Abstract
We found previously that immunosuppressive macrophages (Mφs) induced by Mycobacterium intracellulare infection (MI-Mφs) transmitted their suppressor signals to target T cells through cell contact with target T cells. In this study, we examined what kinds of Mφ surface molecules are required for such cell–to–cell interaction. First, it was found that a B7-1-like molecule (B7–1LM) recognizable with one of three test clones of anti-B7-1 monoclonal antibodies (mAbs) was required for expression of the Mφ suppressor activity. Neither anti-B7-2, anti-ICAM-1, nor anti-VCAM-1 mAb blocked the Mφ suppressor activity. Second, MI-Mφs increased the expression of B7–1LM in parallel with the acquisition of the suppressor activity. Moreover, MI-Mφs bound with target T cells in a B7–1LM-dependent fashion. Third, mAb blocking of CTLA-4 on target T cells did not reduce the suppressor activity of MI-Mφs, suggesting the role of a putative molecule on target T cells other than CTLA-4 as the receptor for B7–1LM of MI-Mφs. Fourth, concanavalin A (Con A) stimulation of MI-Mφs was needed for effective cell contact with target T cells and subsequent expression of the suppressor activity of MI-Mφs. Fifth, the Con A-induced increase in the suppressor activity of MI-Mφs was inhibited by KN-62 but not by herbimycin A, H-7, nor H-88, indicating that Con A-induced up-regulation of MI-Mφ function is mediated by calmodulin-dependent protein kinase II or ATP/P2Z receptors, but independent of protein tyrosine kinase, protein kinase C, and protein kinase A. These findings indicate that a B7/CTLA-4-independent mechanism is needed for the transmission of the suppressor signals from MI-Mφs to target T cells.
Keywords: suppressor macrophage, T cell mitogenesis, B7-1 molecules, cell contact, Mycobacterium intracellulare
INTRODUCTION
Intractable mycobacterioses including multidrug resistant tuberculosis and Mycobacterium avium complex (MAC) infections are frequently encountered in AIDS patients [1,2]. During the course of mycobacterioses in humans and experimental animals, generation of immunosuppressive macrophages (Mφs) is frequently encountered [3,4]. These Mφs suppress T cell functions, including proliferative response and Th1 cytokine production responding to T cell receptor ligation, causing suppression of cellular immunity in the advanced stages of infection [5–7]. Previously, we found that immunosuppressive Mφs were induced in the spleens of M. intracellulare-infected mice and that such Mφ populations (designated MI-Mφs) displayed potent suppressive activity against Con A-induced mitogenesis of splenic T cells [8,9]. The suppressor activity of the MI-Mφs was mediated by humoral mediators including reactive nitrogen intermediates, transforming growth factor-β, prostaglandin E2, free fatty acids, which were produced by MI-Mφs themselves in response to Con A stimulation [10–12].
Recently, we found that cell contact of MI-Mφs with target T cells is required for efficacious manifestation of the suppressor activity of MI-Mφs [12,13]. The suppressor signals of MI-Mφs, which are transmitted to the target T cells via cell contact, principally cross-talk with the early signalling events before the activation of protein kinase C and/or intracellular calcium mobilization [13]. In the present study, we examined which kinds of Mφ cell surface molecules are responsible for the cell–to–cell interaction between MI-Mφs and target T cells. We found that a B7-1-like molecule (designated B7–1LM) on MI-Mφs plays important roles in the transmission of suppressor signals from MI-Mφs to target T cells through a cell–to–cell interaction.
MATERIALS AND METHODS
Microorganisms
M. intracellulare N-260 strain isolated from a patient with MAC infection was used.
Mice
Eight to 10-week-old male BALB/c (Japan Clea Co., Osaka, Japan) were used.
Special agents
Special agents used in this study were as follows: Con A (Sigma Chemical Co., St. Louis, MO, USA), genistein (Sigma), herbimycin A (Sigma), H-7 (Sigma), H-88 (Seikagaku Industry Co., Tokyo, Japan), KN-62 (Sigma), hamster anti-mouse B7-1 (CD80) monoclonal antibody (mAb) (clone 16–10A1) (Pharmingen Co., San Diego, CA, USA), rat anti-mouse B7-1 mAb (clone RMMP-1) (PBL Biomedical Laboratories, New Brunswick, NJ, USA), rat anti-mouse B7-1 mAb (clone 1G10) (Southern Biotechnology Associates, Inc., Birmingham, AL, USA), rat anti-mouse B7-2 (CD86) mAb (Pharmingen), hamster anti-mouse CTLA-4 mAb (Pharmingen), rat anti-mouse CD54 (ICAM-1) mAb (Seikagaku), rat anti-mouse CD106 (VCAM-1) mAb (Serotec Ltd, Oxford, UK), rat anti-mouse CD3 mAb (Serotec), alkaline phosphatase (ALP)-conjugated goat anti-hamster IgG antibody (Ab) (Southern Biotechnology Associates), ALP-conjugated mouse anti-rat IgG Ab (Jackson Immuno Research Laboratories, West Grove, PA, USA), rat IgG (Organon Teknika Corp., Durham, NC, UK), hamster IgG (Organon Teknika), horseradish peroxidase (HRP) conjugated-goat anti-hamster IgG mAb (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), HRP conjugated-goat anti-rat IgG mAb (Santa Cruz Biotechnology, Inc.) and [3H] thymidine (3H-TdR) (NEN Life Science Products Inc., Boston, MA, USA),
Medium
RPMI 1640 medium supplemented with 25 mm HEPES, 2 mm glutamine, 100 µg/ml of streptomycin, 100 units/ml of penicillin G, 5 × 10−5 M 2-mercaptoethanol and 5% (v/v) heat-inactivated fetal bovine serum (FBS) was used for cell culture.
Suppressor activity of MI-Mφs
Spleen cells (SPCs) were harvested from mice infected intravenously with 1 × 108 CFUs of M. intracellulare at 2–3 weeks after infection and cultured in 0·2 ml of the medium in four wells each of flat-bottom 96 well microculture plates (Corning, NY, USA) at the cell densities of 5 × 105−2 × 106 cells/well at 37°C in a CO2 incubator (5% CO2-95% humidified air) for 2 h. The wells were vigorously rinsed with Hanks’ balanced salt solution containing 2% (v/v) FBS by pipetting and then 0·1 ml of the medium was poured onto the resulting wells. This procedure usually gave more than 90% pure Mφ monolayer cultures, with active pinocytic ability of neutral red and with phagocytic ability against latex particles, containing about 6 × 104 cells per culture well from 2 × 106 of M. intracellulare-induced SPCs (MI-SPCs). Then, 2·5 × 105 of normal SPCs in 0·2 ml of the medium containing 2 µg/ml Con A were poured onto the resultant Mφ cultures. SPCs were then cultivated at 37°C in a CO2 incubator for 72 h and pulsed with 0·5 µCi of 3H-TdR (2 Ci/mmol) for the final 6–8 h. Cells were harvested onto glass fibre filters and counted for radioactivity using a 1450 Microbeta Trilux scintillation spectrometer (Wallac Co., Turku, Finland). Suppressor activity of MI-Mφs was calculated as:
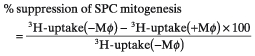 |
Western blotting
SPCs (4 × 107) harvested from normal or MI-infected mice in 8 ml of 5% FBS-RPMI1640 were cultured at 37°C for 2 h in an 90-mm plastic culture dish which precoated with FBS. After rinsing with 2% FBS-HBSS (six times), adherent cells were gently scraped off using rubber policemen into 20% FBS-HBSS and collected by subsequent centrifugation at 250 × g for 5 min. The obtained Mφs were suspended in an appropriate volume (1 × 107 cells/ml) of cell lysis buffer which consist of 50 mm Tris-HCl (pH 7·4) containing 150 mm NaCl, 1% Triton X-100, 1% (v/v) sodium deoxycholate, 0·1% (w/v) sodium dodecyl sulphate (SDS), 1 mm Na3VO4, 1 mm phenylmethanesulphonyl fluoride (PMSF) and 5% (v/v) protease inhibitor cocktail (SIGMA). Samples were centrifuged at 22 000 × g to remove insoluble matter. Equal volume of cell lysate were subjected to SDS – 10% polyacrylamide gel electrophoresis, and transferred to ImmobilonPSQ PVDF membranes (Millipore Corp. Bedford, MA, USA). Membranes were first incubated for over night at 4°C in 1% bovine serum albumin (BSA) in TBST buffer which consist of 10 mm Tris-HCl (pH 7·5) containing 100 mm NaCl and 0·1% Tween 20. The blot was then incubated with anti-B7-1 mAb (clone 1G10 or 16–10A1), diluted in 1% BSA-TBST buffer (1 : 500 dilution) for 4 h at room temperature. After rinsing, membranes were exposed to a HRP-conjugated anti-rat IgG mAb or HRP-conjugated anti-hamster IgG mAb which diluted in 1% BSA-TBST buffer (1 : 7500 dilution) for 2 h at room temperature. After rinsing, membranes were incubated in ECL plus (Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA) for 5 min, and then exposed to X-ray film until a signal was detected.
B7-1 expression on MI-Mφs
B7-1 expression on MI-Mφs was measured by ELISA and flow cytometry as follows.
ELISA
MI-Mφs (5 × 104−2 × 105 cells) were cultured in four wells each of flat-bottom 96 well microculture plates (Corning) at 37°C for 1 h, washed with HBSS, fixed with 0·5% (v/v) glutaraldehyde, and washed again with phosphate-buffered saline (PBS). After blocking with PBS containing 1% (w/v) BSA for over night and subsequent washing with 0·05% (v/v) Tween 20-PBS, the resultant cells were stained with hamster anti-mouse B7-1 mAb (clone 16–10A1) at a concentration of 1 : 200 at room temperature for 2 h, and washed again with 0·1% BSA-PBS. The resultant Mφs were further stained with ALP-conjugated goat anti-hamster IgG Ab for 2 h and washed with 0·1% BSA-PBS. Colour development was achieved by using p-nitrophenyl phosphate (p-NPP) tablets (Sigma) as the substrate.
Flow cytometric analysis
MI-SPCs (4 × 107) suspended in 8 ml of the culture medium were incubated in FBS-coated 90-mm cell culture dish at 37°C for 2 h. After washing with 2% FBS-HBSS, adherent cells were scraped off using a rubber policemen and collected into polypropylene tube (17 × 100 mm) by subsequent centrifugation. After washing with 1% BSA-PBS by centrifugation, the resultant Mφs (>90% pure) (2 × 106) were subjected blocking with 1% BSA-PBS containing 10% (v/v) inactivated BALB/c mouse serum at 0°C for 30 min and then washed with 1% BSA-PBS. The resultant cells were reacted with FITC-conjugated hamster anti-mouse B7-1 mAb (clone 16–10A1) at a concentration of 1 : 500 at 0°C for 3 h, washed with 1% BSA-PBS and thereafter with PBS, and fixed with 1% paraformaldehyde in PBS (pH 7·2) for over night. The resulting Mφ cells were subjected to flow cytometry using FACStar (Becton Dickinson, Mountain View, CA, USA).
Assay for binding of MI-Mφs with T cells
The monolayer cultures of MI-Mφs prepared by seeding 4 × 106 of MI-SPCs on 16-mm culture wells (Corning) were preincubated in the medium containing 2 µg/ml of Con A at 37°C for 4 h. After the addition of anti-B7-1 mAb (final 20 or 50 µg/ml), 1·25 × 106 of nylon wool column-purified splenic T cells suspended in the medium free from Con A were added, subjected to brief centrifugation, and allowed to bind to MI-Mφs by cultivating at 37°C for 6 h. After gentle washing with PBS to remove T cells nonadherent to MI-Mφs and subsequent fixation with 0·1% glutaraldehyde followed by rinsing with PBS, the resultant wells were subjected to blocking with 1% BSA-PBS for 3 h. Then, T cells which were binding to MI-Mφs on the wells were stained with rat anti-mouse CD3 mAb at 37°C for 1 h, washed with 0·1% BSA-PBS, and further stained with ALP-conjugated mouse anti-rat IgG Ab at 37°C for 1 h. After rinsing with 0·1% BSA-PBS, colour development was achieved by using p-NPP tablets as the substrate.
Statistical analysis
Statistical analysis was performed using Bonferroni's multiple t-test.
RESULTS
B7-1-mediated expression of the suppressor activity by MI-Mφs
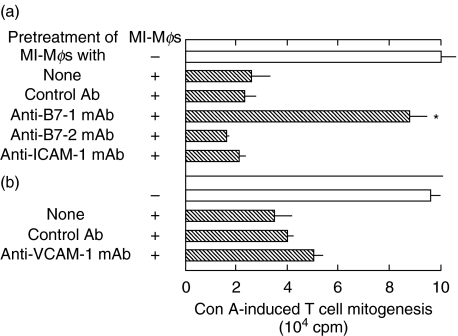
Figure 1 shows the effects of anti-B7-1 (clone 16–10A1), anti-B7-2, anti-ICAM-1, and anti-VCAM-1 mAbs on the suppression by MI-Mφs of SPC mitogenesis. It was found that anti-B7-1 mAb (clone 16–10A1) markedly reduced the suppressor activity of MI-Mφs (P < 0·01), whereas the other mAbs did not. Anti-VCAM-1 mAb slightly decreased the suppressor activity of MI-Mφs. Notably, in separate experiments indicated that the two different clones of anti-B7-1 mAbs (clones RMMP-1 and 1G10) failed to display such significant efficacies in blocking the suppressor activity of MI-Mφs as in the case of the anti-B7-1 mAb (clone 16–10A1) (data not shown). These findings suggest that a B7-1-like molecule (B7–1LM), which shared in part the same epitopes with B7-1 molecules, is required for expression of the suppressor activity of MI-Mφs through cell-to-cell contact with target T cells. This concept is supported by the following findings.
Fig. 1.
Blocking of the suppressor activity of M. intracellulare-induced macrophages (MI-Mφs) with anti-B7-1 mAb. MI-Mφs on microculture wells were pretreated with (a) the indicated mAb anti-B7-1 mAb (clone no. 16–10A1), anti-B7-2 mAb, anti-ICAM-1 mAb, or control Ab (hamster IgG) at 50 µg/ml each or (b) treated with 25 µg/ml of anti-VCAM-1 mAb, or control Ab (rat IgG) at 25 µg/ml each for 2 h. After washing with 2% FBS-HBSS, SPCs were added onto the resultant Mφs (4 × 104/well) and measured for their Con A mitogenic response. Each bar indicates the mean ± SEM (n = 4). *Significantly reduced compared to the control value (+control Ab) (P < 0·01). Results are representative of five independent experiments.
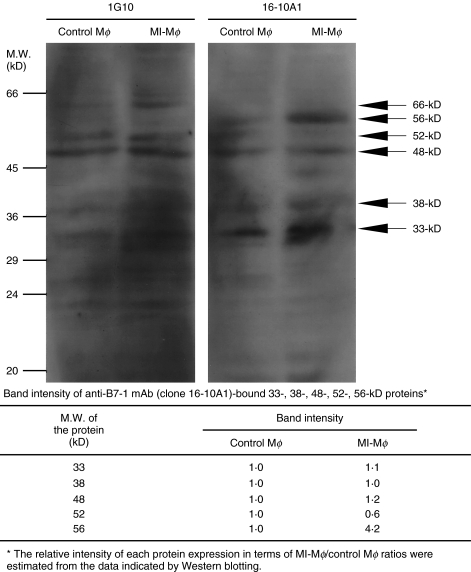
As shown in Fig. 2, Western blotting experiments using the two clones of anti-B7-1 mAb (1G10, 16–10A1) revealed that clone 1G10 mAb bound to 33-kD, 38-kD, 48-kD, 52-kD and 62-kD proteins in MI-Mφ cell lysate. The multiplicity of detected bands is not enigmatic, because B7-1 protein is expressed in various molecular weights due to differential glycosylation and mRNA splicing among various types of cells [14–16]. For instance, it has been reported that 1G10 mAb binds to a 46- and 52-kD proteins in the cell lysates of B cell lineages [15]. As shown in Fig. 2, 16–10A1 mAb bound to 33-, 38-, 48-, 52- and 56-kD proteins in MI-Mφ cell lysate. Notably, Western blotting experiment indicated that 16–10A1 mAb specifically bound to 56-kD protein. In addition, the expression of 56-kD protein was markedly increased in MI-Mφs compared to the control Mφs. On the contrary, the expression of 33-, 38-, 48- and 52-kD proteins did not increase in MI-Mφs compared to that in control Mφs. These findings indicate that the 56-kD protein recognized by 16–10A1 mAb corresponds to B7–1LM protein.
Fig. 2.
Profiles of B7-1 protein expression in M. intracellulare-induced macrophages (MI-Mφs). MI-Mφ cell lysate was analysed by Western blotting using anti-B7-1 mAbs (clone 1G10 and 16–10A1). Cell lysate of control Mφs was prepared from splenic Mφs without MI infection. In the bottom table, band intensities of the 33-, 38-, 48-, 52- and 56-kD proteins which are recognized by the 16–10A1 mAb using densitometer are indicated.
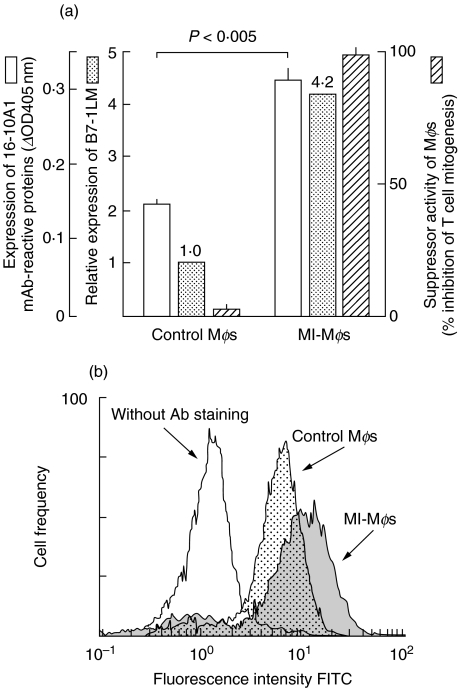
Figure 3a shows the relationship between Mφ suppressor activity and the extent of Mφ expression of whole B7-1 (B7-1 and related proteins) and B7–1LM. The level of expression of whole B7-1 and particularly B7–1LM were increased in MI-Mφs than in control Mφs, and this increase clearly paralleled with the increase in their suppressor activity. Furthermore, when the level of whole B7-1 expression by MI-Mφs was determined by flow cytometric analysis, significantly increased whole B7-1-highly positive cell populations were detected in MI-Mφs (Fig. 3b). The mean fluorescence intensity of MI-Mφs (11·7) was almost double that of control Mφs (6·5). These findings confirm the concept that the suppressor activity of MI-Mφs is associated with the increase in their B7–1LM expression.
Fig. 3.
Expression of whole B7-1 and B7–1LM by M. intracellulare-induced macrophages (MI-Mφs). (a) MI-Mφs or control Mφs (splenic Mφs from normal mice) were measured for the expression of whole B7-1 and B7–1LM by ELISA and Western blotting analysis, respectively, using 16–10A1 mAb (□ and  , respectively) and suppressor activities in terms of percentage inhibition of Con A-induced SPC mitogenesis (
, respectively) and suppressor activities in terms of percentage inhibition of Con A-induced SPC mitogenesis ( ). Each bar indicates the mean ± SEM (n = 4). Results are representative of three or four independent experiments. (b) Whole-B7-1 expression on MI-Mφs (
). Each bar indicates the mean ± SEM (n = 4). Results are representative of three or four independent experiments. (b) Whole-B7-1 expression on MI-Mφs ( ) and control Mφs (
) and control Mφs ( ) were measured by flowcytometric analysis using FITC-conjugated anti-B7-1 mAb (clone no. 16–10A1). Open histogram indicates the basal level of fluorescence on MI-Mφs without anti-B7-1 mAb staining. Results are representative of two independent experiments.
) were measured by flowcytometric analysis using FITC-conjugated anti-B7-1 mAb (clone no. 16–10A1). Open histogram indicates the basal level of fluorescence on MI-Mφs without anti-B7-1 mAb staining. Results are representative of two independent experiments.
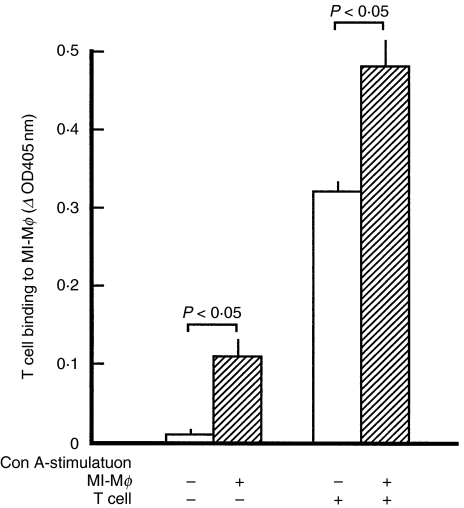
Profiles of B7–1LM-dependent cell contact between MI-Mφs and target T cells
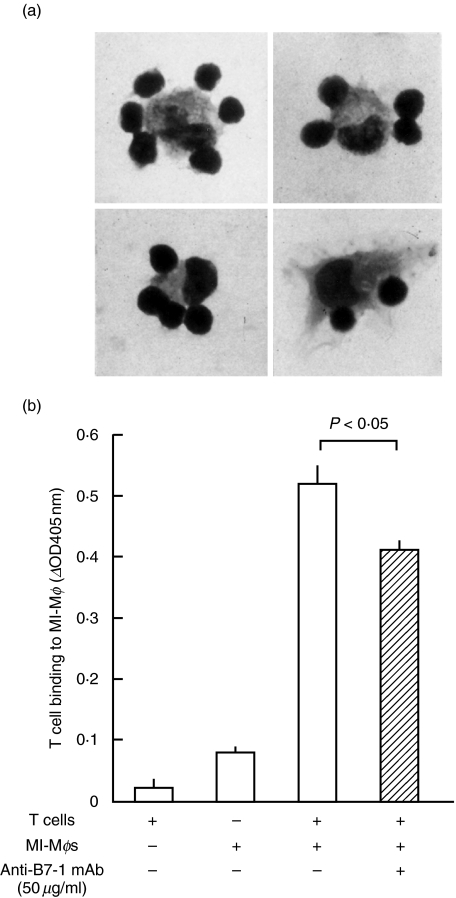
Next, we examined profiles of the binding of MI-Mφs with target T cells. As shown in Fig. 4a, the binding of splenic T cells to MI-Mφs (cluster formation of T cells around MI-Mφs) was observed by microscopy, when MI-Mφs had been stimulated with Con A for 4 h prior to cocultivation with T cells. In this case, the average number of T cells binding to MI-Mφs was 2·7 ± 0·2 (range 0–8). As indicated in Fig. 4b. T cell binding to MI-Mφs was significantly inhibited by the addition of the anti-B7-1 mAb (clone 16–10A1) in a dose-dependent manner. In this case, the inhibition rate due to the treatment with 50 µg/ml of the anti-B7-1 mAb was 25·8 ± 2·8% (n = 3) (P < 0·05). These findings indicate that MI-Mφs are capable of binding to target T cells through B7–1LM.
Fig. 4.
T cell-binding ability of M. intracellulare-induced macrophages (MI-Mφs). (a) Cluster formation of MI-Mφs and target T cells. MI-Mφs are tightly surrounded by T cells. MI-Mφs were stimulated with Con A for 2 h and then co-cultured with SPCs for 3 h. After gentle rinsing, the culture wells were fixed with glutaraldehyde and subjected to Giemsa staining. Representative aspects are shown. (b) MI-Mφs were stimulated with Con A for 4 h, then cocultured with purified splenic T cells in the presence or absence of anti-B7-1 Ab (clone no. 16–10A1) at 50 µg/ml for 6 h, and measured for T cell binding ability by ELISA as described in ‘Materials and methods’. Each bar indicates the mean ± SEM (n = 3). Results are representative of three independent experiments.
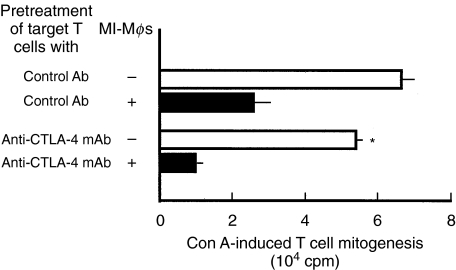
Receptor on target T cells for B7–1LM
It is known that B7 molecule-mediated suppressor signals are transmitted to T cells through B7/CTLA−4 interaction [17,18]. It is thus of interest to examine whether or not MI-Mφ derived suppressor signals are transmitted to target T cells through an interaction between B7–1LM (MI-Mφs) and CTLA-4 (T cells). As shown in Fig. 5, pretreatment of target T cells with anti-CTLA-4 mAb failed to block the expression of MI-Mφ suppressor activity. Moreover, in separate experiments, CTLA-4 Ig was incapable of blocking the suppressor activity of MI-Mφs even when added at 20 µg/ml (data not shown). Therefore, the possibility is excluded that CTLA-4 acts as a B7–1LM receptor on target T cells and plays crucial roles in the transmission of MI-Mφ suppressor signals through the interaction with B7–1LM. It thus appears that there exists unknown receptor(s) on for B7–1LM on target T cells other than CTLA-4.
Fig. 5.
Failure of treatments of target splenic T cells with anti-CTLA-4 mAbs in blocking the suppressor activity of M. intracellulare-induced macrophages (MI-Mφs). SPCs were pretreated with the indicated mAb (100 µg/ml each) for 2 h. After washing with 2% FBS-HBSS, the resultant SPCs were co-cultured with MI-Mφs (2 × 104/well) and measured for their Con A mitogenic response. Each bar indicates the mean ± SEM (n = 4). *Significantly different from the value of T cells treated with control Ab (hamster IgG) (P < 0·05). Results are representative of four independent experiments.
Profiles of Con A stimulation of MI-Mφs required for cell contact with target T cells
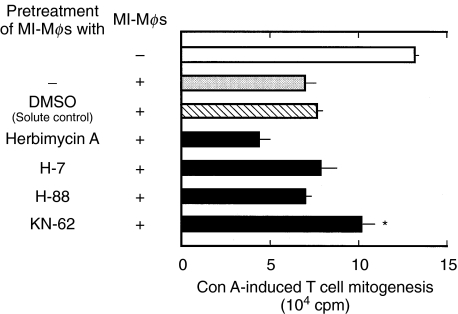
As shown in Fig. 6, binding of MI-Mφs with target T cells was markedly enhanced when MI-Mφs had been stimulated with Con A. It thus appears that Con A stimulation of MI-Mφs is required for the effective cell contact between MI-Mφs and target T cells. In this context, we previously found that Con A stimulation of MI-Mφs is required for the effective expression of their suppressor activity, since TNF-α and IFN-γ produced by Con A stimulated MI-Mφs augment the Mφ suppressor activity in an autocrine fashion [11]. Figure 7 shows the effects of some metabolic inhibitors on the suppressor activity of MI-Mφs. A panel of metabolic inhibitors was used in attempt to identify the signalling pathways responsible for Mφ Con A-stimulation. These included herbimycin A (an inhibitor of protein tyrosine kinase (PTK)), H-7 (an inhibitor of protein kinase C (PKC)), H-88 (an inhibitor of protein kinase A (PKA)) and KN-62 (an inhibitor of Ca2+/calmodulin-dependent protein kinase II (CaMKII)). MI-Mφs were pretreated with them at the concentration of 10 µm, which can inhibit these protein kinases without exhibiting cytotoxicity against Mφs [19]. As shown in Fig. 7, KN-62 partly but significantly diminished expression of the suppressor activity by Con A-stimulated MI-Mφs (P < 0·05). In contrast, the other protein kinase inhibitors did not exhibit such an inhibitory effect. It thus appears that CaMKII may play important roles in signalling pathways of Con A-stimulated MI-Mφs to exhibit their suppressor activity.
Fig. 6.
Enhancement of binding of M. intracellulare-induced macrophages (MI-Mφs) with target T cells by Con A-stimulation. MI-Mφs were stimulated with Con A at 2 µg/ml for 4 h, then co-cultured with purified splenic T cells in the presence or absence of Con A at 2 µg/ml for 16 h, and measured for T cell binding ability by ELISA as described in ‘Materials and methods’. Each bar indicates the mean ± SEM (n = 3).
Fig. 7.
Effects of inhibitors of various types of protein kinases (PTK, PKC, PKA, and CaMKII) on the expression of the suppressor activity by Con A-stimulated M. intracellulare-induced macrophages (MI-Mφs). MI-Mφs were pretreated with indicated inhibitors at 10 µg/ml at 37°C for 2 h. After washing with 2% FBS-HBSS, the resultant MI-Mφs were cocultured with SPCs (2·5 × 105) in the presence of 2 µg/ml of Con A in order to measure their suppressor activity. Each bar indicates the mean ± SEM (n = 4). *Significantly greater than the value of the solute control [MI-Mφs were pretreated with 0·1% dimethyl sulphoxide (DMSO)] (P < 0·05). Results are representative of four independent experiments.
DISCUSSION
In the present study, we examined which kinds of surface molecules on MI-Mφs are responsible for cell–to–cell interaction between MI-Mφs and target T cells, and the following findings were obtained. First, MI-Mφ suppressor activity was blocked by an anti-B7-1 mAb but not by anti-B7-2, anti-ICAM-1, nor anti-VCAM-1 mAb. Notably, only one (clone 16–10A1) of the three test clones of anti-B7-1 mAbs was capable of blocking expression of the suppressor activity by MI-Mφs. These findings suggest that transmission of the suppressor signals from MI-Mφs to target T cells via cell contact was dependent on a novel B7-1-like molecule (B7–1LM), which shares in part the same epitope with B7-1. This concept is further supported by the following findings. First, only 16–10A1 mAb binds 56-kD protein, the expression of which is markedly increased in MI-Mφs. Second, the expression of B7–1LM on MI-Mφs was correlated with their suppressor activity. Third, cell-to-cell binding of MI-Mφs with target T cells was inhibited by the anti-B7-1 mAb (clone 16–10A1). Fourth, the mAb blocking of CTLA-4 molecules on target T cells did not attenuate the MI-Mφ suppressor activity, indicating that CTLA-4 does not act as a B7–1LM receptor, and that MI-Mφ-derived suppressor signals are transmitted to target T cells through the interaction of B7–1LM with unknown receptor(s) on T cells other than CTLA-4. Separate experiments indicated that CD28 also does not act as a B7–1LM receptor (data not shown). As shown in Fig. 7, neither herbimycin A, H-7, nor H-88 exerted inhibitory effects against the expression of the suppressor action by MI-Mφs in response to Con A stimulation. The negative results obtained with these metabolic inhibitors suggest that Con A signal-associated expression of the suppressor activity by MI-Mφs does not involve signalling pathways which are mediated by PTK, PKC, or PKA. On the other hand, the CaMKII inhibitor KN-62 partially attenuated the suppressor activity of MI-Mφs, indicating that CaMKII-mediated signalling events may play important roles in the activation of MI-Mφs to exhibit their suppressor activity in response to Con A signals.
In this context, it is noteworthy that KN-62 has an inhibitory activity against ATP/P2Z (P2X7) receptors [19,20]. Recently, it has been reported that ATP-induced stimulation of P2Z receptors on Mφs is associated with marked increase in the activity of phospholipase D, causing potentiation of Mφ antimycobacterial activity [21–23]. Notably, it has been reported that ATP-induced microbicidal activity of Mφs is attenuated by KN-62 but not by inhibitors of PTK, PKC, and adenylate cyclase [19]. Therefore, it is possible that ATP/P2Z interaction on MI-Mφs is needed for efficacious expression of their suppressor activity against target T cells.
In summary, the present study indicated that suppressive signals from MI-Mφs are transmitted to target T cells through cell contact between a novel B7–1LM on MI-Mφs and certain receptor(s) on target T cells other than CTLA-4. Further studies are currently underway to identify the B7–1LM.
Acknowledgments
This study was supported in part by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
REFERENCES
- Hart CA, Beeching NJ, Duerden BI. Tuberculosis into the next centry. J Med Microbiol. 1996;44:1–34. [Google Scholar]
- Falkinham JO. Epidemiology of infection by nontuberculous mycobacteria. Clin Microbiol Rev. 1996;9:177–215. doi: 10.1128/cmr.9.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner JJ. Suppressor adherent cells in human tuberculosis. J Immunol. 1978;121:2573–9. [PubMed] [Google Scholar]
- Edwards CK, Hedegaar HB, Zlotnik A, et al. Chronic infection due to Mycobacterium intracellulare in mice: association with macrophage release of prostaglandin E2 and reversal by injection of indomethacin, muramyl dipeptide, and interferon-γ. J Immunol. 1986;136:1820–7. [PubMed] [Google Scholar]
- Turcotte R, Lemieux S. Mechanisms of action of Mycobacterium bovis BCG-induced suppressor cells in mitogen induced blastogenesis. Infect Immun. 1982;36:263–70. doi: 10.1128/iai.36.1.263-270.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D, Kirkpatrik CH. The immunology of mycobacterial diseases. Am Rev Respir Dis. 1986;134:1062–71. doi: 10.1164/arrd.1986.134.5.1062. [DOI] [PubMed] [Google Scholar]
- VanHeyningen TK, Collin HL, Russell DG. IL-6 produced by macrophages infected with Mycobacterium species suppresses T cell responses. J Immunol. 1997;158:330–7. [PubMed] [Google Scholar]
- Tomioka H, Saito H, Yamada Y. Characteristics of immunosuppressive macrophages induced in spleen cells by Mycobacterium avium complex infections in mice. J General Microbiol. 1990;136:965–74. doi: 10.1099/00221287-136-5-965. [DOI] [PubMed] [Google Scholar]
- Tomioka H, Saito H. Characterization of immunosuppressive functions of murine peritoneal macrophages induced with various agents. J Leukoc Biol. 1992;51:24–31. doi: 10.1002/jlb.51.1.24. [DOI] [PubMed] [Google Scholar]
- Tomioka H, Sato K, Maw WW, et al. The role of tumor necrosis factor, interferon-γ, transforming growth factor-β, and nitric oxide in the expression of immunosuppressive functions of macrophages induced by Mycobacterium avium complex infection. J Leukoc Biol. 1995;58:704–12. doi: 10.1002/jlb.58.6.704. [DOI] [PubMed] [Google Scholar]
- Tomioka H, Kishimoto T, Maw WW. Phospholipids and reactive nitrogen intermediates collaborate in expression of the T cell mitogenesis inhibitory activity of immunosuppressive macrophages induced in mycobacterial infection. Clin Exp Immunol. 1996;103:219–25. doi: 10.1046/j.1365-2249.1996.d01-614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maw WW, Shimizu T, Sato K, et al. Further study on the roles of the effector molecules on immunosuppressive macrophages induced by mycobacterial infection in expression of their suppressor function against mitogen-stimulated T cell proliferation. Clin Exp Immunol. 1997;108:26–33. doi: 10.1046/j.1365-2249.1997.d01-980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara K, Tomioka H, Shimizu T, et al. Profiles of cell–to–cell interaction of Mycobacterium intracellulare-induced immunosuppressive macrophages with target T cells in terms of suppressor signal transmission. Clin Exp Immunol. 2002;129:272–80. doi: 10.1046/j.1365-2249.2002.01890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman GJ, Gray GS, Gimmi CD, et al. Structure, expression, and T cell costimulatory activity of the murine homologue of the human B lymphocyte activation antigen B7. J Exp Med. 1991;174:625–31. doi: 10.1084/jem.174.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Faherty DA, Gault A, et al. Monoclonal antibody 2D10 recognizes a novel T cell costimulatory molecule on activated murine B lymphocytes. J Immunol. 1994;152:2105–14. [PubMed] [Google Scholar]
- Inobe M, Linsley PS, Ledbetter JA, et al. Identification of an alternatively spliced form of the murine homologue of B7. Biochem Biophys Res Commun. 1994;200:443–9. doi: 10.1006/bbrc.1994.1469. [DOI] [PubMed] [Google Scholar]
- Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–13. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Tivol EA, Boyd SD, McKeon S, et al. CTLA4 Ig prevents lymphoproliferation and fatal multiorgan tissue destruction in CTLA-4-deficient mice. J Immunol. 1997;158:5091–4. [PubMed] [Google Scholar]
- Lammas DA, Stober C, Harvey CJ, et al. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z (P2X7) receptors. Immunity. 1997;7:433–44. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- Humphreys BD, Dubyak GR. Induction of the P2Z/P2X7 nucleotide receptor and associated phospholipase D activity by lipopolyshaccharide and IFN-γ in the human THP-1 monocytic cell line. J Immunol. 1996;157:5627–37. [PubMed] [Google Scholar]
- el-Moatassim C, Dubyak GR. A novel pathway for the activation of phospholipase D by P2Z purinergic receptors in BAC1.2F5 macrophages. J Biol Chem. 1992;267:23664–73. [PubMed] [Google Scholar]
- Exton JH. Phospholipase D. enzymology, mechanisms of regulation, and function. Physiol Rev. 1997;77:303–20. doi: 10.1152/physrev.1997.77.2.303. [DOI] [PubMed] [Google Scholar]
- Kusner DJ, Adams J. ATP-induced killing of virulent Mycobacterium tuberculosis within human macrophages requires phospholipase D. J Immunol. 2000;164:379–88. doi: 10.4049/jimmunol.164.1.379. [DOI] [PubMed] [Google Scholar]