Abstract
Asthma is characterized by an eosinophilic inflammation and a subepithelial fibrosis in the airways. Eosinophils contain several cytotoxic substances, such as eosinophil cationic protein (ECP), which can promote inflammation and cause tissue damage. This has generated the hypothesis that eosinophils may drive remodelling of extracellular matrix (ECM). To investigate the role of eosinophils we used an in vitro model for remodelling, the three-dimensional collagen gel contraction assay. Two sources of eosinophils were used in this study, isolated human peripheral eosinophils (purity > 95%) and stimulated [interleukin (IL)-5, IL-3 and granulocyte macrophage–colony stimulating factor (GM-CSF)] HL-60 clone 15 cells. Human eosinophils or HL-60 cells were cast together with human lung fibroblasts (HFL1) in type I collagen gels. Both types of eosinophils augmented fibroblast-mediated collagen gel contraction in a time and concentration-dependent manner. At 48 h, the gel area in HFL1/eosinophil co-culture was 46·5% ± 0·5 (mean ± s.e.m.) of initial area and in HFL1 culture 52·3% ± 0·1 (P < 0·001). Respective figures for HFL1/stimulated HL-60 co-culture and HFL1 culture only were 44·1% ± 0·5 and 52·4% ± 0·4 (P < 0·001). The release of ECP was increased when fibroblasts were cultured with eosinophils compared to eosinophils cultured alone. In addition, native ECP added to fibroblast gel cultures also augmented contraction. Our results suggest that eosinophils may interact with mesenchymal cells, promoting remodelling of ECM and that ECP constitutes one potential eosinophil-derived mediator driving this process. We conclude that this may be one important mechanism by which eosinophil–ECM interactions will lead to airway tissue remodelling in asthma.
Keywords: airway remodelling, asthma, collagen gel contraction, ECP, eosinophil
INTRODUCTION
Asthma is characterized by a chronic eosinophil inflammation combined with a subepithelial fibrosis in the airways [1–3]. The fibrosis is considered as an irreversible part of the airway obstruction and may be a hallmark of a more chronic and severe disease.
The occurrence of eosinophils in the asthma disease has long been recognized. Eosinophils persist after the acute inflammatory response, and the concept has emerged that these cells might play a role in the tissue remodelling and fibrosis. In line with this concept is data from a murine model of asthma, which showed eliminated eosinophil infiltration and suppressed subepithelial fibrosis after anti-interleukin (IL)-5 treatment [4]. Further, fibrotic tissue have been linked to the chronic presence of eosinophils, both in the lung [5–9] and in other organs [10–12]. Therefore eosinophils have been given a potential role in fibrosis.
Several potential mechanisms can be identified that may be responsible for an extrinsic causal link between tissue eosinophils and fibrosis. First, eosinophils contain several cytotoxic substances and express cytokines, which can promote inflammation and cause tissue injury [13]. Eosinophil cationic protein (ECP) is one of many cytotoxic proteins secreted at tissue sites with eosinophilia [14]. In addition, eosinophils may also secrete a number of inflammatory mediators for example transforming growth factor-β (TGF-β), which have the potential to drive fibrotic tissue remodelling [15]. Finally, several data in the literature indicate that eosinophils and fibroblasts may interact in the tissues [13]. For example, Takafuji et al. [16] has demonstrated augmented ECP release when eosinophils were co-cultured with fibroblasts and Levi-Schaffer et al. [17] showed that lysate from eosinophils could stimulate proliferation, collagen synthesis and lattice contraction of lung and skin fibroblasts.
The fact that asthma is characterized by eosinophilia and fibrosis in the airways has generated the question of whether a causal link exists between these two characterizes of asthma. We therefore aimed to investigate the role of two types of eosinophils (human blood eosinophils and eosinophil-like HL-60 clone 15 cells) and ECP in remodelling of lung tissue in vitro. We used a model where fibroblasts are cultured in a three-dimensional native type I collagen gel. This is used to model the contractile process considered to be one aspect of fibrotic tissue remodelling [18–23].
MATERIALS AND METHODS
Materials
Type I collagen (rat-tail tendon collagen, RTTC) was extracted according to a previously published method [24]. Briefly, tendons were excised from rat-tails, and the tendon sheath and other connective tissues were carefully removed. After repeated washing with TRIS-buffered saline (0·9% NaCl, 10 mm TRIS, pH 7·5) the tendons were washed in increasing concentrations of ethanol. Type I collagen was then extracted in 6 mm acetic acid. Protein concentration was determined by weighing a lyophilized aliquot from each lot of collagen. The RTTC was stored at 4°C until use.
Cell cultures
Human fetal lung fibroblasts (HFL1) were obtained from the American Type Culture Collection (Rockville, MD, USA). The cells were cultured on 100-mm tissue culture dishes (Falcon; Franklin Lakes, NJ, USA) with Dulbecco's modified Eagle's medium (DMEM; Gibco/BRL Life Technologies, Rockville, MD, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco/BRL Life Technologies), 50 U/ml penicillin G sodium, 50 µg/ml streptomycin sulphate (penicillin–streptomycin, Gibco/BRL Life Technologies) and 1 µg/ml amphotericin B (fungizone, Gibco/BRL Life Technologies). The fibroblasts were passaged twice a week and were used between the 15th and 20th passage. Confluent fibroblasts were trypsinized (Trypsin-EDTA; Gibco/BRL Life Technologies, 0·05% trypsin 0·53 mm EDTA), resuspended in DMEM without serum and used for collagen gel culture.
The promyelocytic cell line HL-60 clone 15 was originally obtained from the American Type Culture Collection (Rockville, MD, USA). The cells were cultured in RPMI-1640 medium (Sigma-Aldrich, St Louis, MO, USA) with 10% FBS (Gibco/BRL Life Technologies). Cells were stimulated to differentiate into eosinophil-like cells using 0·3 mm sodium butyrate (Sigma-Aldrich), 10 ng/ml granulocyte macrophage–colony stimulating factor (GM-CSF, Immunokontact, Frankfurt, Germany), 1 ng/ml IL-5 (Immunokontact) and 10 ng/ml IL-3 (Immunokontact) for 5 days as described previously [25–27]. Clone 15 cells incubated at pH 7·8 alone were run in parallel and are referred to as undifferentiated cells. An eosinophilic phenotype was judged by assessing CD9 expression by flow cytometry (see below) and morphology by assessment of May–Grünewald–Giemsa-stained cells. Viability was measured by trypan blue exclusion.
Peripheral blood eosinophils
Buffy coat was obtained from non-allergic healthy blood donors. First, the erythrocytes were sedimented by adding a solution of 4·5% dextran T-500 (Amersham Bioscience, Uppsala, Sweden) phosphate buffered saline (PBS), pH 7·4 as described previously [28]. After 30–60 min in room temperature (RT) the leucocyte-rich plasma was collected, washed with PBS and centrifuged at 200 g for 10 min.
The cell pellet was resuspended in PBS and layered onto isotonic Percoll solution (1·082 g/ml, Amersham Bioscience, Uppsala, Sweden) as described previously [29]. Briefly, after centrifugation (1000 g, 30 min, RT), the mononuclear cells at the interface were removed. The cell pellet containing mainly granulocytes and some erythrocytes was suspended in NH4Cl-EDTA to haemolyse the remaining erythrocytes. The purified granulocytes were then washed and resuspended in PBS.
The number of granulocytes was counted according to their light-scattering properties by flow cytometry before anti-CD16 magnetic beads (MACS, Miltenyi Biotech, Bergisch Gladbach, Germany) were added. After incubation for 25 min, 4°C, the cell/bead suspension was layered on top of a separation column placed in a magnetic field. Magnetically CD16-labelled cells (neutrophils) were trapped in the column and unlabelled cells (eosinophils) were eluted and washed in PBS (300 g, 7 min, 4°C). Cells were kept on ice until further analysis.
Immunostaining and flow cytometry analysis to assess purity of eosinophil population
Cells were immunostained with FITC-conjugated monoclonal antibodies to platelets, red blood cells and neutrophils, respectively: antihuman platelet glykoprotein IIIa, CD61 (IgG1, mouse, Dako A/S, Denmark), antiglycophorin A (IgG1, mouse, Immunotech, France) and CD16 (IgG1, mouse, Becton Dickinson, San Jose, CA, USA). FITC-conjugated mouse IgG1 antibody (Dako) was used as negative control. Immunostained cells were analysed in an EPICS® XL-MCL flow cytometer (Coulter Inc., Hialeh, FL, USA). The analysis showed no significant contamination of either platelets (10 per 100 eosinophils), red blood cells (3/100) or neutrophils (2/100), concentrations of which have shown previously not to effect fibroblast-mediated collagen gel contraction [30–32].
Collagen gel contraction assay
Collagen gels were prepared as described previously [33]. Briefly, distilled water, RTTC, 4× DMEM, fibroblast and eosinophil or HL-60 suspensions were mixed together to a final concentration of 0·75 mg/ml of collagen, 3 × 105 fibroblasts/ml and physiological ionic strength of 1× DMEM. Eosinophils were added in various concentrations from 104 to 106 eosinophils/ml gel. The different mixtures were kept on ice during the preparation. Five hundred and fifty µl of the gel solution were then cast into each well of a 24-well tissue culture plate with a 2-cm2 growth area (Falcon). Gelation occurred within 30 min at 37°C.
The ability of co-cultured eosinophils to affect fibroblast-mediated collagen gel contraction was determined by the slow contraction assay of Bell et al. [18]. Fibroblasts and eosinophils in co-culture were prepared as described above. After gelation the gels were released from the surface of the culture well using a sterile spatula and transferred into 60-mm tissue culture dishes (Falcon) containing 5 ml of serum-free DMEM. The floating gels were incubated at 37°C and 5% CO2 for 4 days and the gel areas were measured daily.
Measurement of gel area
In all the experiments, the area of the collagen gels was measured by an image analyser system (LEICA Microsystems AG; Wetzlar, Germany). The images were recorded by a video camera comprising a zoom lens mounted approximately 15 cm above a lighted stage; 60-mm culture dishes rested on this stage, and the gels were observed directly. No special mounting was required for stability. The area of the gels was then captured and processed by computer software from LEICA Microsystems AG and collagen gel contraction in the horizontal axes was determined. Thickness was not measured and contraction in the vertical area was not assessed. With the described method, the area of each gel could be measured while sterility was preserved.
Eosinophilic cationic protein
The amounts of released ECP in collagen gel samples and surrounding medium were measured. The gels were dissolved in 0·5 ml collagenase (Gibco/BRL Life Technologies, 0·25 mg/ml) for 2–3 h at 37°C [34]. The supernatants were collected by centrifugation (1500 g, 10 min) and cetyldimethylethyl ammonium bromide (CTAB) was added (final concentration 0·2%) before ECP was measured by ECP-CAP-FEIA (Pharmacia, Uppsala, Sweden).
The role of ECP was investigated further by adding ECP (1–10 µg/ml) to the fibroblast-mediated collagen gel contraction assay. Native ECP was purified in high yields from buffy coats of leucocytes from healthy blood donors as described (Trulson et al. in preparation). Briefly, granules were prepared by nitrogen cavitation of the buffy coat leucocytes and separated from other organelles by ultracentrifugation. The granules were extracted by acid NaAc and the extract subjected to gelfiltration on a Sephadex G-75 followed by ion-exchange chromatography on Mono-S (Amersham Biotech, Uppsala, Sweden) and RPC (reversed-phase chromatography). The purity was checked by SDS-PAGE electrophoresis, Western blot analysis and N-terminal amino acid sequencing.
Cell proliferation
In order to estimate cell numbers in the gels, the DNA content was assayed fluorometrically with Hoechst dye 33258 (Sigma-Aldrich, St Louis, MO, USA) by a modification of a previously published method [35]. Briefly, the gels were dissolved by adding collagenase as described above. Cells were then collected by centrifugation (1500 g, 10 min). After freezing and thawing once, 1 ml of ddH2O was added to each sample and cells were sonicated. The DNA content was determined by adding 2 ml of TNE buffer (3 m NaCl, 10 mm TRIS and 1·5 mm EDTA, pH 7·4) containing 2 µg/ml of Hoechst dye 33258. Fluorescence intensities were measured with a fluorescence spectrometer (Fluroscan 2; Labsystems, Finland) with an excitation wavelength at 355 nm and an emission wavelength at 460 nm.
Cell proliferation was also assayed by the cell proliferation reagent WST-1 (Roche Diagnostics Scandinavia AB, Bromma, Sweden), which is a colourimetric assay based on the cleavage of the tetrazolium salt WST-1 by mitochondrial dehydrogenase in viable cells. An expansion in the number of viable cells results in an increase in the overall activity of mitochondrial dehydrogenase. In the assay, 5 × 104 fibroblasts/ml was cultured in 96-well plates in DMEM, 1% FBS together with 1–3 × 105 eosinophils for 2 days. To remove the eosinophils samples were washed three times with serum-free medium, before incubating with WST-1 for 2 h. Absorbance was measured at 450 nm in a SPECTRAmax® 340 microplate spectrophotometer (GMI, Albertville, MN, USA).
Statistical methods
Data are presented as mean of three replicate gels for each condition and standard error of mean (± s.e.m.), unless stated otherwise. As the batch of RTTC, the number of cell passages and culture conditions can affect the gel contraction, the data shown in each figure are taken from a representative experiment of which each experiment was repeated on multiple occasions. Groups of data were evaluated by analysis of variance (anova). Differences between two groups of data were analysed by Student's t-test.
RESULTS
Effects of eosinophils on fibroblast-mediated collagen gel contraction
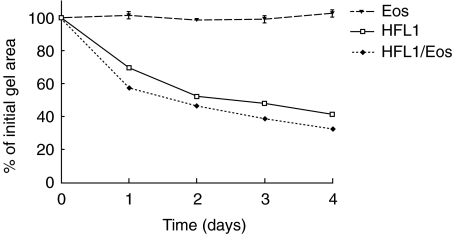
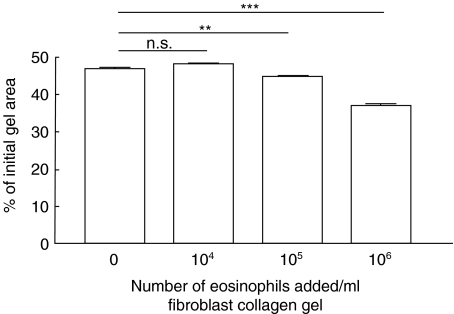
Repeated experiments, containing co-culture of fibroblasts (3 × 105 cells/ml) and human peripheral blood eosinophils (1 × 106 cells/ml), demonstrated a significant (P < 0·05) augmentation of fibroblast-mediated collagen gel contraction (Table 1). When gel area was measured on four consecutive days a time-dependent augmentation of contraction was observed (Fig. 1). Thus, gels containing fibroblasts and eosinophils contracted more at each single point than control gels, containing only fibroblasts. Eosinophils alone did not induce any contraction (Fig. 1). The augmentation of the gel contraction was dependent on the number of eosinophils added, with 105 eosinophils/ml gel being the lowest concentration resulting in an augmenting effect (Fig. 2).
Table 1.
Eosinophils augment fibroblast-mediated contraction of collagen gels
| Cells in collagen gels | Area of gels (% of initial gel area) | s.e.m. | n |
|---|---|---|---|
| Fibroblasts | 51·5 | ±4·1 | 9 |
| Fibroblasts/eosinophils | 40·0* | ±3·3 | 12 |
| Eosinophils | 97·0 | ±0·7 | 5 |
Area was determined after 2 days of culture. Concentration of fibroblasts and eosinophils was 3 × 105 and 1 × 106 cells/ml gel, respectively. Values are means ± s.e.m.; each experiment includes triplicate of gels; n = number of individual experiments.
P < 0·05 versus fibroblasts.
Fig. 1.
Time-dependent augmentation of fibroblast-mediated collagen gel contraction by eosinophils (Eos). Fibroblasts (HFL1: 3 × 105/ml gel) and Eos (106/ml gel) or the two cell types together (HFL1/Eos) were cast into a 24-well tissue culture plate. The assay was performed by releasing the gels into medium immediately after gelation. The area of each gel was measured by an image analyser on 4 consecutive days. Vertical axis: gel area expressed as percentage of original gel size. Data presented as mean of triplicate and standard error of mean (± s.e.m.) from one representative experiment. P < 0·001: HFL1 versus HFL1/Eos at all data points.
Fig. 2.
Eosinophil concentration-dependent augmentation of fibroblast-mediated collagen gel contraction. Increasing numbers of eosinophils were mixed together with fibroblasts in a three-dimensional collagen gel. After gelation, the gels were released and the area of the floating gels was measured after 48 h of culture. Vertical axis: gel area expressed as percentage of original gel size. Horizontal axis: number of eosinophils (Eos) added to gels. Data shown as mean of triplicate and ± s.e.m. ***P < 0·001, **P < 0·01, n.s. = P > 0·05.
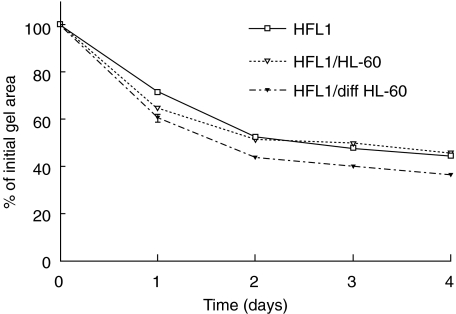
Eosinophil-like differentiated clone 15 HL-60 cells augmented fibroblast-mediated gel contraction in a time-dependent manner, with the most pronounced effect after 2 days of culture (Fig. 3). Co-culture with undifferentiated clone 15 HL-60 cells had no effect over time compared to the control.
Fig. 3.
Differentiated clone 15 HL-60 cells (diff HL-60) augment fibroblast-mediated collagen gel contraction. Clone 15 HL-60 cells were differentiated (IL-5, IL-3, GM-CSF) and cast together with fibroblasts in collagen gels. Undifferentiated clone 15 HL-60 cells (HL-60) were used as control cells. Vertical axis: gel area expressed as percentage of original gel size. Data presented as mean of triplicate and standard error of mean (± s.e.m.). P < 0·001: HFL1/HL-60 versus HFL1/diff HL-60 on days 2, 3 and 4.
Cell proliferation
The amount of DNA in collagen gels was determined to assess whether the increased fibroblast-mediated contraction induced by eosinophils was due to cell proliferation in the culture system. The OD value after 2 days of culture was for fibroblasts 354 ± 20 (mean ± s.e.m.), blood eosinophils 98 ± 3 and co-culture of the two cell types 450 ± 42 (Fig. 4). This indicates that the higher DNA level in co-culture compared to fibroblast culture can be related to eosinophil DNA, and not necessarily eosinophil-induced proliferation of fibroblasts. To confirm this, cell proliferation was also assayed by WST-1, where mitochondrial activity is measured. There was no significant difference between fibroblasts and fibroblast/eosinophil co-culture (OD value: 0·79 ± 0·05 (mean ± s.e.m.) versus 0·80 ± 0·05; n.s.).
Fig. 4.
Cell numbers in collagen gels estimated by DNA content. Floating collagen gels containing fibroblasts (HFL1), co-culture of fibroblasts and eosinophils (HFL1/Eos) or eosinophils (Eos) alone were cultured for 2 days. The gels were then dissolved in collagenase and DNA contents in pellets were determined by a fluorometric assay. Vertical axis: optical density (OD value, arbitrary units) measured at wavelength 355/460 nm. Data shown as means ± s.e.m., n = 6.
ECP release in three-dimensional gel cultures
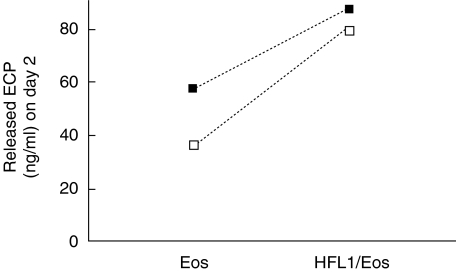
The total ECP release was analysed both in gels and medium. Higher concentrations of ECP were detected in co-culture (83 ± 4 ng/ml) than in samples with eosinophils alone (47 ± 11 ng/ml) (Fig. 5). In fibroblast culture only, no detectable levels of ECP was found.
Fig. 5.
ECP is released in co-culture. Fibroblasts together with eosinophils (HFL1/Eos) or eosinophils (Eos) alone were cast into collagen gels. After 2 days of culture, the gels and surrounding medium were pooled and analysed for ECP (ECP-CAP-FEIA). Vertical axis: concentration of ECP (ng/ml). Horizontal axis: data are given as individual results from two independent experiments. □ = Experiment 1, ▪ = experiment 2.
Effects of native ECP on fibroblast-mediated collagen gel contraction
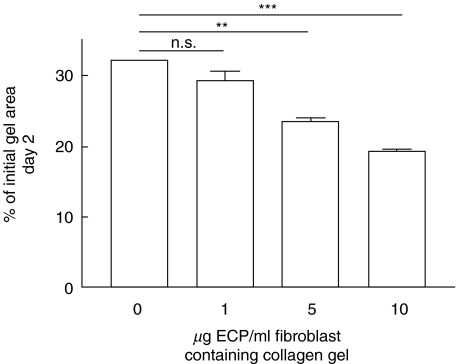
Native ECP in various concentrations (1–10 µg/ml gel) was added to fibroblast-populated collagen gels. One µg ECP/ml gel was enough to augment fibroblast-mediated collagen gel contraction with approximately 5% (Fig. 6). Higher concentrations of ECP increased the stimulating effect on gel contraction in a dose-dependent manner.
Fig. 6.
Native ECP augment fibroblast-mediated collagen gel contraction in a concentration-dependent manner. ECP in various concentrations were added to fibroblast/collagen gels. After gelation, the gels were released and the area of the floating gels was measured after 2 days of culture. Vertical axis: gel area expressed as percentage of original gel size. Horizontal axis: ECP concentration added to gels. Data shown as mean of triplicate ± s.e.m. ***P < 0·001, **P < 0·01, n.s. = P > 0·05.
DISCUSSION
In the present study we demonstrate that both human blood eosinophils and eosinophil-like differentiated HL-60 clone 15 cells stimulate fibroblast-mediated collagen gel contraction. In addition, we identified ECP as a potential mediator in this process. These data suggest a potential and specific role for eosinophils in the long-term fibrotic process that features asthma.
Besides being a structural cell, the fibroblast can secrete a number of inflammatory mediators and is appointed a key cell in tissue remodelling and formation of fibrosis. This is a complex process consisting of recruitment and proliferation of fibroblasts and production of extracellular matrix components. Part of this process includes contraction of matrix. To study this we used fibroblast-mediated collagen gel contraction, an in vitro model considered to be one aspect of fibrotic tissue remodelling [18,19]. Even though the culture system is a simplification of in vivo conditions, the system has been used to elucidate effects of potential fibrotic mediators [15,20–22,36,37]. In the three-dimensional collagen gel system the ability of fibroblasts to contract the gels is dependent on a variety of factors including fibroblast strain, cell density, collagen concentration and the presence of soluble factors [15,38].
In a study by Shock et al. [39], it was demonstrated that conditioned media from guinea pig-derived eosinophils could promote fibroblast proliferation. However, our results indicate that there is no cell proliferation in fibroblast/eosinophil co-culture. A number of methodological differences can explain this discrepancy between the two studies. For instance, we used human eosinophils purified from peripheral blood and co-cultured whole eosinophils, not conditioned media, and fibroblasts. To avoid serum-related effects, such as fibroblast proliferation, we also used a serum-free culture system.
Because red blood cells, platelets and neutrophils are known to affect gel contraction [30–32], the purity of isolated eosinophils was tested by flow cytometry. The estimated number of these contaminating cells in the eosinophil preparation was well below the number that exerts an effect fibroblast-mediated collagen gel contraction. Therefore, we excluded the possibility that the effect was caused by either of these cells.
In a previous study by Levi-Shaffer et al. [17], it was demonstrated that lysate from eosinophils could stimulate fibroblast-mediated lattice contraction. There are a number of differences between our study and the study by Levi-Shaffer et al. First, we cultured whole eosinophils from two sources together with lung fibroblasts in the three-dimensional collagen gel while Levi-Schaffer et al. used lysate from sonicated eosinophils. The rationale for our approach is that eosinophil lysate consists of a mixture of potential profibrogenic factors that are not mandatory released under physiological conditions. The use of whole eosinophils, permitting a physiological degranulation, is more in conformity with in vivo-conditions. Secondly, Levi Shaffer et al. cultured their cells in medium supplemented with serum, which is a factor known to augment collagen gel contraction and to stimulate fibroblast proliferation [40]. Thirdly, the eosinophils used in the study by Levi-Schaffer et al. were from atopic individuals, while we studied cells from non-allergic blood donors. An intriguing question is whether eosinophils from patients with an eosinophil-driven inflammation reveal additional characteristics in the model. Differences in the capacity of normal and light density eosinophils to survive in culture account for variations in the severity of disease seen in patients with persistent eosinophilia [41]. In addition, we used circulating eosinophils and not eosinophils migrated into the airway tissue. The question of whether tissue and primed eosinophils act in a different way compared to blood eosinophils is being addressed currently in our laboratory.
Eosinophils are rarely present in normal lung tissue. The most notable disease linked with eosinophilia of the lung is asthma [42,43]. However, a number of other fibrotic diseases characterized by eosinophilic inflammation are also correlated with a poor prognosis [5–9]. For instance, in idiopathic pulmonary fibrosis an increased number of eosinophils in bronchoalveolar lavage fluid are associated with disease progression [5,6]. Fibrotic lesions in other organs, such as endomyocardial fibrosis [11] and eosinophilia–myalgia syndrome [10], also show a strong association with eosinophilia. However, it has not been established whether there is a causative link or just a covariation between accumulation of eosinophils and the fibrotic response.
Studies have revealed that eosinophil infiltration in tissue is associated commonly with release of granule proteins [44]. We demonstrated that ECP is released when fibroblasts and eosinophils are co-cultured in three-dimensional collagen gels. In addition, ECP added to fibroblast-populated collagen gels were able to augment gel contraction. These data are consistent with Takafuji et al. [16], who showed that cell–cell contact between eosinophils and fibroblasts enhance the release of ECP and that this is an integrin-dependent process. Furthermore, ECP has also been shown to affect several fibroblast activities including proteoglycan metabolism [45] and DNA synthesis [46].
The measured ECP concentration in gel and medium supernatants were lower than the amount of ECP required to augment fibroblast-mediated gel contraction. This could be due to an incomplete ECP recovery, because the protein is strongly basic and therefore may be difficult to extract in in vitro models. Another possibility could be that eosinophils release other factors that may facilitate the effect on eosinophil-induced gel contraction. Besides ECP, a number of additional potential factors that mediate tissue eosinophilia associated to the fibrotic processes exist. Growth factors such as TGF-β and the Th2-like cytokines IL-4 and IL-13 have been attributed to mediate this effect. Eosinophils have been demonstrated to be the major source of TGF-β in severe asthmatic airways [47], and TGF-β stimulates proliferation of fibroblasts [48]. TGF-β, IL-4 and IL-13 can also switch fibroblasts into a myofibroblastic phenotype and stimulate matrix protein production; for example, pro-collagen I and tenascin [48,49].
The role of eosinophils in asthma has recently been a matter of debate. Because IL-5 has been identified as a key cytokine in eosinophilic inflammation [50], it was expected that treatment with anti-IL-5 antibodies would improve symptoms in asthmatic individuals. Accordingly, it was rather confusing when Leckie et al. [51] demonstrated that treatment with anti-IL-5 antibodies in patients with mild allergic asthma resulted in a decreased number of eosinophils in blood and sputum without any concomitant effect on hyperreactivity and the late asthmatic reaction. However, this study did not address the question of whether anti-IL-5 treatment depleted tissue eosinophils. Recently, Flood-Page and coworkers [52] reported that anti-IL-5 treatment reduced airway eosinophils only partially and had no effect on deposition of eosinophilic granule proteins in the airways. These data may explain the minimal clinical effects of anti-IL-5 treatment, and do not rule out a potential role of tissue eosinophils in asthma pathophysiology.
To summarize, we suggest that eosinophils may interact with mesenchymal cells, promoting remodelling of extracellular matrix. The increased release of ECP in co-culture suggests a role for this mediator in the process. This may be an important mechanism by which interactions between eosinophil, fibroblasts and matrix proteins will lead to remodelling of airway tissue in asthma.
Acknowledgments
The authors would like to thank Professor Rolf Lewensohn at the Cancer Centrum Karolinska, for allowing us to use equipment for DNA analysis. The study was supported by grants from the Swedish Heart–Lung Foundation, the Cancer and Allergy Foundation, the Swedish Foundation for Health Care Science and Allergy Research, the Hesselman Foundation, Boehringer-Ingelheim/Pfizer and Karolinska Institutet.
REFERENCES
- Roche WR, Beasley R, Williams JH, Holgate ST. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989;1:520–4. doi: 10.1016/s0140-6736(89)90067-6. [DOI] [PubMed] [Google Scholar]
- Jeffery PK, Wardlaw AJ, Nelson FC, Collins JV, Kay AB. Bronchial biopsies in asthma. An ultrastructural, quantitative study and correlation with hyperreactivity. Am Rev Respir Dis. 1989;140:1745–53. doi: 10.1164/ajrccm/140.6.1745. [DOI] [PubMed] [Google Scholar]
- Wilson JW, Bamford TL. Assessing the evidence for remodelling of the airway in asthma. Pulm Pharmacol Ther. 2001;14:229–47. doi: 10.1006/pupt.2001.0294. [DOI] [PubMed] [Google Scholar]
- Blyth DI, Wharton TF, Pedrick MS, Savage TJ, Sanjar S. Airway subepithelial fibrosis in a murine model of atopic asthma: suppression by dexamethasone or anti-interleukin-5 antibody. Am J Respir Cell Mol Biol. 2000;23:241–6. doi: 10.1165/ajrcmb.23.2.3999. [DOI] [PubMed] [Google Scholar]
- Rudd RM, Haslam PL, Turner-Warwick M. Cryptogenic fibrosing alveolitis. Relationships of pulmonary physiology and bronchoalveolar lavage to response to treatment and prognosis. Am Rev Respir Dis. 1981;124:1–8. doi: 10.1164/arrd.1981.124.1.1. [DOI] [PubMed] [Google Scholar]
- Peterson MW, Monick M, Hunninghake GW. Prognostic role of eosinophils in pulmonary fibrosis. Chest. 1987;92:51–6. doi: 10.1378/chest.92.1.51. [DOI] [PubMed] [Google Scholar]
- Watters LC, Schwarz MI, Cherniack RM, et al. Idiopathic pulmonary fibrosis. Pretreatment bronchoalveolar lavage cellular constituents and their relationships with lung histopathology and clinical response to therapy. Am Rev Respir Dis. 1987;135:696–704. doi: 10.1164/arrd.1987.135.3.696. [DOI] [PubMed] [Google Scholar]
- Hallgren R, Bjermer L, Lundgren R, Venge P. The eosinophil component of the alveolitis in idiopathic pulmonary fibrosis. Signs of eosinophil activation in the lung are related to impaired lung function. Am Rev Respir Dis. 1989;139:373–7. doi: 10.1164/ajrccm/139.2.373. [DOI] [PubMed] [Google Scholar]
- Schwartz DA, Helmers RA, Dayton CS, Merchant RK, Hunninghake GW. Determinants of bronchoalveolar lavage cellularity in idiopathic pulmonary fibrosis. J Appl Physiol. 1991;71:1688–93. doi: 10.1152/jappl.1991.71.5.1688. [DOI] [PubMed] [Google Scholar]
- Martin RW, Duffy J, Engel AG, et al. The clinical spectrum of the eosinophilia–myalgia syndrome associated with 1-tryptophan ingestion. Clinical features in 20 patients and aspects of pathophysiology. Ann Intern Med. 1990;113:124–34. doi: 10.7326/0003-4819-113-2-124. [DOI] [PubMed] [Google Scholar]
- Tai PC, Ackerman SJ, Spry CJ, Dunnette S, Olsen EG, Gleich GJ. Deposits of eosinophil granule proteins in cardiac tissues of patients with eosinophilic endomyocardial disease. Lancet. 1987;1:643–7. doi: 10.1016/s0140-6736(87)90412-0. [DOI] [PubMed] [Google Scholar]
- Gustafsson R, Fredens K, Nettelbladt O, Hallgren R. Eosinophil activation in systemic sclerosis. Arthritis Rheum. 1991;34:414–22. doi: 10.1002/art.1780340406. [DOI] [PubMed] [Google Scholar]
- Gharaee-Kermani M, Phan SH. The role of eosinophils in pulmonary fibrosis [Review] Int J Mol Med. 1998;1:43–53. [PubMed] [Google Scholar]
- Venge P, Bystrom J, Carlson M, Hakansson L, Karawacjzyk M, Peterson C, Seveus L, Trulson A. Eosinophil cationic protein (ECP): molecular and biological properties and the use of ECP as a marker of eosinophil activation in disease. Clin Exp Allergy. 1999;29:1172–86. doi: 10.1046/j.1365-2222.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- Montesano R, Orci L. Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc Natl Acad Sci USA. 1988;85:4894–7. doi: 10.1073/pnas.85.13.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takafuji S, Miyakuni Y, Nakagawa T, et al. Effects of human lung fibroblasts on eosinophil degranulation. Allergy. 2000;55:1170–8. doi: 10.1034/j.1398-9995.2000.00623.x. [DOI] [PubMed] [Google Scholar]
- Levi-Schaffer F, Garbuzenko E, Rubin A, et al. Human eosinophils regulate human lung- and skin-derived fibroblast properties in vitro: a role for transforming growth factor beta (TGF-beta) Proc Natl Acad Sci USA. 1999;96:9660–5. doi: 10.1073/pnas.96.17.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci USA. 1979;76:1274–8. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401–4. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi Y, Mio T, Takigawa K, et al. Fibronectin production by cultured human lung fibroblasts in three-dimensional collagen gel culture. In Vitro Cell Dev Biol Anim. 1998;34:203–10. doi: 10.1007/s11626-998-0125-7. [DOI] [PubMed] [Google Scholar]
- Ohga E, Matsuse T, Teramoto S, Ouchi Y. Activin receptors are expressed on human lung fibroblast and activin A facilitates fibroblast-mediated collagen gel contraction. Life Sci. 2000;66:1603–13. doi: 10.1016/s0024-3205(00)00480-x. [DOI] [PubMed] [Google Scholar]
- Mio T, Liu X, Toews ML, et al. Bradykinin augments fibroblast-mediated contraction of released collagen gels. Am J Physiol Lung Cell Mol Physiol. 2001;281:L164–71. doi: 10.1152/ajplung.2001.281.1.L164. [DOI] [PubMed] [Google Scholar]
- Liu X, Kohyama T, Wang H, et al. Th2 cytokine regulation of type I collagen gel contraction mediated by human lung mesenchymal cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1049–56. doi: 10.1152/ajplung.00321.2001. [DOI] [PubMed] [Google Scholar]
- Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972;54:626–37. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischkoff SA, Condon ME. Switch in differentiative response to maturation inducers of human promyelocytic leukemia cells by prior exposure to alkaline conditions. Cancer Res. 1985;45:2065–9. [PubMed] [Google Scholar]
- Hutt-Taylor SR, Harnish D, Richardson M, Ishizaka T, Denburg JA. Sodium butyrate and a T lymphocyte cell line-derived differentiation factor induce basophilic differentiation of the human promyelocytic leukemia cell line HL-60. Blood. 1988;71:209–15. [PubMed] [Google Scholar]
- Lundahl J, Sehmi R, Hayes L, Howie K, Denburg JA. Selective upregulation of a functional beta7 integrin on differentiating eosinophils. Allergy. 2000;55:865–72. doi: 10.1034/j.1398-9995.2000.00574.x. [DOI] [PubMed] [Google Scholar]
- Cramer R, Dri P, Zabucchi G, Patriarca P. A simple and rapid method for isolation of eosinophilic granulocytes from human blood. J Leukoc Biol. 1992;52:331–6. doi: 10.1002/jlb.52.3.331. [DOI] [PubMed] [Google Scholar]
- Moshfegh A, Halldén G, Lundahl J. Methods for simultaneous quantitative analysis of eosinophil and neutrophil adhesion and transmigration. Scand J Immunol. 1999;50:262–9. doi: 10.1046/j.1365-3083.1999.00594.x. [DOI] [PubMed] [Google Scholar]
- Fredriksson K, Lundahl J, Fernvik E, Liu XD, Rennard SI, Skold CM. Red blood cells stimulate fibroblast-mediated contraction of three dimensional collagen gels in co-culture. Inflamm Res. 2002;51:245–51. doi: 10.1007/pl00000300. [DOI] [PubMed] [Google Scholar]
- Skold CM, Liu X, Umino T, et al. Human neutrophil elastase augments fibroblast-mediated contraction of released collagen gels. Am J Respir Crit Care Med. 1999;159:1138–46. doi: 10.1164/ajrccm.159.4.9805033. [DOI] [PubMed] [Google Scholar]
- Zagai U, Fredriksson K, Rennard SI, Lundahl J, Skold CM. Platelets stimulate fibroblast-mediated contraction of collagen gels. Respir Res. 2003;4:13. doi: 10.1186/1465-9921-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mio T, Adachi Y, Romberger DJ, Ertl RF, Rennard SI. Regulation of fibroblast proliferation in three-dimensional collagen gel matrix. In Vitro Cell Dev Biol Anim. 1996;32:427–33. doi: 10.1007/BF02723005. [DOI] [PubMed] [Google Scholar]
- Liu X, Umino T, Cano M, et al. Human bronchial epithelial cells can contract type I collagen gels. Am J Physiol. 1998;274:L58–65. doi: 10.1152/ajplung.1998.274.1.L58. [DOI] [PubMed] [Google Scholar]
- Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980;102:344–52. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Fukamizu H, Grinnell F. Spatial organization of extracellular matrix and fibroblast activity: effects of serum, transforming growth factor beta, and fibronectin. Exp Cell Res. 1990;190:276–82. doi: 10.1016/0014-4827(90)90197-i. [DOI] [PubMed] [Google Scholar]
- Wen FQ, Skold CM, Liu XD, et al. Glucocorticoids and TGF-beta1 synergize in augmenting fibroblast mediated contraction of collagen gels. Inflammation. 2001;25:109–17. doi: 10.1023/a:1007170622699. [DOI] [PubMed] [Google Scholar]
- Gullberg D, Tingstrom A, Thuresson AC, et al. Beta 1 integrin-mediated collagen gel contraction is stimulated by PDGF. Exp Cell Res. 1990;186:264–72. doi: 10.1016/0014-4827(90)90305-t. [DOI] [PubMed] [Google Scholar]
- Shock A, Rabe KF, Dent G, et al. Eosinophils adhere to and stimulate replication of lung fibroblasts ‘in vitro’. Clin Exp Immunol. 1991;86:185–90. doi: 10.1111/j.1365-2249.1991.tb05793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijnen P, Petrov V, Fagard R. In vitro assay of collagen gel contraction by cardiac fibroblasts in serum-free conditions. Meth Find Exp Clin Pharmacol. 2001;23:377–82. doi: 10.1358/mf.2001.23.7.662122. [DOI] [PubMed] [Google Scholar]
- Tai PC, Sun L, Spry CJ. Effects of IL-5, granulocyte/macrophage colony-stimulating factor (GM-CSF) and IL-3 on the survival of human blood eosinophils in vitro. Clin Exp Immunol. 1991;85:312–6. doi: 10.1111/j.1365-2249.1991.tb05725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AB. Asthma and inflammation. J Allergy Clin Immunol. 1991;87:893–910. doi: 10.1016/0091-6749(91)90408-g. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Chanez P, Lacoste JY, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323:1033–9. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- Noguchi H, Kephart GM, Colby TV, Gleich GJ. Tissue eosinophilia and eosinophil degranulation in syndromes associated with fibrosis. Am J Pathol. 1992;140:521–8. [PMC free article] [PubMed] [Google Scholar]
- Hernnas J, Sarnstrand B, Lindroth P, Peterson CG, Venge P, Malmstrom A. Eosinophil cationic protein alters proteoglycan metabolism in human lung fibroblast cultures. Eur J Cell Biol. 1992;59:352–63. [PubMed] [Google Scholar]
- Birkland TP, Cheavens MD, Pincus SH. Human eosinophils stimulate DNA synthesis and matrix production in dermal fibroblasts. Arch Dermatol Res. 1994;286:312–8. doi: 10.1007/BF00402221. [DOI] [PubMed] [Google Scholar]
- Ohno I, Nitta Y, Yamauchi K, et al. Transforming growth factor beta 1 (TGF beta 1) gene expression by eosinophils in asthmatic airway inflammation. Am J Respir Cell Mol Biol. 1996;15:404–9. doi: 10.1165/ajrcmb.15.3.8810646. [DOI] [PubMed] [Google Scholar]
- Phipps S, Ying S, Wangoo A, Ong YE, Levi-Schaffer F, Kay AB. The relationship between allergen-induced tissue eosinophilia and markers of repair and remodeling in human atopic skin. J Immunol. 2002;169:4604–12. doi: 10.4049/jimmunol.169.8.4604. [DOI] [PubMed] [Google Scholar]
- Malmstrom J, Westergren-Thorsson G, Marko-Varga G. A proteomic approach to mimic fibrosis disease evolvement by an in vitro cell line. Electrophoresis. 2001;22:1776–84. doi: 10.1002/1522-2683(200105)22:9<1776::AID-ELPS1776>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Capron M, Desreumaux P. Immunobiology of eosinophils in allergy and inflammation. Res Immunol. 1997;148:29–33. doi: 10.1016/s0923-2494(97)86271-2. [DOI] [PubMed] [Google Scholar]
- Leckie MJ, ten Brinke A, Khan J, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2144–8. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003;167:199–204. doi: 10.1164/rccm.200208-789OC. [DOI] [PubMed] [Google Scholar]