Abstract
The acute phase of alopecia areata (AA) is characterized by an increase in CD44v3+ and CD44v10+ skin-infiltrating leucocytes (SkIL). Induction of a contact eczema, one of the therapeutic options in AA, can be mitigated strongly by a blockade of CD44v10. The observation that induction of a delayed type hypersensitivity (DTH) reaction abrogates an autoimmune reaction, where both responses apparently use similar effector mechanisms, is surprising and prompted us to search for the underlying mechanisms. AA-affected C3H/HeJ mice were treated with the contact sensitizer SADBE (squaric acid dibutylester) and leucocyte subpopulations and their activation state was evaluated in SkIL and draining lymph nodes. AA-affected mice exhibited an increased number of SkIL with a predominance of T lymphocytes. After treatment with the contact sensitizer SADBE recovery of SkIL was reduced and monocytes predominated. However, a significantly increased number of leucocytes was recovered from draining lymph nodes. Draining lymph node cells from untreated and treated AA mice exhibited all signs of recent activation with high-level expression of co-stimulatory and accessory molecules and an increased percentage of CD44v3+ and CD44v10+ leucocytes. In contrast, SkIL of SADBE-treated AA mice contained relatively few activated T cells and reduced numbers of CD44v3+ and CD44v10+ cells. Thus, the activation state and the distribution of leucocyte subsets in SADBE-treated AA mice are consistent with a blockade of leucocyte extravasation. Accordingly, the therapeutic effect of long-term SADBE treatment may rely on impaired leucocyte traffic.
Keywords: adhesion molecules, autoimmune disease, delayed type hypersensitivity, leucocyte recruitment
INTRODUCTION
Alopecia areata (AA) is an autoimmune disease of the hair follicles [1–3] that can be transferred by skin transplants as well as by CD4+ plus CD8+ T cells [4–7]. It has been shown that induction of AA is accompanied by major histocompatibility complex (MHC) class I and class II expression on epithelial cells [8–10] that leads to a loss of hair follicle immune privilege [11]. Like other autoimmune diseases, AA is treated typically with corticosteroids [12,13], but at present, treatment with a contact sensitizer is regarded to be the most effective for extensive scalp hair loss [14–18]. In particular, squaric acid dibutylester (SADBE) has been shown to induce hair regrowth very efficiently in an animal model of spontaneously developing AA [10,19]. The underlying mechanism has not yet been explored. We expected that induction of an eczema may possibly restore immune homeostasis by expansion of CD4+ CD25+ regulatory T cells (T-reg) or by restoration of activation-induced cell death (AICD), defects in T-reg [20–22] and in AICD [23–25] being associated frequently with autoimmune disorders, including AA [26].
As well as the possible involvement of regulatory T cells and AICD, there has been another aspect that appeared of particular interest. We had noted that CD44v10 is important for the development of granulomas and for dinitrofluorobenzene-induced delayed-type hypersensitivity (DTH) reactions [27]. In addition, induction of AA can be prevented by anti-CD44v10 [28], although anti-CD44v10 did not exert a therapeutic effect on overt AA. CD44v10 is expressed in the basal cell layer of the skin and of squamous epithelia [27,29]. On haematopoietic cells its expression is restricted mainly to monocytes [29,30]. Notably, it is the extravasation of monocytes into the sensitized skin in particular that could be prevented by anti-CD44v10 with high efficacy, i.e. CD44v10 is required mainly for the egress of monocytes [27]. It has been decribed that CD44, particularly CD44 variant isoforms including CD44v10 (CD44v), bind osteopontin ([31–33] and M. Zöller, unpublished). Osteopontin exerts chemotactic and haptotactic activity [34–36] and binding of osteopontin to CD44 supports inflammatory TH1 reactions [32,33].
Another CD44 variant isoform, CD44v3, is also known to bind chemokines, such as basic fibroblast growth factor, that facilitate leucocyte recruitment and extravasation [37–39]. CD44v3 is expressed by T cells, although at a low level, and by activated endothelial cells [40]. CD44v3 is the only CD44 variant isoform that is expressed by skin infiltrating lymphocytes (SkIL) and expression on SkIL is up-regulated significantly in most autoimmune diseases affecting the skin. Anti-CD44v3, similar to anti-CD44v10, inhibits the development of DTH reactions. Thus, CD44v10 and CD44v3 function apparently as homing receptors for SkIL, where CD44v10 probably affects predominantly monocyte egress and CD44v3 T cell infiltration [27,31,34,40,42,43].
The fact that both induction of a DTH reaction as well as induction of a skin-appendage autoimmune disease are associated with up-regulation of the same homing receptors, CD44v10 and CD44v3, and that antibody blockade prevents or mitigates both pathological reactions, suggests similar modes of action in each disease mechanism. However, the fact that one of these pathological reactions (AA) can be treated by induction of the other (eczema) [10,14,44] seems to contradict the apparently shared mechanism of leucocyte recruitment. Our study shows that the therapeutic effect of SADBE is unlikely due to restoration of immune homeostasis by expansion of CD4+ CD25+ T-reg or AICD. Instead, the chronic inflammatory reaction was accompanied by a a retention of CD44v10+ and CD44v3+ leucocytes in draining lymph nodes and an apparent impairment of activated leucocyte migration.
MATERIALS AND METHODS
Mice and treatment
All C3H/HeJ mice were supplied from stocks at the Jackson Laboratory (Bar Harbor, ME, USA), a specific pathogen-free production facility. Mice received conventional low soy oil diet (altromin 1434, Altromin GmBH, Lage, Germany) and acidified water (pH 2·8–3·0) ad libitum. Five mice developed AA spontaneously and in 12 mice AA was induced by grafting lesional skin from AA-affected mice [4]. These 17 mice, which displayed total hair loss at the age of 11–20 months, were treated with the contact sensitizer SADBE [10]. A 1·0 × 1·0 cm area on the left dorsal skin was treated with an initial application of 2% SADBE in acetone followed by weekly application of 0·5% or 1% SADBE in acetone over the entire left dorsal and ventral side of the body. The concentration was chosen such that a moderately severe contact eczema was induced, which persisted for 2–3 days post-application. Treatment was terminated after 8–12 weeks, when hair regrowth was observed on the treated skin. All mice showed complete hair regrowth on the treated side, whereas no hair regrowth was observed on the right, untreated side.
Tissue preparation
Mice were killed by cervical dislocation and skin was collected from AA-affected and control mice. From SADBE-treated mice only the treated half showing hair regrowth was collected. Dorsal skin samples were fixed in Fekete's acid–alcohol–formalin solution and paraffin-embedded. For the isolation of SkIL, fat and subcutaneous muscle tissue was removed and the skin was layered epidermis uppermost on sterile gauze. Skin was then incubated three times for 30 min with a 1 mg/ml trypsin (Sigma, Deisenhofen, Germany)/EDTA solution. After each incubation, the skin was pressed against the sterile gauze and the isolated cells were collected in RPMI-1640 medium containing 10% fetal calf serum (Sigma, Deisenhofen, Germany). After the final trypsin treatment cells were pooled, washed and resuspended in RPMI-1640 containing 10% fetal calf serum plus 10−3m HEPES buffer. The cells were incubated at 37°C, 5% CO2 in a humidified atmosphere for 2 h for the recovery of cell surface molecule expression. For the analysis of T lymphocyte subsets, SkIL from five animals were pooled and SkIL were enriched further by Ficoll-Hypaque gradient centrifugation. Viability was determined by Trypan blue exclusion and was in the range of 70% in SkIL preparations. After Ficoll-Hypaque centrifugation > 95% of cells were viable.
All skin-draining lymph nodes were collected from controls and from AA-affected mice. From SADBE-treated mice, lymph nodes were collected only from the treated side. Single leucocyte cell suspensions were prepared by pressing lymph nodes through fine gauze. Lymph node cells (LNC) from five mice were pooled. Where indicated, LNC were separated into CD4-depleted and CD4+ cells. The latter were subdivided further into the CD4+ CD25− and CD4+ CD25+ subsets using a CD4–CD25 mouse isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) and the appropriate columns. Separation was performed according to the manufacturer's instructions. It should be mentioned that separation of CD25+ cells was achieved by binding of a phycoerythrin (PE)-labelled anti-CD25 monoclonal antibody followed by the addition of magnetic beads coated with an anti-PE antibody, i.e. purified CD25+ cells were PE-labelled. Where such labelling could have created difficulties, cells were separated by panning on antibody-coated Petri dishes [45]. Cells were first depleted of monocytes by 2 × 1 h plastic adherence. Thereafter CD4+ cells were isolated by incubating the cell suspension with anti-CD4 (YTA 3·2.1, rIgG) and subsequent adhesion to antirat IgG-coated Petri dishes. The adherent cells were collected and were incubated with an anti-CD25 monoclonal antibody (7D4, rIgM) and panned on antirat IgM-coated Petri dishes. The non-adherent cells (CD4+/CD25−) and the adherent cells (CD4+/CD25+) were collected. The purity of the CD4+/CD25+ population, whether separated by magnetic bead sorting or by panning, was in the range of 90–95%. Viability of unseparated LNC was in the range of 90% and the viability of the CD4+ CD25+ population was > 95%.
Antibodies
The following primary antibodies were used: antimouse CD4 (clone YTA 3·2.1), unconjugated, fluoresceinisothiocyanate (FITC)- or biotin-labelled, CD8 (clone YTS 169·4.2·1), CD11b (clone YBM 6·6.10) (all European Animal Cell Culture Collection, Porton Down, UK), antimouse CD25 (clone 7D4) and antipanCD44 (clone IM7) (both American Type Culture Collection, Manassus, VA, USA), unconjugated or biotinylated anti-CD44v3 (clone PTS33) [40] and anti-CD44v10 (clone K926) [27], biotinylated antimouse IL10 (clone JES3–16E3), IL12 (clone C17·8), interferon (IFN)-γ (clone XMG1·2), transforming growth factor (TGF)-β (clone A75-3·1), unlabelled, FITC-, biotin- or PE-labelled antimouse CD25 (clone PC61), CD28 (clone 37·51), CD40 (clone 3/23), CD80 (clone 16–10A1), CD86 (clone GL1), CD152 (clone 1B8), CD154 (clone MR1), antipanNK (clone DX5) and FITC-labelled annexin V (all Pharmingen, Hamburg, Germany). Secondary reagents were biotinylated or PE-labelled antirat IgG, streptavidin (strep)-FITC, strep-PE (all Dianova, Hamburg, Germany) and strep-allophycocyanin (APC) (Becton Dickinson, Heidelberg, Germany).
Flow cytometry
Each cell sample was aliquoted and incubated with 1–10 µg ml−1 of the primary antibody. Cells incubated with unconjugated monoclonal antibody (MoAb) were incubated subsequently with the appropriate antimouse or antirat PE-labelled secondary antibody. Cells labelled with biotinylated MoAb were detected with FITC-, PE- or APC-labelled streptavidin. Negative controls were incubated with a non-binding primary antibody and the same secondary reagents. For intracellular staining of cytokines and CD152, cells were fixed and permeabilized in advance. For triple fluorescence analysis cells were stained first with an unconjugated antibody followed by the secondary FITC or APC-labelled antibody. After washing they were stained with a directly PE-labelled antibody. The third antibody was biotinylated and was counterstained with strep-APC or strep-FITC. Flow cytometry followed routine procedures using a FACSCalibur (Becton Dickinson, Heidelberg, Germany). Contaminating keratinocytes in SkIL preparation and cell debris were excluded by gating. Analysis was performed by the CellQuest program.
Lymphocyte proliferation
Unseparated LNC, CD4+ CD25− and CD4+ CD25+ LNC subpopulations (1 × 105−1·25 × 104) were seeded in triplicate in U-shaped 96-well microtitre plates and were cultured in the presence of 7·5 µg/ml concanavalin A (ConA) or were seeded in flat-bottomed 96-well microtitre plates which had been coated with anti-CD3 (10 µg/ml). Where indicated, unseparated LNC and CD4+ CD25- LNC were co-cultured with CD4+ CD25+ LNC at a ratio of 2 : 1. Cells were cultured for 48 h in a humidified atmosphere at 37°C, 5% CO2 in air, adding 1 µCi [3H]-thymidine during the last 16 h of culture. Plates were harvested and [3H]-thymidine incorporation was evaluated using a β-counter.
Statistical evaluation
Significance of differences was evaluated using the two-tailed Student's t-test.
RESULTS
Leukocyte distribution in the skin of SADBE-treated AA mice
As described previously [10], SADBE treatment of AA mice is curative inasmuch as hair starts to regrow and after 8–12 weeks of treatment, mice present with a fully recovered pelage within the treated left side of skin (Fig. 1). Both DTH reactions and AA have been described as accompanied by dermal infiltrates consisting of monocytes, leucocytes, CD4+ and CD8+ T cells [9,46–53]. Lymphocytes predominate in AA [9,46,47,53]. DTH reactions are characterized by a shift from a monocyte-dominated infiltrate in the initial phase to a mixed monocyte-lymphocyte infiltrate in the acute phase and during the resolution [48–51]. Thus, the cure of AA by induction of a chronic DTH reaction is surprising.
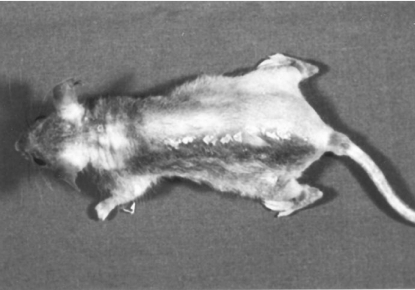
Fig. 1.
SADBE treatment induces hair regrowth in AA affected mice: C3HeJ mice, that spontaneously developed AA were treated weekly with 0·5–1% SADBE in ethanol for 8–12 weeks only on the left side of the ventral and dorsal skin. As can be seen, these mice responded with hair regrowth such that a complete pelage developed in the area of skin under treatment, whereas the untreated right side remained devoid of hair.
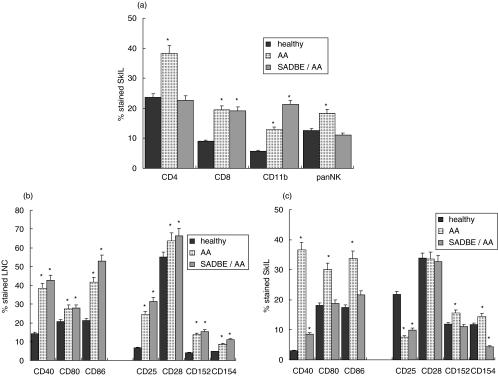
To unravel the underlying mechanism, we evaluated the composition of SkIL in AA and SADBE-treated AA mice (Fig. 2a). SkIL of untreated and SADBE-treated AA mice contained an increased percentage of CD8+ T cells and of monocytes. In SkIL of SADBE-treated AA mice the percentage of monocytes was increased significantly, even in comparison to AA mice. It should be mentioned that a relatively low number of cells could be eluted by trypsin treatment, although previous immunohistology revealed strong infiltrates in the skin of SADBE-treated AA mice [10]. The low recovery of SkIL was in striking contrast to the recovery of cells from draining LNC. While 21·2 ± 2·58 × 106 cells were recovered from healthy mice, there were 31·6 ± 3·4 × 106 (P = 0·009) in AA mice and 46·1 ± 4·58 × 106 (P < 0·0001) in SADBE-treated AA mice. However, the distribution between CD4+, CD8+, sIgM+ and CD11b+ cells was unchanged (data not shown). Instead, significant differences were seen when analysing the activation state of draining LNC. In untreated and SADBE-treated AA mice, the percentage of CD25+, CD154+ (CD40L) and CD152+ (CTLA4) cells was increased and expression of the co-stimulatory molecules CD40 and CD86 was also strongly up-regulated (Fig. 2b). With the exception of CD25, which was expressed only by a few SkIL of AA mice, a corresponding profile of accessory molecules and their ligands was seen in SkIL of AA mice; that is, a higher percentage of cells than in control mice expressed CD40, CD80, CD86, CD154 and CD152. This was in striking contrast to SkIL of SADBE-treated AA mice, where none of these markers were expressed at an increased level and expression of CD40L was reduced strongly. In addition, the reduction in CD25+ cells was not as strong as in AA mice (Fig. 2c).
Fig. 2.
Leukocyte subpopulations in the skin and draining lymph nodes in SADBE-treated AA mice: (a) SkIL of healthy, AA and SADBE-treated AA mice were stained with anti-CD4, anti-CD8, anti-CD11b and an antibody that recognizes NK cells; (b) draining LNC and (c) SkIL of healthy, AA and SADBE-treated AA mice were stained with anti-CD40, anti-CD80, anti-CD86, anti-CD25, anti-CD28, anti-CD152 and anti-CD154. The percentage of stained cells (mean ± s.d. of four independently performed experiments) is shown. Significant differences (P < 0·01) are indicated by an asterisk.
Thus, in comparison to AA mice, there was a striking reduction in the expression of SkIL activation markers from SADBE-treated AA mice. Furthermore, although CD25+ cells were reduced compared to healthy mice, the reduction was less pronounced than in SkIL of AA mice. Because we had noted previously that AA SkIL are largely devoid of CD4+ CD25+ T cells [26,52,54], we wondered whether the curative effect of SADBE treatment may have been due to a (partial) restoration in T-reg.
SADBE treatment has no impact on regulatory T cells, cytokines and activation induced cell death
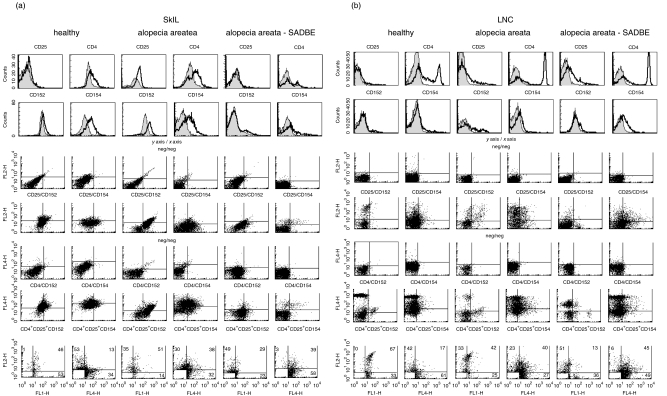
T-reg are a small population of CD4+ CD25+ T cells that can be differentiated from freshly activated T cells by the intracellular expression of CD152 [55]. To define this subpopulation, CD4+ CD25+ LNC were stained with anti-CD152 and, for comparison, with anti-CD154 (Table 1) or SkIL and draining LNC were triple-stained with anti-CD4, anti-CD25 and anti-CD152 or anti-CD154 (Fig. 3). SkIL from untreated and SADBE-treated AA mice contained few CD4+ CD25+ T cells and even most of these few CD4+ CD25+ cells did not express CD152 (Table 1). Triple fluorescence staining confirmed that SkIL of untreated and SADBE-treated AA mice contained very few CD4+ CD25+ cells which expressed predominantly CD154 (Fig. 3a). LNC of healthy mice expressed almost no CD152. However, the few CD152+ cells belonged mainly to the CD4+ CD25+ T-reg population. SADBE-treated AA mice displayed an increased number of CD4+ CD25+ draining LNC. However, the majority of these cells were activated freshly, i.e. they co-expressed CD154. LNC from untreated AA mice were partially CD154+ (Fig. 3b). Taken together, there was no evidence for restoration of T-reg within the skin of SADBE-treated AA mice. In the lymph nodes, the percentage of CD4+ CD25+ cells was increased, but they did not display the T-reg phenotype.
Table 1.
The majority of CD4+ CD25+ cells in the draining lymph nodes of SADBE-treated AA mice are freshly activated
| % of CD4+ CD25+ cells (P-value) | ||||
|---|---|---|---|---|
| Donor | Organ | % of totala CD4+/CD25+ | CD4+/CD25+/CD152+ | CD4+/CD25+/CD154+ |
| Healthy | LNC | 5·9 | 66·1 | 11·9 |
| AA | DrLNC | 8·1 (0·062) | 18·5 (0·002) | 23·5 (0·087) |
| SADBE-treated AAb | DrLNC | 9·8 (0·015) | 6·1 (<0·001) | 54·1 (0·013) |
| Healthy | SkIL | 5·9 | 32·2 | 5·1 |
| AA | SkIL | 4·1 | 12·2 | 19·5 |
| SADBE-treated AAb | SkIL | 3·3 | 7·0 | 33·3 |
CD4+ CD25+ cells were isolated by sorting with antibody-coated magnetic beads (see Material and methods). LNC were pooled from two to three mice; mean values ± s.d. from three experiments are shown. SkIL were pooled from five mice (one experiment)
mice were treated weekly with 0·5–1% SADBE in acetone for 8–12 weeks.
Fig. 3.
Differentiation between activated and regulatory CD4+ CD25+ SkIL and LNC: (a) SkIL and (b) draining LNC of healthy, AA and SADBE-treated AA mice were stained with anti-CD25, -CD4, -CD152 and -CD154 and were triple-stained with anti-CD4 (FITC or APC), anti-CD25 (PE) and either anti-CD152 (FITC) or anti-CD154 (APC). The 1st and 2nd rows show single-staining overlays for CD25, CD4, CD152 and CD154. The 3rd, 4th, 5th and 6th rows show negative controls and double-staining with anti-CD25/anti-CD152 or anti-CD154 and anti-CD4/anti-CD152 or anti-CD154. In the 7th row the population of CD4+ CD25+ cells has been gated. It shows all CD152+ and all CD154+ cells, but only CD4+ CD25+ double-positive cells (as defined by the gating). Thus, cells in the upper right quadrant are CD4+ CD25+ CD152+, respectively, CD4+ CD25+ CD154+.
To confirm that induction of T-reg by SADBE treatment cannot be considered as the therapeutic principle behind SADBE treatment, CD4+ CD25+ draining LNC of healthy, AA and SADBE-treated AA mice were stimulated with the mitogen ConA or by cross-linking of CD3. This investigation was conducted to evaluate the cells’ proliferative capacity and also to see whether they were able to inhibit the proliferative activity of unseparated LNC or of CD4+ CD25- LNC. CD4+ CD25+ LNC of healthy mice exhibited low proliferative activity themselves and suppressed proliferative activity of unseparated and of CD4+ CD25− LNC. In SADBE-treated AA mice, CD4+ CD25+ LNC showed high proliferative activity. They suppressed proliferation of unseparated cells weakly and did not suppress proliferation of CD4+ CD25- cells. CD4+ CD25+ LNC of AA mice also exhibited reduced inhibitory activity (Table 2).
Table 2.
CD4+ CD25+ LNC of SADBE-treated AA mice display weak inhibitory potential
| cpm/1 × 105 or 1·5 × 105 cellsa (% inhibition)b | ||||
|---|---|---|---|---|
| LNC Subpopulation | Stimulus | Healthy | AA | SADBE-treated AAc |
| Unseparated | ConA | 14·244 | 9·706 | 14·009 |
| CD4+ CD25− | ConA | 10·713 | 8·180 | 11·012 |
| CD4+ CD25+ | ConA | 7·215 | 7·244 | 12·410 |
| Unseparated + CD4+ CD25+ | ConA | 7·593 (57·5) | 7·972 (40·2) | 16·189 (20·0) |
| CD4+ CD25- + CD4+ CD25+ | ConA | 7·309 (49·0) | 9·288 (21·3) | 16·389 (4·8) |
| Unseparated | anti-CD3 | 35·282 | nt | 22·680 |
| CD4+ CD25- | anti-CD3 | 31·220 | nt | 27·955 |
| CD+ CD25+ | anti-CD3 | 4·868 | nt | 22·717 |
| Unseparated + CD4+ CD25+ | anti-CD3 | 10·392 (72·4) | nt | 29·398 (13·6) |
| CD4+ CD25− + CD4+ CD25+ | anti-CD3 | 6·408 (81·0) | nt | 34·904 (11·2) |
Counts per minute (cpm) are derived from 1 × 105 unseparated, CD4+ CD25- and CD4+ CD25+ lymphocytes and from 1 × 105 unseparated plus 0·5 × 105 CD4+ CD25+ and 1 × 105 CD4+ CD25− plus 0·5 × 105 CD4+ CD25+ lymphocytes, respectively; s.d. of triplicates were in the range of 2–5% and are not shown for clarity of presentation.
% Inhibition has been calculated from the sum of cpm obtained with the individual populations.
Mice were treated weekly with 0·5–1% SADBE in acetone for 8–12 weeks.
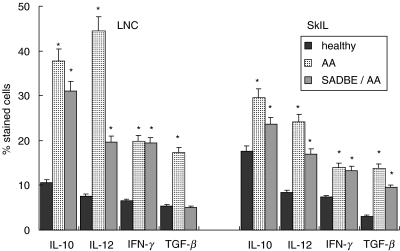
High-level expression of regulatory cytokines, particularly of TGF-β or IL-10, could be another mechanism whereby SADBE treatment interferes with autoimmune reactions in AA. Therefore, expression of TGF-β, IL-10 and for comparison, IL-12 and IFN-γ, was evaluated in draining LNC and SkIL (Fig. 4). As described previously [14,56,57], the percentage of IL-10 and TGF-β, but also of IL-12 and IFN-γ expressing draining LNC and SkIL, was increased in AA mice. In draining LNC of SADBE-treated AA mice, IL-10, and in SkIL IL-10 and TGF-β expression, was high compared to healthy mice, but remained below those of untreated AA mice. The same was true for IL-12 expression. Comparable levels of IFN-γ expression were seen in untreated and SADBE-treated AA mice. Hence, the cytokine expression profile did not provide an explanation for the therapeutic effect of SADBE.
Fig. 4.
Cytokine expression in SkIL and draining LNC: SkIL and draining LNC of healthy, AA and SADBE-treated AA mice were fixed and permeabilized and stained with anti-IL-10, anti-IL-12, anti-IFN-γ and anti-TGF-β. The percentage of stained cells (mean ± s.d. of four independently performed experiments) is shown. Significant differences (P < 0·01) are indicated by an asterisk.
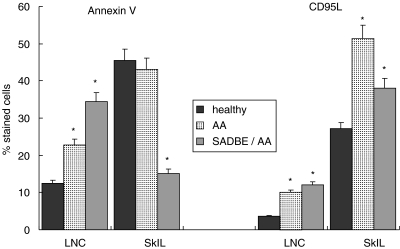
Resistance towards AICD is also believed to sustain autoimmune reactions [23–25]. This aspect was studied by annexin V staining and examination of CD95L expression after culturing cells for 24 h on plates coated with subthreshold levels of anti-CD3. There was no evidence for a distortion of AICD in AA mice or SADBE-treated AA mice. CD95L expression was slightly up-regulated in draining LNC of untreated and SADBE-treated AA mice. A high number of SkIL expressed CD95L and the percentage of CD95L-expressing cells was increased further in AA mice. Compared to the latter, CD95L was reduced in SADBE-treated AA mice. Also, draining LNC of untreated and treated AA mice displayed increased levels of annexin V-stained cells, which corresponded well with the higher percentage of activated T cells found in draining LNC of these mice. There was a slight decrease in the percentage of annexin V-stained SkIL in AA mice, but a striking decrease in SADBE-treated AA mice. The former could be indicative of a slight increase in apoptosis resistance in AA mice. The strong decrease of apoptotic SkIL in SADBE-treated AA mice was unexpected. It should, however, be remembered that: (1) SkIL of SADBE-treated AA mice contained a low percentage of activated T cells, i.e. AICD should not be pronounced and (2) SkIL of SADBE-treated AA mice contained a high percentage of monocytes. Thus, the strong reduction in annexin V-stained SkIL may be due partly to the lower percentage of T cells (Fig. 5).
Fig. 5.
CD95L expression and AICD in SkIL and draining LNC of SADBE-treated AA mice: SkIL and draining LNC of healthy, AA and SADBE-treated AA mice were cultured for 24 h on plates coated with subthreshold levels of anti-CD3 (1 µg/ml). Thereafter cells were stained with annexin V-FITC or with anti-CD95L. The percentage of stained cells (mean ± s.d. of three independently performed experiments) is shown. Significant differences (P < 0·01) are indicated by an asterisk.
Taken together, in SADBE-treated AA mice there was a strong increase in the percentage of monocytes in SkIL. LNC were enlarged and contained a high percentage of activated T cells. In AA mice, but not in SADBE-treated AA mice, activated T cells were also recovered in SkIL. The failure to detect activated (possibly autoreactive) T cells in SkIL of SADBE-treated AA mice was not due to high numbers of T-reg, increased levels of regulatory cytokines or sustained AICD. This leaves us with the option that SADBE treatment may interfere with the recruitment of autoreactive T cells towards their target.
Impaired leucocyte recruitment in the skin of SADBE-treated AA mice
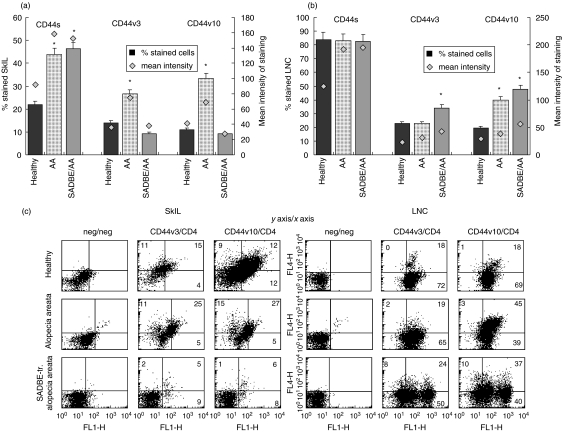
CD44 is known to be important for leucocyte traffic [58–60], and particularly CD44v10 and CD44v3 in particular have been described as up-regulated in SkIL of AA affected mice as well as during DTH reactions [27,40]. PanCD44 expression was high in SkIL of untreated and SADBE-treated AA mice. However, in SADBE-treated AA SkIL expression of CD44v3 and CD44v10 was reduced compared to healthy mice, whereas it was up-regulated in untreated AA SkIL (Fig. 6a). The expression profile of CD44v3 and CD44v10 in draining LNC differed significantly from that of SkIL. The percentage of CD44v3+ and CD44v10+ cells was increased significantly in draining LNC of SADBE-treated AA mice and cells expressed CD44v3 and CD44v10 at an elevated level (Fig. 6b). The majority of CD44v10+ and CD44v3+ draining LNC and most of the few CD44v10+ and CD44v3+ SkIL of SADBE-treated AA mice were CD4+. In contrast, in SkIL of AA and healthy mice only 60–70% of CD44v3+ and CD44v10+ cells expressed CD4 (Fig. 6c). Thus, there was a significant decrease of CD44v3+ and CD44v10+ leucocytes in SkIL, but a strong enrichment of CD44v3+ and CD44v10+ T cells in the draining lymph nodes of SADBE-treated AA mice. Taking into account the high-level expression of activation markers/accessory molecules on LNC of SADBE-treated AA mice (Fig. 2b) and the low level of activated lymphocytes in the skin (Fig. 2c), we interpret the findings in the sense that SADBE treatment may be therapeutic for AA by preventing the recruitment of activated (autoreactive) lymphocytes.
Fig. 6.
CD44v3 and CD44v10 expression in SkIL and draining LNC of SADBE-treated AA mice: (a) SkIL and (b) draining LNC of healthy, AA and SADBE-treated AA mice were stained with anti-CD44v3 and anti-CD44v10. The percentage of stained cells (mean ± s.d. of four independently performed experiments) and the mean intensity of expression are shown. Significant differences (P < 0·01) in the percentage of stained cells are indicated by an asterisk. (c) SkIL, and draining LNC of healthy, AA and SADBE-treated AA mice were double stained with anti-CD4 (FITC) and anti-CD44v3 (biotinylated plus strep-APC) or anti-CD44v10 (biotinylated plus strep-APC). The percentage of CD4−/CD44v3+ and of CD4−/CD44v10+ (upper left quadrant) as well as the percentage of CD4+/CD44v3+ and CD4+/CD44v10+ cells (upper right quadrant) are indicated.
DISCUSSION
AA is treated commonly with corticosteroids [12,13], but treatment with contact sensitizers, particularly SADBE, is regarded as the most effective therapeutic approach for extensive scalp hair loss, with relatively few side-effects [8,25,47–51]. Trying to elucidate the underlying mechanism revealed that the curative effect of SADBE relies most probably on altered leucocyte traffic as a consequence of repeated DTH reactions.
A first hint towards our interpretation was derived from the extraction and analysis of SkIL in SADBE-treated AA mice. In comparison to normal-haired mice, increased numbers of SkIL were recovered from AA mice, but the recovery was relatively limited in SADBE-treated mice despite the fact that immunohistology revealed high numbers of infiltrating cells in the upper dermis of SADBE-treated AA mice [10,14,44]. As might have been expected in a contact eczema [50,51,61,62], the percentage of monocytes was increased strongly in SkIL of SADBE-treated AA mice. Furthermore, compared to normal-haired mice, the percentage of activated T cells was increased in the draining lymph nodes and the skin of AA mice, but in SADBE-treated AA mice activated T cells were recovered only from the draining lymph nodes, and not from the skin. Thus, in contrast to the skin of AA mice, there was no evidence for a locally ongoing autoimmune reaction, the finding being in line with the therapeutic effect of SADBE in AA mice, i.e. hair regrowth.
However, SADBE is a contact sensitizer and one would have expected a dermal infiltrate of the TH1 type [63,64]. In fact, skin-draining lymph nodes were enlarged massively and displayed features of freshly activated T cells with high level expression of CD25, CD28 and CD40L. Hence, the question arose as to what may hamper the expected strong inflammatory reaction of the skin.
Because we had noted a relative paucity in CD4+ CD25+ cells in mice developing AA after the transfer of AA-affected skin [26], we speculated that induction of a contact eczema may be accompanied/followed by an overshooting balancing reaction with expansion of T-reg. By stimulating TH2 and IL-10 secretion [65,66] such T-reg might support not only the resolution of the DTH, but also reduction of the AA reaction. This was not the case. There were very few CD4+ CD25+ T cells in the skin of SADBE-treated mice and these were not stained by CD152. In the lymph nodes, the number of CD4+ CD25+ T cells was increased strongly but, again, these were not CD4+ CD25+ CD152+ T-reg. Thus, the absence of an inflammatory reaction in the skin was not a consequence of an excess of regulatory elements.
Taking into account the massive enlargement of draining lymph nodes, it was possible that the resolution of the SADBE-induced eczema might be accompanied by local tissue alterations which could prevent lymphocyte homing into the skin. SkIL express mainly CD44v3 [37,40,41] and CD44v10 has been described as strongly up-regulated in DTH reactions [27,29]. Furthermore, CD44v3 was also reported to be expressed by vessel endothelium of the skin and to be of major importance for leucocyte extravasation [37,40]. However, SADBE-treated skin contained very few CD44v3+ cells and the number of CD44v10+ cells was reduced compared to healthy mice. As both CD44v3 and CD44v10 were up-regulated in the draining lymph nodes, it could be excluded that SADBE interfered with the up-regulation of these skin-homing receptors on activated leucocytes. This leaves us with the possible explanation that lymphocyte extravasation into the lower dermis and subcutaneous fat was hampered severely in SADBE-treated AA mice. The activation state of vessel endothelium, i.e. up-regulated expression of proteoglycans acting as chemokine catchers [67–69] and harbouring of chemokines are known to facilitate T cell and monocyte recruitment towards the skin [70–75]. In particular, osteopontin is known to exert chemotactic and haptotactic activity [34–36]. Osteopontin also is known to bind to CD44 variant isoforms [31–33] and binding of osteopontin to CD44 is known to support inflammatory TH1 reactions [32,33] and to stimulate the cell survival pathway via activation of PI3 kinase and Akt [76]. Hence, we hypothesize that tissue repair in chronic eczema [77,78] may account for a hindrance in lymphocyte extravasation and migration into the lower dermis. Although the epidermis appeared unaltered in the skin of SADBE-treated AA mice, the lower dermis was thickened considerably and contained reduced numbers of lymphocytes (data not shown).
Taken together, we interpret our findings in the sense that AA can be cured by the repeated induction of a contact eczema, the resolution of which may by accompanied by tissue repair that hampers the recruitment of autoreactive T cells. If SADBE-induced hair regrowth is in fact based on hampered T cell recruitment, less encumbering therapeutic modalities such as the local application of chemokine neutralizing antibodies should be therapeutically efficient.
Acknowledgments
This investigation was supported by the Deutsche Forschungsgemeinschaft, grant Zo40/9–1 (MZ) and Ho 1598/8–1 (RH), the National Alopecia Areata Foundation (KJM, RH). KJM is a recipient of the Alfred Blaschko Memorial Fellowship, Marburg.
REFERENCES
- Kalish RS, Johnson KL, Hordinsky MK. Alopecia areata. Autoreactive T cells are variably enriched in scalp lesions relative to peripheral blood. Arch Dermatol. 1992;128:1072–7. doi: 10.1001/archderm.128.8.1072. [DOI] [PubMed] [Google Scholar]
- Randall VA. Is alopecia areata an autoimmune disease? Lancet. 2001;358:1922–4. doi: 10.1016/S0140-6736(01)06943-4. [DOI] [PubMed] [Google Scholar]
- McElwee KJ, Tobin DJ, Bystryn JC, et al. Alopecia areata: an autoimmune disease? Exp Dermatol. 1999;8:371–9. doi: 10.1111/j.1600-0625.1999.tb00385.x. [DOI] [PubMed] [Google Scholar]
- McElwee KJ, Boggess D, King LE, Sundberg JP. Experimental induction of alopecia areata-like hair loss in C3H/HeJ mice using full-thickness skin grafts. J Invest Dermatol. 1998;111:797–803. doi: 10.1046/j.1523-1747.1998.00380.x. [DOI] [PubMed] [Google Scholar]
- Gilhar A, Ullmann Y, Berkutzki T, et al. Autoimmune hair loss (alopecia areata) transferred by T lymphocytes to human scalp explants on SCID mice. J Clin Invest. 1998;101:62–7. doi: 10.1172/JCI551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JM, McElwee KJE, King L, et al. Gene array profiling and immunomodulation studies define a cell-mediated immune response underlying the pathogenesis of alopecia areata in a mouse model and humans. J Invest Dermatol. 2002;119:392–402. doi: 10.1046/j.1523-1747.2002.01811.x. [DOI] [PubMed] [Google Scholar]
- Gilhar A, Landau M, Assy B, et al. Melanocyte-associated T cell epitopes can function as autoantigens for transfer of alopecia areata to human scalp explants on Prkdc (scid) mice. J Invest Dermatol. 2001;117:1357–62. doi: 10.1046/j.0022-202x.2001.01583.x. [DOI] [PubMed] [Google Scholar]
- Paus R, Slominski A, Czarnetzki BM. Is alopecia areata an autoimmune-response against melanogenesis-related proteins, exposed by abnormal MHC class I expression in the anagen hair bulb? Yale J Biol Med. 1993;66:541–54. [PMC free article] [PubMed] [Google Scholar]
- Zhang JG, Oliver RF. Immunohistological study of the development of the cellular infiltrate in the pelage follicles of the DEBR model for alopecia areata. Br J Dermatol. 1994;130:405–14. doi: 10.1111/j.1365-2133.1994.tb03371.x. [DOI] [PubMed] [Google Scholar]
- Freyschmidt-Paul P, Sundberg JP, Happle R, et al. Successful treatment of alopecia areata-like hair loss with the contact sensitizer squaric acid dibutylester (SADBE) in C3H/HeJ mice. J Invest Dermatol. 1999;113:61–8. doi: 10.1046/j.1523-1747.1999.00640.x. [DOI] [PubMed] [Google Scholar]
- Christoph T, Muller-Rover S, Audring H, et al. The human hair follicle immune system: cellular composition and immune privilege. Br J Dermatol. 2000;142:862–73. doi: 10.1046/j.1365-2133.2000.03464.x. [DOI] [PubMed] [Google Scholar]
- MacDonald N, Wiseman MC, Shapiro J. Alopecia areata: topical immunotherapy − application and practical problems. J Cutan Med Surg. 1999;3:36–40. [PubMed] [Google Scholar]
- Papadopoulos AJ, Schwartz RA, Janniger CK. Alopecia areata. Pathogenesis, diagnosis, and therapy. Am J Clin Dermatol. 2000;1:101–5. doi: 10.2165/00128071-200001020-00004. [DOI] [PubMed] [Google Scholar]
- Hoffmann R, Wenzel E, Huth A, et al. Growth factor mRNA levels in alopecia areata before and after treatment with the contact allergen diphenylcyclopropenone. Acta Derm Venereol. 1996;76:17–20. doi: 10.2340/00015555761720. [DOI] [PubMed] [Google Scholar]
- Happle R, Echternacht K. Induction of hair growth in alopecia areata with D. N. C. B. Lancet. 1997;2:1002–3. doi: 10.1016/s0140-6736(77)92896-3. [DOI] [PubMed] [Google Scholar]
- Happle R, Kalveram KJ, Buchner U, et al. Contact allergy as a therapeutic tool for alopecia areata: application of squaric acid dibutylester. Dermatologica. 1980;161:289–97. doi: 10.1159/000250380. [DOI] [PubMed] [Google Scholar]
- Stricker RB, Goldberg B. Significance of cytokine patterns in alopecia areata before and after therapeutic allergic contact dermatitis. J Invest Dermatol. 1996;106:379–80. doi: 10.1111/1523-1747.ep12343179. [DOI] [PubMed] [Google Scholar]
- Buckley DA, Du Vivier AW. The therapeutic use of topical contact sensitizers in benign dermatoses. Br J Dermatol. 2001;145:385–405. doi: 10.1046/j.1365-2133.2001.04399.x. [DOI] [PubMed] [Google Scholar]
- Freyschmidt-Paul P, Hoffmann R, Levine E, et al. Current and potential agents for the treatment of alopecia areata. Curr Pharm Des. 2001;7:213–30. doi: 10.2174/1381612013398266. [DOI] [PubMed] [Google Scholar]
- McHugh RS, Shevach EM. The role of suppressor T cells in regulation of immune responses. J Allergy Clin Immunol. 2002;110:693–702. doi: 10.1067/mai.2002.129339. [DOI] [PubMed] [Google Scholar]
- Horwitz DA, Gray JD, Zheng SG. The potential of human regulatory T cells generated ex vivo as a treatment for lupus and other chronic inflammatory diseases. Arthritis Res. 2002;4:241–6. doi: 10.1186/ar414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinen J, Shearer WT. Basic and clinical immunology. J Allergy Clin Immunol. 2003;111:813–8. doi: 10.1067/mai.2003.154. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–56. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- O'Reilly LA, Strasser A. Apoptosis and autoimmune disease. Inflamm Res. 1999;48:5–21. doi: 10.1007/s000110050369. [DOI] [PubMed] [Google Scholar]
- Mountz JD, Zhou T, Su X, Wu J, Cheng J. The role of programmed cell death as an emerging new concept for the pathogenesis of autoimmune diseases. Clin Immunol Immunopathol. 1996;80:2–14. doi: 10.1006/clin.1996.0136. [DOI] [PubMed] [Google Scholar]
- Zöller M, McElwee KJ, Engel P, Hoffmann R. Transient CD44 variant isoform expression and reduction in CD4(+)/CD25(+) regulatory T cells in C3H/HeJ mice with alopecia areata. J Invest Dermatol. 2002;118:983–92. doi: 10.1046/j.1523-1747.2002.01745.x. [DOI] [PubMed] [Google Scholar]
- Rösel M, Seiter S, Zöller M. CD44v10 expression in the mouse and functional activity in delayed type hypersensitivity. J Cell Physiol. 1997;171:305–17. doi: 10.1002/(SICI)1097-4652(199706)171:3<305::AID-JCP9>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Freyschmidt-Paul P, Seiter S, Zöller M, et al. Treatment with an anti-CD44v10-specific antibody inhibits the onset of alopecia areata in C3H/HeJ mice. J Invest Dermatol. 2000;115:653–7. doi: 10.1046/j.1523-1747.2000.00113.x. [DOI] [PubMed] [Google Scholar]
- Wagner SN, Wagner C, Reinhold U, et al. Predominant expression of CD44 splice variant v10 in malignant and reactive human skin lymphocytes. J Invest Dermatol. 1998;111:464–71. doi: 10.1046/j.1523-1747.1998.00302.x. [DOI] [PubMed] [Google Scholar]
- Zöller M. CD44v10 in hematopoiesis and stem cell mobilization. Leuk Lymph. 2000;38:463–80. doi: 10.3109/10428190009059265. [DOI] [PubMed] [Google Scholar]
- Katagiri YU, Sleeman J, Fujii H, et al. CD44 variants but not CD44s cooperate with beta1-containing integrins to permit cells to bind to osteopontin independently of arginine–glycine–aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res. 1999;59:219–26. [PubMed] [Google Scholar]
- Ashkar S, Weber GF, Panoutsakopoulou V, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–4. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- Denhardt DT, Noda M, O'Regan AW, et al. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–61. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan JW, Hota C, Shigeyama Y, et al. Site-directed mutagenesis of the arginine–glycine–aspartic acid sequence in osteopontin destroys cell adhesion and migration functions. J Cell Biochem. 1995;57:680–90. doi: 10.1002/jcb.240570413. [DOI] [PubMed] [Google Scholar]
- Nau GJ, Guilfoile P, Chupp GL, et al. A chemoattractant cytokine associated with granulomas in tuberculosis and silicosis. Proc Natl Acad Sci USA. 1997;94:6414–9. doi: 10.1073/pnas.94.12.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber GF, Zawaideh S, Hikita S, et al. Phosphorylation-dependent interaction of osteopontin with its receptors regulates macrophage migration and activation. J Leukoc Biol. 2002;72:752–61. [PubMed] [Google Scholar]
- Grimme HU, Termeer CC, Bennett KL, et al. Colocalization of basic fibroblast growth factor and CD44 isoforms containing the variably spliced exon 3 (CD44v3) in normal skin and in epidermal skin cancers. Br J Dermatol. 1999;141:824–32. doi: 10.1046/j.1365-2133.1999.03154.x. [DOI] [PubMed] [Google Scholar]
- Bennett KL, Jackson DG, Simon JC, et al. CD44 isoforms containing exon V3 are responsible for the presentation of heparin-binding growth factor. J Cell Biol. 1995;128:687–98. doi: 10.1083/jcb.128.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumova S, Woods A, Couchman JR. Heparan sulfate proteoglycans on the cell surface: versatile coordinators of cellular functions. Int J Biochem Cell Biol. 2000;32:269–88. doi: 10.1016/s1357-2725(99)00116-8. [DOI] [PubMed] [Google Scholar]
- Seiter S, Engel P, Föhr N, Zöller M. Mitigation of delayed-type hypersensitivity reactions by a CD44 variant isoform v3-specific antibody: blockade of leukocyte egress. J Invest Dermatol. 1999;113:11–21. doi: 10.1046/j.1523-1747.1999.00635.x. [DOI] [PubMed] [Google Scholar]
- Seiter S, Schadendorf D, Tilgen W, Zöller M. CD44 variant isoform expression in a variety of skin-associated autoimmune diseases. Clin Immunol Immunopathol. 1998;89:79–93. doi: 10.1006/clin.1998.4565. [DOI] [PubMed] [Google Scholar]
- Kuniyasu H, Oue N, Tsutsumi M, et al. Heparan sulfate enhances invasion by human colon carcinoma cell lines through expression of CD44 variant exon 3. Clin Cancer Res. 2001;7:4067–72. [PubMed] [Google Scholar]
- Weiss JM, Renkl AC, Maier CS, et al. Osteopontin is involved in the initiation of cutaneous contact hypersensitivity by inducing Langerhans and dendritic cell migration to lymph nodes. J Exp Med. 2001;194:1219–29. doi: 10.1084/jem.194.9.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotellessa C, Peris K, Caracciolo E, et al. The use of topical diphenylcyclopropenone for the treatment of extensive alopecia areata. J Am Acad Dermatol. 2001;44:73–6. doi: 10.1067/mjd.2001.109309. [DOI] [PubMed] [Google Scholar]
- Wysocki LJ, Sato VL. ‘Panning’ for lymphocytes: a method for cell selection. Proc Natl Acad Sci USA. 1978;75:2844–8. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peret C, Wiesner-Menzel L, Happle R. Immunohistochemical analysis of T-cell subsets in the peribulbar and intrabulbar infiltrates of alopecia areata. Acta Derm Venerol. 1984;64:26–30. [PubMed] [Google Scholar]
- Ghersetich I, Campanile G, Lotti T. Alopecia areata: immunohistochemistry and ultrastructure of infiltrate and identification of adhesion molecule receptors. Int J Dermatol. 1996;35:28–33. doi: 10.1111/j.1365-4362.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Ptak W, Herzog WR, Askenase PW. Delayed-type hypersensitivity initiation by early-acting cells that are antigen mismatched or MHC incompatible with late-acting, delayed-type hypersensitivity effector T cells. J Immunol. 1991;146:469–75. [PubMed] [Google Scholar]
- Gibbs JH, Grange JM, Beck JS, et al. Early delayed hypersensitivity responses in tuberculin skin tests after heavy occupational exposure to tuberculosis. J Clin Pathol. 1991;44:919–23. doi: 10.1136/jcp.44.11.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren F, Anderson C. The spectrum of inflammatory cell response to dimethyl sulfoxide. Contact Dermatitis. 2000;42:216–21. doi: 10.1034/j.1600-0536.2000.042004216.x. [DOI] [PubMed] [Google Scholar]
- Buchanan KL, Murphy JW. Kinetics of cellular infiltration and cytokine production during the efferent phase of a delayed-type hypersensitivity reaction. Immunology. 1997;90:189–97. doi: 10.1046/j.1365-2567.1997.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- McElwee KJ, Silva K, Boggess D, et al. Alopecia areata in C3H/HeJ mice involves leukocyte mediated sheath disruption in advance of overt hair loss. Vet Pathol. in press. [DOI] [PubMed]
- Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach EM. Certified professionals: CD4+CD25+ suppressor T cells. J Exp Med. 2001;193:41–5. doi: 10.1084/jem.193.11.f41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwee KJ, Hoffmann R, Freyschmidt-Paul P, et al. Resistance to alopecia areata in C3H/HeJ mice is associated with increased expression of regulatory cytokines and a failure to recruit CD4(+) and CD8(+) cells. J Invest Dermatol. 2002;119:1426–33. doi: 10.1046/j.1523-1747.2002.19620.x. [DOI] [PubMed] [Google Scholar]
- Teraki Y, Imanishi K, Shiohara T. Cytokines in alopecia areata: contrasting cytokine profiles in localized form and extensive form (alopecia universalis) Acta Derm Venereol. 1996;76:421–3. doi: 10.2340/0001555576421423. [DOI] [PubMed] [Google Scholar]
- DeGrendele HC, Estess P, Siegelman MH. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science. 1997;278:672–5. doi: 10.1126/science.278.5338.672. [DOI] [PubMed] [Google Scholar]
- Estess P, Nandi A, Mohamadzadeh M, Siegelman MH. Interleukin 15 induces endothelial hyaluronan expression in vitro and promotes activated T cell extravasation through a CD44-dependent pathway in vivo. J Exp Med. 1999;190:9–19. doi: 10.1084/jem.190.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DG, Bell JI, Dickinson R, et al. Proteoglycan forms of the lymphocyte homing receptor CD44 are alternatively spliced variants containing the v3 exon. J Cell Biol. 1995;128:673–85. doi: 10.1083/jcb.128.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring L, Gosling J, Chensue SW, et al. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–61. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler WJ, Yawalkar N, Britschgi M, et al. Cellular and molecular pathophysiology of cutaneous drug reactions. Am J Clin Dermatol. 2002;3:229–38. doi: 10.2165/00128071-200203040-00001. [DOI] [PubMed] [Google Scholar]
- Ohmen JD, Hanifin JM, Nickoloff BJ, et al. Overexpression of IL10 in atopic dermatitis. Contrasting cytokine patterns with delayed-type hypersensitivity reactions. J Immunol. 1995;154:1956–63. [PubMed] [Google Scholar]
- Lu B, Rutledge BJ, Gu L, et al. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187:601–8. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonuleit H, Schmitt E, Kakirman H, et al. Infectious tolerance: human CD25+ regulatory T cell convey suppressor activity on conventional CD4+ T helper cells. J Exp Med. 2002;196:255–60. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann D, Bruett CH, Plöttner H, et al. Human CD4+CD25+ regulatory contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells. J Exp Med. 2002;196:247–53. doi: 10.1084/jem.20020642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Adams DH, Hubscher S, et al. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1 beta. Nature. 1993;361:79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- Witt DP, Lander AD. Differential binding of chemokines to glycosaminoglycan subpopulations. Curr Biol. 1994;4:394–400. doi: 10.1016/s0960-9822(00)00088-9. [DOI] [PubMed] [Google Scholar]
- Gallo RL. Proteoglycans and cutaneous vascular defense and repair. J Invest Dermatol Symp Proc. 2000;5:55–60. doi: 10.1046/j.1087-0024.2000.00008.x. [DOI] [PubMed] [Google Scholar]
- Yu X, Barnhill RL, Graves DT. Expression of monocyte chemoattractant protein-1 in delayed type hypersensitivity reactions in the skin. Lab Invest. 1994;71:226–35. [PubMed] [Google Scholar]
- Santamaria-Babi LF, Moser B, Perez-Soler MT, et al. The interleukin-8 receptor B and CXC chemokines can mediate transendothelial migration of human skin homing T cells. Eur J Immunol. 1996;26:2056–61. doi: 10.1002/eji.1830260914. [DOI] [PubMed] [Google Scholar]
- Santamaria-Babi LF, Perez-Soler MT, Hauser C, Blaser K. Skin-homing T cells in human cutaneous allergic inflammation. Immunol Res. 1995;14:317–24. doi: 10.1007/BF02935627. [DOI] [PubMed] [Google Scholar]
- Egan PJ, Kimpton W, Seow HF, et al. Inflammation-induced changes in the phenotype and cytokine profile of cells migrating through skin and afferent lymph. Immunology. 1996;89:539–46. doi: 10.1046/j.1365-2567.1996.d01-776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand ML, Warren JS, Mansour MK, et al. Inhibition of T cell recruitment and cutaneous delayed-type hypersensitivity-induced inflammation with antibodies to monocyte chemoattractant protein-1. Am J Pathol. 1996;148:855–64. [PMC free article] [PubMed] [Google Scholar]
- Sebastiani S, De Albanesi C, PO, et al. The role of chemokines in allergic contact dermatitis. Arch Dermatol Res. 2002;293:552–9. doi: 10.1007/s00403-001-0276-9. [DOI] [PubMed] [Google Scholar]
- Lin YH, Yang-Yen HF. The osteopontin-CD44 survival signal involves activation of the phosphatidylinositol 3-kinase/Akt signaling pathway. J Biol Chem. 2001;276:46024–30. doi: 10.1074/jbc.M105132200. [DOI] [PubMed] [Google Scholar]
- Kimura R, Hu H, Stein-Streilein J. Delayed-type hypersensitivity responses regulate collagen deposition in the lung. Immunology. 1992;77:550–5. [PMC free article] [PubMed] [Google Scholar]
- Wangoo A, Cook HT, Taylor GM, Shaw RJ. Enhanced expression of type 1 procollagen and transforming growth factor-beta in tuberculin induced delayed type hypersensitivity. J Clin Pathol. 1995;48:339–45. doi: 10.1136/jcp.48.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]