Abstract
Plant extracts have been implicated in various immunoregulatory effects that are poorly understood. Thus, we investigated the modulatory activity of PureCell Complex (PCT)-233, an active molecular complex from mesophyll tissue of Spinacia oleacea on the inflammatory process. Alveolar macrophages (AM) were treated with PCT-233 and/or budesonide, a well-known anti-inflammatory agent, before or after being stimulated with lipopolysaccharides (LPS). Pro- and anti-inflammatory cytokine production, tumour necrosis factor (TNF) and interleukin (IL)-10, respectively, were measured in cell-free supernatants at different times after the treatment. PCT-233 increased unstimulated AM release of both TNF and IL-10, whereas heat- and light-inactivated PCT-233 stimulated only the release of TNF without affecting IL-10 production, suggesting that different mechanisms are involved in the modulation of TNF and IL-10 release by PCT-233. The presence of LPS did not modify PCT-233-stimulated TNF production, but the ratio TNF/IL-10 production by LPS-stimulated AM was reduced significantly in the presence of PCT-233. Pretreatment of AM with PCT-233 and budesonide before LPS stimulation reduced TNF production at both protein and mRNA levels, whereas IL-10 production was increased. Moreover, TNF/IL-10 ratio was reduced further with the combination PCT-233/budesonide. Interestingly, AM treatment with PCT-233 and budesonide 18 h after LPS stimulation did not modulate TNF release significantly but it did increase IL-10 production, and a synergistic effect was observed with the combination PCT-233/budesonide. These exciting data suggest that PCT-233 possesses some anti-inflammatory properties, even when added during the inflammatory process, and could potentiate the effect of other anti-inflammatory agents.
Keywords: alveolar macrophages, budesonide, IL-10, PCT-233, TNF
INTRODUCTION
Glucocorticoids are potent anti-inflammatory drugs used widely in the treatment of various inflammatory diseases such as asthma, rheumatoid arthritis, atopic dermatitis and inflammatory bowel disease [1,2]. They control inflammation by increasing the transcription of anti-inflammatory genes and decreasing the transcription of inflammatory genes [3]. Glucocorticoids such as budesonide (BUD) have been shown to induce production of interleukin (IL)-10 and inhibit macrophage inflammatory protein 1α (MIP-1α), granulocyte macrophage–colony stimulating factor (GM-CSF), IL-6 and tumour necrosis factor (TNF) production by macrophages [4,5]. However, long-term corticosteroid use is associated with serious sequel including osteoporosis, adrenal insufficiency, gastrointestinal effects, hyperlipidaemia, growth suppression and neurological effects [6,7]. Furthermore, there is evidence suggesting that BUD decreases phagocytosis of alveolar macrophages (AM) [8]. Thus, given the side effects of glucocorticoids, concomitant agents that can potentiate their anti-inflammatory properties allowing the reduction of concentration used could have a tremendous impact on therapeutic treatment.
Plant extracts have been shown to have anti-inflammatory activities [9] and several active components have been suggested [10,11]. PureCell Complex (PCT)-233 corresponds to the isolation and stabilization [12] of the photosynthetic machinery (photosystems I and II, the cytochrome b6f complex and the proton-translocating ATP synthetase) [13] of the thylakoid membrane from mesophyll tissue of spinach leaves (Spinacia olecea). PCT-233 is still active and can dissipate energy repeatedly through an electron transport system [14]. The functional quality of the molecular complex can be measured by fluorescence based on its capacity to react to light and dissipate its energy (Fv/Fm ratio) [15].
We have demonstrated recently that PCT-233 is able to protect IMR-32 cells (human neuroblastoma) from apoptosis caused by the generation of reactive oxygen species (ROS) and singlet oxygen [16]. In this model, PCT-233 efficacy was linked directly to its intrinsic activity (Fv/Fm ratio), as shown in comparison with heat ‘de-activated’ PCT-233 [16]. Furthermore, intraperitoneal injection of PCT-233 reduces in a concentration-dependent fashion 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced ear oedema in mice as well as carrageenan paw oedema in rats (unpublished data). Because ROS intermediates may act as signal transduction messengers and modulate gene transcription, particularly for proinflammatory cytokines [17], and given the redox potential and antioxidant properties of the molecule complexes of PCT-233, we postulated that PCT-233 modulates pro- and anti-inflammatory cytokine production in AM.
AM play a central role in the regulation of immune and inflammatory activities as well as in tissue remodelling in the lung [18]. These cells are the first line of defence against infectious agents and other immunologic insults and they can produce both pro- and anti-inflammatory cytokines such as TNF and IL-10 [19,20]. Whereas TNF is involved in numerous inflammatory diseases including septic shock, inflammatory bowel disease, rheumatoid arthritis and allergic airway inflammation, IL-10 plays a protective role in these diseases [21,22]. A good balance in the production of these cytokines is crucial to maintaining lung homeostasis. There is increasing evidence suggesting that AM participate to the production and maintenance of airway inflammation in asthma and allergic diseases [18], and one of the mechanisms of corticosteroid efficacy is to down-regulate the release of inflammatory mediators and cytokines from AM [23,24]. Thus, given the importance of AM in maintaining lung homeostasis, we investigated the modulatory effect of PCT-233 on cytokine production of LPS-stimulated AM.
MATERIALS AND METHODS
PCT-233 preparation
PCT-233 was extracted from spinach leaves (Spinacia oleacea), as described in a Canadian patent [12] and a stock solution of 100 mg/ml (5%) was prepared in phosphate buffered saline (PBS). Further dilutions (0·05% and 0·005%) were prepared in RPMI medium. PCT-233 integrity was evaluated by spectrophotometry (Beckman DU 640, Brea, CA, USA) [25] and fluorimetry (Hansatech Instruments Ltd, UK) [15]. Endotoxin contamination was <1 endotoxin unit according to Sigma's E-Toxate semiquantitative assay (Sigma Chemical Co., St Louis, MO, USA). Furthermore, PCT-233 did not inhibit the binding of LPS to LPS-binding protein as measured using endoblock test (Cedarlane Laboratories limited, Hornby, ON, Canada) according to the manufacturer's protocol. In some experiments, heat- and light-inactivated PCT-233 was used. To confirm that the sample had been correctly deactivated (20% of the initial activity), the maximum quantum efficiency of photosystem II was measured with the Hansatech fluorescence monitoring system (Hansatech Instruments Ltd, UK).
Cell
NR8383 is an AM cell line isolated from Sprague–Dawley rats, used previously in vitro to study macrophage-related activities [20,26]. NR8383 cells were maintained in Ham's F-12 media (Invitrogen Canada Inc, Burlington, ON, Canada) as we have described previously [26]. All experiments were performed in RPMI-1640 medium with 5% FBS, 1% Hepes buffer, 1% penicillin–streptomycin (Invitrogen Canada Inc.). After 2 h adherence in 96-well plates (Falcon; Becton Dickinson Labware, Lincoln Park, NJ, USA) at 37°C, AM were treated with PCT-233 (0·005% and 0·05% w/v) for various periods of time (6, 18, 24, 72, 96 and 120 h) with and without LPS (10 and 100 ng/ml) (Salmonella enteritidis; Sigma Chemical Co.). At the end of the treatment, cell-free supernatants were recovered and stored at − 70°C for future analysis. Cells were also treated with LPS for 18 h before being treated with PCT-233 and BUD (Sigma Chemical Co.) or pretreated with PCT-233 and BUD for 18 h before LPS stimulation.
AM from Sprague–Dawley rats were isolated as described previously [27]. Briefly, animals were anaesthetized and exsanguinated by cutting the abdominal aorta. The trachea was catheterized with a polyethylene tube and airways were washed with 50 ml of cold PBS by repeated instillation of 8–10 ml. Cells from rat bronchoalveolar lavage contained 96·6% ± 0·5% AM according to May–Grünwald–Giemsa and non-specific esterases staining. The viability always exceeded 95%.
Cytokine release and assay
IL-10 and TNF levels were measured in cell-free supernatants using enzyme-linked immunosorbent assay (ELISA) kits for rat (Pharmingen, San Diego, CA, USA). Plates were read on a Thermomax microplate reader (Molecular Devices, Menlo Park, CA, USA). The sensitivity of ELISA was 5 pg/ml for IL-10 and 4 pg/ml for TNF. The ratio of proinflammatory on anti-inflammatory cytokines from the same supernatant has been calculated as followed: level of TNF (ng/ml) divided by level of IL-10 (ng/ml).
Reverse transcription-polymerase chain reaction (RT-PCR)
AM were treated with and without PCT-233 (0·005%) and BUD (1 nm) for 18 h before being stimulated with LPS (10 ng/ml) for different periods of time. Cells were collected and total RNA was extracted using TRIzol reagent (Invitrogen Canada Inc.). Total RNA was quantified using RiboGreen™ RNA quantification reagent (Molecular Probes Inc., Eugene, OR, USA). For complementary DNA synthesis, 1 µg of total RNA of each sample was reverse transcribed by Moloney murine leukaemia virus RT enzyme (Invitrogen Canada Inc.) according to the manufacturer's protocol. PCR was performed using Qiagen Taq DNA polymerase protocol and reaction was performed in 30 µl final volume. Thirty cycles were performed which corresponded to the linear portion of the curve. The primers used were: (1) rat GAPDH sense: 5′-GAC AAG ATG GTC AAG GTC GG-3′, and antisense: 5′-CAT GGA CTG TGG TCA TGA GC-3′ (537 bp); (2) rat TNF sense: 5′-TTC TGT CTA CTG AAC TTC GGG GTG ATC GGT CC-3′ and antisense: 5′-GTA TGA GAT AGC AAA TCG GCT GAC GGT GTG GG-3′ (354 bp); (3) rat IL-10 sense: 5′-TGC CAA GCC TTG TCA GAA ATG-3′, and antisense: 5′-TGA GTG TCA CGT AGG CTT CTA-3′[286 base pairs (bp)]. Products were run on a 2% agarose gel and stained with ethidium bromide (5 µg/ml).
Statistical analysis
Different statistical approaches were used to analyse data. A multifactor analysis of variance was performed to investigate the simultaneous effects of three factors for time-course data. We defined the LPS factor with two levels: with and without LPS; the PCT-233 factor with three levels: 0, 0·005, and 0·05%; the time factor with seven levels: 6, 18, 24, 48, 72, 96, and 120 h. Main and interaction factor effects were included in the models (fixed effects only). Important 2 × 2 interactions existed; the analyses of factor effects were conducted within one factor and two-way anova was performed for the other two factors. The univariate normality assumptions were verified with the Shapiro–Wilk tests. The Brown–Foresythe tests were used to verify the homogeneity of variances. Pre- and post-treatment data were analysed using a four-way anova. The results were considered significant with P-values <0·05. All analyses were conducted using the statistical package SAS (SAS Institute Inc., Cary, NC, USA).
RESULTS
Time-course analysis of IL-10 and TNF production in presence of PCT-233
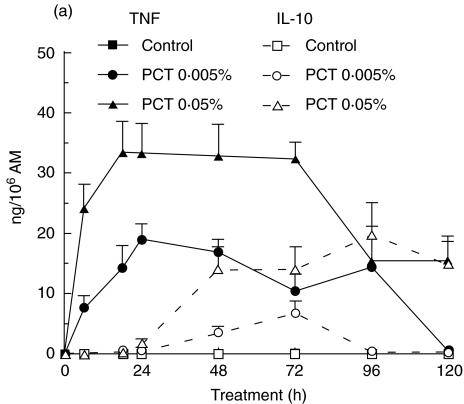
Toxicity tests were done using different concentrations of PCT-233 (0·005, 0·05 and 0·1%). None of the concentrations used affected AM viability as measured by trypan blue exclusion. To determine the modulatory effect of PCT-233 on AM cytokine production, NR8383 were treated with two concentrations of PCT-233 (0·005% and 0·05%) for different periods of time (6, 18, 28, 48, 72, 96, and 120 h) in the presence or not of LPS (10 ng/ml), and levels of TNF and IL-10 were measured in cell-free supernatants. AM spontaneous release of TNF increased over time from 68·8 ± 9·2 to 166·2 ± 28·0 pg/106 AM from 6 h to 120 h, respectively. PCT-233 significantly (P < 0·001) increased TNF release in a concentration- and time-dependent manner (Fig. 1a). The maximum TNF release was observed between 18 and 24 h and it decreased at different rate depending on PCT-233 concentration. AM released spontaneously very limited amounts of IL-10 (0·6 ± 0·3 to 5·8 ± 2·3 pg/106 AM from 6 to 120 h, respectively). However, both PCT-233 concentrations (0·005 and 0·05%) significantly (P < 0·05 and P < 0·001, respectively) stimulated the release of IL-10 in a concentration- and time-dependent manner (Fig. 1a). In contrast to TNF, the maximum IL-10 release was observed at 72 and 96 h at 0·005 and 0·05% PCT-233, respectively. Higher concentration of PCT-233 (0·1%) showed similar results than 0·05% PCT-233 (data not shown).
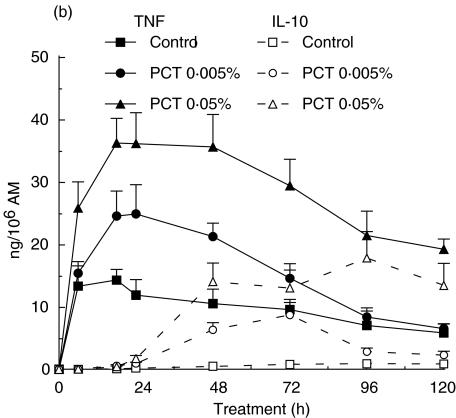
Fig. 1.
Production of TNF and IL-10 by alveolar macrophages (AM). AM were treated with PCT-233 (0·005% and 0·05%) without (a) and with (b) LPS (10 ng/ml) for different periods of time (6, 18, 24, 48, 72, 96 and 120 h) and cell-free supernatants were tested for TNF and IL-10 content. The release of both TNF and IL-10 was significantly (P < 0·001) stimulated by LPS and PCT-233 compared with control, but with different timing. Mean ± s.e.m. of eight experiments.
LPS is well known to stimulate macrophages. Significant amount of TNF was released within the first 6 h (13·3 ± 3·2 ng/106 AM) of LPS stimulation (10 ng/ml) with a maximum at 18 h (14·3 ± 1·7 ng/106 AM), whereas lower amounts of IL-10 were released at these times (0·011 ± 0·003 ng/106 AM and 0·091 ± 0·020 ng/106 AM, respectively) (Fig. 1b). The maximum release of IL-10 by LPS-stimulated AM was observed at 96 h with 0·90 ± 0·18 ng/106 AM. The stimulation of TNF and IL-10 release by 0·05% PCT-233 was similar in the presence and absence of LPS (Fig. 1a,b). However, LPS-stimulated TNF and IL-10 release was significantly (P < 0·001) increased by the presence of 0·005% PCT-233 compared with PCT-233 or LPS alone. Interestingly, PCT-233 induced a further reduction of TNF/IL-10 ratio over time (Table 1) compared with LPS alone, suggesting a shift toward anti-inflammatory cytokine production by PCT-233. Different concentrations of LPS (100 and 1000 ng/ml) were used in combination with PCT-233 (0·005 and 0·05%) and similar results were observed (data not shown). Intriguingly, a high concentration of PCT-233 (0·05%) increased TNF release in the presence of 10 ng/ml LPS, whereas it inhibited TNF release in the presence of 100 ng/ml LPS, suggesting a modulation depending on the intensity of the stimulus.
Table 1.
Modulation of TNF/IL-10 ratio by PCT-233 over time
| PCT-233 without LPS | PCT-233 with LPS | |||||
|---|---|---|---|---|---|---|
| Time (h) | 0 | 0·005% | 0·05% | 0 | 0·005% | 0·05% |
| 6 | 122·3 | 454·8 | 860·5 | 1176·3 | 723·4 | 909·4 |
| 18 | 19·7 | 24·2 | 85·9 | 155·9 | 42·9 | 88·4 |
| 24 | 15.6 | 22·0 | 18·8 | 59·0 | 26·7 | 20·9 |
| 48 | 13·6 | 4·7 | 2·3 | 21·6 | 3·4 | 2·5 |
| 72 | 22·7 | 1·5 | 2·3 | 12·1 | 1·7 | 2·3 |
| 96 | 70·5 | 2·1 | 0·8 | 7·8 | 2·9 | 1·2 |
| 120 | 28·5 | 2·0 | 1·0 | 6·7 | 2·8 | 1·4 |
Alveolar macrophages were treated with PCT-233 (0·005% and 0·05%) without and with LPS (10 ng/ml) for different periods of time (6, 18, 24, 48, 72, 96 and 120 h) and cell-free supernatants were tested for TNF and IL-10 content. The ratio was calculated by dividing TNF levels by IL-10 levels (ng/ml).
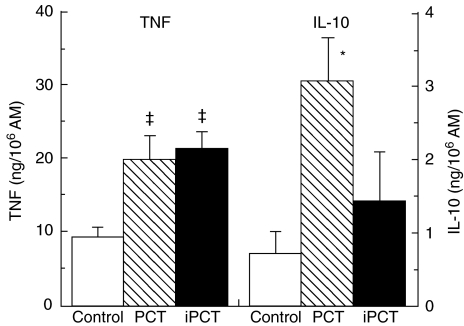
To determine whether active PCT-233 was necessary for modulating AM cytokine production, AM were treated with light- and heat-deactivated PCT-233 (0·05%) for 48 h in the presence of LPS (10 ng/ml). Active PCT-233 significantly increased both TNF and IL-10 release from AM, whereas inactive PCT-233 did not modulate AM IL-10 release (Fig. 2), suggesting that the modulation of TNF and IL-10 release by PCT-233 may be mediated by different mechanisms. To understand further the role of TNF in the increase of IL-10 release, AM were treated with different concentrations of anti-TNF antibody (0·1, 0·5, 1·0, and 1·5 µg/ml) at the same time than PCT-233 and both TNF and IL-10 levels were measured. TNF release was inhibited significantly in a concentration-dependent manner (28, 65, 92 and 98%, respectively). However, only 1·0 and 1·5 µg/ml anti-TNF significantly inhibited IL-10 release (76% and 78%, respectively), suggesting that IL-10 production was mediated by both TNF-dependent and -independent mechanisms.
Fig. 2.
Modulation of alveolar macrophage (AM) cytokine production by PCT-233 (PCT) and inactive PCT-233 (iPCT). AM were treated with PCT-233 and iPCT-233 (0·05%) and LPS (10 ng/ml) for 48 h and TNF and IL-10 levels were measured in cell-free supernatants. PCT-233 significantly increased both TNF and IL-10 production (‡P < 0·002 and *P < 0·02, respectively) compared with control, whereas inactive PCT-233 significantly (P < 0·002) increased TNF release only. Mean ± s.e.m. of six experiments.
To confirm the effect of PCT-233 on primary cells, freshly isolated AM from Sprague–Dawley rats were incubated with PCT-233 for 48 h with and without LPS. Levels of TNF and IL-10 were lower than levels from the NR8383 cell line, but both PCT-233 and LPS stimulated the release of TNF and IL-10 (Table 2). Furthermore, the ratio TNF/IL-10 was reduced by PCT-233 in the presence of LPS in both cells. However, there was a small difference observed between freshly isolated AM and NR8383 AM. High PCT-233 concentration, 0·05%, did not increase IL-10 release further in AM, in contrast to NR8383 AM. Thus, given the similarity of the data and to minimize the use of animals, rat N8383 cell line was used to investigate further the modulation of cytokine production by PCT-233.
Table 2.
Production of TNF and IL-l0 by freshly isolated rat alveolar macrophages (AM)
| Treatment | TNF (pg/106 AM) | IL-l0 (pg/106 AM) |
|---|---|---|
| Sham | 55·6 ± 13·3 | 3·3 ± 1·4 |
| PCT -233 (0.005%) | 4519·5 ± 934·8† | 430·3 ± 112·8* |
| PCT-233 (0.05%) | 12895·0 ± 2460·8† | 251·4 ± 92.0* |
| LPS (10 ng/ml) | 4095·1 ± 998·6† | 38·7 ± 13·7* |
| LPS + PCT-233 (0.005%) | 7119·8 ± 1577·9 | 352·5 ± 91·1 |
| LPS + PCT-233 (0.05%) | 13354·1 ± 2038·8 | 246·5 ± 108·8 |
Rat alveolar macrophages were purified and treated with PCT-233 with and without LPS for 48 h and levels of TNF and IL-l0 were measured. Both concentrations of PCT-233 and LPS significantly increased TNF and IL-10 release
P < 0·01
P < 0·05, respectively.
The combination of LPS and PCT-233 did not significantly increase PCT-233-stimulated cytokine release.
Modulation of LPS-stimulated TNF and IL-10 production by pretreatment with PCT-233 and BUD
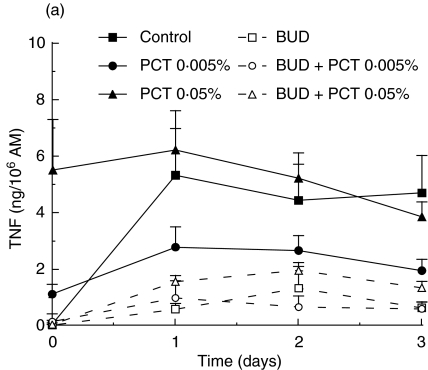
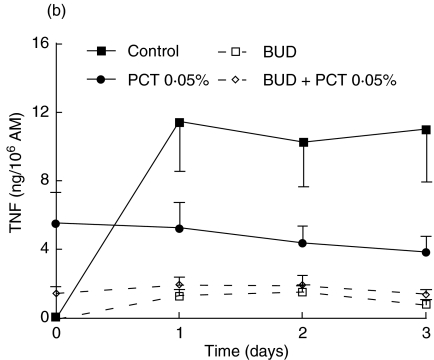
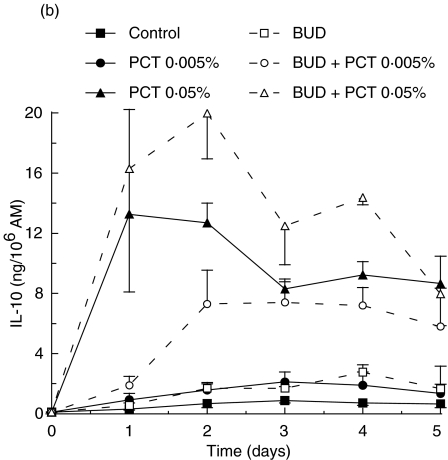
To investigate the anti-inflammatory properties of PCT-233, its effect on LPS-stimulated AM was compared to a well-known anti-inflammatory agent, BUD. Preliminary experiments using different concentrations of BUD (1, 10 and 100 nm) were performed and no significant difference was observed between the concentrations used (data not shown). Thus, 1 nm BUD was used in further experiments in combination with two concentrations of PCT-233 (0·005 and 0·05%). AM were treated with PCT-233 and/or BUD for 18 h before being stimulated with LPS (10 and 100 ng/ml). Day 0 corresponds to cytokine levels prior to LPS stimulation. Treatments were stopped at different times (1, 2 and 3 days) after LPS stimulation (Fig. 3). Both TNF and IL-10 were measured in cell-free supernatants. Pretreatment with PCT-233 (0·005%) and BUD significantly (P < 0·001) inhibited LPS-stimulated AM TNF release compared with control (Fig. 3a). The inhibition caused by the combination BUD/PCT-233 (0·005%) was similar to BUD alone, given the efficacy of BUD to inhibit TNF release.
Fig. 3.
Effect of pretreatment of alveolar macrophages (AM) with PCT-233 and budesonide (BUD) on cytokine production. AM were treated with BUD (1 nm) and/or PCT-233 for 18 h before being stimulated with 10 ng/ml LPS (a) and 100 ng/ml LPS (b) and TNF was measured in cell-free supernatants. IL-10 was also measured in cell-free supernatants when cells were stimulated with 10 ng/ml LPS (c). Control represents AM pretreatment in medium and stimulated with LPS at day 0. Data at day 0 correspond to cytokine levels just prior to LPS stimulation. Mean ± s.e.m. of five to six experiments.
Pretreatment (18 h) with higher concentration of PCT-233 (0·05%) stimulated TNF release (day 0). However, the addition of LPS at day 0 did not significantly increase the release of TNF (day 1 compared with day 0) that decreased over time (Fig. 3a). Interestingly, the presence of BUD and PCT-233 reduced PCT-233-stimulated TNF release by AM prior to LPS stimulation (day 0) and continued to inhibit TNF release at 1, 2 and 3 days after LPS stimulation. Similar data were observed with the combination BUD/PCT-233 (0·05%) using LPS 100 ng/ml (Fig. 3b). However, when this concentration of LPS was added at day 0, PCT-233 (0·05%) significantly (P < 0·001) inhibited LPS-stimulated TNF release. Thus, although PCT-233 alone increases TNF release, it inhibits LPS-stimulated TNF release.
Pretreatment with BUD did not significantly increase LPS-stimulated IL-10 release (Fig. 3c). However, the production of IL-10 was maintained at day 3 in the presence of BUD/PCT-233. Pretreatment with PCT-233, alone and in combination with BUD, significantly (P < 0·001) increased IL-10 release by AM compared with LPS or BUD alone. However, there was no significant difference between the combination of BUD/PCT-233 and PCT-233 alone. Stimulation with higher concentrations of LPS (100 ng/ml) gave similar results (data not shown).
BUD was more efficient than PCT-233 to inhibit TNF release, whereas PCT-233 was more potent to stimulate IL-10 release than BUD. Furthermore, TNF/IL-10 ratio demonstrated that PCT-233 was more effective at reducing the TNF/IL-10 ratio than was BUD, and the lowest ratios was obtained using the BUD/PCT-233 combination (Table 3). Our results suggest that PCT-233 may potentiate the anti-inflammatory effects of glucocorticoids.
Table 3.
Modulation of TNF/IL-10 ratio by PCT-233 and BUD pretreatment
| Time after LPS stimulation (days) | |||
|---|---|---|---|
| Pretreatment | 1 | 2 | 3 |
| Sham | 49·8 | 12·6 | 16·2 |
| PCT-233 (0·005%) | 3·0 | 1·1 | 1·6 |
| PCT-233 (0·05%) | 1·3 | 0·5 | 0·5 |
| BUD (1 nm) | 4·4 | 2·9 | 0·9 |
| BUD + PCT-233 (0·005%) | 0·9 | 0·2 | 0·2 |
| BUD + PCT-233 (0·05%) | 0·3 | 0·2 | 0·1 |
Alveolar macrophages were treated with PCT-233, BUD alone or in combination for 18 h before being stimulated with LPS (10 ng/ml) and cell-free supernatants were collected at different times after LPS stimulation. The ratio was calculated by dividing TNF levels by IL-10 levels (ng/ml).
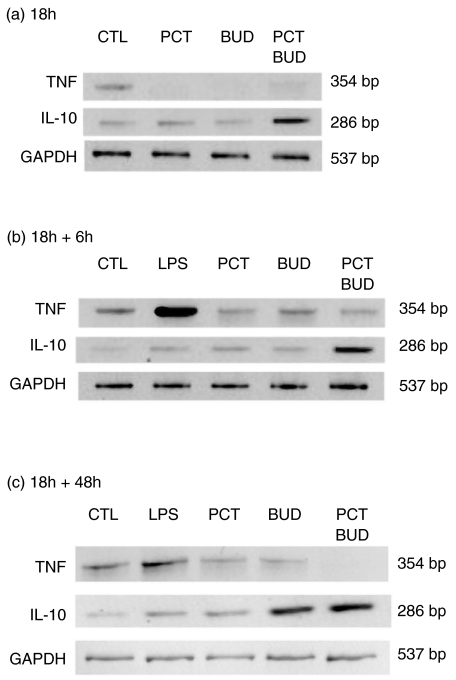
To determine whether the reduction of TNF and the increase of IL-10 production were reflected at mRNA level, RT-PCR was performed on RNA isolated from sham-treated cells and cells treated with PCT-233 and BUD. mRNA levels were investigated at the end of the pretreatment (18 h), and 6 and 48 h after LPS stimulation corresponding to maximum levels of TNF mRNA and protein, respectively. Pretreatment with PCT-233 and BUD (18 h) inhibited TNF mRNA levels in unstimulated AM (Fig. 3a). LPS increased TNF mRNA level, which was inhibited by the presence of PCR-233 and BUD for 6 and 48 h (Fig. 4b,c). An increase in IL-10 mRNA levels was observed with the combination of PCT-233 and BUD at all times studied (Fig. 4). These data suggest that PCT-233 and BUD modulate AM cytokine production at both mRNA and protein levels.
Fig. 4.
Difference in mRNA levels for TNF and IL-10 in alveolar macrophages (AM) treated with PCT-233 and LPS. AM were treated with PCT-233 (0·005%) and budesonide (BUD, 1 nm) for 18 h in the absence of LPS and RT-PCR was performed for TNF, IL-10 and GAPDH (a). AM were treated with PCT-233 and BUD for 18 h in the absence of LPS and stimulated with LPS for 6 h (b) or 48 h (c) and RT-PCR was performed. Controls (CTL) represent AM without stimulation or treatment. Results of a representative experiment of three experiments are presented.
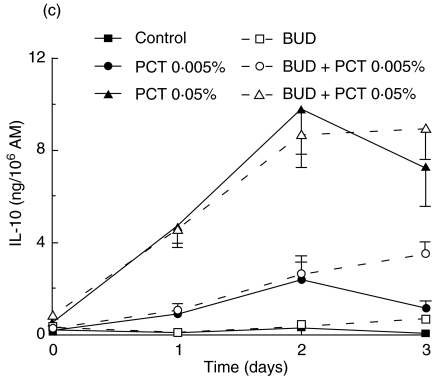
Modulation of LPS-stimulated TNF and IL-10 production by post-treatment with PCT-233 and BUD
To investigate further the possibility that PCT-233 contributes to the resorption of inflammation, AM were stimulated with LPS for 18 h before being treated with PCT-233 and BUD alone or in combination (day 0). BUD and low concentration of PCT-233 (0·005%) did not modulate TNF release significantly (Fig. 5a). In contrast, high concentration of PCT-233 (0·05%) added 18 h after LPS stimulation (10 ng/ml) (P < 0·01) increased AM TNF release significantly, but the combination of BUD and PCT-233 (0·05%) abrogated PCT-233-stimulated TNF release. However, 0·05% PCT-233 did not significantly increase TNF release when AM were stimulated with 100 ng/ml LPS (data not shown).
Fig. 5.
Effect of post-treatment of alveolar macrophages (AM) with PCT-233 and budesonide (BUD) on cytokine production. AM were stimulated with 10 ng/ml LPS for 18 h before being treated with PCT-233 and BUD (1 nm) alone or in combination and TNF (a) and IL-10 (b) were measured in cell-free supernatants. Control represents AM stimulated with LPS without other treatment. Data at day 0 correspond to cytokine levels just prior to PCT-233 and BUD treatment. Mean ± s.e.m. of six experiments.
IL-10 levels were measured in the same cell-free supernatants. Both PCT-233 (0·005%) and BUD alone significantly (P < 0·01) increased LPS-stimulated IL-10 release (Fig. 5b) compared with LPS alone (control). However, higher concentration of PCT-233 (0·05%) significantly (P < 0·001) stimulated more IL-10 release than PCT-233 0·005% and BUD. Interestingly, the BUD/PCT-233 combination potentiated IL-10 release compared with both PCT-233 and BUD alone, suggesting that BUD/PCT-233 may be more potent at inhibiting the inflammation than either agent alone. Similar results were observed with higher concentration of LPS (100 ng/ml) (data not shown).
The TNF/IL-10 ratio after LPS stimulation was 90·3. This ratio was reduced to 17·9, 7·2 and 1·2 2 days after the addition of 0, 0·005 and 0·05% PCT-233, respectively, showing the efficacy of PCT-233 to inhibit the inflammatory response (Table 4). BUD reduced the TNF/IL10 ratio to 6·2 two days after its addition to AM and this ratio was reduced further by the presence of 0·005% and 0·05% PCT-233 to 1·5 and 0·7, respectively (Table 4) showing the additive anti-inflammatory effect of PCT-233.
Table 4.
Modulation of TNF/IL-10 ratio by PCT-233 and BUD post-treatment over time
| Time (days) | LPS | PCT-233 0·005% | PCT-233 0·05% | BUD | BUD + PCT-233 0·005% | BUD + PCT-233 0·05% |
|---|---|---|---|---|---|---|
| 1 | 34·5 | 11·8 | 12·1 | 18·9 | 5·6 | 0·6 |
| 2 | 17·9 | 7·2 | 1·2 | 6·2 | 1·5 | 0·7 |
| 3 | 9·0 | 3·8 | 1·5 | 4·5 | 1·0 | 0·7 |
| 4 | 7·4 | 2·7 | 1·0 | 2·2 | 0·6 | 0·4 |
| 5 | 4·4 | 3·2 | 0·9 | 2·9 | 1·0 | 0·7 |
Alveolar macrophages were treated stimulated with LPS (10 ng/ml) for 18 h before being treated with PCT-233 and BUD (1 nm) alone or in combination and cell-free supernatants were collected at different times. The ratio was calculated by dividing TNF levels by IL-10 levels (ng/ml).
DISCUSSION
IL-10, discovered more than a decade ago, represents one of the most important immunomodulatory cytokines. IL-10 suppresses inflammatory responses by blocking the production of proinflammatory mediators such as TNF, IL-1β, IL-6, IL-8, MIP-1α and GM-CSF [17]. Furthermore, IL-10 enhances the production of IL-1 receptor antagonist and the expression of soluble p55 and p75 TNF receptors, suggesting that IL-10 induces a shift from production of proinflammatory to anti-inflammatory mediators. Administration of IL-10 has been shown to ameliorate many inflammatory diseases in murine models [28,29]. Thus, agents that induce IL-10 production, such as PCT-233, may be useful in anti-inflammatory therapy.
We have demonstrated that PCT-233 increases the production of IL-10 by AM in a concentration- and time-dependent manner. Given that TNF increases IL-10 production [21] and that PCT-233 induces TNF production, it was essential to determine whether TNF was responsible for IL-10 production. Our data with anti-TNF suggest that PCT-233-induced IL-10 release is partly independent of PCT-233-induced TNF production. Furthermore, light- and heat-deactivated PCT-233 stimulated TNF release without modulating IL-10 release (Fig. 2) suggesting that PCT-233-induced IL-10 production is partly independent on PCT-233-stimulated TNF release.
Although PCT-233 induced TNF production the addition of LPS did not increase this production further, suggesting that PCT-233 does not potentiate an inflammatory response. In contrast, PCT-233 (0·005%) increased LPS-stimulated IL-10 production further, reducing the TNF/IL-10 ratio (Table 1). These results indicate that PCT-233 should accelerate the resorption of inflammation. Thus, our data suggest strongly that PCT-233 causes a shift in cytokine balance toward anti-inflammatory cytokines in inflammatory responses. Intriguingly, high concentration of PCT-233 (0·05%) stimulated TNF release in presence of low concentration of LPS. However, when AM were stimulated with higher concentration of LPS causing increased level of TNF release, PCT-233 inhibited TNF release. These data suggest that PCT-233 can modulate the inflammatory response depending on the intensity of the stimulus. The increased level of IL-10 release by higher concentrations of PCT-233 and LPS may be responsible for the inhibition of TNF production. However, further investigations are needed to better understand this mechanism.
BUD has been demonstrated to inhibit TNF release from human AM when cells were treated before the addition of the stimulus [5], whereas there are some controversies about the modulation of IL-10 by BUD treatment [4,30,31]. Our data show that both BUD and PCT-233 inhibit TNF production at both mRNA and protein levels when given in pretreatment (Figs 3 and 4). Although PCT-233 increased TNF release after 18 h treatment (Fig. 1a) it inhibited TNF mRNA level (Fig. 4a), suggesting that TNF release may not be related to new synthesis. PCT-233 and BUD treatment (18 h) did not modulate IL-10 production. However, the combination of PCT-233 and BUD increased IL-10 mRNA levels, which may be observed later at protein level. Thus, PCT-233 and BUD modulate the production of TNF and IL-10 at both mRNA and protein levels.
Although pretreatment with BUD and PCT-233 inhibited LPS-stimulated TNF release, post-treatment did not modulate TNF production. However, IL-10 release was increased by both pre- and post-treatments (Fig. 5). Furthermore, a potentiation was observed with the combination BUD/PCT-233. Although PCT-233 does not inhibit TNF release when added 18 h after LPS stimulation, it may inhibit an ongoing inflammatory response by reducing the TNF/IL-10 ratio. Furthermore, PCT-233 can potentiate the efficacy of other anti-inflammatory agents such as BUD, suggesting that PCT-233 is a good additive to anti-inflammatory therapy.
Inflammatory processes are crucial for the destruction of pathogens and virus-infected cells by activated immune cells, which secrete a variety of proinflammatory cytokines. However, prolonged and/or inappropriate inflammation contributes to the pathogenesis of many diseases. Our data suggest that PCT-233 can modulate the inflammatory disequilibrium mainly by stimulating anti-inflammatory cytokine production thus, reducing the ratio TNF/IL-10. It is well known that spinach is an important source of antioxidant activity [32]; it has been suggested that such activity is responsible for the reduced levels of proinflammatory cytokines in animals fed with an enriched spinach diet [33]. PCT-233 complex contains some flavonoids which are well known to possess antioxidant and anti-inflammatory properties [10,11]. However, PCT-233 has to be active to stimulate IL-10 release, suggesting that flavonoids contained in PCT-233 are not responsible for the stimulation of IL-10. Furthermore, antioxidants have been shown to inhibit LPS-stimulated TNF release in macrophages [34], whereas an increase in TNF production was observed with PCT-233. Thus, the antioxidant property of PCT-233 may not be involved in the modulation of TNF. However, antioxidant has been shown to stimulate IL-10 production [35] and IL-10 has been suggested to act as an antioxidant [36]. Thus, it will be extremely difficult to discriminate between these two functions of PCT-233. More investigations are needed to further understand the mechanisms involved in the modulation of AM cytokine production by PCT-233.
Given the side effects of corticosteroid therapy [6,37], PCT-233 could be a good addition to corticosteroids, reducing the required concentration and increasing their efficacy. PCT-233 does not contain any toxic products or conservation ingredients, nor does it have any toxic effects. The important properties of PCT-233 are the low concentration needed to observe its anti-inflammatory effects and efficacy when given after the inflammatory response has begun. However, further investigations are needed to understand better its anti-inflammatory properties. Research is in progress in our laboratory in order to elucidate the mechanism of action.
Acknowledgments
This work was supported by PureCell Technologies. E.Y.B. is a Senior FRSQ Scholar. The authors would like to thank Éric Daneau for technical assistance.
REFERENCES
- Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond) 1998;94:557–72. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- Norbiato G, Bevilacqua M, Vago T, Clerici M. Glucocorticoids and Th-1, Th-2 type cytokines in rheumatoid arthritis, osteoarthritis, asthma, atopic dermatitis and AIDS. Clin Exp Rheumatol. 1997;15:315–23. [PubMed] [Google Scholar]
- Barnes PJ, Pedersen S, Busse WW. Efficacy and safety of inhaled corticosteroids. New developments. Am J Respir Crit Care Med. 1998;157:S1–S53. doi: 10.1164/ajrccm.157.3.157315. [DOI] [PubMed] [Google Scholar]
- John M, Lim S, Seybold J, et al. Inhaled corticosteroids increase interleukin-10 but reduce macrophage inflammatory protein-1alpha, granulocyte-macrophage colony-stimulating factor, and interferon-gamma release from alveolar macrophages in asthma. Am J Respir Crit Care Med. 1998;157:256–62. doi: 10.1164/ajrccm.157.1.9703079. [DOI] [PubMed] [Google Scholar]
- Ek A, Larsson K, Siljerud S, Palmberg L. Fluticasone and budesonide inhibit cytokine release in human lung epithelial cells and alveolar macrophages. Allergy. 1998;54:691–9. doi: 10.1034/j.1398-9995.1999.00087.x. [DOI] [PubMed] [Google Scholar]
- Buchman AL. Side effects of corticosteroid therapy. J Clin Gastroenterol. 2001;33:289–94. doi: 10.1097/00004836-200110000-00006. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Exp Gerontol. 1999;34:721–32. doi: 10.1016/s0531-5565(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Zetterlund A, Larsson PH, Muller-Suur C, Palmberg L, Larsson K. Budesonide but not terbutaline decreases phagocytosis in alveolar macrophages. Respir Med. 1998;92:162–6. doi: 10.1016/s0954-6111(98)90089-0. [DOI] [PubMed] [Google Scholar]
- Miller AL. The etiologies, pathophysiology, and alternative/complementary treatment of asthma. Altern Med Rev. 2001;6:20–47. [PubMed] [Google Scholar]
- Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- Hippeli S, Elstner EF. Inhibition of biochemical model reactions for inflammatory processes by plant extracts: a review on recent developments. Free Radic Res. 1999;31(Suppl.):S81–7. doi: 10.1080/10715769900301361. [DOI] [PubMed] [Google Scholar]
- Purcell M. 1999. Procedure for preparing active plant extracts used to trap free radicals; the extracts and compounds and devices containing them. Canadian patent: CA 2293852.
- Vothknecht UC, Westhoff P. Biogenesis and origin of thylakoid membranes. Biochim Biophys Acta. 2001;1541:91–101. doi: 10.1016/s0167-4889(01)00153-7. [DOI] [PubMed] [Google Scholar]
- Allen JF, Forsberg J. Molecular recognition in thylakoid structure and function. Trends Plant Sci. 2001;6:317–26. doi: 10.1016/s1360-1385(01)02010-6. [DOI] [PubMed] [Google Scholar]
- Maxwell K. Chlorophyl fluorescence − a practical guide. J Exp Bot. 2000;51:659–68. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- Purcell M, Daneau E, Siddiqi SJ, Phipps J. Photosynthesis in health care? New thylakoid pigments complex against cell death. Photosynthesis Res. 2001;69:24. [Google Scholar]
- Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996;10:709–20. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- Bissonnette E. Alveolar macrophages in the pathogenesis of asthma. Recent Res Devel Allergy Clin Immunol. 2000;1:129–41. [Google Scholar]
- Kelley J. Cytokines of the lung. Am Rev Respir Dis. 1990;141:765–88. doi: 10.1164/ajrccm/141.3.765. [DOI] [PubMed] [Google Scholar]
- Sirois J, Menard G, Moses AS, Bissonnette EY. Importance of histamine in the cytokine network in the lung through H2 and H3 receptors: stimulation of IL-10 production. J Immunol. 2000;164:2964–70. doi: 10.4049/jimmunol.164.6.2964. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. Tumor necrosis factor. In: Remik DG, Friedland JS, editors. Cytokines in health and disease. New York: Marcek Dekker Inc.; 1997. pp. 223–39. [Google Scholar]
- de Wall Malefyt R. IL-10. In: Oppenhein JJ, Feldmann M, editors. Cytokine reference. San Diego: Academic Press; 2000. pp. 165–85. [Google Scholar]
- Linden M, Brattsand R. Effects of a corticosteroid, budesonide, on alveolar macrophage and blood monocyte secretion of cytokines: differential sensitivity of GM-CSF, IL-1 beta, and IL-6. Pulm Pharmacol. 1994;7:43–7. doi: 10.1006/pulp.1994.1004. [DOI] [PubMed] [Google Scholar]
- Fuller RW, Kelsey CR, Cole PJ, Dollery CT, MacDermot J. Dexamethasone inhibits the production of thromboxane B2 and leukotriene B4 by human alveolar and peritoneal macrophages in culture. Clin Sci (Lond) 1984;67:653–6. doi: 10.1042/cs0670653. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. In: Packer L, Douce R, editors. Methods in enzymology. London: Academic Press; 1987. pp. 350–82. [Google Scholar]
- Ménard G, Bissonnette EY. Priming alveolar macrophages by leukotriene D4. Potentiation of inflammation. Am J Respir Cell Mol Bio. 2000;23:572–7. doi: 10.1165/ajrcmb.23.4.4152. [DOI] [PubMed] [Google Scholar]
- Déry RE, Bissonnette EY. IFN-γ potentiates the release of TNF-α and MIP-1α by alveolar macrophages during allergic reactions. Am J Respir Cell Mol Biol. 1999;20:407–12. doi: 10.1165/ajrcmb.20.3.3252. [DOI] [PubMed] [Google Scholar]
- Zhang YC, Pileggi A, Agarwal A, et al. Adeno-associated virus-mediated IL-10 gene therapy inhibits diabetes recurrence in syngeneic islet cell transplantation of NOD mice. Diabetes. 2003;52:708–16. doi: 10.2337/diabetes.52.3.708. [DOI] [PubMed] [Google Scholar]
- Lindsay JO, Ciesielski CJ, Scheinin T, Brennan FM, Hodgson HJ. Local delivery of adenoviral vectors encoding murine interleukin 10 induces colonic interleukin 10 production and is therapeutic for murine colitis. Gut. 2003;52:363–9. doi: 10.1136/gut.52.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrelds IM, van Hal PT, Haakmat RC, Hoogsteden HC, Saxena PR, Zijlstra FJ. Time dependent production of cytokines and eicosanoids by human monocytic leukaemia U937 cells; effects of glucocorticosteroids. Med Inflamm. 1999;8:229–35. doi: 10.1080/09629359990397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly RP, Dickensheets H, Finbloom DS. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J Interferon Cytokine Res. 1999;19:563–73. doi: 10.1089/107999099313695. [DOI] [PubMed] [Google Scholar]
- Ou B, Huang D, Hampsch-Woodill M, Flanagan JA, Deemer EK. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: a comparative study. J Agric Food Chem. 2002;50:3122–8. doi: 10.1021/jf0116606. [DOI] [PubMed] [Google Scholar]
- Cartfor MC, Gemma C, Bickford PC. Eighteen-month-old Fischer 344 rats fed a spinach-enriched diet show improved delay classical eyeblink conditioning and reduced expression of tumor necrosis factor alpha (TNFalpha) and TNFbeta in the cerebellum. J Neurosci. 2002;22:5813–6. doi: 10.1523/JNEUROSCI.22-14-05813.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Kinneer K. Chemoprotection by phenolic antioxidants. Inhibition of tumor necrosis factor alpha induction in macrophages. J Biol Chem. 2002;277:2477–84. doi: 10.1074/jbc.M106685200. [DOI] [PubMed] [Google Scholar]
- Kotake Y, Sang H, Tabatabaie T, Wallis GL, Moore DR, Stewart CA. Interleukin-10 overexpression mediates phenyl-N-tert-butyl nitrone protection from endotoxemia. Shock. 2002;17:210–6. doi: 10.1097/00024382-200203000-00009. [DOI] [PubMed] [Google Scholar]
- Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174:1549–55. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkness CA. Establishing a therapeutic index for the inhaled corticosteroids: part II. Comparisons of systemic activity and safety among different inhaled corticosteroids. J Allergy Clin Immunol. 1998;102:S52–64. doi: 10.1016/s0091-6749(98)70005-3. [DOI] [PubMed] [Google Scholar]