Abstract
In attempt to investigate the stimulatory effect of Pseudomonas aeruginosa on innate immunity and to correlate it to its level of resistance to antimicrobials, 20 isolates were applied; 8 isolates were susceptible and 12 multidrug-resistant. Genetic diversity was defined by PFGE. Human monocytes of two healthy volunteers were in vitro stimulated by the isolates for the production of pro-inflammatory (TNF-α, IL-1β, IL-6, IL-8 and IL-12) and anti-inflammatory cytokines (IL-10), of malondialdehyde and of procalcitonin. Cytokines were estimated by EIA, malondialdehyde by the thiobarbiturate assay and procalcitonin by an immunochemiluminometric assay. Survival of 48 Wistar rats was recorded after induction of sepsis by the intraperitoneal injection of three susceptible and three multidrug-resistant isolates. To test whether comparative effect of the latter isolates on survival correlates with any difference of monocyte-mediated release of pro-inflammatory mediators, monocytes of two rats were in vitro stimulated for the production of TNF-α and of malondialdehyde. In vitro stimulation of human monocytes by the susceptible isolates elicited elevated production of malondiadeheyde, of IL-1β and of IL-6 compared to stimulation by multidrug-resistant isolates. Similar differences were found for TNF-α and IL-8, but they were not statistically significant. Production of IL-10 and IL-12 was not detected after stimulation with any isolate. Levels of procalcitonin were similar after induction with either susceptible or multidrug-resistant isolates. Mean survival of animals was 7·56, 21·80 and 55·20 h, respectively, after challenge by the susceptible isolates and 28·89, 61·8 and more than 120 h, respectively, after challenge by the multidrug-resistant isolates. Differences of survival were accompanied by greater rodent monocyte-release of TNF-α and malondialdehyde after stimulation by the susceptible isolates compared to multidrug-resistant ones. It is concluded that considerable differences are encountered on the stimulation of human monocytes by susceptible and resistant isolates of Pseudomonas aeruginosa. These results correlate with in vivo evidence and might influence decision on therapeutics.
Keywords: Pseudomonas aeruginosa, cytokines, sepsis, resistance
INTRODUCTION
Pseudomonas aeruginosa is a common nosocomial pathogen of immunocompromised and severely ill patients. It causes disseminated infections in neutropenic patients, in patients with cystic fibrosis and it constitutes a common cause of nosocomial sepsis in Intensive Care Units [1]. Pathogens are often multidrug-resistant; their incidence is continuously increasing [2].
Since treatment of an infection by a multidrug-resistant isolate is a challenge for the physician, detailed knowledge of the interaction between host's innate immunity and the pathogen is of particular importance. However, data on the effect of multidrug-resistant isolates on host monocytes are limited; their importance is accentuated by the introduction of immunomodulation as an attractive alternative for the management of these infections [3].
Virulence of P. aeruginosa is attributed to the production of various exotoxins (exotoxin A, exoenzyme S, elastase etc.) as well as to outer membrane lipopolysaccharide (LPS) [4]. However pathogenesis is not determined only by the production of exotoxins or endotoxins but mainly by the interaction of the bacterial cell with the nonspecific immunity host cell [5].
There are limited data in the literature for the correlation of multidrug-resistance with the ability of the pathogens to elicit immune responses. The present study was aimed at differences between susceptible and multidrug-resistant isolates of P. aeruginosa to trigger human monocytes. It involved the comparative effect on (a) the release of pro-inflammatory and anti-inflammatory cytokines as well as of malondialdehyde (MDA) by monocytes, the latter being a marker of the oxidative burst [6]; (b) on the biosynthesis of procalcitonin which is a novel marker of systemic infection [7]; and (c) on survival, in an experimental model of sepsis.
MATERIALS AND METHODS
Bacterial isolates
Twenty isolates of P. aeruginosa were studied derived from the following clinical specimens (urine: 7; purulent aspirate from cavitary lesions: 5; bronchial secretions/sputum: 5; blood: 3). Each one was implicated as pathogen for one nosocomial infection in a separate individual. Multidrug-resistance was defined as resistance to two or more groups of antimicrobials of different chemical structure [8]. Resistance was determined by measurement of minimal inhibitory concentrations (MIC). MICs of 12 antimicrobials were measured by a microdilution technique of the antimicrobial to a final volume of 0·1 ml with a log-phase inoculum of 5 × 105 cfu/ml. MIC was defined as the minimal concentration of the antimicrobial that limited visible bacterial growth after 18 h of incubation at 35°C. MIC breakpoints for determination of resistance for the studied antimicrobials were: ticarcillin and piperacillin 64 µg/ml; ceftazidime and cefepime 8 µg/ml; imipenem and meropenem 4 µg/ml; ciprofloxacin 2 µg/ml; gentamicin and tobramycin 4 µg/ml and amikacin 16 µg/ml [9].
Pulse field gel electrophoresis (PFGE) of the total DNA of each isolate was performed to confirm whether all isolates were genetically discrete [10]. Briefly, after bacterial cell lysis by sonication genomic DNA was isolated, digested by restriction endonuclease Spe I and subjected to electrophoresis on 1·5% agarose gel; voltage was adjusted to 200 Volt, temperature to 12°C and both phases of ramping at 15 and 23 h in a Gene Navigator apparatus (Pharmacia, Upsala, Sweden).
Release of endotoxins (LPS)
A log-phase inoculum of each isolate was grown after incubation of single colonies for 12 h in 10 ml of Mueller-Hinton broth (Oxoid, London, UK). Grown colonies were washed three times with Mueller-Hinton broth to remove any free LPS, diluted 1 : 100, re-suspended in 10 ml of Mueller-Hinton broth and incubated at 37°C in a shaking water bath. The resulting inoculum was equal to 5 × 106 cfu/ml. After incubation for 0·5, 2 and 4 h, 1 ml was removed and centrifuged at 1700 g for 7 min. LPS of the supernatant was estimated with the colourimetric QCL-1000 LAL assay (BioWhitaker, Maryland, USA, lower limit of detection 0·1 EU/ml). Free LPS concentrations were expressed as EU/106 cfu of bacterial cells.
In vitro stimulation of human monocytes
Human monocytes were isolated from two healthy adult volunteers, as already described [11]. Briefly, heparinized venous blood was layered over Ficoll Hypaque (Biochrom, Berlin, Germany) and centrifuged. The separated mononuclear cells were washed three times with PBS (pH 7·2) (Merck, Darmstadt, Germany) and re-suspended in RPMI 1640 (Biochrom, Berlin, Germany) supplemented with 10% Fetal Bovine Serum (Biochrom) and 2 mm of glutamine (Biochrom) in the presence of 100 U/ml penicillin G and 0·1 mg/ml of streptomycin (Sigma, St. Louis, USA). After incubation for 1 h at 37°C in 5% CO2, nonadherent cells were removed while adherent monocytes were washed three times with Hank's solution (Biochrom). Cells were then harvested by 0·25% trypsin/0·02% EDTA (Biochrom) and re-suspended in RPMI 1640 supplemented with 10% FBS and 2 mm glutamine in a 12-well plate at a density of 1 × 105 cells/well. The final volume of fluid per well was 2·4 ml and the purity of monocytes was above 95%, as assessed by Giemsa staining. Cells were left to incubate for 30 min at 37°C in 5% CO2, when 20 µl of two different inocula of a logarithmic growth of each isolate of P. aeruginosa were added in each well; the final inocula per well were 5 × 104 cfu/ml and 5 × 106 cfu/ml, respectively. Prior to addition in the wells, bacterial cells were washed three times with PBS (pH: 7·2) so that free endotoxin was removed. Cells were subsequently incubated for two and four more hours at 37°C in 5% CO2 when culture supernatants were collected and stored at −70°C until assayed.
Each separate isolate was added to wells containing monocytes of each one of the two volunteers, so that eight wells corresponded to each isolate for stimulation with both inocula and for both periods of incubation. The procedure was performed in duplicate for each isolate; a total of six wells of monocytes of each individual left unstimulated served as controls.
Measurements of cytokines, MDA and procalcitonin
Tumour necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), IL-6, IL-8, IL-10 and IL-12 were measured in culture supernatants with an enzyme immunoassay (EIA, Amersham, London, UK). Lowest limits of detection were 15·1 pg/ml for TNF-α, 10 pg/ml for IL-1β and IL-6 and 30 pg/ml for IL-8, IL-10 and IL-12. All measurements were performed in duplicate and cytokine concentrations were expressed as pg/104 monocytes.
Concentrations of MDA were measured by the thiobarbiturate method, as already described [12]. Briefly, a 0·1 ml aliquot of culture supernatant was diluted 1 : 10 in trichloracetic acid 20% (Merck, Darmstadt, Germany) and centrifuged at 12000 g in 4°C for 10 min. The supernatant was removed and incubated with 1 ml PBS pH 7·0 and 1 ml of thiobarbituric acid 0·6% (Merck) for 20 min at 90°C. Optical density was read at 535 nm (Hitachi Spectrophotometer, Tokyo, Japan). A sample of water treated in the same way was used as blank. Concentrations of MDA were estimated by a standard curve designed by known concentrations of 1,1,3,3-tetramethoxy-propane (Merck) and expressed as mmol/104 monocytes. All measurements were performed in duplicate and the lower limit of detection was 0·03 mmol/ml. The intraday and the interday coefficients of variation of the assay were 5·45% and 11·45%, respectively.
Estimation of procalcitonin (PCT) in culture supernatants was performed in duplicate with an assay based on immunochemoluminescence (BRAHMS Diagnostica GmbH, Berlin, Germany, lower limit of detection 0·08 ng/ml). Concentrations were expressed as ng/104 monocytes.
Animal study
Forty-eight male Wistar rats of a mean (± SD) weight of 309·3 ± 80·2 g were used. Animals were kept in metal cages and had access to tap water and food ad libitum. Temperature ranged 18–22°C, relative humidity 55–65% and the light/dark cycle was 6am/6 pm. The study received permission from the Veterinary Directorate of the Perfecture of Athens according to Greek legislation in conformance with the Council Directive of the EU. Six isolates of P. aeruginosa, three susceptible (8, 12 and 19) and three multidrug-resistant (6, 15 and 17) were used. Singles colonies of each isolate were incubated in Mueller-Hinton broth at 37°C for 18 h and bacterial cells were washed three times in PBS (pH: 7·2) to remove any free endotoxins. A total inoculum of 1 × 108/kg from each isolate in a final volume of one ml was injected intraperitoneally, according to the model described elsewhere [13], as follows: isolates 8 and 17 in 10 rats each, isolates 6 and 19 in nine rats each and isolates 12 and 15 in five rats each. Survival of the animals was recorded every four hours for a total of five days.
To investigate relevance of the animal model to in vitro findings on human monocytes, rodent monocytes were in vitro stimulated with the above six isolates applied for the animal study. After light ether anaesthesia (Abbott Laboratories, Chicago, USA), a midline incision was performed in two Wistar rats weighting 290 and 325 g, respectively; heparinized whole blood was collected after removal of the gut and puncture of the inferior vena cava under aseptic conditions. Rat monocytes were isolated from peripheral blood mononuclear cells and subsequently stimulated by the six isolates, as already described for human monocytes. Concentrations of TNF-α and of malondialdehyde in cell supernatants were estimated as above.
Statistical analysis
Results for LPS were expressed as medians and for cytokines as means and 95% confidence intervals. Comparisons among susceptible and multidrug-resistant isolates were performed by Mann–Whitney rank sum test.
The rate of variation of pro-inflammatory mediators elicited by the monocytes of each volunteer was assessed by dividing estimated concentrations of each tested pro-inflammatory mediator separately for each time interval and for each applied bacterial inoculum. Results were expressed by their medians.
Survival of the animals was estimated after Kaplan-Meyer analysis; comparisons were performed by log-rank test. Any value of P≤ 0·05 was considered as significant.
RESULTS AND DISCUSSION
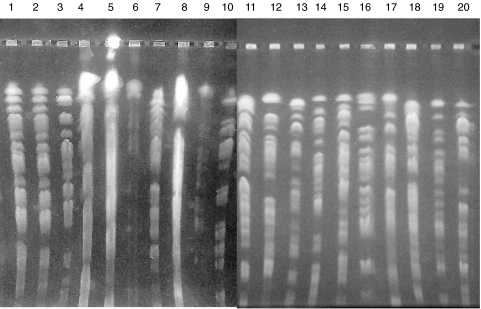
Eight isolates were susceptible to all studied antimicrobials and 12 were multidrug-resistant. PFGE (Fig. 1) confirmed that bacterial isolates were genetically discrete. MIC50 of ticarcillin, piperacillin, ceftazidime, cefepime, imipenem, meropenem, ciprofloxacin, gentamicin, tobramycin and amikacin were 4, 4, 2, 2, 2, 0·25, 0·12, 1, 1 and 1µg/ml, respectively, for the susceptible isolates and >256, >256, 128, 64, 64, 64, 128, 256, 256 and 125µg/ml, respectively, for the multidrug-resistant isolates.
Fig. 1.
Pulse Field Gel Electrophoresis (PFGE) of the 20 studied isolates of P. aeruginosa. All isolates are genetically discrete. Isolates 5, 8, 9, 11, 12, 13, 16 and 19 are susceptible to all tested antimicrobials with anti-pseudomonadal activity; isolates 1, 2, 3, 4, 6, 7, 10, 14, 15, 17, 18 and 20 are multidrug-resistant.
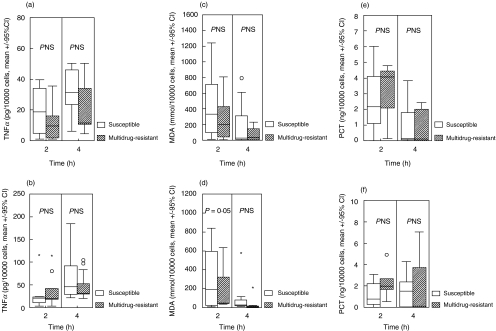
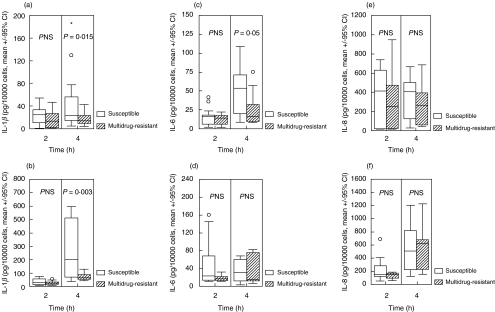
Concentrations of all measured cytokines, of malondialdehyde and of procalcitonin in cell supernatant of control monocytes were below detection limits. Concentrations of TNF-α, of MDA and of procalcitonin in the supernatants of monocytes stimulated by susceptible and multidrug-resistant isolates are shown in Fig. 2. Respective concentrations of IL-1β, of IL-6 and of IL-8 are given in Fig. 3. Median rates of variation between the two volunteers for the secretion of TNF-α, IL-1β, IL-6, IL-8, MDA and procalcitonin were 3·08, 2·16, 2·22, 1·62, 1·25 and 0·88. Results revealed that susceptible isolates induced a statistically significant increased release of IL-1β, IL-6 and MDA by human monocytes compared to multidrug-resistant isolates.
Fig. 2.
Concentrations of (a, b) TNF-α, (c, d) malondialdehyde (MDA) and (e, f) procalcitonin (PCT) in the cell supernatant of human monocytes after incubation with eight susceptible and 12 multidrug-resistant isolates of P. aeruginosa. (a, c, e) show results after incubation with a 5 × 104 cfu/ml inoculum and (b, d, f) show results after incubation with a 5 × 106 cfu/ml incoculum. ○ denote outliers and * denote extremes.
Fig. 3.
Concentrations of (a, b) interleukin-1β (IL-1β), (c, d) interleukin-6 (IL-6) and (e, f) interleukin-8 (IL-8) in the cell supernatant of human monocytes after incubation with eight susceptible (□) and 12 multidrug-resistant ( ) isolates of P. aeruginosa. (a, c, e) show results after incubation with a 5 × 104 cfu/ml inoculum and (b, d, f) show results after incubation with a 5 × 106 cfu/ml incoculum. ○ denote outliers and * denote extremes.
) isolates of P. aeruginosa. (a, c, e) show results after incubation with a 5 × 104 cfu/ml inoculum and (b, d, f) show results after incubation with a 5 × 106 cfu/ml incoculum. ○ denote outliers and * denote extremes.
Concentrations of IL-10 and of IL-12 remained undetectable in monocyte supernatant after stimulation with either susceptible or multidrug-resistant isolates (data not shown). IL-10 is an anti-inflammatory cytokine that is often released later than pro-inflammatory cytokines [14]. This may explain why it remained undetectable during the four-hour duration of the incubation of monocytes with bacterial cells. Although the latter hypothesis might be tested by further extension of the incubation period, this was not performed since bacterial growth would lead to consumption of the cell culture medium thus affecting results.
P. aeruginosa is a common pathogen causing nosocomial infections that is characterized by a high frequency of multidrug-resistance [1]. Difficulties in treatment of these infections due to the limited therapeutic options render any data on host–pathogen interaction of importance; the latter are particularly limited in case of multidrug-resistant pathogens. The present study was designed to compare the host–parasite interaction of susceptible and multidrug-resistant isolates of P. aeruginosa.
The studied interaction is a complex situation due to the interplay of various factors. Purified human monocytes were applied contrary to the majority of studies on the sepsis cascade applying peripheral mononuclear cells [14]. Although, results revealed that susceptible isolates induced a greater release of pro-inflammatory cytokines and of MDA by human monocytes compared to multidrug-resistant isolates, that phenomenon should be examined in correlation to the release of endotoxins by the bacterial cells since the latter stimulate monocyte receptors [14,15]. Respective median LPS released in broth after 0·5, 2 and 4 h of incubation were 20·11, 23·84 and 65·45 EU/106 susceptible bacterial cells and 62·74, 62·74 and 64·60 EU/106 multidrug-resistant isolates. LPS release appears to be greater by multidrug-resistant isolates when compared to susceptible ones. However the release of LPS by susceptible isolates is gradually increased over incubation contrary to multidrug-resistant isolates; during incubation of the latter isolates release of endotoxins remained stable. This observation parallels to differences of cytokine release after stimulation of monocytes by susceptible and resistant isolates.
MDA is a marker of lipid peroxidation that is significantly increased in blood during sepsis [6] and that is correlated with the severity of illness in critically ill patients [16]. Increased lipid peroxidation induced by susceptible P.aeruginosa isolates might be further evidence of an exaggerated inflammatory response induced by these bacteria when compared to multidrug-resistant ones (Fig. 2).
No difference was observed for procalcitonin, which is prognostic marker of severe sepsis and septic shock [7]. Data on its role and site of production are lacking. Our results are consistent with limited data that identify monocytes as one probable source of procalcitonin release in inflammation [17].
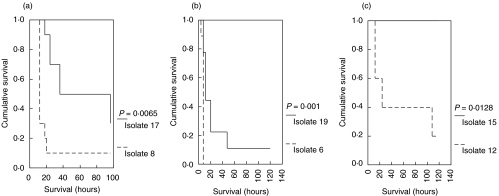
In vitro differences between susceptible and multidrug-resistant isolates to elicit monocyte responses are of limited value if not correlated to an experimental model. Progression to death evolved rapidly in rats challenged by susceptible isolates compared to those challenged by multidrug-resistant isolates (Fig. 4). Mean (± SE) survival of animals after challenge by the susceptible isolates 6, 7 and 12 was 7·56 (± 0·44), 21·80 (± 7·87) and 55·20 (± 21·63) hours, respectively; survival after challenge with the multidrug-resistant isolates 19, 17 and 15 was 28·89 (± 11·44), 61·8 ± 11·81 and more than 120 h, respectively.
Fig. 4.
Comparative survival of a total of 48 Wistar rats challenged intraperitoneally by three susceptible and three multidrug-resistant isolates of P. aeruginosa as follows: (a) isolates 8 (susceptible) and 17 (multidrug-resistant) inoculated in 10 rats each; (b) isolates 6 (susceptible) and 19 (multidrug-resistant) inoculated in nine rats each; and (c) isolates 12 (susceptible) and 15 (multidrug-resistant) inoculated in 10 rats each
These results are in accordance with in vitro data on human monocytes. When rat monocytes were incubated in the presence of the six isolates applied for the animal study (Table 1), release of TNF-α and of MDA was higher after stimulation by the susceptible isolates compared with the multidrug-resistant ones. Similar differences were found after stimulation of human monocytes by these six isolates. As a consequence, it might be hypothesized that differences in survival could be correlated with the different potential of susceptible and multidrug-resistant isolates to elicit monocyte responses.
Table 1.
Comparative release of malondialdehyde (MDA) and tumour necrosis factor-alpha (TNF-α) by rat and human monocytes after in vitro stimulation with three susceptible and three multidrug-resistant isolates of P. aeruginosa applied for the animal study
| MDA (mmol/104 cells) | TNF-α (pg/104 cells) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rat monocytes | Human monocytes | Rat monocytes | Human monocytes | ||||||
| No of isolate Applied inoculum (cfu/ml) | Time of incubation (h) | 5 × 104 | 5 × 106 | 5 × 104 | 5 × 106 | 5 × 104 | 5 × 106 | 5 × 104 | 5 × 106 |
| 8 Susceptible | |||||||||
| 2 | 13·9 | 13·9 | * | 616·8 | * | * | 47·15 | 13·87 | |
| 4 | 13·7 | 5·2 | 432·0 | 115·0 | * | 71·36 | 138·96 | 112·03 | |
| 17 Multidrug-resistant | |||||||||
| 2 | 9·9 | 14·5 | 476·1 | 48·9 | * | * | 0·46 | 11·60 | |
| 4 | 4·4 | 5·0 | 18·1 | 4·3 | * | * | 22·26 | 29·73 | |
| 6 Susceptible | |||||||||
| 2 | 9·2 | 19·1 | 309·1 | 204·2 | 11·52 | * | 17·37 | 67·90 | |
| 4 | 9·4 | 8·6 | 19·4 | 28·8 | 121·21 | 21·42 | 14·96 | 78·71 | |
| 19 Multidrug-resistant | |||||||||
| 2 | 4·1 | 7·1 | 426·6 | 48·0 | 7·2 | * | 24·48 | 19·02 | |
| 4 | 14·4 | 8·1 | 80·8 | 302·4 | * | 36 | 36·54 | 27·14 | |
| 12 Susceptible | |||||||||
| 2 | 29·1 | * | 413·2 | 492·0 | * | 21·42 | 19·72 | 17·19 | |
| 4 | 4·7 | 8·4 | 34·6 | 576·0 | 12·00 | 33·42 | 27·30 | 25·00 | |
| 15 Multidrug-resistant | |||||||||
| 2 | 15·4 | 17·5 | 640·7 | 739·2 | * | * | 17·09 | * | |
| 4 | 4·3 | 4·5 | 59·1 | 432·0 | * | * | 2·30 | 22·22 | |
undetectable.
The results of the present study demonstrate the existence of a significant difference in host–pathogen interaction of multidrug-resistant P.aeruginosa isolates in comparison to susceptible ones. These data need to be confirmed by further experimental data on various microorganisms since they might lead to significant considerations regarding the application of immunomodulatory therapy in sepsis [18].
REFERENCES
- 1.Tacconelli E, Tumbarello M, Bertagnolio S, Citton R, Spanu T, Fadda G, Cauda R. Multidrug-resistant Pseudomonas aeruginosa bloodstream infection: Analysis of trends in prevalence and epidemiology. Emerg Infect Dis. 2002;8:220–1. doi: 10.3201/eid0802.010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gales AC, Jones RN, Turnidge J, Rennie R, Ramphal R. Characterization of Pseudomonas aeruginosa isolates: Occurrence rates, antimicrobial susceptibility patterns, and molecular typing in the global SENTRY antimicrobial surveillance program, 1997–99. Clin Infect Dis. 2001;32:S146–55. doi: 10.1086/320186. [DOI] [PubMed] [Google Scholar]
- 3.Kurahashi K, Kajigawa O, Sawa T, Ohara M, Gropper MA, Frank DW, Martin TR, Wiener-Kronish JP. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J Clin Invest. 1999;104:743–50. doi: 10.1172/JCI7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reinhart K, Karzai W. Anti-tumor necrosis factor therapy in sepsis: update on clinical trials and lessons learned. Crit Care Med. 2001;29(Suppl. 1):S121–5. doi: 10.1097/00003246-200107001-00037. [DOI] [PubMed] [Google Scholar]
- 5.Rumbaugh KP, Colmer JA, Griswold JA, Hamood AN. The effects of infection of thermal injury by Pseudomonas aeruginosa PAO1 on the murine cytokine response. Cytokine. 2001;16:160–8. doi: 10.1006/cyto.2001.0960. [DOI] [PubMed] [Google Scholar]
- 6.Goode HF, Cowley HC, Walker BE, Howdle PD, Webster NR. Decreased antioxidant status and increased lipid peroxidation in patients with septic shock and secondary organ dysfunction. Crit Care Med. 1995;23:646–51. doi: 10.1097/00003246-199504000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Giamarellos-Bourboulis EJ, Mega A, Grecka P, Scarpa N, Koratzanis G, Thomopoulos G, Giamarellou H. Procalcitonin: a marker to clearly differentiate systemic inflammatory response syndrome and sepsis in the critically ill patient? Intensive Care Med. 2002;28:1351–6. doi: 10.1007/s00134-002-1398-z. [DOI] [PubMed] [Google Scholar]
- 8.Hirakata Y, Srikumar R, Poole K, et al. Multidrug efflux systems play an important role in the invasiveness of Pseudomonas aeruginosa. J Exp Med. 2002;196:109–18. doi: 10.1084/jem.20020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Committee for Clinical and Laboratory Standards. Performance standards for antimicrobial susceptibility testing. 12th Int Supplement. 2002;22:94–5. [Google Scholar]
- 10.Giamarellos-Bourboulis EJ, Karnessis L, Galani I, Giamarellou H. In vitro killing effect of moxifloxacin on clinical isolates of Stenotrophomonas maltophilia resistant to trimethoprim-sulfamethoxazole. Antimicrob Agents Chemother. 2002;46:3997–9. doi: 10.1128/AAC.46.12.3997-3999.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liel Y, Rudich A, Nagauker-Shriker O, Yermiyahu T, Levy R. Monocyte dysfunction in patients with Gaucher disease: evidence for interference of glucocerebroside with superoxide generation. Blood. 1994;83:2646–53. [PubMed] [Google Scholar]
- 12.Bégin ME, Ells G, Horrobin DF. Polyunsaturated fatty acid-induced cytotoxicity agaist tumour cells and its relationship to lipid peroxidation. J Natl Cancer Inst. 1988;80:188–94. doi: 10.1093/jnci/80.3.188. [DOI] [PubMed] [Google Scholar]
- 13.Short BL, Gardiner WM, Walker RI, Fletcher JR, Rogers JE. Rat intraperitoneal sepsis. A clinically relevant model. Circ Shock. 1983;10:351–9. [PubMed] [Google Scholar]
- 14.van der Poll T, van Deventer SJH. Cytokines and anticytokines in the pathogenesis of sepsis. Infect Dis Clin Nor Amer. 1999;13:413–26. doi: 10.1016/s0891-5520(05)70083-0. [DOI] [PubMed] [Google Scholar]
- 15.Misfeldt ML, Legaard PK, Howell SE, Fornella MH, LeGrand RD. Induction of interleukin-1 from murine peritoneal macrophages by Pseudomonas aeruginosa exotoxin A. Infect Immun. 1990;58:978–82. doi: 10.1128/iai.58.4.978-982.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alonso de Vega JM, Diaz J, Serrano E, Carbonell LF. Plasma redox status relates to severity in critically ill patients. Crit Care Med. 2000;28:1812–4. doi: 10.1097/00003246-200006000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Oberhoffer M, Stonans I, Russwurm S, Stonane E, Vongelsang H, Hunker U, Jager L, Reinhart K. Procalcitonin expression in human peripheral blood mononuclear cells and its modulation by lipopolysaccharides and sepsis-related cytokines in vitro. J Laboratory Clin Med. 1999;134:49–55. doi: 10.1016/s0022-2143(99)90053-7. [DOI] [PubMed] [Google Scholar]
- 18.Cohen ML. Epidemiology of drug resistance: implications for a post-antibiotic era. Science. 1992;257:1050–5. doi: 10.1126/science.257.5073.1050. [DOI] [PubMed] [Google Scholar]