Abstract
We have shown previously that both humoral and cellular immune responses to heat shock protein 60 (HSP60) are elevated in chronic periodontitis patients compared with non-diseased subjects. The aim of the present study was to determine whether periodontal treatment could influence the level of serum antibodies to human HSP60 and Porphyromonas gingivalis GroEL, a bacterial homologue of human HSP60. Sera were obtained from 21 patients with moderate to advanced chronic periodontitis at the baseline examination and again after completion of treatment. Antibody levels were determined using an enzyme-linked immunosorbent assay. The mean anti-P. gingivalis GroEL antibody levels were down-regulated significantly by periodontal treatment when recombinant P. gingivalis GroEL was used as an antigen, whereas antibody levels to P. gingivalis GroEL-specific peptide were significantly elevated following successful periodontal therapy. The mean level of anti-human HSP60 antibody remained unchanged although individual levels of antibody either increased or decreased after periodontal treatment, suggesting that synthesis of these antibodies might be regulated independently during the course of periodontal infection. Although their regulatory mechanisms in chronic infection are not understood, further study would provide insight not only into the role of these antibodies in the pathogenesis of periodontitis but also into the possible link between periodontitis and systemic diseases such as coronary heart disease.
Keywords: atherosclerosis, HSP60, periodontitis, treatment
INTRODUCTION
Heat shock protein 60 (HSP60) belongs to a family of related proteins which have been conserved during evolution. Despite being highly homologous between prokaryotic and eukaryotic cells, HSP60s are strongly immunogenic and immune responses to microbial HSP60s are speculated to initiate chronic inflammatory diseases in humans [1]. HSP60 has been reported to be involved in the pathogenesis of a number of chronic diseases including periodontal disease. We have demonstrated previously that the frequency of sero-positivity and the antibody titre to human HSP60 and Porphyromonas gingivalis GroEL, a bacterial homologue of human HSP60, were significantly higher in periodontitis patients compared with periodontally healthy control subjects. Furthermore, affinity purified serum antibodies to human HSP60 and P. gingivalis GroEL cross-reacted with P. gingivalis GroEL and human HSP60, respectively [2]. Recently, we demonstrated that the proliferative response of peripheral blood T cells to autologous HSP60 was significantly higher in periodontitis patients compared with gingivitis patients. Furthermore, clonal analysis, using single-strand conformation polymorphism, demonstrated clearly that HSP60-specific T cells accumulated in the gingival lesions of periodontitis patients but not in gingivitis patients and that the T cell clones with an identical specificity to those in peripheral blood existed in periodontitis lesions [3]. In addition, human HSP60 is expressed abundantly in periodontitis lesions and, similar to bacterial lipopolysaccharide (LPS), is able to stimulate tumour necrosis factor (TNF)-α production from macrophages [4]. Thus, immune responses to HSP60 derived from either inflammatory tissue or bacteria were thought to play an important role in the periodontal disease process. However, as yet there are no reports describing the effect of periodontal treatment on the humoral immune response to HSP60s.
Recent cross-sectional epidemiological studies have shown that individuals with chronic periodontitis have a significantly increased risk of developing coronary heart disease (CHD) [5–7]. However, while the evidence linking periodontitis with an increased risk for CHD is limited [8] and any causal relationship between periodontal disease and coronary heart disease has not been clarified, there is much evidence linking chronic infection per se to CHD. It is therefore not unreasonable to suggest that chronic periodontitis could contribute to the total burden of infection and as such contribute to the development of atherosclerosis. Support for this has come from the concept that immune responses targeted to self-proteins located in the vessel wall are a result of molecular mimicry with bacterial antigens. As a number of studies have demonstrated that the immune response to either endogenous (human) or bacterial HSP60 may be involved in the pathogenesis of atherosclerosis and subsequent coronary heart disease and cerebrovascular disease [9–12], we hypothesized that elevated serum antibodies to periodontopathic bacterial HSP60 during the course of periodontal infection cross-reacts with human HSP60s expressed in either the periodontal tissues or on arterial endothelial and smooth muscle cells and hence could deteriorate pre-existing atherosclerotic lesions further. Therefore, the aim of the present study was to determine whether periodontal treatment leads to a reduction in the serum levels of antibodies to P. gingivalis GroEL and, in turn, in the serum levels of anti-human HSP60 antibodies.
MATERIALS AND METHODS
Patients
A total of 21 patients with moderate to advanced chronic periodontitis were included in the study. In order to exclude the confounding effects of smoking, all patients were non-smokers. The mean age of the patients was 40·6 years at the baseline examination. The institutional review boards of Niigata University Graduate School of Medical and Dental Sciences approved this study and written informed consent was obtained from all the patients before inclusion in the study. Periodontal tissue destruction was assessed as described previously [3]. Clinical examination included plaque control record [13], probing depths, attachment levels and alveolar bone resorption. Probing depths and attachment levels were recorded at six sites around each tooth. Mean probing depth and attachment level at baseline and at reassessment were calculated by dividing the mean probing depth and attachment level of each subject by the number of subjects. Radiographs were used to measure the alveolar bone resorption on the proximal surface of each tooth [14]. Mean alveolar bone resorption was calculated the same as mean probing depth and attachment level. All patients had no record of periodontal treatment and had not taken antibiotics at least 3 months prior to the baseline examination. The clinical profile of the study population is presented in Table 1.
Table 1.
Clinical profile at baseline and reassessment
| Baseline | Reassessment | |
|---|---|---|
| Mean percentage sites with plaque | 51·9 ± 24·0 | 16·0 ± 13·6* |
| Mean pocket depth (mm) | 4·3 ± 1·3 | 2·2 ± 0·4* |
| % sites with probing depth <4 mm | 48·5 ± 25·5 | 90·0 ± 21·0* |
| % sites with probing depth 4–6 mm | 35·8 ± 15·5 | 8·9 ± 16·2* |
| % sites with probing depth >6 mm | 14·2 ± 16·6 | 1·1 ± 2·4* |
| Mean attachment level (mm) | 4·9 ± 1·7 | 3·7 ± 1·2* |
| Mean bone loss (%) | 42·2 ± 16·2 | n.d.** |
| Number of teeth | 26·4 ± 3·4 | 23·2 ± 5·2 |
Significantly different from baseline values
n.d.: not determined.
Treatment
Initial therapy included mechanical plaque control together with scaling and root planing under local anaesthesia. The effect of the initial therapy was assessed 1 month after the therapy and surgical procedures (flap surgery) were carried out where there were residual periodontal pockets. As the number of sites receiving surgical intervention varied from patient to patient (all the patients received surgery at one site at least), the time interval between examinations at baseline and reassessment also varied (4–7 months). All patients were assessed again 2 months after completion of the surgery and followed subsequently monthly or 3-monthly depending upon individual patient requirements. Antibiotics were usually prescribed for 4 days after periodontal surgery. Serum samples were taken at baseline and at 3 months after completion of active therapy (i.e. 2 months after periodontal surgery).
Production of antibody to P. gingivalis GroEL-specific peptide
Based on the data base search (P42375, BLASTP, GenomeNet, Kyoto, Japan), we selected P. gingivalis-specific amino acid sequence (460 KEGAVMVQKVKEGK 473) within the entire sequence consisting of 545 amino acids and rabbit polyclonal antibody against this peptide was raised. For this purpose, cysteine was inserted at the C-terminus of lysine residue at position 473 and keyhole limpet haemocyanin as a carrier protein was coupled using m-maleimidobenzoyl-N-hydroxysuccinimide ester. After completion of standard immunization protocol, the IgG fraction was obtained from the antisera by ammonium sulphate precipitation.
Confirmation of antigen-specificity of the antibody to P. gingivalis GroEL-specific peptide
In order to confirm the specificity of the antibody to P. gingivalis-specific peptide, recombinant human HSP60 (Stressgen Biotechnologies Corp., Victoria, Canada), P. gingivalis GroEL and Actinobacillus actinomycetemcomitans GroEL produced in our laboratory [15] were subjected to 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membrane using Trans-Blot SD (Bio-Rad Laboratories; Hercules, CA, USA) for Western blotting. The membranes were reacted with rabbit polyclonal anti-P. gingivalis GroEL peptide antibody and monoclonal anti-human HSP60 antibody (LK-1; Stressgen) only reactive with mammalian HSP60 [16] followed by biotinylated bovine antirabbit IgG (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) or biotinylated horse antimouse IgG (Vector, Burlingame, CA, USA) and finally with avidin–biotin–immunoperoxidase (Vector). The peroxidase was developed using 0·005% 3,3′-diaminobenzidine in TRIS-HCl buffer, pH 7·2 containing 0·01% hydrogen peroxide.
ELISA for anti-P. gingivalis GroEL antibody and P. gingivalis GroEL-specific antibody levels in sera
Microtitre plates were coated with either recombinant P. gingivalis GroEL or P. gingivalis GroEL-specific peptide (100 µl/well; 5 µg/ml in 0·05 m carbonate buffer, pH 9·6) prepared in our laboratory overnight at 4°C [2]. After washing three times with phosphate-buffered saline (PBS) containing 0·05% Tween-20 (PBS-T; pH 7·4), non-specific binding sites were blocked with PBS-T containing 1% bovine serum albumin for 1·5 h at room temperature (RT). The plates were washed with PBS-T and 100 µl of test samples diluted with blocking reagent (1 : 100) were added and incubated for 1 h at RT. After washing three times with PBS-T, horseradish peroxidase-conjugated goat anti-human IgG (Sigma Chemical Co., St Louis, MO, USA) were added and incubated further for 1 h at RT. The o-phenylene diamine diluted to 0·5 mg/ml in 0·1 m citrate buffer containing 0·015% hydrogen peroxide was added. Colour development was stopped by the addition of 4 m sulphuric acid and absorbance was read at a wavelength of 490 nm using an automated enzyme-linked immunosorbent assay (ELISA) reader (Labsystems Oy, Helsinki, Finland) and analysed using genesis lite software (Labsystems).
Measurement of serum antibody levels to human HSP60
Serum antibody levels to human HSP60 were determined by ELISA using commercial kits (Stressgen).
Statistical analysis
Clinical parameters and levels of serum antibodies at baseline and reassessment were compared using a paired t-test. A value of P < 0·05 was considered to indicate a significant difference in statistical analyses.
RESULTS
Clinical effect of periodontal treatment
Mean plaque control record at the initial examination was 51·9 ± 24·0% and declined to 16·0 ± 13·6% at reassessment. There was a significant improvement in the periodontal condition following treatment, as evidenced by the reduction in probing depths and clinical attachment gain. Mean interproximal bone loss at baseline was 42·2%, while the mean probing depth was 4·3 mm at baseline and at reassessment 2·2 mm (Table 1).
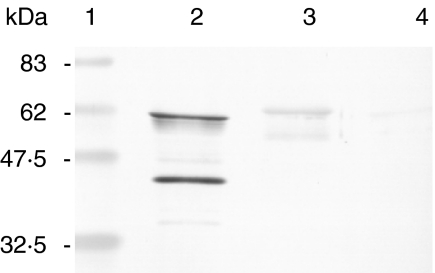
Specificity of anti-P. gingivalis GroEL antibody
Western blot analysis demonstrated that rabbit anti-P. gingivalis GroEL-specific peptide antibody reacted strongly with recombinant P. gingivalis GroEL and reacted very weakly with A. actinomycetemcomitans GroEL but not with recombinant human HSP60 (Fig. 1). LK-1 reacted only with recombinant human HSP60, but not with recombinant P. gingivalis GroEL, P. gingivalis GroEL-specific peptide or recombinant A. actinomycetemcomitans GroEL (data not shown).
Fig. 1.
Reactivity of rabbit polyclonal anti-P. gingivalis GroEL-specific peptide antibody. Two µg of each antigen were subjected to electrophoresis in SDS-polyacrylamide gels and blotted onto nitrocellulose, and reactivities to rabbit anti-P. gingivalis GroEL-specific peptide antibody was examined. (a) P. gingivalis GroEL showed strong reactivity, whereas A. actinomycetemcomitans showed very weak reactivity. Human recombinant HSP60 did not react with the antibody. Lane 1, marker; lane 2, recombinant P. gingivalis GroEL; lane 3, recombinant A. actinomycetemcomitans GroEL; lane 4, recombinant human HSP60.
Serum antibody levels to human HSP60
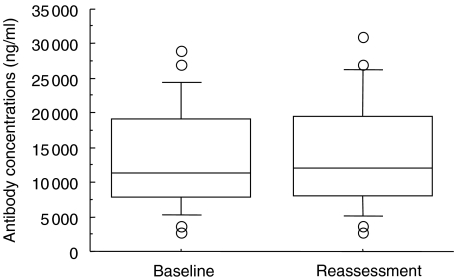
Serum antibody levels to HSP60 were highly variable among the patients before periodontal treatment. Individual anti-HSP60 antibody levels did not correlate with disease severity as shown by mean pocket depth or mean alveolar bone loss (data not shown). Although individual levels of the antibody either increased or decreased after periodontal treatment, the mean concentration of anti-HSP60 antibody did not change as a result of treatment (13·97 µg/ml following treatment compared with 13·75 µg/ml before treatment) (Fig. 2).
Fig. 2.
Serum antibody levels to human HSP60 at baseline examination and reassessment. Data are determined by commercial ELISA kit (box plots show medians, 25th and 75th percentiles as boxes, 10th and 90th percentiles as whiskers. Outside values are shown as open circles). No significant difference was observed between baseline examination and reassessment (paired t-test).
Serum antibody levels to P. gingivalis GroEL
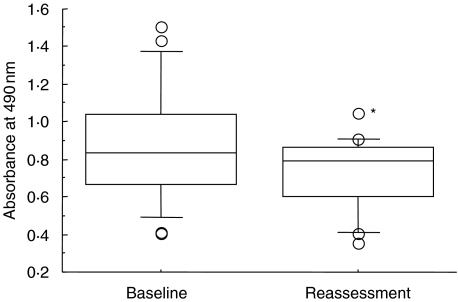
As with antibodies to HSP60, the serum levels of anti-P. gingivalis GroEL antibody before treatment did not correlate with any clinical parameters. In contrast to anti-HSP60 antibody, however, the levels of anti-P. gingivalis GroEL antibody decreased significantly after periodontal therapy (paired t-test; P = 0·0015) (Fig. 3). However, individual analysis showed that slight elevation of serum antibody level was present in five of the 21 patients.
Fig. 3.
Serum antibody levels to P. gingivalis GroEL at baseline examination and reassessment. Data are determined by ELISA using recombinant P. gingivalis GroEL as an antigen (box plots show medians, 25th and 75th percentiles as boxes, 10th and 90th percentiles as whiskers. Outside values are shown as open circles). *Significant difference was observed between baseline examination and reassessment (paired t-test, P = 0·0015).
Serum antibody levels to P. gingivalis GroEL-specific peptide
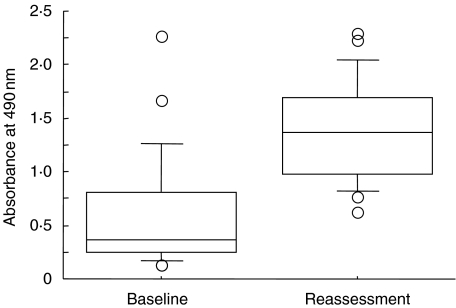
In contrast to the serum levels of antirecombinant P. gingivalis GroEL antibody, the levels of anti-P. gingivalis GroEL-specific peptide antibody increased significantly after periodontal therapy (paired t-test; P < 0·0001) (Fig. 4). The mean values of antibody level were 0·58 at baseline and 1·37 at reassessment, respectively. However, only one patient demonstrated the reduction of antibody after therapy.
Fig. 4.
Serum antibody levels to P. gingivalis GroEL-specific peptide at baseline examination and reassessment. Data are determined by ELISA using synthetic P. gingivalis GroEL-specific peptide as an antigen (box plots show medians, 25th and 75th percentiles as boxes, 10th and 90th percentiles as whiskers. Outside values are shown as open circles). *Significant difference was observed between baseline examination and reassessment (paired t-test, P < 0·0001).
DISCUSSION
In the present study we investigated the effect of periodontal treatment on the antibody responses to recombinant human HSP60, recombinant P. gingivalis GroEL that includes an entire amino acid sequence and to synthetic peptide that includes only a P. gingivalis GroEL-specific amino acid sequence. The results showed that whereas antibody to P. gingivalis GroEL decreased significantly after periodontal treatment, antibody levels to P. gingivalis GroEL-specific peptide were, unexpectedly, elevated significantly following therapy. The antibody to recombinant P. gingivalis GroEL has been shown to cross-react with human HSP60 [2] and possibly with other bacterial HSP60. On the other hand, antibody to the synthetic peptide is specific to P. gingivalis GroEL and has only marginal reactivity with other bacterial HSP60. Although explanation of the results found in the present study is speculative, several mechanisms can be postulated. First, as discussed by Mooney et al., periodontal treatment may result in an inoculation effect leading to an increase in antibody to P. gingivalis GroEL-specific peptide. At the same time the reduction in antigen load resulting from improved oral hygiene and the use of antibiotics post-surgically, or from a combination of these mechanisms [17], may result in a reduction in the total burden of infection and a subsequent reduction in antibody to all bacterial GroEL which includes antibody to P. gingivalis GroEL. The combination of this reduction in the total burden of infection together with the inoculation effect may explain why some patients show an increase in antibody to human HSP60 while others show a decrease. Secondly, periodontitis patients could be considered to be immunized with components derived from periodontopathic bacteria such as P. gingivalis GroEL and, as discussed above, periodontal treatment may result in an inoculation effect similar to vaccination. In this context, if the processed peptide from P. gingivalis GroEL contains a regulatory epitope it could possibly stimulate T cells to synthesize Th2/3 cytokines [interleukin (IL)-10 and transforming growth factor (TGF)-β] and the antibody response to this peptide would be up-regulated. On the other hand, if processed antigen presented by antigen-presenting cells (APCs) does not contain a regulatory epitope, effector T cells could be stimulated to produce Th1 cytokines. Thus, the antibody response to cross-reactive antigens such as bacterial HSP60 may depend upon the nature of the antigen, the nature of the APC and the nature of the cytokine profile elicited [18]. The mean levels of antibody to HSP60, however, showed no change, even though some of the patients showed a decrease of the antibody level. Alternatively, this lack of change in antibody to human HSP60 may suggest that periodontal infection and subsequent periodontal inflammation, which is responsible for enhanced expression of endogenous HSP60 [4], are of little relevance to systemic levels of anti-human HSP60 antibody. HSP60 can be identified in the circulation of normal individuals [19,20] and HSP60 derived from other parts of the body may be responsible for the circulating antibody to HSP60. The variation seen in individual patients, however, would seem to argue against this; however, this again remains to be clarified.
It is difficult to relate our findings to actual periodontal infection with P. gingivalis as we did not examine the presence of P. gingivalis in the periodontal pockets. However, there is an overwhelming number of reports which have shown a strong correlation between the presence of P. gingivalis and periodontal disease. This bacterium has been detected in 84·6% of Japanese periodontitis patients at baseline and in as high as 50·0% after scaling and root planing [21]. At the same time it is found in only 27·1% of periodontally healthy Japanese adults [22]. Therefore, it is reasonable to assume that most, if not all, patients in this study had P. gingivalis in their periodontal pockets and that the proportion of P. gingivalis-positive patients as well as P. gingivalis-positive pockets decreased after treatment.
There are several implications of the results of the present study to the pathogenesis of periodontitis itself and the possible role of periodontal infection to systemic conditions. As antibody levels to human HSP60, containing both specific and cross-reactive antibodies, showed a high degree of variability after treatment, but P. gingivalis GroEL-specific antibody increased, this must mean that the relative proportion of cross-reactive antibody capable of reacting with human HSP60 decreased, thus suggesting that periodontal disease may contribute to the total burden of infection. The extent to which it does, however, would vary from patient to patient. Although there is no indisputable biological evidence so far that periodontal infection increases atherogenesis and risk for CHD, a number of studies have demonstrated epidemiologically that the subjects having periodontal disease are at higher risk of developing CHD [23,24]. One possible mechanism linking chronic infection (including periodontal disease) and atherosclerosis is the immune response to HSPs, such that antibodies to chlamydial or mycobacterial HSPs [25], antibodies to either periodontopathic bacterial HSP60s or human HSP60 induced by periodontal infection and the subsequent inflammatory response may react with injured endothelial cells, resulting in further development of athersclerosis.
In summary, the present study has shown that the humoral immune response to periodontopathic bacteria may result in both specific and cross-reactive antibodies. In contrast to the antibodies to bacterial HSP60, mean antibody levels to HSP60 showed little change after periodontal treatment, although there was significant variation among individuals, suggesting that the contribution of periodontal disease to the total burden of infection is variable between individuals. Further study is necessary to provide a gereater insight into the role of these antibodies in the pathogenesis of periodontitis and as a possible link between periodontitis and CHD.
Acknowledgments
This study was supported by grants from the Ministry of Education, Science Sports and Culture of Japan (13470462, 14657553, 14771219) and Grants for Promotion of Niigata University Research Projects (To KY and TN).
REFERENCES
- Kiessling R, Gronberg A, Ivanyi J, et al. Role of hsp60 during autoimmune and bacterial inflammation. Immunol Rev. 1991;121:91–112. doi: 10.1111/j.1600-065x.1991.tb00824.x. [DOI] [PubMed] [Google Scholar]
- Tabeta K, Yamazaki K, Hotokezaka H, Yoshie H, Hara K. Elevated humoral immune response to heat shock protein 60 (hsp60) family in periodontitis patients. Clin Exp Immunol. 2000;120:285–93. doi: 10.1046/j.1365-2249.2000.01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Ohsawa Y, Tabeta K, et al. Accumulation of human heat shock protein 60-reactive T cells in the gingival tissues of periodontitis patients. Infect Immun. 2002;70:2492–501. doi: 10.1128/IAI.70.5.2492-2501.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki K, Tabeta K, Yoshie H, Yamazaki K. Self-heat shock protein 60 induces tumour necrosis factor-α in monocyte-derived macrophage: possible role in chronic inflammatory periodontal disease. Clin Exp Immunol. 2002;127:72–7. doi: 10.1046/j.1365-2249.2002.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeStefano F, Anda RF, Kahn HS, Williamson DF, Russell CM. Dental disease and risk of coronary heart disease and mortality. Br Med J. 1993;306:688–91. doi: 10.1136/bmj.306.6879.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila KJ, Valtonen VV, Nieminen M, Huttunen JK. Dental infection and the risk of new coronary events: prospective study of patients with documented coronary artery disease. Clin Infect Dis. 1995;20:588–92. doi: 10.1093/clinids/20.3.588. [DOI] [PubMed] [Google Scholar]
- Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67:1123–37. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- Madianos PN, Bobetsis GA, Kinane DF. Is periodontitis associated with an increased risk of coronary heart disease and preterm and/or low birth weight births? J Clin Periodontol. 2002;29(Suppl. 3):22–36. doi: 10.1034/j.1600-051x.29.s3.2.x. [DOI] [PubMed] [Google Scholar]
- Xu Q, Willeit J, Marosi M, et al. Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis. Lancet. 1993;341:255–9. doi: 10.1016/0140-6736(93)92613-x. [DOI] [PubMed] [Google Scholar]
- Kol A, Sukhova GK, Lichtman AH, Libby P. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-α and matrix metalloproteinase expression. Circulation. 1998;98:300–7. doi: 10.1161/01.cir.98.4.300. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, Kundsin R, Shih J. Baseline IgG antibody titers to Chlamydia pneumoniae, Helicobacter pylori, herpes simplex virus, and cytomegalovirus and the risk for cardiovascular disease in women. Ann Intern Med. 1999;131:573–7. doi: 10.7326/0003-4819-131-8-199910190-00004. [DOI] [PubMed] [Google Scholar]
- Roivainen M, Viik-Kajander M, Palosuo T, et al. Infections, inflammation, and the risk of coronary heart disease. Circulation. 2000;101:252–7. doi: 10.1161/01.cir.101.3.252. [DOI] [PubMed] [Google Scholar]
- O'Leary TJ, Drake RB, Naylor JE. The plaque control record. J Periodontol. 1972;43:38. doi: 10.1902/jop.1972.43.1.38. [DOI] [PubMed] [Google Scholar]
- Schei O, Waerhaug J, Lovdal A, Arno A. Alveolar bone loss as related to oral hygiene and age. J Periodontol. 1959;30:7–16. [Google Scholar]
- Tabeta K, Yoshie H, Yamazaki K. Characterization of serum antibody to Actinobacillus actinomycetemcomitans GroEL-like protein in periodontitis patients and healthy subjects. Oral Microbiol Immunol. 2001;16:290–5. doi: 10.1034/j.1399-302x.2001.016005290.x. [DOI] [PubMed] [Google Scholar]
- Boog CJP, de Graeff-Meeder ER, Lucassen MA, et al. Two monoclonal antibodies generated against human hsp60 show reactivity with synovial membranes of patients with juvenile chronic arthritis. J Exp Med. 1992;175:1805–10. doi: 10.1084/jem.175.6.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moony J, Adonogianaki E, Riggio MP, Takahashi K, Haerian A, Kinane DF. Initial serum antibody titer to Porphyromonas gingivalis influences development of antibody avidity and success of therapy for chronic periodontitis. Infect Immun. 1995;63:3411–6. doi: 10.1128/iai.63.9.3411-3416.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana FJ, Carmi P, Mor F, Cohen IR. DNA fragments of the human 60-kDa heat shock protein (HSP60) vaccinate against adjuvant arthritis: identification of a regulatory HSP60 peptide. J Immunol. 2003;171:3533–41. doi: 10.4049/jimmunol.171.7.3533. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Bulmer J, Hanks BM, Wright BH. Identification of human heat shock protein 60 (Hsp60) and anti-Hsp60 antibodies in the peripheral circulation of normal individuals. Cell Stress Chaperones. 1999;4:29–35. doi: 10.1054/csac.1998.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Schett G, Perschinka H, et al. Serum soluble heat shock protein 60 is elevated in subjects with atherosclerosis in a general population. Circulation. 2000;102:14–20. doi: 10.1161/01.cir.102.1.14. [DOI] [PubMed] [Google Scholar]
- Takamatsu N, Yano K, He T, Umeda M, Ishikawa I. Effect of initial periodontal therapy on the frequency of detecting Bacteroides forsythus, Porphyromonas gingivalis, and Actinobacillus actinomycetemcomitans. J Periodontol. 1999;70:574–80. doi: 10.1902/jop.1999.70.6.574. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Suzuki N, Nakano Y, Shibuya K, Ogawa Y, Koga T. Distribution of Actinobacillus actinomycetemcomitans serotypes and Porphyromonas gingivalis in Japanese adults. Oral Microbiol Immunol. 2003;18:135–9. doi: 10.1034/j.1399-302x.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- Beck JD, Pankow J, Tyroler HA, Offenbacher S. Dental infection and atherosclerosis. Am Heart J. 1999;138:S528–S33. doi: 10.1016/s0002-8703(99)70293-0. [DOI] [PubMed] [Google Scholar]
- Pussinen PJ, Jousilahti P, Alfthan G, Palosuo T, Asikainen S, Salomaa V. Antibodies to periodontal pathogens are associated with coronary heart disease. Arterioscler Thromb Vasc Biol. 2003;23:1250–4. doi: 10.1161/01.ATV.0000072969.71452.87. [DOI] [PubMed] [Google Scholar]
- Pockley AG. Heat shock proteins, inflammation, and cardiovascular disease. Circulation. 2002;105:1012–7. doi: 10.1161/hc0802.103729. [DOI] [PubMed] [Google Scholar]