Abstract
This study tested the hypothesis that in patients with HIV-associated lipodystrophy, adiponectin levels were related to insulin resistance, TNF-α and IL-6 and treatment with nucleoside analaogues. HIV seropositive men undergoing highly active antiretroviral treatment were enrolled into three predetermined clinical groups: lipodystrophy with central fat accumulation (n = 12); lipodystrophy without central fat accumulation (n = 15); no lipodystrophy (n = 15). HIV-negative healthy men served as controls (n = 12). Both lipodystrophic groups had a low percentage of limb fat compared to the two control groups. Patients with lipodystrophy with fat accumulation had increased truncal fat compared with controls. Levels of adiponectin did not correlate with either TNF-α or IL-6. Low levels of adiponectin were found in both lipodystrophic groups and were associated with current or previous treatment with stavudine. Furthermore, the adiponectin level correlated with the percentage of limb fat. Patients with lipodystrophy with fat accumulation were more insulin resistant, measured by HOMA-IR, compared with controls. However, HOMA-IR did no correlate to adiponectin or other cytokines. In conclusion, the finding of no difference between the two lipodystrophic groups with regard to adiponectin, indicates that low levels of adiponectin reflects fat atrophy, whereas the insulin resistance was best explained by increased truncal fat mass.
Keywords: HIV, lipodystrophy, insulin resistance, adiponectin, cytokines
INTRODUCTION
Antiretroviral therapy of HIV-infected patients has been associated with the development of lipodystrophy (LD). LD is characterized by peripheral fat loss (lipoatrophy) and central fat accumulation [1,2], but a recent prospective study has demonstrated that highly active antiretroviral therapy (HAART) may induce selective loss of limb fat [3]. The changes of fat distribution are often associated with dyslipidaemia [1,4,5], increased lipolysis [6], insulin resistance [1,4], and less often with diabetes mellitus [1,7], recently rewieved by Chen et al. [8]. Initially this syndrome was regarded as an effect of protease inhibitors (PI) [1,7], but recent reports have also demonstrated fat redistribution in nucleoside reverse transcriptase inhibitors (NRTI-treated), PI-naïve patients [9–13]. In a number of studies stavudine has been associated with a higher risk to develop lipodystrophy [9–11,14,15].
Recently, the adipose tissue has been identified as an important metabolic organ, which plays an important role in synthesizing and secreting a number of hormones and cytokines, including TNF-α[16], IL-6 [17], leptin and adiponectin [18].
Adiponectin is a 30-kD protein, synthesized and secreted exclusively by adipose tissue and shares homology to complement factor C1q and to collagen [19,20]. The functional role of adiponectin is not known, but recent evidence has suggested an important role for adiponectin in the regulation of insulin action and energy homeostasis [21]. In vitro and in vivo studies in rodents have shown that adiponectin reduces plasma levels of glucose [22–25], increases the ability of subphysiological levels of insulin to suppress gluconeogenesis [24], accelerates the oxidation of nonesterified fatty acids by muscle [22,23,26], lowers circulating fatty acid [22,23], reduces the plasma levels of triglycerides [22–24] and prevents lipid accumulation in skeletal muscle and liver [23]. Adiponectin might be regulated by TNF-α and IL-6. Thus, adiponectin knockout mice furthermore display high levels of TNF-α mRNA in adipose tissue and high concentrations in plasma [27]. Incubation of adipocytes with TNF-α result in a decrease of adiponectin [28], suggesting that TNF-α is a regulator of adiponectin [29] although it should be noted that the levels of TNF-α used were 1000-fold greater than those reported in other studies investigating the role of TNF-α in metabolic conditions. In addition, a recent study demonstrated that adiponectin gene expression and secretion is inhibited by IL-6 [30]. Finally, plasma adiponectin concentration is decreased in humans with obesity [31,32], type 2 diabetes [32,33], coronary artery disease [33,34] and dyslipidaemia [35]. Plasma level of adiponectin is also low in humans with congenital and acquired lipodystrophies, characterized by selective loss of body fat [36].
Recently, a relationship between low circulating levels of adiponectin and lipodystrophy in HIV patients has been described [37]. The purpose of this study was to test the hypothesis that in patients with HIV-associated lipodystrophy, adiponectin levels were related to the cytokines TNF-α and IL-6 as well as treatment with nucleoside analaogues. In addition, by defining two lipodystrophy groups, one with and one without central fat accumulation, we would obtain information about whether it was the peripheral fat loss or the central fat accumulation which would be linked with the low adiponectin levels.
MATERIALS AND METHODS
Subjects
Forty-two HIV-positive men receiving antiretroviral therapy were recruited from the outpatient clinic of the Department of Infectious Diseases, Rigshospitalet in Copenhagen. Lipodystrophy (LD) was defined clinically by physical examination of peripheral lipoatrophy (fat loss from face, arms, buttocks or legs) with or without central fat accumulation (abdomen, dorso-cervical fat pad) [38]. Patients were enrolled into the following three predetermined clinical groups of fat distrubution: Patients with lipodystrophy with central fat accumulation (LD-FA) (n = 12); Patients with lipodystrophy without central fat accumulation (lipoatrophy) (LD-LA) (n = 15); Patients without lipodystrophy (no-LD) (n = 15). Twelve age-matched HIV-seronegative healthy individuals served as controls. Body mass index (BMI) were similar for the groups except for the HIV-patients with lipodystrophy without central fat accumulation (LD-LA), who had a significantly lower BMI (Table 1). Demographic data were colleted for each patient: age, duration of HIV infection, weight, height, CD4 count and HIV-RNA copies. Information was obtained regarding duration and type of antiretroviral therapy. All patients were taking nucleoside reverse transcriptase inhibitors (NRTI). In the LD-FA group 11 patients were taking protease inhibitors (PI), and 4 were taking non-nucleoside reverse transcriptase inhibitors (NNRTI). In the LD-LA group 10 patients were taking PI, and 4 were taking NNRTI. In the no-LD group 9 patients were taking PI, and 5 were taking NNRTI.
Table 1.
Charateristics of the study groups
| Characteristics | LD-FA n=12 | LD-LA n=15 | No-LD n=15 | Healthy controls n=12 |
|---|---|---|---|---|
| Age (years) | 46·5 (33–63) | 48 (33–72) | 45 (31–62) | 45 (27–52) |
| Duration of HIV-infection (years) | 8·5 (6–12·5) | 11 (8–14·5) | 9 (6·0–14·5) | |
| HIV RNA, undetectable HIV RNA, No. (%) | 10 (83) | 12 (80) | 14 (93) | |
| CD4 cell count (cells/µl) | 645 (365–815) | 460 (375–615) | 450 (315–700) | |
| Antiretroviral therapy | ||||
| Duration of antiretroviral therapy (months) | 71·0 (48·0–82·0) | 90·0 (79·0–111·0)†† | 67·0 (50·5–71·0) | |
| Duration of PI (months) | 53·0 (32·0–69·0) | 65·0 (59·0–69·5) | 65·0 (61·0–69·0) | |
| Duration of NNRTI (months) | 27·5 (11·5–41·5) | 18·0 (8·5–27·5) | 25·0 (13·0–29·0) | |
| Duration of NRTI (months) | 71·0 (48·0–82·0) | 90·0 (79·0–111·5)†† | 67·0 (50·5–71·0) | |
| Duration of stavudine (month) | 27·0 (17·0–47·0)* | 61·0 (49·0–70·0) | 25·0 (22·0–49·5) | |
| Stavudine: current/previous, no (%) | 2.0 (16·7)/3(25) | 7 (46·7)/7(46·7) | 2 (13·3)/0 | |
| Body composition | ||||
| Weight (kg) | 80 (74·5–84·3)** | 66 (61·8–73)‡‡‡ | 70·5 (57·3–75·5) | 81 (74–90·1) |
| BMI (kg/m2)(22·5–27·1) | 23·7 (23·1–25·8)** | 21·3 (20·4–21·9)‡‡‡‡ | 23·0 (22·3–23·4)24·9 | |
| Total fat mass (kg) | 16·7 (15·9–22·3)** | 7·23 (7·0–10·4)‡‡‡‡ | 14·1 (11·5–17·1)‡ | 19·1 (15·1–22·9) |
| Total fat mass (%) | 21 (19–26)*** | 13 (10·5–15)†‡‡‡ | 19·5 (17–23) | 23 (20–24) |
| Total lean mass (kg)(57·8–66·4) | 59·3 (57·3–61·0) | 53·3 (51·9–58·6) | 51·9 (47·4–59·2) | 59·7 |
| Truncal fat mass (kg) | 11·2 (9·7–13·2)**†† | 4·7 (3·5–6·9)‡‡ | 6·67 (5·7–9·6) | 9·9 (7·1–12·5) |
| Truncal fat mass (%) | 61·9 (59·3–64·3)††‡‡ | 59·9 (52·5–65·9) | 52·7 (46·7–55·6) | 50·9 (45·9–54·4) |
| Limb fat mass (kg) | 5·57 (4·53–7·65)**‡‡ | 2·49 (2·23–2·76)††‡‡‡‡ | 4·65 (3·89–0·10)‡‡ | 8·12 (7·1–9·6) |
| Limb fat mass (%) | 33·3 (28·5–35·7)††‡‡‡ | 28·3 (26·2–36·4)††‡‡‡‡ | 40·2 (37·8–44·9) | 45·3(41·6–47·2) |
| Trunk fat mass/limb fat mass | 1·76 (1·73–1·26)††‡‡ | 2·17 (1·45–2·54)††‡‡‡ | 1·32 (1·08–1·48) | 1·13 (0.97–1·29) |
Data expressed are median and 25% and 75% quartiles/[range]. LD, lipodystrophy. LD-FA, lipodystrophy with fat accumulation; LD-LA, lipodystrophy without fat accumulation; no-LD, patients without lipodystrophy. NRTI, nucleoside reversetranscriptase inhibitors; NNRTI, non-nucleoside reversetranscriptase inhibitors; PI, protease inhibitors. Previous indicate previous therapy.
indicates significant difference between the indicated subgroup and LD-LA (*: P < 0·05,
indicates significant difference between the indicated subgroup and LD-LA P < 0·01)
indicates significant difference between the indicated subgroup and no-LD (†:P < 0·05,
indicates significant difference between the indicated subgroup and no-LD P < 0·01)
indicates significant difference between the indicated subgroup and controls (‡: P < 0·05,
indicates significant difference between the indicated subgroup and controls P < 0·01,
indicates significant difference between the indicated subgroup and controls P < 0·001,
indicates significant difference between the indicated subgroup and controls P < 0·0001).
All HIV-positive patients received a stable HAART regimen with no changes in antiretroviral therapy during the preceding 8 weeks, had no signs of ongoing infections and had fasting glucose under 7 mmol/l. Informed consent was obtained from all patients according to the requirements of the local ethical committee.
Blood sampling
Peripheral blood samples were obtained after an overnight fasting. Measurements of plasma glucose (mmol/l), insulin (pmol/l), total cholesterol (mmol/l), HDL-cholesterol (mmol/l), LDL-cholesterol (mmol/l) and triglycerides (mmol/l) were determined immediately using routine methods.
Laboratory tests
Cytokines were measured in plasma. Ethylenediaminetetraacetate (EDTA) was used as an anticoagulant. Plasma was stored at −80°C until analysed. TNF-α and IL-6 were determined by enzyme-linked immunosorbent assay (ELISA) kits (Quantikine High Sensitivity, R & D systems, Minneapolis, USA). Thresholds of detection were 0·094 pg/ml for TNF-α and 0·18 pg/ml for IL-6. Adiponectin was determined by a human adiponectin Radio Immuno Assay (RIA) kit (The LINCO Research, Inc., St. Charles, MO, USA). Samples were diluted 1 : 500 in assay-buffer prior to measurements. Threshold of dectection was 1 ng/ml. The intra- and inter-assay coefficients of variation were between 1·78% and 6·21% and 9·25 and 6·90%, respectively. All samples and standards were run as duplicates and the mean of duplicates was used in the statistical analyses.
CD4 cell counts were calculated by flowcytometry and HIV-RNA copies were measured by the Amplicor Hiv Monitor (Roche Molecular Systems, Branchburg, NJ, USA) (lower limit of dectection: 20 copies/ml).
Insulin resistance
Insulin resistance was assessed from fasting plasma insulin and glucose by using the homeostasis model (HOMA) [39]. The model assumes that normal weight subjects younger than 35 years have an insulin resistance of 1. The formulae for HOMA method is as follow: HOMA-IR = insulin (µU/ml) × glucose (mmol/l)/22·5 [39].
Body composition analysis
Fat and fat-free tissue masses for the whole body, trunk and extremities were measured using dual-energy X-ray absorptiometry (DEXA) scanner, Norland XR 36 (Norland Corporation, Fort Atkinson, WI, USA). The pixel size is 6·5 × 13·0 mm2 (width × height). Software version 3·94. Patients were placed as previously described [40]. The scanner was calibrated daily against the standard calibration block supplied by the manufacturer. All measurements were done in a single laboratory. Whole-body and regional fat measurements (trunk and extremity) were determined as previously described [40,41].
Statistics
Statistical calculations were performed using systat statistical software 8·0 (systat, Evanston, IL, USA). Data are presented as medians and 25% and 75% quartiles. P < 0·05 was considered significant in all analyses. Differences between independent groups were evaluated by Fischer's exact test (categorical variables) and a two–tailed t-test (continuous variables) or an analysis of variance (anova) if more than two groups were compared. If the anova had a significant P-value, pair-wise comparisons were subsequently performed by a Tukey test. In all statistical analyses, groups were considered independent because they were included only by the presence of an HIV diagnosis and the presence of lipodystrophy. The HIV-controls and the healthy controls were randomly selected according to their age and lack of lipodystrophy. Adiponectin, TNF-α and IL-6 were normally distributed after log transformations. Correlations were evaluated by Spearman's rank correlation coefficient (Rs).
RESULTS
The demographic and body compostion characteristics and antiretroviral therapy are shown in Table 1. There were no significant differences in the duration of the HIV infection, CD4 + cell count and HIV amount between the three HIV-infected groups. The subjects in the LD-LA group had received therapy (NRTI-therapy) for a longer period than subjects in the no-LD group and had a lower BMI compared to patients in the LD-FA group and the healthy controls. The dual PI therapy was saquinavir-ritonavir, indinavir-saquinavir or lopinavir-ritonavir and one patient had indinavir-nelfinavir. The LD-LA group compared to the no-LD group and the LD-FA group were more likely to receive stavudine and abacavir (46·7%versus 13·3% (no-LD) and versus 16·7% (LD-FA)) and had higher cumulative months of stavudine use (Table 1). Nearly all patients currently or previously receiving stavudine had LD (90%) and among LD patients 70% received or had received stavudine (P = 0·001). 77% of patients ever treated with abacavir had LD, but all of these also had received or received stavudine. Only 37% of LD patients had received or received abacavir. Two patients received abacavir without stavudine and none had LD (data not shown).
Patients in the LD-LA group had reduced percentage fat compared to the LD-FA group, the no-LD group and the controls. The lean body mass did not differ between the groups. The LD-LA group had reduced total limb fat (kg) compared to the LD-FA group, the no-LD group and the healthy controls. The LD-FA group had increased total truncal fat (kg) compared to the LD-LA group and the no-LD group, but not compared to healthy controls. However, the LD-FA group had higher percentage of truncal fat compared to healthy subjects, whereas the percentage of truncal fat did not differ between the LD-LA group, the no-LD group and the healthy controls. The percentage of limb fat did not differ between the two LD groups, but it was lower than both the no-LD group and controls. The truncal fat to limb fat ratio did not differ between the two LD groups, but was increased compared to both the no-LD group and the controls (Table 1).
Adiponectin, TNF-α and IL-6
Levels of plasma adiponectin, TNF-α or IL-6 did not differ between the LD-FA group and the LD-LA group. Accordingly, the two groups were pooled into one group, named lipodystrophy (LD-group).
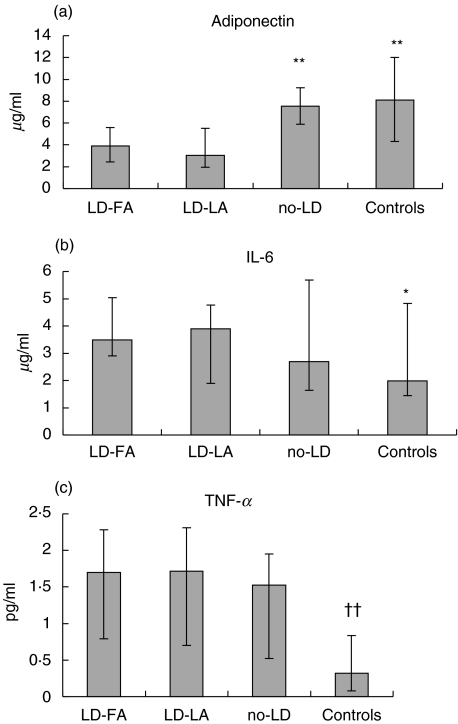
The LD-group had lower levels of adiponectin compared with the no-LD group and compared with controls. There was no significant difference between the no-LD group and the healthy subjects (Fig. 1a).
Fig. 1.
Levels of circulating adiponectin, IL-6 and TNF-α in HIV-infected patients with lipodystrophy with fat accumulation (LD-FA), HIV-infected patients with lipodystrophy without fat accumulation (LD-LA), HIV-infected patients without lipodystrophy (no-LD) and in healthy, HIV negative controls (controls). LD-FA and LD-LA did not differ with regard to any parameters. Medians and quartiles are shown. *indicates significant difference (*P < 0·05) (**P < 0·01) between LD-FA/LD-LA and the indicated subgroup; †indicates significant difference (†† = P < 0·01) between the total HIV group and controls. LD-FA n = 12; LD-LA n = 15; no-LD n = 15; Controls n = 12.
The IL-6 level was higher in the LD-group compared with the healthy control group (Fig. 1b). Furthermore, the TNF-α level was higher in the total HIV-positive group compared with controls, but there was no difference between the different HIV-positive groups (Fig. 1c).
Adiponectin and highly active antiretroviral therapy
Regarding the effect of antiretroviral drugs, the patients were divided into three new groups: (a) currently receiving the drug; (b) previously treated with the drug and not receiving it today; or (c) never received the drug. Group a versus c was compared by a two-tailed t-test for independent groups. Futhermore, a + b (a and b pooled) versus c was compared. Groups were considered independent because they included different subjects, who were not matched by any epidemiological, clinical or chemical parameters. For PI the comparison between a + b versus c were not made because only two patients never received PI.
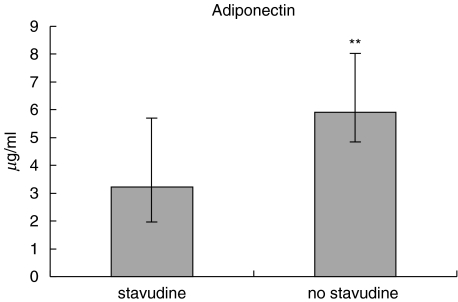
Statistical comparisons were performed for groups of antiretroviral drugs: protease inhibitors, nucleoside analogues and non nucleoside analogues. Thereafter, the effect of individual drugs was examined (n > 8): indinavir, saquinavir, ritonavir, stavudine, abacavir, lamivudine/zidovudine, didanosine, efavirenz. Regarding PIs, we also investigated the effect of dual PI treatment. Patients currently or previously treated with stavudine (group a +b) had significantly lower levels of adiponectin compared with patients, who had never received stavudine (group c) (P = 0·004) (Fig. 2). When this result was adjusted for a possible type I error due to multiple comparisons (eight different drugs) by a Bonferoni correction the P-value was still significant (P = 0·03). Furthermore, if we adjusted the P-value by a factor 16 due to that a versus c as well as a + b versus c were compared for the eight different drugs the P-value still tended to be significant (P = 0·06). There was also a significant difference between patients currently or previously treated with abacavir (3·04 µg/ml (1·98–4·91) versus 5·59 µg/ml (3·42–7·85), P = 0·02) (Data not shown). Since all patients in the abacavir group, received or had received stavudine, it was assumed that the findings of low adiponectin level in the abacavir group could be ascribed to an effect of stavudine. Stavudine was also strongly associated with the clinical diagnosis of lipodystrophy (Fisher's exact test). If the association between stavudine and adiponectin was adjusted for the effect of lipodystrophy in an anova (including logadiponectin as the dependent variable) the effect of stavudine was no longer significant (P = 0·10) whereas lipodystrophy still had a significant effect (P = 0·01). The category of clinical lipodystrophy explained 26% (adjusted R2) of the variability in adiponectin and the addition of stavudine intake added only 3% extra to this model (Data not shown). Accordingly, stavudine seemed to contribute importantly to lipodystrophy but it was not the only causative factor.
Fig. 2.
Levels of circulating adiponectin in HIV-infected patients currently or previously treated with stavudine (stavudine) and HIV-infected patients never received stavudine (No stavudine) (**P < 0·01).
Metabolic parameters
The LD-FA group had increased level of insulin and HOMA-IR in comparison with the healthy group, whereas such a difference was not found for the LD-LA group. Plasma-triglycerides did not differ between the two LD groups, but was elevated compared to the healthy control group. Both LD groups had lower HDL compared to controls (Table 2).
Table 2.
Biochemical and metabolic determinations
| Patients with LD | ||||
|---|---|---|---|---|
| Characteristics | LD-FA (n = 12) | LD-LA (n = 15) | no LD (n = 15) | Healthy controls (n = 12) |
| Cholesterol(mmol/l) | 6·3 (5·2–7·0) | 6·0 (5·3–7·1) | 5·9 (4·7–6·6) | 5·8 (4·5–6·5) |
| HDL (mmol/l) | 1·0 (0·9–1·1) | 1·1 (0·9–1·3) | 1·2 (1·0–1·5) | 1·3 (1·15–1·65)†† |
| LDL (mmol/l) | 4·3 (3·3–4·9) | 4·0 (3·1–5·0) | 3·9 (2·9–4·9) | 3·7 (3·0–4·6) |
| Triglycerides(mmol/l) | 2·5 (1·9–3·5) | 2·5 (2·3–3·5) | 2·5 (1·3–2·5)* | 1·1 (0·7–2·4)††† |
| Glucose (mmol/l) | 5·0 (4·7–5·4) | 4·9 (4·7–5·3) | 5·0 (4·8–5·5) | 4·8 (4·6–5·0) |
| Insulin (ρmol/l) | 97·5 (64·5–110·5) | 55·0 (38·0–90·0) | 55·0 (43·5–74·0) | 34·5 (20·0–94·0)‡‡ |
| IR-HOMA | 3·74 (2·15–4·58) | 2·0 (1·55–3·6) | 2·02 (1·67–2·84) | 1·12 (0·71–3·36)‡‡ |
Results expressed are median and 25% and 75% quartiles; LD, lipodystrophy; LD-FA, HIV-infected patients with lipodystrophy with fat accumulation; LD-LA, HIV-infected patients with lipodystrophy without fat accumulation; no-LD, HIV-infected patients without lipodystrophy; Controls IR-HOMA, insulin resistance as measured by homeostasis mode of assessment [39]; LDL, low density lipoprotein; HDL, high density lipoprotein. (controls). LD-FA and LD-LA did not differ with regards to the lipid profile.
denotes significant difference (*P < 0·05) between LD-FA/LD-LA and no-LD;
denotes significant difference († = P < 0·05;
denotes significant difference = P < 0·01,
denotes significant difference = P < 0·001) between LD-FA/LD-LA and controls;
denotes significant difference (
denotes significant difference = P < 0·01) between LD-FA and controls.
Regarding LDL and glucose, there were no significant differences between any of the four groups.
Relationships between adiponectin and TNF-α, IL-6, lipid profile or insulin resistance
There was a negative correlation between adiponectin and triglycerides (Rs = −0·41, n = 26, P < 0·05) in the LD-group and in the total HIV positive group (Rs = −0·52, n = 39, P = 0·002), but not within the control group. A positive correlation was found between adiponectin and HDL in the total HIV positive group (Rs = 0·43, n = 39, P < 0·02) and in all subjects (Rs = 0·44, n = 48, P < 0·02).
There was no correlation between adiponectin on one hand and insulin, glucose and insulin resistance on the other, within neither the LD-group nor within the no-LD group. However, in the healthy HIV negative controls, a negative correlation existed between adiponectin and insulin (Rs = −0·79, n = 11, P < 0·01) and between adiponectin and HOMA-IR (Rs = −0·78, n = 11, P < 0·01).
Relationship between adiponectin, insulin resistance and body composition
In the total HIV positive group the adiponectin level correlated negatively with the percentage of truncal fat (Rs = −0·51, n = 31, P < 0·01) and with truncal to limb fat ratio (Rs = −0·48, n = 31, P < 0·01) and positively with the percentage of limb fat (Rs = 0·46, n = 31, P < 0·01).
HOMA-IR correlated positively with total trunk fat (Rs = 0·39, n = 41, P < 0·05) and with percentage trunk fat (Rs = 0·38, n = 41, P < 0·05).
DISCUSSION
The major finding in this study was that low level of adiponectin was found in patients with lipodystrophy, both with and without central fat accumulation. Low level of adiponectin was associated with low limb fat mass in accordance with previous findings [37]. In addition, we found an association between low adiponectin and high percentage of truncal fat. However, the causal relationship is most likely between adiponectin and limb fat, as truncal and visceral fat accumulation is probably a compensatory response to the loss of peripheral fat [8].
Low level of adiponectin was found especially in patients with previous or current stavudine treatment. Multiple linear regression analysis revealed that the variation in adiponectin was explained primarily by the clinical lipodystrophy diagnosis, reflecting an altered fat distribution rather than stavudine treatment per se. However, the low number of subjects in the different groups limits the final conclusion that can be made. Stavudine has been associated with a higher risk of developing lipodystrophy [9–11,14,15] and drug-induced mitochondrial dysfunction in adipocytes has been suggested as a potentiel mechanism for the induction of lipodystrophy by NRTI [42,43].
In a study by Hadigan et al. [6] it was demonstated that the rates of lipolysis were significantly increased in HIV-subjects with LD and use of stavudine was predictive of increased free fatty acid flux. Moreover, use of stavudine and the rate of lipolysis were strong predictors of insulin resistance. This effect could be promoted by low adiponectin, which in our study is correlated with LD and stavudine use. Stavudine could mediate this effect by increasing fatty acid β-oxidation as demonstrated in mice [44] and/or by enhancing the expression of the malic enzyme [45].
The TNF-α levels were elevated in the total HIV groups compared with healthy controls consistent with other findings [46,47], but there were no differences between HIV subgroups. However, other studies of HIV-infected patients with LD have shown that sTNFR are elevated compared to patients without LD [46,47] and that TNF-α gene polymorphism is a determinant in the development of HIV-related lipodystrophy [48]. In addition, experimental research suggests that the regulation of adiponectin and TNF-α are related [27–29]. STNFR binds TNF-α with high affinity and may act either as inhibitors of TNF-α or carriers of this cytokine [49]. Plasma levels of sTNFR are strongly linked to the local as well as the systemic production of TNF-α but sTNFR are more stable in the circulation and accordingly they are often a more consistent marker in the TNF system than circulating levels of TNF-α[49]. This may explain why there was no difference between plasma TNF-α levels in HIV subgroups in the present study. Measurement of insulin resistance was obtained using the homeostasis model HOMA-IR, which exclusively reflects liver insulin resistance. With this limitation, only patients with LD-FA differed from the healthy controls with regard to insulin resistance. In addition, truncal fat mass (and not limb fat mass) was associated with HOMA-IR. In agreement, a recent study also found that lipoatrophy was not associated with insulin resistance [50].
Low levels of adiponectin have been associated with insulin resistance in subjects without HIV [32,36,36,51,52]. In the present study, we confirmed a negative relationship between adiponectin and insulin resistance measured by HOMA in the healthy controls, whereas we were not able to confirm such a relationship within the HIV patients. In patients with LD, the level of adiponectin is linked to fat atrophy rather than to insulin resistance. Although, TNF-α has been suggested to induce insulin resistance [53] and IL-6 to counteract the effects of TNF-α[54,55], altered levels of these cytokines did not explain the insulin resistance in the LD-FA group.
We further found a positive correlation between adiponectin and HDL in HIV-infected patients and a negative correlation between adiponectin and triglycerides in patients with lipodystrophy as has been shown before in patients with lipodystrophy [36], dyslipidaemia [35] and in healthy humans [52].
In conclusion, low levels of adiponectin are associated with lipodystrophy and linked to treatment with stavudine. The finding of no difference between LD-FA and LD-LA with regard to adiponectin indicates that it is the low limb, more than the truncal fat mass, that is the link to the low adiponectin level. Only patients with LD-FA had insulin resistance measured by HOMA. The latter was related to truncal fat mass, but not explained by adiponectin or other cytokines.
Acknowledgments
We thank the patients for their participation in this study. Ruth Rousing, Hanne Willumsen, Lene Pors and Bente Baadegaard are thanked for excellent technical help. The study was supported by the Danish Medical Research Council (22 01 0019) and H:S Rigshospitalet.
REFERENCES
- 1.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–F58. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Lo JC, Mulligan K, Tai VW, Algren H, Schambelan M. ‘Buffalo hump’ in men with HIV-1 infection. Lancet. 1998;351:867–70. doi: 10.1016/S0140-6736(97)11443-X. [DOI] [PubMed] [Google Scholar]
- 3.Mallon PW, Miller J, Cooper DA, Carr A. Prospective evaluation of the effects of antiretroviral therapy on body composition in HIV-1-infected men starting therapy. AIDS. 2003;17:971–9. doi: 10.1097/00002030-200305020-00005. [DOI] [PubMed] [Google Scholar]
- 4.Kosmiski LA, Kuritzkes DR, Lichtenstein KA, et al. Fat distribution and metabolic changes are strongly correlated and energy expenditure is increased in the HIV lipodystrophy syndrome. AIDS. 2001;19:1993–2000. doi: 10.1097/00002030-200110190-00012. [DOI] [PubMed] [Google Scholar]
- 5.Meininger G, Hadigan C, Laposata M, et al. Elevated concentrations of free fatty acids are associated with increased insulin response to standard glucose challenge in human immunodeficiency virus-infected subjects with fat redistribution. Metabolism. 2002;51:260–6. doi: 10.1053/meta.2002.29999. [DOI] [PubMed] [Google Scholar]
- 6.Hadigan C, Borgonha S, Rabe J, Young V, Grinspoon S. Increased rates of lipolysis among human immunodeficiency virus-infected men receiving highly active antiretroviral therapy. Metabolism. 2002;51:1143–7. doi: 10.1053/meta.2002.34704. [DOI] [PubMed] [Google Scholar]
- 7.Carr A, Samaras K, Chisholm DJ, Cooper DA. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 1998;20:1881–3. doi: 10.1016/S0140-6736(98)03391-1. [DOI] [PubMed] [Google Scholar]
- 8.Chen D, Misra A, Garg A. Clinical review 153: Lipodystrophy in human immunodeficiency virus-infected patients. J Clin Endocrinol Metab. 2002;87:4845–56. doi: 10.1210/jc.2002-020794. [DOI] [PubMed] [Google Scholar]
- 9.Saint-Marc T, Partisani M, Poizot-Martin I, et al. A syndrome of peripheral fat wasting (lipodystrophy) in patients receiving long-term nucleoside analogue therapy. AIDS. 1999;13:1659–67. doi: 10.1097/00002030-199909100-00009. [DOI] [PubMed] [Google Scholar]
- 10.Madge S, Kinloch -d, e-Loes S, Mercey D, Johnson MA, Weller IV. Lipodystrophy in patients naive to HIV protease inhibitors. AIDS. 1999;13:735–7. doi: 10.1097/00002030-199904160-00020. [DOI] [PubMed] [Google Scholar]
- 11.Mallal SA, John M, Moore CB, James IR, McKinnon EJ. Contribution of nucleoside analogue reverse transcriptase inhibitors to subcutaneous fat wasting in patients with HIV infection. AIDS. 2000;14:1309–16. doi: 10.1097/00002030-200007070-00002. [DOI] [PubMed] [Google Scholar]
- 12.Carr A, Miller J, Law M, Cooper DA. A syndrome of lipoatrophy, lactic acidaemia and liver dysfunction associated with HIV nucleoside analogue therapy: contribution to protease inhibitor–related lipodystrophy syndrome. AIDS. 2000;14:F25–F32. doi: 10.1097/00002030-200002180-00001. [DOI] [PubMed] [Google Scholar]
- 13.Tsekes G, Chrysos G, Douskas G, et al. Body composition changes in protease inhibitor-naive HIV-infected patients treated with two nucleoside reverse transcriptase inhibitors. HIV Medical. 2002;3:85–90. doi: 10.1046/j.1468-1293.2002.00105.x. [DOI] [PubMed] [Google Scholar]
- 14.Saint-Marc T, Partisani M, Poizot-Martin I, et al. Fat distribution evaluated by computed tomography and metabolic abnormalities in patients undergoing antiretroviral therapy: preliminary results of the LIPOCO study. AIDS. 2000;14:37–49. doi: 10.1097/00002030-200001070-00005. [DOI] [PubMed] [Google Scholar]
- 15.Joly V, Flandre P, Meiffredy V, et al. Increased risk of lipoatrophy under stavudine in HIV-1-infected patients: results of a substudy from a comparative trial. AIDS. 2002;16:2447–54. doi: 10.1097/00002030-200212060-00010. [DOI] [PubMed] [Google Scholar]
- 16.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 17.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–50. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 18.Mora S, Pessin JE. An adipocentric view of signaling and intracellular trafficking. Diabetes Metab Res Rev. 2002;18:345–56. doi: 10.1002/dmrr.321. [DOI] [PubMed] [Google Scholar]
- 19.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 20.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–9. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 21.Tsao TS, Lodish HF, Fruebis J. ACRP30, a new hormone controlling fat and glucose metabolism. Eur J Pharmacol. 2002;440:213–21. doi: 10.1016/s0014-2999(02)01430-9. [DOI] [PubMed] [Google Scholar]
- 22.Fruebis J, Tsao TS, Javorschi S, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA. 2001;98:2005–10. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 24.Berg AH, Du Combs TPX, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–53. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 25.Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108:1875–81. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 27.Maeda N, Shimomura I, Kishida K, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–7. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 28.Kappes A, Loffler G. Influences of ionomycin, dibutyryl-cycloAMP and tumour necrosis factor-alpha on intracellular amount and secretion of apM1 in differentiating primary human preadipocytes. Horm Metab Res. 2000;32:548–54. doi: 10.1055/s-2007-978684. [DOI] [PubMed] [Google Scholar]
- 29.Maeda N, Takahashi M, Funahashi T, et al. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–9. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 30.Fasshauer M, Kralisch S, Klier M, et al. Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2003;301:1045–50. doi: 10.1016/s0006-291x(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 31.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 32.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–5. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 33.Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–9. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 34.Kumada M, Kihara S, Sumitsuji S, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–9. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 35.Matsubara M, Maruoka S, Katayose S. Decreased plasma adiponectin concentrations in women with dyslipidemia. J Clin Endocrinol Metab. 2002;87:2764–9. doi: 10.1210/jcem.87.6.8550. [DOI] [PubMed] [Google Scholar]
- 36.Haque WA, Shimomura I, Matsuzawa Y, Garg A. Serum adiponectin and leptin levels in patients with lipodystrophies. J Clin Endocrinol Metab. 2002;87:2395. doi: 10.1210/jcem.87.5.8624. [DOI] [PubMed] [Google Scholar]
- 37.Mynarcik DC, Combs T, McNurlan MA, Scherer PE, Komaroff E, Gelato MC. Adiponectin and leptin levels in HIV-infected subjects with insulin resistance and body fat redistribution. J Acquir Immune Defic Syndr. 2002;31:514–20. doi: 10.1097/00126334-200212150-00009. [DOI] [PubMed] [Google Scholar]
- 38.Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999;19:2093–9. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 39.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment. insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 40.Carr A, Emery S, Law M, Puls R, Lundgren JD, Powderly WG. An objective case definition of lipodystrophy in HIV-infected adults: a case-control study. Lancet. 2003;361:726–35. doi: 10.1016/s0140-6736(03)12656-6. [DOI] [PubMed] [Google Scholar]
- 41.Hadigan C, Miller K, Corcoran C, Anderson E, Basgoz N, Grinspoon S. Fasting hyperinsulinemia and changes in regional body composition in human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 1999;84:1932–7. doi: 10.1210/jcem.84.6.5738. [DOI] [PubMed] [Google Scholar]
- 42.Kakuda TN, Brundage RC, Anderson PL, Fletcher CV. Nucleoside reverse transcriptase inhibitor-induced mitochondrial toxicity as an etiology for lipodystrophy. AIDS. 1999;13:2311–2. doi: 10.1097/00002030-199911120-00019. [DOI] [PubMed] [Google Scholar]
- 43.Brinkman K, Smeitink JA, Romijn JA, Reiss P. Mitochondrial toxicity induced by nucleoside-analogue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophy. Lancet. 1999;354:1112–5. doi: 10.1016/S0140-6736(99)06102-4. [DOI] [PubMed] [Google Scholar]
- 44.Gaou I, Malliti M, Guimont MC, et al. Effect of stavudine on mitochondrial genome and fatty acid oxidation in lean and obese mice. J Pharmacol Exp Ther. 2001;297:516–23. [PubMed] [Google Scholar]
- 45.Roche R, Poizot-Martin I, Yazidi CM, et al. Effects of antiretroviral drug combinations on the differentiation of adipocytes. AIDS. 2002;16:13–20. doi: 10.1097/00002030-200201040-00003. [DOI] [PubMed] [Google Scholar]
- 46.Ledru E, Christeff N, Patey O, et al. Alteration of tumor necrosis factor-alpha T-cell homeostasis following potent antiretroviral therapy: contribution to the development of human immunodeficiency virus-associated lipodystrophy syndrome. Blood. 2000;95:3191–8. [PubMed] [Google Scholar]
- 47.Mynarcik DC, McNurlan MA, Steigbigel RT, Fuhrer J, Gelato MC. Association of severe insulin resistance with both loss of limb fat and elevated serum tumor necrosis factor receptor levels in HIV lipodystrophy. J Acquir Immune Defic Syndr. 2000;25:312–21. doi: 10.1097/00042560-200012010-00004. [DOI] [PubMed] [Google Scholar]
- 48.Maher B, Alfirevic A, Vilar FJ, Wilkins EG, Park BK, Pirmohamed M. TNF-alpha promoter region gene polymorphisms in HIV-positive patients with lipodystrophy. AIDS. 2002;16:2013–8. doi: 10.1097/00002030-200210180-00005. [DOI] [PubMed] [Google Scholar]
- 49.Richards C, Gauldie J. Role of cytokines in the acute-phase response. In: Aggarwal BB, Aggarwal PR, editors. Human Cytokines. Their Roles in Disease and Therapy. Cambridge, MA: Blackwell Science; 1995. pp. 253–69. [Google Scholar]
- 50.Worm D, Kirk O, Andersen O, et al. Clinical lipoatrophy in HIV-1 patients on HAART is not associated with increased abdominal girth, hyperlipidaemia or glucose intolerance. HIV Medical. 2002;3:239–46. doi: 10.1046/j.1468-1293.2002.00125.x. [DOI] [PubMed] [Google Scholar]
- 51.Stefan N, Vozarova B, Funahashi T, et al. Plasma adiponectin concentration is associated with skeletal muscle insulin receptor tyrosine phosphorylation, and low plasma concentration precedes a decrease in whole-body insulin sensitivity in humans. Diabetes. 2002;51:1884–8. doi: 10.2337/diabetes.51.6.1884. [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto Y, Hirose H, Saito I, et al. Correlation of the adipocyte-derived protein adiponectin with insulin resistance index and serum high-density lipoprotein-cholesterol, independent of body mass index, in the Japanese population. Clin Sci. 2002;103:137–42. doi: 10.1042/cs1030137. [DOI] [PubMed] [Google Scholar]
- 53.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–15. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J. 2002;16:1335–47. doi: 10.1096/fj.01-0876rev. [DOI] [PubMed] [Google Scholar]
- 55.Walker UA, Brinkman K. NRTI induced mitochondrial toxicity as a mechanism for HAART related lipodystrophy: fact or fiction? HIV Medical. 2001;2:163–5. doi: 10.1046/j.1464-2662.2001.00073.x. [DOI] [PubMed] [Google Scholar]