Abstract
To gain an insight into the mechanisms of chronic and acute inflammation, the production of neutrophil chemokines in different types of tonsillitis – hyperplastic tonsillitis (HT), recurrent tonsillitis (RT) and peritonsillar abscesses (PA) – was investigated. The chemokines interleukin-8 (IL-8), growth-related oncogene-α (GRO-α), epithelial cell-derived neutrophil attractant-78 (ENA-78) and granulocyte chemotactic protein-2 (GCP-2) were detected and shown to have different biological activities. With respect to the biological properties of CXC chemokines, the biological activity of the chemokines was identified using a three-step high-performance liquid chromatography (HPLC) technique, a bioassay involving measurement of neutrophil chemotaxis in a single Boyden chamber in tissue of HT, RT and PA. Using reverse transcription-polymerase chain reaction (RT-PCR), the chemokine concentrations were determined in the different tonsillitis entities. The chemokine pattern was dominated in PA by IL-8 and GRO-α and in RT by GRO-α. Hyperplastic tonsils of patients without a history of infection generated about five times lower IL-8 than PA. A protein concentration of GCP-2 was induced in PA and RT, whereas ENA-78 remained the same in all entities. In conclusion, it would appear that IL-8 was up-regulated in acute inflammation, whereas GRO-α dominated in chronic inflammation. ENA-78 seems not to play a pivotal role in inflammatory processes in tonsils. GCP-2 may serve as a substitute chemokine in certain inflammatory conditions as its quantity of mRNA and protein was higher in RT and PA than in HT.
Keywords: ENA-78, IL-8, GRO-α, GCP-2, neutrophil chemokines, tonsillitis
INTRODUCTION
The synthesis of inflammatory mediators is a fundamental mechanism for leucocyte recruitment to an inflamed tissue. The successful delivery of the appropriate population of leucocytes to a restricted site of acute inflammation will depend on the repertoire of inducible chemokines synthesized locally and on the temporal expression of chemokine receptors on the leucocytes. Neutrophil–chemokine interactions are important to this process, because this cell population expresses chemokine receptors, including CXCR2 and CCR1 [1].
Based on the position of conserved cysteines, chemokines are divided into four subfamilies: CXC, CC, C and CX3C chemokines [2–6]. CXC chemokines containing one amino acid between the two NH2-terminal cysteine residues can be divided further into two subgroups depending on the presence or absence of the sequence Glu-Leu-Arg (ELR) immediately in front of the first cysteine residue. The ELR/CXC chemokines GRO-α/CXCL1, GRO-β/CXCL2, GRO-γ/CXCL3, epithelial cell-derived neutrophil attractant 78 (ENA-78)/CXCL5, granulocyte chemotactic protein-2 (GCP-2)/CXCL6, neutrophil-activating protein-2 (NAP-2)/CXCL7 and interleukin (IL)-8/CXCL8 chemoattract and activate neutrophils, whereas the ELR negative CXC chemokines are mainly active on lymphocytes [1,5,6].
Within this context, and in order to gain an insight into the mechanisms of chronic and acute inflammation, the leucocyte chemotactic factor-synthesizing ability in different types of tonsillitis – hyperplastic tonsillitis (HT), recurrent tonsillitis (RT) and peritonsillar abscesses (PA) – were investigated.
The healthy palatine tonsils are sites of continuous stimulation of lymphoid cells, and this has been interpreted as a permanent activation (so-called ‘physiological inflammation’ of tonsils) [7]. Only few systematic studies comparing recurrent or chronically inflamed tonsils with ‘normal’ ones have been published to date [8]. Tonsillitis occurs if the activity and proliferation of pathogens in the tonsillar lymphoid tissue exceeds the protective potency of activated lymphoid and immunoglobulin-producing cells [9]. In this situation, especially in chronic or recurrent cases, surgical tonsillectomy is a common therapeutic approach [10].
Tonsillar hypertrophy or hyperplastic tonsils (HT) is characterized by massively enlarged tonsils in children without a history of throat infections [11] which may lead to obstructive sleep apnoea [12]. The pathogenesis of HT is poorly understood, although obstructive sleep apnoea is an indication in children for tonsillectomy and adenoidectomy [13].
METHODS
Patients and methods
Prior written informed consent was obtained from all patients suffering from HT, RT and peritonsillar abscesses (PA). HT was defined clinically as kissing tonsils in children without a history of inflammation. Tonsils of patients who had had acute tonsillitis 3–4 times and showed clinical signs of chronic tonsillitis were investigated as RT. Tonsils removed during routine surgery from patients with PA were also subjected to investigation. The study was approved by the Ethics Committee of the University of Münster, Germany.
Chemokine extraction from tonsillar tissue
Twenty g of HT, RT and PA tissue, respectively, was suspended in 350 ml of 0·1 m citric acid. After adding 350 ml of 96% ethanol, the suspension was homogenized in an ice–water mixture. The homogenized tissue was centrifuged at 1000 g for 30 min at 4°C, and the opalescent supernatant was concentrated with ultrafilters (YM3 filters, cutoff of 3 kDa, Amicon, Danvers, MA, USA), followed by diafiltration against the buffers required for further experiments.
Purification of chemoattractants by high-performance liquid chromatography (HPLC)
Chemoattractants were purified by sequential heparin-affinity chromatography, reversed-phase chromatography and microbore cation exchange chromatography.
Supernatants of 400 ml were concentrated initially to a volume of less than 5 ml using ultrafilters (Amicon YM3 filters, cut-off of 3 kDa), followed by diafiltration against buffer A (0·01 m Tris, 0·01 m citric acid) at pH 8·0, required for the heparin–sepharose cartridge.
Concentrated supernatants were passed through a heparin–sepharose cartridge (Hi-Trap, 10 × 5 mm, Pharmacia, Freiburg, Germany) at 0·5 ml/min. Proteins bound to the column were then eluted using 2 m NaCl in equilibration buffer. The eluate was collected manually as one fraction (15 ml) and tested for neutrophil chemotactic activity.
Proteins stripped from the heparin column were acidified to pH 2–3 with formic acid, concentrated (in the Amicon chamber) and diafiltrated against 0·1% trifluoroacetic acid (TFA) using Amicon YM3 filters (cut-off 3 kDa), and then applied to a preparative RP-8 HPLC column (C8 nucleosil with end capping, 250 × 12·6 mm, 7 µm particle size, Marcherey-Nagel, Düren, Germany) equilibrated previously with water : acetonitrile : TFA (90 : 10 : 0·1 v/v/v). UV absorbance was monitored at 215 nm and fractions were taken manually according to peaks or shoulders of UV absorbance at a flow rate of 3 ml/min.
Microbore HPLC purification of attractants
Aqueous ammonia was added to bioactive fractions from RP-8 HPLC until the pH was 4·0. The whole sample was then applied to a microbore Mono-S HPLC (microbore Mono-S PC 1·65, Pharmacia, Freiburg, Germany) attached to Beckmann-Gold HPLC system (Beckmann), which was equilibrated previously with 50 mm ammonium formate, pH 4·0, containing 20% acetonitrile. Proteins were eluted with a gradient of increasing concentrations of NaCl (0–1 m) in equilibration buffer using a flow rate of 100 µl/min. UV absorbance was monitored at 280 nm.
Isolation of neutrophils
Venous blood obtained from healthy donors was collected into citric acid/dextran and centrifuged for 20 min. The plasma as well as the white buffy coat was pipetted away. The remaining blood cell sediment was mixed with a gelatine solution prewarmed to 37°C and stored at 37°C for 30 min. The yellowish supernatant was collected and centrifuged at room temperature. Ammonium chloride (0·15 m) was added in order to lyse red blood cells. The suspension was again centrifuged and the sediment obtained was washed with phosphate buffered saline (PBS). The final cell preparations contained more than 90% PMN (>85% neutrophils) with a viability greater than 97% as assessed by the trypan blue exclusion method.
Neutrophil chemotaxis assay
HPLC fractions from the RP-8 column (30 µl) and from the Mono-S column (10 µl and 60 µl) were lyophilized, dissolved in PBS containing 0·1% bovine serum albumin (BSA) [(c) complete PBS] and assayed for neutrophil chemotactic activity. Neutrophil chemotaxis was performed using the blind-well Boyden chambers. The Boyden chambers were filled with samples at appropriate dilutions and covered with a polyvinylpyrrolidone-containing filter (pore size 3 µm: Costar). Neutrophils were suspended in NaCl containing 0·9 mm CaCl2, 0·5 mm MgCl2 and 0·1% BSA at a density of 1–1·5 × 106 cells/ml 100 µl of each suspension was transferred to each chamber. The Boyden chambers were incubated in a humidified atmosphere at 37°C for 1·5 h. Cells remaining in the upper part of the Boyden chamber and the filters were removed. Migrated cells present in the lower part of the Boyden chamber were lysed by adding Triton-X 100 (final concentration 0·1%). β-glucuronidase activity in the lysates was determined using p-nitrophenyl-β-d-glucuronide (Sigma-Aldrich, Deisenhofen, Germany) as a substrate. Calculation of the number of migrated cells was carried out using known numbers of neutrophils, which were lysed with 0·1% Triton-X 100 and incubated with the β-glucoronidase substrate. In each test series positive as well as negative controls were used. Formyl-Nle-Leu-Phe (FNLP) (10−8m, Sigma-Aldrich) served as a positive control for neutrophil chemotactic activity, whereas (c) PBS was used as a negative control. Neutrophil chemotactic activity was expressed as a chemotactic index, which was calculated as stimulated migration divided by random migration (migration towards buffer).
Enzyme-linked immunosorbent assay (ELISA) measurements
ELISA tests for chemokines IL-8 (detection range >31·2 pg/ml), ENA-78 (detection range >31·2 pg/ml), GRO-α (detection range >31·2 pg/ml) and GCP-2 (detection range >31·2 pg/ml) were carried out according to the manufacturer's recommendations (R&D, Wiesbaden, Germany). After RP-8 column 30 µl of each fraction, and after Mono-S column 10 µl of each fraction were lyophilized for the ELISA test.
Semiquantitative reverse transcriptase polymerase chain reaction (RT-PCR)
RNA extraction from tonsils. We examined 12 cases of chronic tonsillitis, nine cases of HT and nine cases of PA. Isolation of total RNA was performed using RNeasy minikits from Quiagen (Düsseldorf-Ratingen, Germany) according to the manufacturer's specifications. 1 µg RNA was subjected to reverse transcription using the Promega reverse transcription system.
Quantitative TaqMan PCR.
Quantitative PCR analysis was performed on the ABI Prism® 7900HT detection system (Applied Biosystems, Foster City, CA, USA). Gene expression assays (Assay-on-Demand™, 250 µl (20×) suitable for this system are based on 5′ nuclease chemistry and consist of two unlabelled PCR primers for amplifying the sequence of interest and a 6-FAM dye-labelled TaqMan® MGB probe for detecting the sequence of interest.
The analysis of IL-8-and GAPDH-gene expression was performed using commercial gene expression assay from Applied Biosystems (Assay-on-Demand™: IL-8, assay no. Hs 00174103m1; GAPDH, assay no. Hs 99999905m1).
Individual gene expression assays were designed for detection of ENA-78, GRO-α and GCP-2 gene expression. (Assay-by-DesignSM, Applied Biosystems: Gro-α F: CCCCACTGCGC CCAAA, Gro-αR: CAAGCTTTCCGCCCATTCTT, ENA-78F: CCCACAGTGCTCCAAGGT, ENA-78R: TCAAGACAAATT TCCTTCCCGTTCT; GCP-2F: CCGCAGTGCTCCAAGGT, GCP-2R: AGACAAACTTGCTTCCCGTTCTT).
All primers and probes were functionally tested on a human cDNA pool.
Preparation of PCR.
The following components for each single PCR were filled into a single well of a 384 PCR plate, using a robotic pipette system (TECAN Deutschland GmbH, Crailsheim, Germany): 4 µl of 2×TaqMan® Universal PCR Master Mix (Applied Biosystems), 0·4 µl of 20× Assay-Mix (Assay-on-Demand™/Assay-by-DesignSM), 2 µl of diluted cDNA (1 : 100) and 1·6 µl of RNase free water. The reaction itself was performed for all gene expression assays according to the following thermocycler protocol: 2 min 50°C, 10 min 95°C, 40 cycles: (15 s at 95°C, 60 s at 60°C). Double determination was performed for all gene expression samples, and a negative control for each gene expression assay was always included.
Quantifying cDNA.
PCR conditions allowing a reliable comparison of chemokine expression with GAPDH mRNA as housekeeping gene expression in different samples were established using a modified protocol by making serial dilutions of template cDNA at constant cycle numbers for each primer pair, to verify that the subsequent PCR reactions were performed in the linear range of PCR amplification. For quantifying PCR products SDS software (SDS 2·0) was used.
Statistical analysis
The chemokine measurements using the ELISA technique are given as mean value and standard error of the mean. Intergroup comparisons were carried out using the non-parametric Mann–Whitney test, where P < 0·05 was considered statistically significant.
RESULTS
Hyperplastic tonsillitis
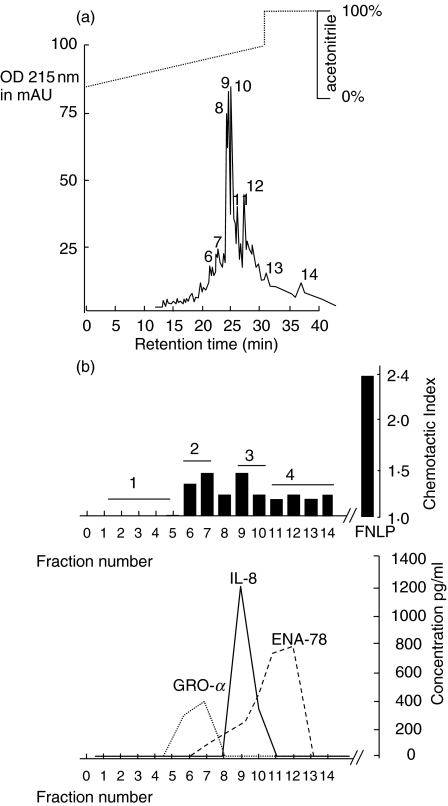
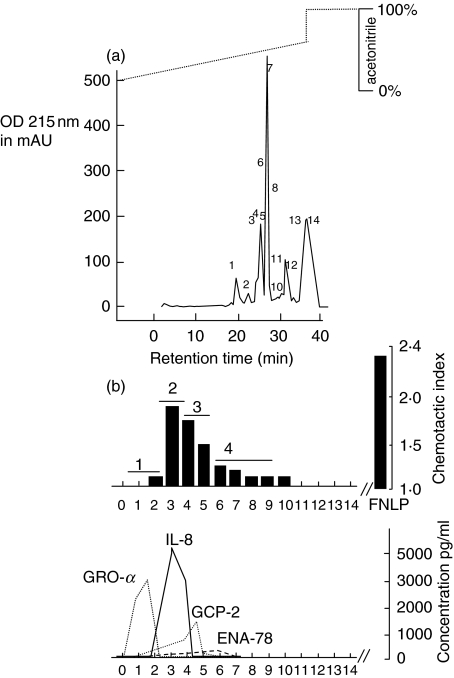
A volume of 400 ml supernatant of tissue homogenate was concentrated in an Amicon chamber, as described above, and buffered for heparin binding chromatography. The heparin binding protein fraction was applied to the chemotaxis assay, and exhibited chemotactic activity (data not shown). The chemotactic active protein sample was then concentrated, buffered and applied to the reversed-phase chromatography (Fig. 1a). Chemotactic activity was shown in protein fractions 6–14 (Fig. 1b). By ELISA, we determined the chemokine concentrations in biologically active fractions using 30 µl of the obtained fractions for the ELISA. According to the data obtained in Fig. 1b, GRO-α dominates in fractions 6–7, whereas IL-8 elutes next, followed by ENA-78. GCP-2 was not detectable within the limits of the ELISA kit.
Fig. 1.
Chemotactic activity of proteins after reversed-phase chromatography (C8 nucleosil RP-8) in hyperplastic tonsils. (a) Chromatogram (RP-8 column). Supernatant collected from tissue homogenization was concentrated and applied to a heparin–sepharose cartridge. The eluate was collected as one fraction and subjected to size exclusion chromatography (RP-8 column). The figure shows the chromatogram and the collected protein fractions labelled with numbers 1–14. (b) Chemotactic index and concentrations of chemokines. The manually collected protein fractions 1–14 were then analysed for chemotactic activity. In each test series, both positive and negative controls were included at appropriate concentrations. FNLP (formyl-Nle-Leu-Phe) served as a positive control for chemotactic responsiveness of PMN, whereas PBS was used as a negative control. The data shown here are those corresponding to 30 µl fraction aliquots transferred to the Boyden chamber. All fraction volumes tested had been freeze-dried prior to testing. In addition, an ELISA was performed to identify the different chemokines. The corresponding fractions were pooled as fractions 1, 2, 3 and 4 and applied to a Mono-S column (see Fig. 2).
Using 30 µl of each fraction for the chemotaxis assay it was found that the biological activity of IL-8 and GRO-α was nearly identical, and higher than that of ENA-78. The groups of fractions showing biological activity and correlating with peaks in the chromatogram were pooled (1–5 = fraction number 1; 6–7 = fraction number 2; 9–10 = fraction number 3; 11–14 = fraction number 4) and applied to the Mono-S column.
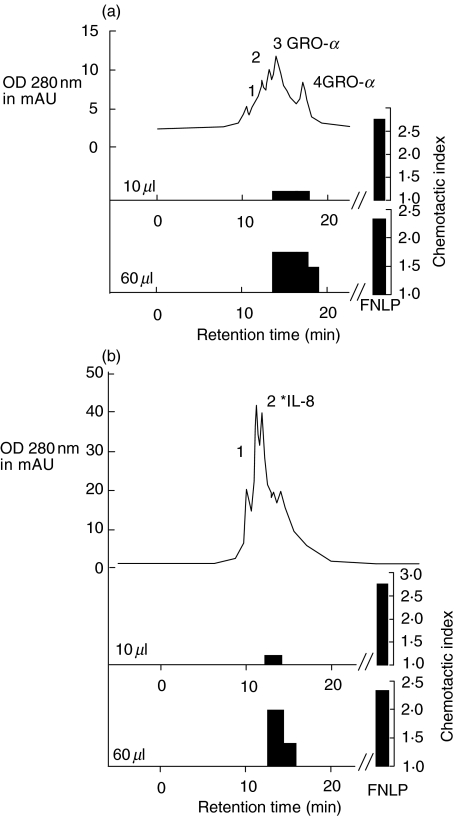
Reversed-phase cation exchange chromatography revealed different protein peaks in the different runs. The chemotactic activity in the Mono-S purified extracts from run 2 (RP-8 run fractions 6–7) and from run 3 (RP-8 run fractions 9–10) are shown in Fig. 2a,b. The chemotactic indices were assessed by inserting 10 and 60 µl of the extracts into the Boyden chamber. Those fractions containing GRO-α and IL-8 showed biological activity at 60 µl-tested volume.
Fig. 2.
Chromatogram of microcation exchange HPLC and chemotactic index of the protein fractions from HT. Bioactive fractions from the RP-8 column (1–5 = fraction number 1; 6–7 = fraction number 2; 9–10 = fraction number 3; 11–14 = fraction number 4) were applied to the Mono-S column. Fractions from Mono-S runs were collected automatically and again tested for chemotactic activity (10 µl and 60 µl aliquot protein fractions were collected for the Boyden chamber test). Two chromatograms are shown as examples: run number 2 in (a) and run number 3 in (b), with chemotactic indices and ELISA measurements identifying the chemokines.
Recurrent tonsillitis
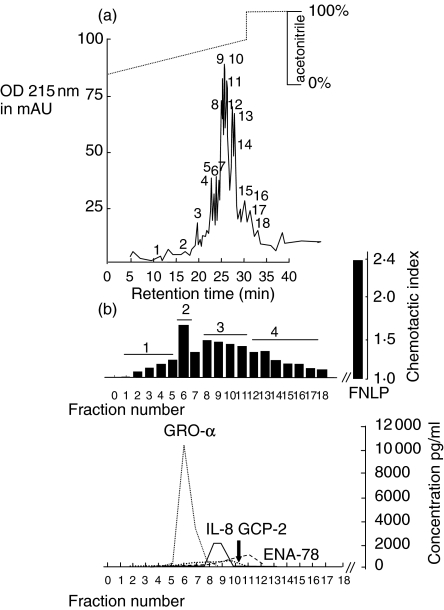
Heparin-bound proteins were stripped from the column and applied as one fraction for the reversed phase chromatography (Fig. 3a). The elution time of the different chemokines was the same as in HT. However, in contrast to HT, GRO-α dominates the biological activity, followed by IL-8. GCP-2 was detected in the range of 750 pg/ml by ELISA using 30 µl aliquots of the RP-8 fractions. ENA-78 protein was measurable, although biological activity was not detected at these concentrations, as shown in Fig. 3b. Fractions with biological activity were again pooled (1–5 = 1; 6–7 = 2; 8–11 = 3; 12–18 = 4) and applied to the Mono-S column. Representative runs are shown in Fig. 4. Figure 4a shows the second peak which is found to contain GRO-α. Biological activity was weak when using 10 µl of the peak volume for the Boyden chamber test. In Fig. 4b the second peak contained IL-8. Ten µl of eluate was lyophilysed and applied to the Boyden chamber test. In this case the concentration was too low to detect biological activity, whereas 60 µl showed biological activity.
Fig. 3.
Chemotactic activity of proteins after reversed-phase chromatography (C8 nucleosil RP-8) in recurrent tonsillitis. (a) Chromatogram (RP-8 column). Supernatant collected from tissue homogenization was concentrated and applied to a heparin–sepharose cartridge. The eluate was collected as one fraction and subjected to size exclusion chromatography. The figure shows the chromatogram and the collected protein fractions labelled with numbers 1–18. (b) Chemotactic index and concentrations of chemokines. The manually collected protein fractions 1–18 were then analysed for chemotactic activity. In each test series, both positive and negative controls were included at appropriate concentrations. FNLP (formyl-Nle-Leu-Phe) served as a positive control for chemotactic responsiveness of PMN, whereas PBS was used as a negative control. The data shown here are those corresponding to 30 µl fraction aliquots transferred to the Boyden chamber. All fraction volumes tested had been freeze-dried prior to testing. In addition, an ELISA was performed to identify the different chemokines. Corresponding fractions were pooled as fractions 1, 2, 3 and 4 and subjected to Mono-S column (see Fig. 4).
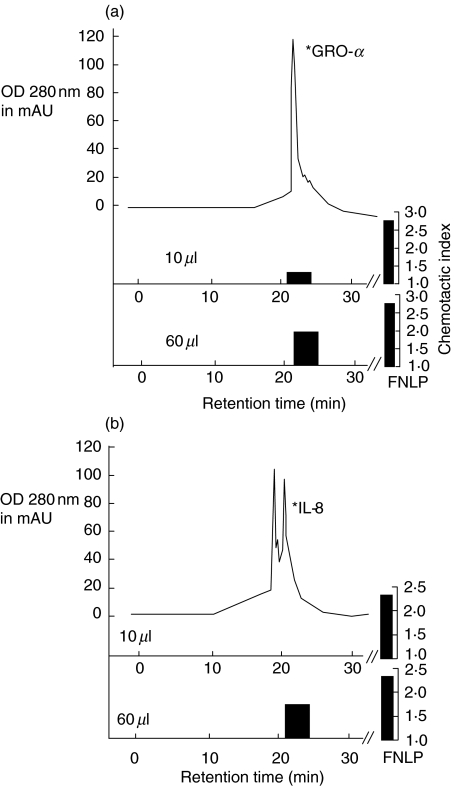
Fig. 4.
Chromatogram of microcation exchange HPLC and chemotactic index of the protein fractions from RT. Bioactive fractions from the RP-8 column were pooled into four fractions and applied to the Mono-S column. Fractions from Mono-S runs were collected automatically and again tested for chemotactic activity (10 µl and 60 µl aliquot protein fraction were collected for the Boyden chamber test). Two chromatograms are shown as examples: run number 2 in (a) and run number 3 in (b), with chemotactic indices and ELISA measurements identifying the chemokines.
Peritonsillar abscesses
After reversed phase chromatography of RP-8, the column elution patterns of neutrophil chemokines from peritonsillar abcess tissue were, in general, no different from those in RT or HT. In contrast to RT, IL-8 dominated the biological activity, although high concentrations of GCP-2 and ENA-78 (up to 1000 pg/30 µl HPLC fraction volume) were measured in other fractions obtained from HPLC (Fig. 5b). Further separation of the pooled fractions 1, 2, 3 and 4 on the Mono-S column gave no new biological activity pattern. IL-8 could be separated from GRO-α. Activity of GCP-2 and ENA-78 could not be measured in the Boyden chamber system (data not shown).
Fig. 5.
Chemotactic activity of proteins after reversed-phase chromatography (C8 nucleosil RP-8) in peritonsillar abscesses. (a) Chromatogram (RP-8 column). Supernatant collected from tissue homogenization was concentrated and applied to a heparin–sepharose cartridge. The eluate was collected as one fraction and subjected to size exclusion chromatography. The manually collected protein fractions 1–14 were then analysed for chemotactic activity. The figure shows the chromatogram and the collected protein fractions labelled with numbers 1–18. (b) Chemotactic index and concentrations of chemokines. In each test series, both positive and negative controls were included at appropriate concentrations. FNLP (formyl-Nle-Leu-Phe) served as a positive control for chemotactic responsiveness of PMN, whereas PBS was used as a negative control. The data shown here are those corresponding to the 30 µl fraction aliquots transferred to the Boyden chamber. All fraction volumes tested were freeze-dried prior to testing. In addition, an ELISA was performed to identify the different chemokines. Corresponding fractions were pooled as fractions 1, 2, 3 and 4 and subjected to a Mono-S column (data are not shown).
Results of PCR in HT, RT and PA
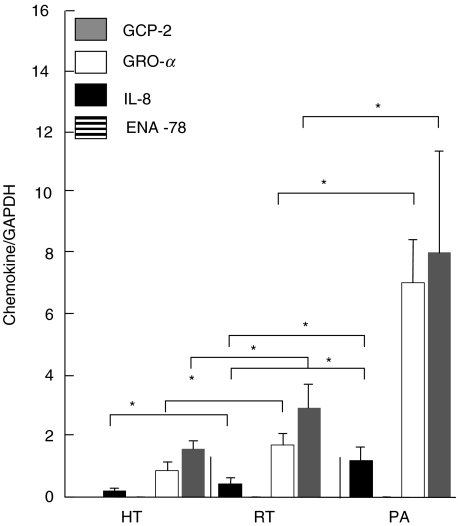
Comparing chemokine mRNA levels in the different tonsillitis forms with one another, we registered the lowest levels of all chemokines in HT, with the exception of ENA-78, which was undetectable in all tonsillitis forms. In RT and PA, we measured significantly up-regulated mRNA levels for GRO-α, GCP-2, and IL-8 in comparison to HT (Fig. 6). In PA the chemokine mRNA levels for GRO-α, GCP-2, and IL-8 were significantly higher than in both RT and HT. The mRNA levels corresponded to the protein levels obtained after elution on RP-8 column with the exception of ENA-78. In addition, in RT, although the GCP-2 mRNA level was higher than in HT, the protein could not be detected in substantial amounts in RT and was undetectable in HT.
Fig. 6.
Chemokine mRNA expression in HT, RT and PA. Chemokine mRNA levels are expressed as a quotient of chemokine CT value/GAPDH CT value (mean ± s.e.m.). Significant results are marked with an asterisk (P < 0·05).
DISCUSSION
Neutrophils represent the first-line defence cells of the innate immune system and are equipped with a battery of effector molecules for the destruction of bacteria and other invading microorganisms. In addition, these cells can respond extremely rapidly (within minutes) to signals such as chemotactic gradients generated by ELR-positive CXC chemokines. The expression of chemokines in various tissues is regulated differentially during the immune response to infection. The production of the ELR-CXC chemokines IL-8 [14], GRO-α, -β and -γ [15], NAP-2 [16], ENA-78 [17] and GCP-2 [18] has already been studied in vitro in great detail. In vivo, the functional role of chemokines has still to be elucidated. Previous reports assumed a functional role for chemokines in arthritis, pancreatitis, colitis, psoriasis and cancer [19–22]
Human palatine tonsils contain 10–30 blind-ending tubular, branched crypts, extending through the full thickness of the organ and enlarging the tonsillar surface up to 300 cm2. The content of the crypts is composed mainly of degenerated cells and cellular debris. The epithelium of the crypts is a modified form of the stratified squamous tissue that covers the remaining oropharynx, including the outer surface of the tonsil. In these crypts, luminal antigens are taken up by specialized cells of the reticulated squamous epithelium [23]. Many chemokines are produced only after appropriate stimulation of the cells with proinflammatory cytokines or bacterial or viral products (inducible chemokines) [4,5]. In addition, the expression of chemokines depends on the cellular source and different proinflammatory stimuli [18]. In cases of acute and chronically infected tonsils, accumulation of neutrophils has been observed in the epithelium [24].
In accordance with the pathophysiological understanding of acute inflammation processes [25] we detected IL-8, the first identified member of the chemokine subgroup, which is the most potent neutrophil chemotactic and activating protein in PA. As under most in vitro stimulation conditions, IL-8 levels and biological activity were most pronounced compared to the other chemokines. Its protein level and biological potency were higher than GRO-α > GCP-2 > ENA-78 following RP-8 chromatography. Both IL-8 and GRO-α are known to be synthesized by primary nasal epithelial cells, nasal fibroblasts and neutrophils in response to various stimuli in vitro [25,26]. In vitro, IL-1β and tumour necrosis factor (TNF)-α seem to be potent stimulators of chemokine production, whereas interferon (IFN)-γ inhibits chemokine production [18]. Here, however, GRO-α protein was less pronounced than IL-8. In addition, the chemotactic potency of GRO-α is weaker than that of IL-8, as shown previously in concentration–response curves.
IL-8 and GCP-2 are the only ELR/CXC chemokines that chemoattract and activate neutrophils by binding to both CXCR1 and CXCR2, whereas the other ELR/CXC chemokines signal only through CXCR2 [27,28]. Despite the weaker biological potency of GCP-2 and the smaller amounts of GCP-2 secreted, compared with those of IL-8, GCP-2 was co-produced with IL-8 and GRO-α in peritonsillar abscesses. It has been suggested that GCP-2 is a typical mesenchymal cell-derived chemokine as shown in stimulation experiments within nasal fibroblasts, whereas IL-8 is also released by blood cells, especially neutrophils [18,26].
In contrast to PA, in recurrent tonsillitis GRO-α predominates IL-8 regarding the level of protein expression and hence biological activity. GRO-α is also produced by a variety of normal cells such as monocytes, endothelial cells, fibroblasts and synovial cells after stimulation with lipopolysaccharide, IL-1 or TNF-α. It is a powerful activator of neutrophils and induces chemotaxis, exocytosis and the respiratory burst in vitro and neutrophil accumulation in vivo [29]. In psoriasis, Schroeder elucidates the role of GRO-α in maintaining the inflammation processes, whereas the other two forms, GRO-β and GRO-γ, could not be detected [19]. GRO-β and GRO-γ were identified in cDNA libraries from activated monocytes and neutrophils. The three GRO proteins share 33–40% identity with IL-8. Information on the expression and biological activities of GRO-β and GRO-γ is scarce [29]. Both cytokines bind to the same receptors as GRO-α and elicit the same pattern of responses in human neutrophils. Similar results were obtained in tonsillitis, i.e. neutrophil chemotactic activity due to GRO-β or GRO-γ was undetectable.
Although the biological activity of NAP-2 has not been characterized in tonsillitis, NAP-2 represents a C-X-C chemokine belonging to the group of platelet basic proteins and is stored in the α-granules of platelets as the 70-amino acid-long C-terminal part of several precursor molecules, by far the most abundant of these being the connective tissue activating peptide III (CTAP-III, 85 amino acids). Proteolytic processing of CTAP-III in platelets is mediated by serine protease activity [30–32].
The different chemokine patterns in PA and RT may be at least in part the result of secretion products from activated neutrophils. The neutrophil protease gelatinase B contributes greatly to the fast response, as it is prepacked in granules and helps the neutrophil to migrate through basement membranes and connective tissues. Gelatinase B processes IL-8 into a 10–30-fold more active chemokine. This results in an important positive feedback loop, because IL-8 induces the rapid release of gelatinase B from the granules [33]. In contrast, the CXC chemokines CTAP-III, GRO-α, PF-4 and ENA-78 are degraded by gelatinase B. The activity of human GCP-2 remains unchanged after cleavage [34].
A hallmark of our results is represented by ENA-78 protein and mRNA level expression. The protein was detected at nearly the same level in the different disease entities, but ENA-78 mRNA was not inducible. One possible explanation may be related to the cell type-specific induction of IL-8 and ENA-78 mRNA, which has been observed in human monocytes [35] and in the human intestinal epithelial cell line Caco-2 [36]. It may depend on the expression of specific transcription factors and the presence of corresponding regulatory elements in the upstream areas of the two promoters regions. While an NF-κB binding site seems to be essential for the induction of both IL-8 and ENA-78 transcription, putative AP-2-like binding sites have been detected only in the ENA-78 promoter region [37]. The transcription factor AP-2 has been shown to play a role in epidermal cell-specific expression of keratin genes [38].
Lymphoid hyperplasia in hyperplastic tonsils appears to emerge due to an increase in lymphoid elements. The tonsil size has been shown to be directly proportional to the mean bacterial load. The bacteriology of hyperplastic tonsils does not differ greatly from that of recurrently infected ones, although Haemophilus infection is somewhat more common in tonsil hyperplasia. These findings also apply in cases without a history of infection. Staphylococcus aureus and Streptococcus pyogenes have been reported to correlate with hyperplasia as well [39]. It has been assumed that hyperplasia of the tonsils is physiological up to a point, but highly hyperplastic tonsils probably harbour a bacterial infection of a more or less chronic nature in the core, even in cases without a history of throat infection. If bacteria are found in great concentrations, these findings correlate with patient history, clinical findings and weight of the tonsils. Conversely, these findings are mostly absent in healthy tonsils [39].
Compared to PA, the level of IL-8 protein expression was about five times lower in HT, and approximately the same as in HPLC fractions of RT. However, IL-8 represented the highest most abundant chemokine, followed by ENA-78, GRO-α and, finally GCP-2. According to the concentrations in the HPLC fractions, biological activity was measurable but not exceptionally expressed. Our investigations demonstrate that hyperplastic tonsillitis is characterized by an acute inflammatory chemokine pattern, as determined by chemokine activity. PCR data also revealed the lowest levels of mRNA for all chemokines in HT compared to RT and PA. Chemokine GCP-2, followed by mRNA levels, were highest for GRO-α and IL-8 and, again, ENA-78 was almost undetectable.
In summary, small amounts of ENA-78 were measured at the protein level in acute, chronic and hyperplastic tonsillitis. The biological activity of ENA-78 was not detectable at this protein concentration. Furthermore, ENA-78 mRNA was not induced in PA or RT. GCP-2 expression in tonsillitis differs from ENA-78; although the GCP-2 protein level was highest in PA, followed by RT, the amount of protein was too low to show biological activity compared with IL-8 or GRO-α. As GCP-2 acts on both the chemokine receptors CCR1 and CCR2, it serves as a substitute chemokine in certain inflammatory conditions. IL-8 was identified as the acute phase chemokine expressed in PA and HT. In RT, GRO-α plays a pivotal role. These results suggest that hyperplastic tonsils may be an inflammatory form of tonsillitis.
REFERENCES
- Luster AD. Mechanisms of disease: chemokines – chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. 675–705. [DOI] [PubMed] [Google Scholar]
- Luster AD, Cardiff RD, Maclean JA, Crowe K, Granstein RD. Delayed wound healing and disorganized neovascularization in transgenic mice expressing the IP-10 chemokine. Proc Assoc Am Phys. 1998;110:183–96. [PubMed] [Google Scholar]
- Mantovani A. Inflammatory cytokines. Recenti Prog Med. 2000;91:422–4. [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Morales J, Hedrick JA. Recent advances in chemokines and chemokine receptors. Crit Rev Immunol. 1999;19:1–47. [PubMed] [Google Scholar]
- Surjan L., Jr Tonsils and lympho-epithelial structures in the pharynx as immuno barriers. Acta Otolaryngol. 1987;103:369–72. [PubMed] [Google Scholar]
- Korsrud FR, Brandtzaeg P. Influence of tonsillar disease on the expression of J chain by immunoglobulin-producing cells in human palatine and nasopharyngeal tonsils. Scand J Immunol. 1981;13:281–7. doi: 10.1111/j.1365-3083.1981.tb00136.x. [DOI] [PubMed] [Google Scholar]
- Scadding GK. Immunology of the tonsil: a review. J Roy Soc Med. 1990;83:104–7. doi: 10.1177/014107689008300216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying MD. Immunological basis of indications for tonsillectomy and adenoidectomy. Acta Otolaryngol Suppl. 1988;454:279–85. doi: 10.3109/00016488809125041. [DOI] [PubMed] [Google Scholar]
- Beck EG, Schmidt P. Environmental medicine group diagnosis of children – studies from 1982 to 1990. Zentralbl Hyg Umweltmed. 1993;193:395–418. [PubMed] [Google Scholar]
- Stradling JR, Douglas NJ. Heart attacks and sleep apnoea. Lancet. 1990;336:1378–9. doi: 10.1016/0140-6736(90)92929-c. [DOI] [PubMed] [Google Scholar]
- Berkowitz RG, Zalzal GH. Tonsillectomy in children under 3 years of age. Arch Otolaryngol Head Neck Surg. 1990;116:685–6. doi: 10.1001/archotol.1990.01870060043006. [DOI] [PubMed] [Google Scholar]
- Wuyts A, Proost P, Lenaerts JP, Ben Baruch A, Van Damme J, Wang JM. Differential usage of the CXC chemokine receptors 1 and 2 by interleukin-8, granulocyte chemotactic protein-2 and epithelial-cell-derived neutrophil attractant-78. Eur J Biochem. 1998;255:67–73. doi: 10.1046/j.1432-1327.1998.2550067.x. [DOI] [PubMed] [Google Scholar]
- Wang D, Richmond A. Nuclear factor-kappa B activation by the CXC chemokine melanoma growth-stimulatory activity/growth-regulated protein involves the MEKK1/p38 mitogen-activated protein kinase pathway. J Biol Chem. 2001;276:3650–9. doi: 10.1074/jbc.M006115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt E, Petersen F, Ludwig A, Ehlert JE, Bock L, Flad HD. The beta-thromboglobulins and platelet factor 4: blood platelet-derived CXC chemokines with divergent roles in early neutrophil regulation. J Leukoc Biol. 2000;67:471–8. doi: 10.1002/jlb.67.4.471. [DOI] [PubMed] [Google Scholar]
- Walz A, Strieter RM, Schnyder S. Neutrophil-activating peptide ENA-78. Adv Exp Med Biol. 1993;351:129–37. doi: 10.1007/978-1-4615-2952-1_14. [DOI] [PubMed] [Google Scholar]
- Wuyts A, Struyf S, Gijsbers K, et al. The CXC chemokine GCP-2/CXCL6 is predominantly induced in mesenchymal cells by interleukin-1beta and is down-regulated by interferon-gamma: comparison with interleukin-8/CXCL8. Lab Invest. 2003;83:23–34. doi: 10.1097/01.lab.0000048719.53282.00. [DOI] [PubMed] [Google Scholar]
- Schroder JM. Identification and structural characterization of chemokines in lesional skin material of patients with inflammatory skin disease. Meth Enzymol. 1997;288:266–97. doi: 10.1016/s0076-6879(97)88019-6. [DOI] [PubMed] [Google Scholar]
- Wang J, Huang M, Lee P, et al. Interleukin-8 inhibits non-small cell lung cancer proliferation: a possible role for regulation of tumor growth by autocrine and paracrine pathways. J Interferon Cytokine Res. 1996;16:53–60. doi: 10.1089/jir.1996.16.53. [DOI] [PubMed] [Google Scholar]
- Grimm MC, Elsbury SK, Pavli P, Doe WF. Interleukin 8: cells of origin in inflammatory bowel disease. Gut. 1996;38:90–8. doi: 10.1136/gut.38.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekanecz Z, Strieter RM, Kunkel SL, Koch AE. Chemokines in rheumatoid arthritis. Springer Semin Immunopathol. 1998;20:115–32. doi: 10.1007/BF00832002. [DOI] [PubMed] [Google Scholar]
- Nave H, Gebert A, Pabst R. Morphology and immunology of the human palatine tonsil. Anat Embryol (Berl) 2001;204:367–73. doi: 10.1007/s004290100210. [DOI] [PubMed] [Google Scholar]
- Ebenfelt A, Ivarsson M. Neutrophil migration in tonsils. J Anat. 2001;198:497–500. doi: 10.1046/j.1469-7580.2001.19840497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudack C, Maune S, Eble J, Schroeder JM. The primary role in biologic activity of the neutrophil chemokines IL-8 and GRO-alpha in cultured nasal epithelial cells. J Interferon Cytokine Res. 2003;23:113–23. doi: 10.1089/107999003321455507. [DOI] [PubMed] [Google Scholar]
- Rudack C, Hermann W, Eble J, Schroeder JM. Neutrophil chemokines in cultured nasal fibroblasts. Allergy. 2002;57:1159–64. doi: 10.1034/j.1398-9995.2002.23748.x. [DOI] [PubMed] [Google Scholar]
- Wolf M, Delgado MB, Jones SA, Dewald B, Clark-Lewis I, Baggiolini M. Granulocyte chemotactic protein 2 acts via both IL-8 receptors, CXCR1 and CXCR2. Eur J Immunol. 1998;28:164–70. doi: 10.1002/(SICI)1521-4141(199801)28:01<164::AID-IMMU164>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Proost P, Wuyts A, Conings R, et al. Human and bovine granulocyte chemotactic protein-2: complete amino acid sequence and functional characterization as chemokines. Biochemistry. 1993;32:10170–7. doi: 10.1021/bi00089a037. [DOI] [PubMed] [Google Scholar]
- Geiserls T, Dewald S, Ehrengruber M, Clark-Lewis I, Baggiolini M. The interleukin-8-related chemotactic cytokines GRO-α, GRO-β, and GRO-γ activate human neutrophil and basophil leukocytes. J Biol Chem. 1993;268:15419–24. [PubMed] [Google Scholar]
- Page CP. Platelet activation. J Lipid Med. 1991;4:1–4. [PubMed] [Google Scholar]
- Car BD, Baggiolini M, Walz A. Formation of neutrophil-activating peptide 2 from platelet-derived connective-tissue-activating peptide III by different tissue proteinases. J Biochem. 1991;275:581–4. doi: 10.1042/bj2750581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AB, Stevens MD, Miller EJ, Atkinson MA, Mullenbach G. Generation of the neutrophil-activating peptide-2 by cathepsin G and cathepsin G-treated human platelets. Am J Physiol. 1992;263:51–6. doi: 10.1152/ajplung.1992.263.2.L249. [DOI] [PubMed] [Google Scholar]
- Van den Steen PE, Wuyts A, Husson J, Proost P, Van Damme J, Opdenakker G. Gelatinase B/MMP-9 and neutrophil collagenase/MMP-8 process the chemokines human GCP-2/CXCL6, ENA-78/CXCL5 and mouse GCP-2/LIX and modulate their physiological activities. Eur J Biochem. 2003;270:3739–49. doi: 10.1046/j.1432-1033.2003.03760.x. [DOI] [PubMed] [Google Scholar]
- Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood. 2000;96:2673–81. [PubMed] [Google Scholar]
- Schnyder-Candrian S, Walz A. Neutrophil-activating protein ENA-78 and IL-8 exhibit different patterns of expression in lipopolysaccharide and cytokine-stimulated human monocytes. J Immunol. 1997;158:3888–94. [PubMed] [Google Scholar]
- Keates S, Keates AC, Mizoguchi E, Bhan A, Kelly CP. Enterocytes are the primary source of the chemokine ENA-78 in normal colon and ulcerative colitis. Am J Physiol. 1997;273:75–82. doi: 10.1152/ajpgi.1997.273.1.G75. [DOI] [PubMed] [Google Scholar]
- Corbett MS, Schmitt I, Riess O, Walz A. Characterization of the gene for human neutrophil-activating peptide 78 (ENA-78) Biochem Biophys Res Commun. 1997;205:612–7. doi: 10.1006/bbrc.1994.2709. [DOI] [PubMed] [Google Scholar]
- Leask A, Byrne C, Fuchs E. Transcription factor AP2 and its role in epidermal-specific gene expression. Proc Natl Acad Sci USA. 1991;88:7948–52. doi: 10.1073/pnas.88.18.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindroos R. Bacteriology of the tonsil core in recurrent tonsillitis and tonsillar hyperplasia – a short review. Acta Otolaryngol Suppl. 2000;543:206–8. doi: 10.1080/000164800454404. [DOI] [PubMed] [Google Scholar]