNγ, IL-12, IL-18 in spleen, and bacterial load in the liver to confirm infection. Consumption of phytoestrogen containing diets significantly reduced IFNγ production in spleens of ERα-deficient mice (P < 0·05; Fig. 3b), indicating phytoestrogen signalling, possibly through ERβ, may have a role in regulating IFNγ production. Soy diets also slightly reduced IFNγ production by wild type spleen cells (Fig. 2b), suggesting the presence of ERα in wild type mice plays a role in diminished IFNγ production. IFNγ production in ERα-deficient mice fed soy-containing diets was not correlated with increased mycobacterial load in the liver (Fig. 3a). Splenic IL-12 and IL-18 levels were not affected in wild type and ERα-deficient mice on the phytoestrogen containing diets (Fig. 2c, 2d, 3c,d), with the exception that whole soy increased IL-12 levels in the tissues of ERα-deficient mice (Fig. 3c).
DISCUSSION
Soy isoflavones are weak oestrogens that may alter the effect of endogenous sex steroids on immune function. In this series of studies, we demonstrate that IFNγ production during chronic infection was significantly higher in mice deficient in one of the major oestrogen receptor signalling pathways (ERα-deficient mice) compared to wild type mice across all of the diets. The effect of loss of signalling via ERα was particularly apparent in the casein control diet. This result is in contrast with studies in uninfected mice in which oestrogen treatment increased IFNγ production by concanavalin-A-stimulated splenocytes from ovariectomized C57BL6 mice [22,23]. However, in a model of experimental colitis, 17β-oestradiol treatments of C57BL6 mice decreased IFNγ expression in colonic tissue [21]. The basis for the differences could be related to several factors including the dose and duration of treatment in addition to the specific tissue and cell type(s) involved. Nonetheless, our findings support a role for ERα in regulating the production of IFNγ during the course of a chronic bacterial infection and inflammation. Ovariectomized mice have relatively low levels of circulating 17β-oestradiol (< 10 pg/ml), suggesting either the repression of IFNγ production mediated by ERα is extremely sensitive to 17β-oestradiol or unliganded ERα can mediate this effect. Interestingly, NFκB regulates IFNγ transcription and has been shown to interact with ERs (reviewed in [26]), however, interaction of NFκB with ER has not previously been shown to modulate IFNγ.
In addition to the effects of loss of signalling via ERα on IFNγ production, we also demonstrated that dietary phytoestrogens, such as genistein and soy can modulate the cytokine response to chronic bacterial infection and inflammation in mice. Secretory IFNγ production in the spleen was reduced on a soy-based diet and by the addition of genistein to a casein-based diet. The effect of a soy-based diet was the same whether genistein was added to the casein diet or part of the whole soy diet. This response was exacerbated in ERα-deficient mice relative to wild type mice. The observed decrease in IFNγ production by infected ERα-deficient mice fed soy phytoestrogens was likely mediated via ERβ given the relative affinity of genistein for ERβ over ERα. However, we cannot exclude the possibility that genistein may be acting through a novel ER.
To further define cytokine networks involved in controlling chronic bacterial infection in mice we measured two cytokines, IL-12 and IL-18, which have important regulatory roles in production of IFNγ by T-cells, B-cells and NK cells [27,28]. M. avium infection of genetically susceptible BALB/C mice is associated with diminished IL-12, IL-18 and ensuing IFNγ production and Th1 responsiveness, whereas DBA/2 mice resistant to M. avium infection exhibit increased production of IL-12, IL-18 and IFNγ[20]. In our studies IL-18 levels, but not IL-12 levels, correlated with elevated IFNγ production in chronically infected ERα-deficient mice relative to wild type mice. IL-12p70 independent pathways, primarily involving IL-23 have been described in M. avium infected mice [29]. To our knowledge, oestrogen-signalling pathways have not been reported to modulate IL-18; however, these data suggest that signalling through ER-α may play a role in secretory IL-18 production. In contrast to the action of genistein on IFNγ levels, however, phytoestrogen signalling through ERβ did not alter IL-18 levels. IL-18 synergizes with IL-12 to stimulate IFNγ production [27] and increased IL-18 secretion may be sufficient to support the greater IFNγ levels in chronically infected ERα-deficient mice.
Collectively, these results suggest that dietary soy isoflavones, such as genistein, may impact resistance or susceptibility to infection. Interestingly, an epidemiological study reported that the vegetarian diet of Asian immigrants in the United Kingdom was a risk factor for tuberculosis [30]. Although, the mechanism behind the increased risk of tuberculosis was not determined, the authors speculated that vitamin D deficiency may in part be responsible. Other dietary factors, such as the phytoestrogen content, were not ruled out. A multitude of natural plant products have been tested for antimycobacterial activity in vitro and many have been used as traditional medicines [31–33], but to our knowledge, phytoestrogens have not been tested extensively for these activities either in vitro or in vivo.
Consumption of soy products in dietary supplements and soy-based infant formula can result in serum levels of phytoestrogens that are actually higher than serum levels in Asian diets [13]. There have been few studies on the immune modulatory effect of soy products. Yellayi et al. [11] showed that injection of genistein into ovariectomized female mice resulted in dramatic reduction in the size of the thymus as well as the percentage of CD4+CD8− thymocytes. In this study, dietary supplementation of genistein produced significant decreases in thymic size, although not as great as with injected genistein. However, in other studies orally administered genistein resulted in an increased CTL and NK cell activity that was correlated with inhibition of tumour growth [34]. Moreover, dietary supplementation of daidzein, another phytoestrogen in soy, resulted in enhanced thymus weight and phagocytic activity by macrophages [35]. Together, our results and the studies listed above suggest that dietary oestrogens play a role in modulation of cell-mediated immunity and type I inflammatory responses and may be a factor in disease resistance and susceptibility.
Acknowledgments
This work was supported by National Institutes of Health grant NIEHS-3 P01 ES10535–03S1. The authors would like to thank Dr Miriam Golomb for her critical reading of the manuscript, David Wendell for expert technical assistance and Dr Ron Tessman for the statistical analysis.
REFERENCES
- 1.Lee HP, Gourley L, Duffy SW, Esteve J, Lee J, Day NE. Dietary effects on breast-cancer risk in Singapore. Lancet. 1991;337:1197–200. doi: 10.1016/0140-6736(91)92867-2. [DOI] [PubMed] [Google Scholar]
- 2.Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr Cancer. 1994;21:113–31. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- 3.Styrt B, Sugarman B. Estrogens and infection. Rev Infect Dis. 1991;13:1139–50. doi: 10.1093/clinids/13.6.1139. [DOI] [PubMed] [Google Scholar]
- 4.Friedman A, Waksman Y. Sex hormones and autoimmunity. Israel J Med Sci. 1997;33:254–7. [PubMed] [Google Scholar]
- 5.Ansar Ahmed S, Talal N. Sex hormones and autoimmune rheumatic disorders. Scand J Rheumat. 1989;18:69–76. doi: 10.3109/03009748909099921. [DOI] [PubMed] [Google Scholar]
- 6.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5. [PubMed] [Google Scholar]
- 7.Martini MC, Dancisak BB, Haggans CJ, Thomas W, Slavin JL. Effects of soy intake on sex hormone metabolism in premenopausal women. Nutr Cancer-an Int J. 1999;34:133–9. doi: 10.1207/S15327914NC3402_2. [DOI] [PubMed] [Google Scholar]
- 8.Kaufmann SH. Immunity to intracellular bacteria. Ann Rev Immunol. 1993;11:129–63. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 9.Curran EM, Berghaus LJ, Vernetti NJ, Saporita AJ, Lubahn DB, Estes DM. Natural killer cells express estrogen receptor-alpha and estrogen receptor-beta and can respond to estrogen via a non-estrogen receptor-alpha-mediated pathway. Cell Immunol. 2001;214:12–20. doi: 10.1006/cimm.2002.1886. [DOI] [PubMed] [Google Scholar]
- 10.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–6. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yellayi S, Naaz A, Szewczykowski MA, et al. The phytoestrogen genistein induces thymic and immune changes: a human health concern? Proc Natl Acad Sci USA. 2002;99:7616–21. doi: 10.1073/pnas.102650199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet. 1997;350:23–7. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- 13.Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342:1209–10. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- 14.Ottenhoff TH, Verreck FA, Lichtenauer-Kaligis EG, Hoeve MA, Sanal O, van Dissel JT. Genetics, cytokines and human infectious disease: lessons from weakly pathogenic mycobacteria and salmonellae. Nat Genet. 2002;32:97–105. doi: 10.1038/ng0902-97. [DOI] [PubMed] [Google Scholar]
- 15.Flynn JL, Ernst JD. Immune responses in tuberculosis. Curr Opin Immunol. 2000;12:432–6. doi: 10.1016/s0952-7915(00)00116-3. [DOI] [PubMed] [Google Scholar]
- 16.Appelberg R, Castro AG, Pedrosa J, Silva RA, Orme IM, Minoprio P. Role of gamma interferon and tumor necrosis factor alpha during T-cell-independent and – dependent phases of mycobacterium avium infection. Infect Immun. 1994;62:3962–71. doi: 10.1128/iai.62.9.3962-3971.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon-gamma gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doherty TM, Sher A. Defects in cell-mediated immunity affect chronic, but not innate, resistance of mice to mycobacterium avium infection. J Immunol. 1997;158:4822–31. [PubMed] [Google Scholar]
- 19.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon-gamma in resistance to mycobacterium-tuberculosis infection. J Exp Med. 1993;178:2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi K, Nakata N, Kai M, Kasama T, Hanyuda Y, Hatano Y. Decreased expression of cytokines that induce type 1 helper T cell/interferon-gamma responses in genetically susceptible mice infected with Mycobacterium avium. Clin Immunol Immunopathol. 1997;85:112–6. doi: 10.1006/clin.1997.4421. [DOI] [PubMed] [Google Scholar]
- 21.Verdu EF, Deng YK, Bercik P, Collins SM. Modulatory effects of estrogen in two murine models of experimental colitis. Am J Physiol – Gastrointestinal Liver Physiol. 2002;283:G27–G36. doi: 10.1152/ajpgi.00460.2001. [DOI] [PubMed] [Google Scholar]
- 22.Karpuzoglu-Sahin E, Hissong BD, Ahmed SA. Interferon-gamma levels are upregulated by 17-beta-estradiol and diethylstilbestrol. J Reprod Immunol. 2001;52:113–27. doi: 10.1016/s0165-0378(01)00117-6. [DOI] [PubMed] [Google Scholar]
- 23.Karpuzoglu-Sahin E, Zhi-Jun Y, Lengi A, Sriranganathan N, Ahmed SA. Effects of long-term estrogen treatment on IFN-gamma, IL-2 and IL-4 gene expression and protein synthesis in spleen and thymus of normal C57BL/6 mice. Cytokine. 2001;14:208–17. doi: 10.1006/cyto.2001.0876. [DOI] [PubMed] [Google Scholar]
- 24.Grasso G, Muscettola M. The influence of beta-estradiol and progesterone on interferon gamma production in vitro. Int J Neurosci. 1990;51:315–7. doi: 10.3109/00207459008999730. [DOI] [PubMed] [Google Scholar]
- 25.Le N, Yousefi S, Vaziri N, Carandang G, Ocariz J, Cesario T. The effect of beta-estradiol, progesterone and testosterone on the production of human leukocyte derived interferons. J Biol Regul Homeostatic Agents. 1988;2:199–204. [PubMed] [Google Scholar]
- 26.McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev. 1999;20:435–59. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- 27.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Ann Rev Immunol. 2001;19:423–74. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 28.Yoshimoto T, Takeda K, Tanaka T, Ohkusu K, Kashiwamura S, Okamura H, Akira S, Nakanishi K. Il−12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells – synergism with IL-18 for IFN-gamma production. J Immunol. 1998;161:3400–7. [PubMed] [Google Scholar]
- 29.Cooper AM, Kipnis A, Turner J, Magram J, Ferrante J, Orme IM. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J Immunol. 2002;168:1322–7. doi: 10.4049/jimmunol.168.3.1322. [DOI] [PubMed] [Google Scholar]
- 30.Strachan DP, Powell KJ, Thaker A, Millard FJC, Maxwell JD. Vegetarian diet as a risk factor for tuberculosis in immigrant South London asians. Thorax. 1995;50:175–80. doi: 10.1136/thx.50.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newton SM, Lau C, Gurcha SS, Besra GS, Wright CW. The evaluation of forty-three plant species for in vitro antimycobacterial activities; isolation of active constituents from Psoralea corylifolia and Sanguinaria canadensis. J Ethnopharmacol. 2002;79:57–67. doi: 10.1016/s0378-8741(01)00350-6. [DOI] [PubMed] [Google Scholar]
- 32.Newton SM, Lau C, Wright CW. A review of antimycobacterial natural products. Phytother Res. 2000;14:303–22. doi: 10.1002/1099-1573(200008)14:5<303::aid-ptr712>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 33.Lall N, Meyer JJM. In vitro inhibition of drug-resistant and drug-sensitive strains of Mycobacterium tuberculosis by ethnobotanically selected South African plants. J Ethnopharmacol. 1999;66:347–54. doi: 10.1016/s0378-8741(98)00185-8. [DOI] [PubMed] [Google Scholar]
- 34.Guo TL, McCay JA, Zhang LX, Brown RD, You L, Karrow NA, Germolec DR, White KL., Jr Genistein modulates immune responses and increases host resistance to B16F10 tumor in adult female B6C3F1 mice. J Nutr. 2001;131:3251–8. doi: 10.1093/jn/131.12.3251. [DOI] [PubMed] [Google Scholar]
- 35.Zhang RQ, Li YP, Wang WQ. Enhancement of immune function in mice fed high doses of soy daidzein. Nutr Cancer – an Int J. 1997;29:24–8. doi: 10.1080/01635589709514597. [DOI] [PubMed] [Google Scholar]