Abstract
In chronic hepatitis B virus (HBV) infection, immune responses to hepatitis B core antigen (HBcAg) are weak. Interleukin (IL)-10 is a potent immunosuppressive cytokine which we reported recently to be secreted in response to HBcAg by peripheral blood mononuclear cells (PBMCs) from patients with chronic HBV infection or healthy controls. Using an enzyme-linked immunospot assay, we compared the ability of HBcAg to stimulate IL-10 production by PBMC with that of lipopolysaccharide (LPS), phytohaemagglutinin-P and hepatitis C virus-derived antigens in 16 patients with chronic HBV infection and six healthy controls. Frequencies of IL-10 spot-forming cells (SFC) in response to HBcAg were comparable to those obtained with LPS in patients with chronic HBV infection. Frequencies of IL-10 SFC in response to HBcAg or to LPS were significantly higher in patients with chronic HBV infection than in healthy controls. IL-10 SFC in response to HBcAg consisted of 26–35% T cells, 62–70% monocytes and less than 1% B cells in patients with chronic HBV infection. Only monocytes contributed to IL-10 production in controls. Frequencies of HBcAg stimulated IL-10 SFC representing T cells and monocytes were significantly higher in patients with elevated serum alanine aminotransferase (ALT) and detectable HBV DNA than in patients with normal ALT and undetectable HBV DNA. The potent ability of HBcAg to stimulate IL-10 production by PBMC may contribute importantly to immune tolerance toward HBV.
Keywords: chronic hepatitis B virus infection, enzyme-linked immunospot assay, interleukin 10, hepatitis B core antigen
INTRODUCTION
In chronic hepatitis B virus (HBV) infection cellular immune responses are weak or absent, except during acute exacerbation [1–4]. The immunological and virological basis for viral persistence in chronic HBV infection has not been elucidated fully, although cytokine imbalance, T cell exhaustion, anergy and viral mutations have been suggested as possible mechanisms [1].
Hepatitis B core antigen (HBcAg) possesses unique immunological features conveying the properties of both T cell-independent and -dependent antigens: as little as 0·025 µg of HBcAg can elicit antibody production without need for an adjuvant; immunization with HBcAg primes preferentially type 1 helper T (Th1) cells, and HBcAg is an effective carrier for heterogeneous epitopes [5–7]. However, we found previously in patients with chronic HBV infection that peripheral blood mononuclear cells (PBMC) and lymphocytes infiltrating the liver secreting the potent immune-suppressor interleukin (IL)-10 [8–14] in response to HBcAg outnumber HBcAg-specific interferon (IFN)-γ-secreting cells [15]. In addition, IL-10-secreting cells were induced upon stimulation of PBMC from healthy controls with HBcAg. Thus HBcAg may promote HBV infection by stimulating production of immunosuppressive IL-10 in excess of immunostimulatory IFN-γ. Relationships between IL-10 production by PBMC stimulated with HBV antigens and disease activities of chronic HBV infection remain unclear [16,17], although IL-10 production by PBMC stimulated with HBcAg or HBeAg has been reported to decrease with successful treatment in patients with chronic HBV infection [18].
In the present study we found that HBcAg stimulated IL-10 production by CD4+ and CD8+ T cells, as well as by monocytes from patients with chronic HBV infection. Frequencies of HBcAg-stimulated IL-10 immunospot-forming cells (SFC) among PBMC were significantly higher in patients with serum alanine aminotransferase (ALT) elevations and detectable HBV DNA than in other patients with normal ALT concentrations and undetectable HBV DNA. Additionally, we found that HBcAg also stimulated IL-10 production by monocytes from healthy controls.
PATIENTS AND METHODS
Subjects
Blood samples were obtained from 16 patients with chronic HBV infection and from six healthy controls who had normal ALT concentrations and no serological markers of hepatitis virus infection. All patients were HBsAg-positive, HBsAb-negative and HBcAb-positive with serum in a 1 : 200 dilution. Patients were separated into two groups: group A, patients with elevated serum ALT and detectable HBV-DNA measured by commercially available transcription-mediated amplification (TMA) assay with a detection limit of 3·7 LEG/ml; and group N, patients with normal serum ALT and undetectable HBV-DNA (Table 1). Patients had not received immunomodulating drugs during the preceding 3 years and had no other cause of chronic liver injury, such as HCV infection or excessive alcohol intake. Informed consent was obtained from all subjects, and the study was approved by the Ethical Review Committee of Jichi Medical School.
Table 1.
Characteristics of chronic hepatitis B patients with elevated serum ALT and detectable HBV DNA (group A), patients with normal ALT and undetectable HBV DNA (group N) and healthy controls
| Group A | Group N | Control | |
|---|---|---|---|
| Number of subjects | 7 | 9 | 6 |
| Age (years) | 42·5 ± 3·5 | 34·7 ± 2·2 | 28·2 ± 0·4 |
| Gender (M:F) | 6 : 1 | 8 : 1 | 4 : 2 |
| ALT (IU/l) | 154·2 ± 44·6 | 23·8 ± 2·6 | 18·9 ± 0·3 |
| HBV DNA (LGE/ml) | 5·7 ± 0·6 | <3·7 | <3·7 |
Values are means ± s.d. ALT, alanine aminotransferase; HBV, hepatitis B virus.
Antigens and mitogens
Recombinant HBcAg purchased from the Institute of Immunology (Tokyo, Japan) was more than 95% pure according to high-performance liquid chromatography. Lipopolysaccharide (LPS) and phytohaemagglutinin (PHA-P) were obtained from Sigma (Atlanta, GA, USA). HCV-derived core antigens (JCC-2 or C7) were generous gifts from the Chemo-Sero-Therapeutic Research Institute (Kumamoto, Japan) and the Advanced Life Science Institute (Saitama, Japan), respectively.
Isolation of PBMC, CD3+ T cells, B cells and monocytes
PBMC isolated from heparinized venous blood by gradient centrifugation using Ficoll-Hypaque (Amersham-Pharmacia Biotech, Uppsala, Sweden) were suspended in RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum (RPMI-1640/10% FCS). The cells were kept cool to avoid adhesion and activation of monocytes.
To determine which cells among PBMC were responsible for IL-10 production, PBMC were incubated for 18 h in a 14 ml polypropylene round-bottomed tube (Falcon 2059, BD Biosciences, San Jose, CA, USA) with 10 µg/ml HBcAg, 1 µg/ml LPS or medium alone at 37°C in a humidified 5% CO2 atmosphere. Then CD3+ T cells, B cells and monocytes were isolated from stimulated PBMC by antibody-coated magnetic beads. CD3+ T cells were isolated with MACS CD3+ microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany), monocytes with a monocyte negative-isolation kit (Dynal, Oslo, Norway) and B cells with CD19-positive isolation beads and Detacha-beads (Dynal), according to the manufacturers’ instructions.
Enzyme-linked immunospot (ELISpot) assay
Frequencies of IL-10-secreting cells among PBMC, isolated T cells, B cells and monocytes were measured using an ELISpot assay as described previously [15]. In brief, 2 × 104 PBMC were added to each well coated with antihuman IL-10 antibody (Mabtech, Nacka, Sweden) for stimulation with 10 µg/ml of HBcAg, 1 µg/ml of LPS, 10 µg/ml PHA-P, 10 µg/ml of HCV derived antigens (JCC-2 and C7) or medium alone. For T cells, B cells and monocytes isolated from HBcAg-stimulated PBMC, 105 T or B cells or 104 monocytes were added to each well coated with antihuman IL-10 antibody. After incubation for 20 h, spots reflecting IL-10-secreting cells were visualized and counted. Responses were considered significant when a minimum of five SFC were present per well and the number was at least twice that in wells without the stimulus. All results are expressed as means of duplicate SFC in the presence of stimulus minus SFC without stimulus.
Phenotype analysis
In selected patients, ELISpot assays were performed using CD4+ or CD8+ cell-depleted non-adherent PBMC to analyse the phenotype of IL-10-secreting T cells. Non-adherent PBMC (lymphocytes) separated readily from monocytes which weakly express CD4 but remain adherent to the culture dish after 4 h of PBMC incubation with or without HBcAg. Non-adherent PBMC then were incubated with magnetic Dynabeads (Dynal A.S) coated with anti-CD4 or -CD8 monoclonal antibody, and cells bound to the two types of beads were depleted from unbound cells. The two types of unbound cells (CD4 depleted or CD8 depleted) were added to each well of 96-well nitrocellulose membrane-bottomed microtitre plates; well bottoms were coated with antihuman IL-10 antibody. Thus frequencies of specific IL-10-secreting cell types could be determined. To evaluate the contribution of B cells to IL-10 SFC, B cell depleted non-adherent PBMC also were analysed.
Statistical analysis
Data were analysed using a Kruskal–Wallis test or a Mann–Whitney U-test. A P-value less than 0·05 was considered indicative of statistical significance. Statistical analyses were carried out using a statistical software package statview version 5·0 for Macintosh (SAS Institute, Cary, NC, USA).
RESULTS
Cells secreting IL-10 in response to stimulation with HBcAg, LPS, PHA-P or HCV-derived antigens
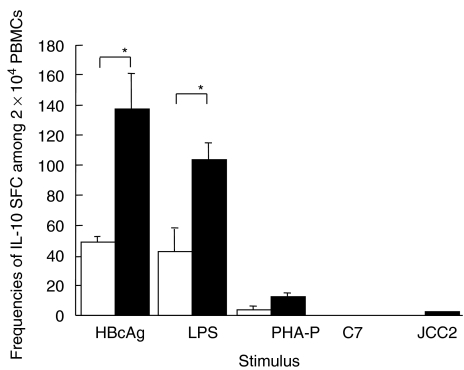
Frequencies of IL-10 SFC in response to different antigens and mitogens are shown in Fig. 1. The median (and range) of frequencies of IL-10 SFC/2 × 104 PBMC upon stimulation with HBcAg in patients with chronic HBV infection and in healthy controls were 126 (56–502) and 45 (31–66), respectively; those with LPS stimulation were 94 (44–166) and 41 (18–70), respectively. IL-10 SFC with PHA-P stimulation were 9 (7–29) and 4 (2–7), respectively. Few IL-10 SFC were detected in response to HCV-derived antigens in either patients with chronic HBV infection or healthy controls. Both HBcAg and LPS induced significantly more IL-10 SFC than PHA-P and HCV-derived antigens in patients with chronic HBV infection (P < 0·05). Frequencies of IL-10 SFC induced by either HBcAg or LPS were significantly higher in patients with chronic HBV infection than in healthy controls (P < 0·05). Frequencies of HBcAg-induced IL-10 SFC were higher, although not significantly, than those of LPS-induced IL-10 SFC in patients with chronic HBV infection.
Fig. 1.
Frequencies of IL-10-secreting cells upon stimulation with HBcAg, LPS, PHA-P and HCV-derived peptides (JCC-2 and C-7) per 2 × 104 PBMC. Open columns are frequencies of IL-10-secreting cells in healthy controls, and filled columns are those in patients with chronic HBV infection. *P < 0·05. IL, interleukin; HBcAg, hepatitis B core antigen; LPS, lipopolysaccharide; PHA-P, phytohaemagglutinin; HCV, hepatitis c virus; PBMC, peripheral blood mononuclear cell.
Frequencies of IL-10 SFC representing T cell, B cell and monocyte fractions of PBMC
In a preliminary study, T cells isolated from PBMC of patients with chronic HBV infection did not respond to HBcAg in the absence of antigen-presenting cells (APC). Thus we stimulated PBMC with HBcAg or LPS before separating them into T cell, B cell and monocyte fractions. The composition of T cells, B cells and monocytes in PBMC stimulated with HBcAg or LPS were 26–45%, 5–9% and 16–28%, respectively.
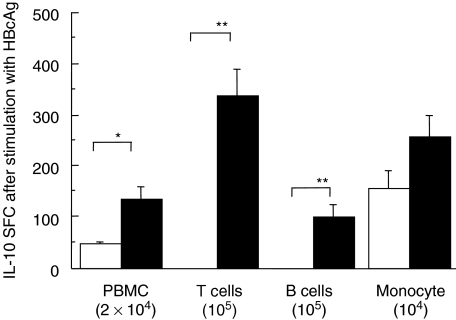
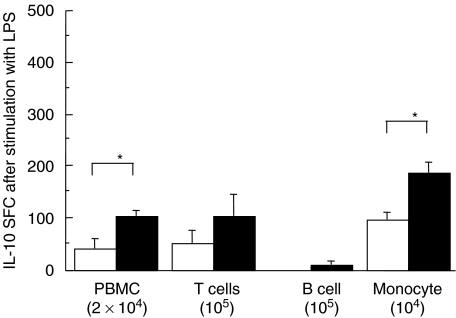
Among PBMC, monocytes, T cells and a few B cells secreted IL-10 in response to stimulation with HBcAg in patients with chronic HBV infection; monocytes were the main IL-10-producing cells in healthy controls (Fig. 2). Patients with increased serum ALT and detectable serum HBV DNA had higher frequencies of HBcAg-induced IL-10-secreting T cells and monocytes than those with normal ALT and undetectable HBV DNA (P < 0·03 and P < 0·01, respectively; Table 2). Frequencies of IL-10 SFC in monocytes stimulated with LPS were higher in patients with chronic HBV infection than in healthy controls (Fig. 3).
Fig. 2.
Frequencies of IL-10-secreting cells in 2 × 104 PBMC, 105 CD3+ T cells, 104 monocytes and 105 B cells separated from PBMC stimulated with HBcAg. Open columns are frequencies of IL-10-secreting cells in healthy controls and filled columns are frequencies in patients with chronic HBV infection.*P < 0·05, **P < 0·01. IL, interleukin; PBMC, peripheral blood mononuclear cell; HBV, hepatitis B virus.
Table 2.
Frequencies of IL-10 SFC among 2 × 104 PBMC, 105 T cells, 105 B cells and 104 monocytes in response to HBcAg in chronic hepatitis B patients with normal ALT levels and undetectable HBV DNA (group N), and patients with elevated ALT and detectable HBV DNA (group A)
| 2 × 104 PBMC | 105 T cells | 105 B cells | 104 monocytes | |
|---|---|---|---|---|
| Group N | 83 (63–146)* | 293 (45–348)** | 70 (25–265) | 124 (38–323)*** |
| Group A | 146 (69–502)* | 496 (395–596)** | 80 (22–178) | 362 (69–502)*** |
Values are medians (and ranges). SFC, spot-forming cell; PBMC, peripheral blood mononuclear cell; HBcAg, hepatitis B core antigen; ALT, alanine aminotransferase; HBV, hepatitis B virus.
P = 0·03,
P = 0·02,
P = 0·007.
Fig. 3.
Frequencies of IL-10-secreting cells in 2 × 104 PBMC, 105 CD3+ T cells, 104 monocytes and 105 B cells separated from PBMC stimulated with LPS. Open columns are frequencies of IL-10-secreting cells in healthy controls, and filled columns are frequencies in patients with chronic HBV infection. *P < 0·05.
T cell subsets secreting IL-10 in response to HBcAg stimulation
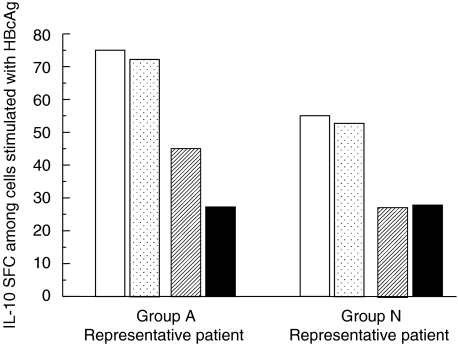
T cell subsets of non-adherent PBMCs that secreted IL-10 in response to HBcAg were analysed by the conventional magnetic bead depletion methods in two patients with increased serum ALT and detectable HBV DNA and in two patients with normal ALT and undetectable HBV DNA. In both patients with increased serum ALT and detectable HBV DNA, the frequency of IL-10 SFC in non-adherent PBMC exposed to HBcAg was reduced more by depletion of CD4+ cells than by depletion of CD8+ cells. In contrast, in both patients with normal serum ALT and undetectable HBV DNA, the frequency of IL-10 SFC among non-adherent PBMC in response to HBcAg was reduced almost equally by depletion of CD4+ cells and depletion of CD8+ cells (Fig. 4).
Fig. 4.
Phenotypes of non-adherent IL-10-secreting cells in a patient with elevated serum ALT and detectable HBV DNA (group A representative patient) and a patient with normal ALT and undetectable HBV DNA (group N representative patient). Two patients were studied from each group and similar results were obtained within each pair of patients with similar laboratory findings. Non-adherent PBMC collected from HBcAg-stimulated PBMC were depleted of CD4+, CD8+ or B cells, and frequencies of IL-10-secreting cells among fractionated and unfractionated non-adherent PBMC were assayed. Frequencies of IL-10 SFC are expressed as numbers among 105 non-adherent PBMC before depletion. Open columns represent IL-10-secreting cells among unfractionated non-adherent PBMC; open columns with black stippling among B cell-depleted non-adherent PBMC; hatched columns among CD8+ T cell-depleted non-adherent PBMC; and filled columns among CD4+ T cell-depleted non-adherent PBMC. IL, interleukin; ALT, alanine aminotransferase; HBV, hepatitis B virus; PBMC, peripheral blood mononuclear cell; SFC, spot-forming cell.
DISCUSSION
In the present study, we showed that HBcAg stimulates IL-10 secretion by peripheral blood T cells, monocytes and B cells in patients with chronic HBV infection. Approximately 0·34% of T cells, 2·5% of monocytes and 0·12% of B cells secreted IL-10 in response to HBcAg at 10 µg/ml. IL-10 SFC among PBMC in response to HBcAg stimulation were estimated to include 62–70% of monocytes, 26–35% of T cells and less than 1% B cells. Not only CD4+ T cells but also CD8+ T cells secreted IL-10 in response to stimulation with HBcAg.
Frequencies of HBcAg-induced IL-10 SFC in peripheral blood T cells and monocytes both were significantly higher in patients with elevated ALT and detectable HBV DNA than in patients with normal ALT and undetectable HBV DNA. We have shown previously that frequencies of HBcAg-stimulated IFN-γ SFC in the peripheral blood are higher in patients with elevated serum ALT and detectable HBV DNA than in patients with normal ALT and undetectable HBV DNA [15]. IL-10 secretion by human monocytes has been reported to be augmented by the proinflammatory cytokine IFN-γ[14]; stimulation of IL-10 secretion by HBcAg and its augmentation by IFN-γ may prevent development of severe liver damage by suppressing immune responses to HBV infection. However, excessive IL-10 production also may contribute to viral persistence and weak cellular immune responses to HBV antigens in patients with chronic HBV infection.
Recently, a concept of anergic CD4+ and CD8+ T cell induction by immature dendritic cells primed by PBMC-derived IL-10 has attracted much attention [19,20]. Deranged APC function has been reported in HBV transgenic mice as well as in humans with chronic HBV infection [21,22]. IL-10 produced in response to HBcAg stimulation may prime dendritic cells and monocytes to induce anergic CD4+ and CD8+ T cells in chronic HBV infection. IL-10-secreting CD8+ T cells are considered to be anergic, non-cytolytic cells that prevent proliferation of cytolytic T cells expressing type 1 cytokines [23–26]. In the present study, we found that a portion of T cells secreting Il-10 in response to HBcAg stimulation were CD8+. Based on negative correlations of serum ALT with frequencies of IL-10-secreting CD8+ T cells and extent of hepatic fibrosis, Prezzi et al. [27] proposed that IL-10-secreting CD8+ T cells act to prevent tissue damage caused by cytolytic T cells expressing type 1 cytokines in chronic HCV infection. This hypothesis may also be applicable to HBV infection, although further studies are necessary.
Our study also showed that HBcAg stimulates IL-10 secretion by monocytes obtained from healthy people upon stimulation with HBcAg. However, acute HBV infection in healthy adults is generally self-limited, eliciting vigorous immune responses. Although IL-10 is thought generally to be immunosuppressive, this cytokine has been reported to induce potent cytotoxic T lymphocytes in the presence of IL-2 [28]. We have reported that frequencies of HBcAg-specific Th1 cells assessed by HBcAg-stimulated IFN-γ SFC increase markedly before peak acute exacerbation in chronic hepatitis B, followed by a decrease after the peak [15]. Thus IL-10 may be bifunctional in HBV infection, participating in strong antiviral T cell responses in the early phase of acute HBV infection or acute exacerbation of a chronic infection and suppressing responses in the recovery phase and in chronic HBV infection.
In conclusion, HBcAg stimulates IL-10 production by T cells as well as monocytes in patients with chronic HBV infection, and by monocytes in healthy people. Both CD4+ and CD8+ T cells in the peripheral blood of patients with chronic HBV infection secrete IL-10 in response to stimulation with HBcAg. IL-10 may play a role in modulating antiviral responses in HBV infection, contributing to protection from severe liver damage but also to persistence of HBV infection.
REFERENCES
- Löhr HF, Gerken G, Schlicht HJ, et al. Low frequency of cytotoxic liver-infiltrating T lymphocytes specific for endogenous processed surface and core proteins in chronic hepatitis B. J Infect Dis. 1993;168:1133–9. doi: 10.1093/infdis/168.5.1133. [DOI] [PubMed] [Google Scholar]
- Ferrari C, Penna A, Bertoletti A, et al. Cellular immune responses to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J Immunol. 1990;145:3442–9. [PubMed] [Google Scholar]
- Tsai SL, Chen PJ, Lai MY, et al. Acute exacerbations of chronic type B hepatitis are accompanied by increased T cell responses to hepatitis B core and e antigens. Implications for hepatitis B e antigen seroconversion. J Clin Invest. 1992;89:87–96. doi: 10.1172/JCI115590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehermann B, Fowler P, Sidney J, et al. The cytotoxic-T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J Exp Med. 1995;181:1047–58. doi: 10.1084/jem.181.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich DR, McLachlan A. The nucleocapsid of hepatitis B virus is both a T cell-independent and T cell-dependent antigen. Science. 1986;234:1398–401. doi: 10.1126/science.3491425. [DOI] [PubMed] [Google Scholar]
- Milich DR, Schödel F, Hughes JL, et al. The hepatitis B virus core and e antigens elicit different Th cell subsets; antigen structure can affect Th cell phenotype. J Virol. 1997;71:2192–201. doi: 10.1128/jvi.71.3.2192-2201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schödel F, Moriarty AM, Peterson DL, et al. The position of heterologous epitopes inserted in hepatitis B virus core particles determines their immunogenicity. J Virol. 1992;66:106–14. doi: 10.1128/jvi.66.1.106-114.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino DF, Zlotnik A, Vieira P, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–51. [PubMed] [Google Scholar]
- Yue FY, Dummer R, Geertsen R, et al. Interleukin-10 is a growth factor for human melanoma cells and down-regulates HLA class Ι, HLA class ΙΙ and ICAM-Ι molecules. Int J Cancer. 1997;71:630–7. doi: 10.1002/(sici)1097-0215(19970516)71:4<630::aid-ijc20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Tsuruma T, Yagihashi A, Torigoe T, et al. Interleukin-10 reduces natural killer sensitivity and downregulates MHC class Ι expression on H-ras-transformed cells. Cell Immunol. 1998;184:121–8. doi: 10.1006/cimm.1998.1266. [DOI] [PubMed] [Google Scholar]
- Kundu N, Fulton AM. Interleukin-10 inhibits tumor metastasis, downregulates MHC class Ι and enhances NK lysis. Cell Immunol. 1997;180:55–61. doi: 10.1006/cimm.1997.1176. [DOI] [PubMed] [Google Scholar]
- Knolle PA, Uhrig A, Hegenbarth S, et al. IL-10 down-regulates T cell activation by antigen-presenting liver sinusoidal endothelial cells through decreased antigen uptake via the mannose receptor and lowered surface expression of accessory molecules. Clin Exp Immunol. 1998;114:427–33. doi: 10.1046/j.1365-2249.1998.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R, Haanan J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class-ΙΙ major histocompatibility complex expression. J Exp Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R, Abrams J, Bennett B, et al. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyodo N, Tajimi M, Ugajin T, et al. Frequencies of interferon-γ and interleukin-10 secreting cells in peripheral blood mononuclear cells and liver infiltrating lymphocytes in chronic hepatitis B virus infection. Hepatol Res. 2003. pp. 109–16. [DOI] [PubMed]
- Vingerhoets J, Michielsen P, Vanham G, et al. HBV-specific lymphoproliferative and cytokine responses in patients with chronic hepatitis B. J Hepatol. 1998;28:8–16. doi: 10.1016/s0168-8278(98)80196-7. [DOI] [PubMed] [Google Scholar]
- Schlaak JF, Tully G, Löhr HF, et al. HBV specific immune defect in chronic hepatitis B (CHB) is correlated with a dysregulation of pro- and anti-inflammatory cytokines. Clin Exp Immunol. 1999;115:508–14. doi: 10.1046/j.1365-2249.1999.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico MA, Quiroga JA, Subira D, et al. Hepatitis B virus-specific T cell proliferation and cytokine secretion in chronic hepatitis B e antibody-positive patients treated with ribavirin and interferon alpha. Hepatology. 2001;33:295–300. doi: 10.1053/jhep.2001.21147. [DOI] [PubMed] [Google Scholar]
- Sato K, Yamashita N, Matsuyama T. Human peripheral blood monocyte-derived interleukin-10-induced semi-mature dendritic cells induce anergic CD4 (+) and CD8 (+) T cells via presentation of the internalized soluble antigen and cross-presentation of the phagocytosed necrotic cellular fragments. Cell Immunol. 2002;215:186–94. doi: 10.1016/s0008-8749(02)00021-7. [DOI] [PubMed] [Google Scholar]
- Steinbrink K, Graulich E, Kubsch S, et al. CD4 (+) and CD8 (+) anergic T cells induced by interleukin-10-treated human dendritic cells display antigen-specific suppressor activity. Blood. 2002;99:2468–76. doi: 10.1182/blood.v99.7.2468. [DOI] [PubMed] [Google Scholar]
- Akbar SM, Onji M, Inaba K, et al. Low responsiveness of hepatitis B virus-transgenic mice in antibody response to T cell-dependent antigen: defect in antigen-presenting activity of dendritic cells. Immunology. 1993;78:468–75. [PMC free article] [PubMed] [Google Scholar]
- Wang FS, Xing LH, Liu MX, et al. Dysfunction of peripheral blood dendritic cells from patients with chronic hepatitis B virus infection. World J Gastroenterol. 2001;7:537–41. doi: 10.3748/wjg.v7.i4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerwenka A, Morgan TM, Harmsen AG, et al. Migration kinetics and final destination of type 1 and type 2 CD8 effector cells predict protection against pulmonary virus infection. J Exp Med. 1999;189:423–34. doi: 10.1084/jem.189.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgame P, Abrams JS, Clayberger C, et al. Differing lymphokine profiles of functional subsets of human CD4+ and CD8+ T cell clones. Science. 1991;254:279–82. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Maggi E, Giudizi MG, Biagiotti R, et al. Th-2 like CD8+ T cells showing B-cell helper function and reduced cytolytic activity in human immunodeficiency virus type I infection. J Exp Med. 1994;180:489–95. doi: 10.1084/jem.180.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth S, van den Broek M, Frossard CP, et al. CD8 (+) T cells secreting type 2 lymphokines are defective in protection against viral infection. Cell Immunol. 2000;202:13–22. doi: 10.1006/cimm.2000.1639. [DOI] [PubMed] [Google Scholar]
- Prezzi C, Casciaro MA, Francavilla V, et al. Virus-specific CD8 (+) T cells with type 1 or type 2 cytokine profile are related to different disease activity in chronic hepatitis C virus infection. Eur J Immunol. 2001;31:894–906. doi: 10.1002/1521-4141(200103)31:3<894::aid-immu894>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Santin AD, Hermonat PL, Ravaggi A, et al. Interleukin-10 increases Th1 cytokine production and cytotoxic potential in human papillomavirus-specific CD8+ cytotoxic T lymphocytes. J Virol. 2000;74:4729–37. doi: 10.1128/jvi.74.10.4729-4737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]