Abstract
In bullous pemphigoid (BP), the binding of BP180-specific antibodies to their hemidesmosomal target antigen is not sufficient for blister formation, but must be accompanied by the release of proteases. Using plasminogen activator (PA) knock-out mice, the PA system has previously been shown to be a prerequisite for blister formation in experimental murine BP. Here, we found elevated levels of plasmin and tPA, but not of uPA, in blister fluid from BP patients (n = 7) compared to blisters from patients with toxic epidermal necrolysis (n = 4) and suction blisters in healthy controls (n = 7). Subsequently, we addressed the question whether keratinocytes release PA in response to the binding of anti-BP180 antibodies. Treatment of cultured normal human keratinocytes with BP IgG, but not with control IgG, led to both increased protein and mRNA levels of tPA, but not of uPA, as determined by ELISA and RT-PCR, respectively. The specificity of this finding was confirmed using BP180-deficient keratinocytes from a patient with generalized atrophic benign epidermolysis bullosa, where no tPA release was observed after stimulation with BP IgG. Our results show the elevated expression and release of tPA from normal human keratinocytes upon stimulation with antibodies to human BP180. Keratinocytes, by secreting tPA, may thus play an active role in blister formation of BP.
Keywords: autoimmunity, blister, hemidesmosome, mRNA, pathogenesis, protease
INTRODUCTION
Bullous pemphigoid (BP) is a subepidermal blistering disease characterized by tissue-bound and circulating antibodies to the hemidesmosomal proteins BP180 and BP230 [1–4]. While BP230 is associated with the intracellular hemidesmosomal plaque [5], BP180 is a transmembrane glycoprotein with a long extracellular tail that spans the lamina lucida of the dermal-epidermal junction [reviewed in 6]. The 16th noncollagenous (NC) domain of the BP180 ectodomain is an antigenic site targeted by the majority of BP sera [7–9]. The pathogenic relevance of anti-BP180 antibodies has previously been demonstrated in various experimental models of BP [10–13]. In addition, titres of circulating anti-BP180 NC16A antibodies were shown to parallel disease activity in BP patients [14]. In both human and experimental BP it was demonstrated that the binding of autoantibodies alone is not sufficient for blister formation but must be accompanied by the infiltration of inflammatory cells and the subsequent release of proteinases. Latter were thought to finally cause dermal-epidermal separation [10,11,15–18].
Recently, the plasminogen activator (PA) system has been shown to be pivotal for blister formation in experimental murine BP [18]. There are two classes of PA, known as urokinase-type PA (uPA) and tissue-type PA (tPA) that are products of different genes and independently regulated [reviewed in 19]. Both uPA and tPA are produced by normal human epidermal keratinocytes (NHEK) [20]. uPA and tPA convert the ubiquitous zymogen plasminogen into the serine proteinase plasmin which has the capacity to cleave many proteins, including extracellular matrix proteins and matrix metalloproteinase precursors such as gelatinase B [19].
In this study, we initially assayed levels of plasmin, tPA, and uPA in blister fluid of BP patients and found abnormally high levels of plasmin and tPA. Subsequently, we investigated the hypothesis that the binding of anti-BP180 antibodies to their cell surface receptor mediates the release of uPA and tPA from cultured NHEK. Indeed, we observed an up-regulation of tPA, but not of uPA, at both protein and mRNA levels in response to the binding of anti-BP180 antibodies.
MATERIALS AND METHODS
Human and rabbit sera
Serum samples were obtained from 3 bp patients with linear deposits of C3 and IgG at the dermal-epidermal junction by direct immunofluorescence (IF) microscopy of perilesional skin before treatment was initiated. By indirect IF microscopy on 1 m NaCl-separated human skin, IgG antibodies bound to the epidermal side of the artificial split in all three IgG preparations (sera). In patient BP-1, the titre was 5120 (2560), in patient BP-2 5120 (11280), and in patient BP-3, it was 2560 (640). By immunoblotting of epidermal extracts [21], all patients’ sera exclusively labelled BP180 and revealed no reactivity with BP230. Antibodies reactive with BP180 were further specified by Western blot analysis of recombinant BP180 NC16A [8] where IgG preparations recognized BP180 NC16A at dilutions of 1 : 60 000 (BP-1), 1 : 10 000 (BP-2), and 1 : 5000 (BP-3), respectively. Rabbit sera 594 [22], 8009, and 2296 [13] were generated against GST-NC16A2-4 containing a 42 amino acid stretch of human BP180 NC16A. Normal human and preimmune rabbit sera were used as controls.
Blister fluid
Blister fluid was taken from 7 bp patients with typical clinical, histological and immunopathological features of BP. Patients with toxic epidermal necrolysis (TEN, n = 4) revealed extensive epidermal necrosis by histopathology and negative direct IF microscopy. In addition, suction blisters were raised on the flexor side of the forearm in 7 healthy volunteers as described [23,24]. All patients were in the acute phase of the disease and had not yet been treated. Blister puncture was performed within the first 6 h of blister formation. After centrifugation, blister fluid supernatants were stored at − 80°C until used.
Recombinant proteins
GST-NC16A fusion proteins were expressed in Escherichia coli strain DH5α and purified by glutathione-agarose affinity-chromatography; SDS-PAGE and immunoblotting were performed as reported [8].
Keratinocyte culture
Normal human epidermal keratinocytes (NHEK) were isolated from human neonatal foreskin and grown in tissue culture flasks (Becton Dickinson Labware, Franklin Lakes, NJ, USA) in keratinocyte growth medium (KGM; Clonetics, La Jolla, CA, USA) containing 0·15 mm Ca2+ at 37°C in a humidified atmosphere with 5% CO2 as described [25]. Feeder layers of lethally irradiated fibroblasts were not used. Keratinocytes from a previously characterized patient with generalized atrophic benign epidermolysis bullosa (GABEB), that lack BP180 expression [26], were also grown. For optimal growth, GABEB keratinocytes were kept in collagen I-coated flasks (Becton Dickinson Labware) in equal parts of KGM and keratinocyte-SFM (Gibco, Breda, the Netherlands) as reported [27].
Isolation of IgG
Total IgG was isolated from human and rabbit sera by Protein G Sepharose 4 Flow affinity column chromatography (Pharmacia AB, Uppsala, Sweden) as described [25]. Human BP180-specific antibodies were affinity purified from BP-3 serum by the use of the AminoLink Plus immobilization kit (Pierce, Rockford, IL, USA) as reported previously [13]. In brief, recombinant GST fusion protein NC16A2-4 (amino acids 507–562) was covalently coupled to 4% beaded agarose matrix before incubation with BP-3 serum or normal human serum. BP180 NC16A2-4-specific antibodies were eluted with 0·1 m glycine buffer, neutralized with Tris-HCl, and shown to have preserved their reactivity with 1 m NaCl-split human skin. Both NC16A2-4-specific IgG and affinity purified total IgG was washed, concentrated, and sterile filtered as described [13,25]. The final protein concentration was determined by photometry at 280 nm and Bradford protein assay (Bio-Rad, Hercules, CA, USA). tPA and uPA levels in IgG preparations were below the detection limit of the ELISAs.
Stimulation of keratinocytes
For stimulation experiments, the same number of keratinocytes (between 10 000 and 15 000 cells/cm2) was added to each well of the 24-well plates without collagen I coating (Becton Dickinson Labware) and grown to 70–80% confluence in KGM. Since collagen I-coating has previously been reported to modulate tPA mRNA expression [28] GABEB keratinocytes were kept in 24-well plates without collagen I-coating for at least 48 h before stimulation experiments were initiated. Hydrocortisone was omitted 12 h prior to stimulation to exclude interference with PA production [29]. In most experiments, keratinocytes were treated with 4 mg/ml purified human or rabbit IgG, an IgG concentration that was previously identified as optimal for the release of IL-6 and IL-8 from NHEK [25]. In some experiments, concentrations of 8 mg/ml IgG were applied; BP180 NC16A-specific human IgG was employed at a concentration of 1 mg/ml, all diluted in KGM without hydrocortisone. In addition, GABEB keratinocytes were stimulated with IL-1β (1 ng/ml), TNFα (40 ng/ml; both Biosource, Fleurus, Belgium), and human serum (1 : 10; all diluted in KGM without hydrocortisone), respectively, that are known to induce tPA release in cultured keratinocytes [30,31]. To account for potentially different cell numbers in individual wells due to different growth rates, each experiment was done in triplicate and culture supernatants/cell extracts from 3 similarly treated wells were pooled before subjected to ELISA or RNA isolation. In addition, all experiments were performed at least twice using keratinocytes from different donors.
Determination of plasmin
For the detection of plasmin, H-D-valyl-L-leucyl-L-lysine-L-p-nitroanilide dihydrochloride S2251 (Chromogenix, Mölndal, Sweden) was used as chromogenic substrate as described [32]. Briefly, 100 µl of a 4 mm substrate solution was added to 240 µl of assay buffer (20 mm Hepes, 280 mm NaCl, 2 mm MgCl2, 0·5 g/l Brij-100, 0·16% gelatine). Absorbance was read at 405 nm. The assay was calibrated using known amounts of plasmin.
ELISA protocols tPA and uPA (both Biopool AB, Umea, Sweden) were determined by ELISA according to the manufacturer's instructions. Samples, negative controls (including medium alone and PBS), and standards (1·5–30 ng/ml tPA and 0·16–4·0 ng/ml uPA) were assayed in quadruplicate. As further negative control of the tPA ELISA, substrates were also tested in normal goat IgG-coated wells; the resulting OD readings were then subtracted from the OD readings obtained after incubation of substrates with goat anti-human tPA IgG. The detection limits of the assays were 1·5 ng/ml (tPA) and 0·1 ng/ml (uPA).
Quantification of tPA and uPA mRNA expression by RT-PCR
Total cytoplasmatic RNA was isolated from stimulated keratinocytes using the RNeasy® Mini Kit Easy (Quiagen, Hilden, Germany) and reversed-transcribed using oligo(dT) provided by SuperScript® II (Life Technologies, Karlsruhe, Germany). The resulting cDNA (5 µl) was used to amplify a segment of the t-PA (234-base pair [bp]) and u-PA (276-bp) cDNA, by polymerase chain reaction (PCR) using specific primers as described previously [33]. A glyceraldehyde phosphate dehydrogenase (GADPH, internal control) was simultaneously amplified using an upstream primer (5′-CCACCCATGGCAAATTCCATG GCA-3′) identical to position 212–235 and a downstream primer (5′-TCTAGACGGCAGGTCAGGTCCACC-3′) complementary to position 786–809 of the human GAPDH mRNA. Amplification was carried out in a Techne Thermal Cycler PHC-3 for 30 cycles. PCR products were analysed on a 1·2% agarose-ethidium bromide gel. The gels were photographed and the intensity of the individual t-PA, u-PA and GADPH mRNA bands measured by laser densitometric scanning, using a Molecular Dynamics Personal Densitometer. Changes in t-PA and u-PA mRNA levels were expressed as a relative ratio of t-PA or u-PA mRNA/GAPDH mRNA [34].
Statistics
For statistical analysis, the Mann–Whitney U-test was applied.
Ethical approval
The ethical approval of the study was obtained by the local Ethic Committee (No. 37/98).
RESULTS
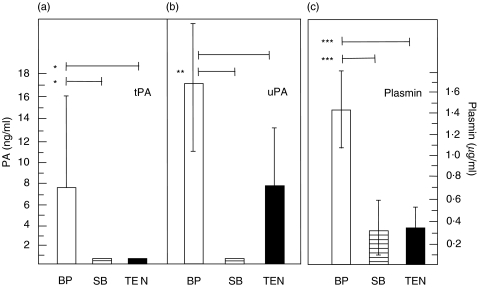
Elevated levels of plasmin and tPA, but not of uPA, were detected in blister fluid of BP patients compared to both suction blisters in healthy controls and patients with toxic epidermal necrolysis (TEN). Levels of plasmin, tPA, and uPA were analysed in blister fluid of BP patients and compared to levels in suction blisters of healthy controls and blisters of patients with TEN. In BP blisters, plasmin levels were significantly higher compared to both suction and TEN blisters (P < 0·001); tPA could only be detected in BP, but not in suction or TEN blisters. Levels of uPA in BP blisters were significantly higher compared to levels in suction blisters but not to those in TEN blisters (P = 0·005 and P = 0·173) (Fig. 1).
Fig. 1.
Elevated levels of plasmin and tPA were present in blister fluid of patients with bullous pemphigoid compared to both suction blisters in healthy controls and blisters of patients with toxic epidermal necrolysis. Blister fluid from patients with bullous pemphigoid (BP; n = 7; □), from suction blisters (SB) raised on the forearm of healthy volunteers (n = 7;  ), and from blisters in patients with toxic epidermal necrolysis (TEN; n = 4; ▪) were analysed for reactivity with (a) tPA, (b) uPA and (c) plasmin in duplicate by ELISA and chromogenic assay, respectively. Bars show mean ± SD (ng/ml for tPA and uPA, µg/ml for plasmin levels). Asterisks indicate statistical significance between levels in BP blister fluid compared to levels in both blister fluid of SB in healthy volunteers and of blisters in TEN patients (*P < 0·05, **P > 0·01, ***P < 0·001).
), and from blisters in patients with toxic epidermal necrolysis (TEN; n = 4; ▪) were analysed for reactivity with (a) tPA, (b) uPA and (c) plasmin in duplicate by ELISA and chromogenic assay, respectively. Bars show mean ± SD (ng/ml for tPA and uPA, µg/ml for plasmin levels). Asterisks indicate statistical significance between levels in BP blister fluid compared to levels in both blister fluid of SB in healthy volunteers and of blisters in TEN patients (*P < 0·05, **P > 0·01, ***P < 0·001).
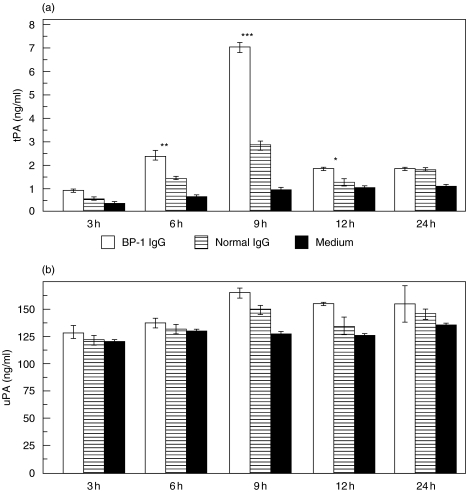
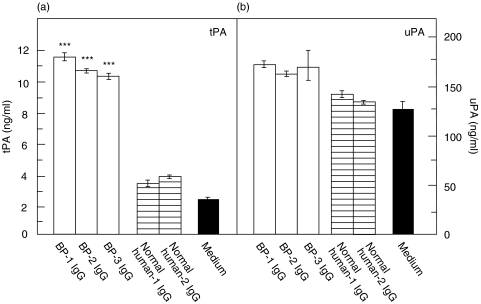
IgG from BP patients induced a time-dependent secretion of tPA from NHEK. Third passage NHEK were grown to 70–80% confluence and stimulated with 4 mg/ml IgG generated from patient BP-1 and a healthy control subject, respectively. After incubation periods of 6 h, 9 h, and 12 h, significantly elevated levels of tPA were detected in culture supernatants of NHEK stimulated with BP IgG compared to stimulation with normal IgG (P < 0·01, P < 0·001, and P < 0·05, respectively). After 9 h, tPA levels in supernatants reached a maximum (2·3-fold), whereas after a 3 h and 24 h incubation time, no increase of tPA concentrations were found compared to treatment with normal human IgG (P = 0·15 and P = 1·0, respectively) (Fig. 2a). In contrast, no elevated uPA secretion was observed under these conditions (Fig. 2b). Similar data were obtained using IgG obtained from R2296 and R8009 (data not shown). Subsequent experiments were therefore performed using an incubation time of 9 h. When NHEK were treated with 4 mg/ml affinity-purified IgG from patients BP-1, BP-2, and BP-3, tPA levels were increased by 2·7- to 3·0-fold compared to incubation with normal human IgG (P < 0·001). Again, uPA levels in the culture medium were not elevated compared to treatment with normal human IgG (Fig. 3).
Fig. 2.
Cultured normal human epidermal keratinocytes (NHEK) released tPA, but not uPA, in a time-dependent fashion after incubation with BP IgG. NHEK were incubated with 4 mg/ml IgG affinity-purified from the serum of a patient with bullous pemphigoid (BP-1 IgG; □), from a healthy control (normal IgG;  ), and with medium alone (▪), respectively. After incubation times of 3 h, 6 h, 9 h, 12 h, and 24 h, culture supernatants were analysed for (a) tPA and (b) uPA reactivity in quadruplicate by ELISA. Bars show mean ± SD (ng/ml). Asterisks indicate a statistically significant difference between BP IgG- and normal IgG-treated cells (*P < 0·05, **P > 0·01, ***P < 0·001). This pattern is representative of the pattern seen in 2 separate experiments with keratinocytes from different donors.
), and with medium alone (▪), respectively. After incubation times of 3 h, 6 h, 9 h, 12 h, and 24 h, culture supernatants were analysed for (a) tPA and (b) uPA reactivity in quadruplicate by ELISA. Bars show mean ± SD (ng/ml). Asterisks indicate a statistically significant difference between BP IgG- and normal IgG-treated cells (*P < 0·05, **P > 0·01, ***P < 0·001). This pattern is representative of the pattern seen in 2 separate experiments with keratinocytes from different donors.
Fig. 3.
Cultured normal human epidermal keratinocytes (NHEK) released tPA but not uPA in response to IgG purified from the serum of patients with bullous pemphigoid. Cultured NHEK were incubated with 4 mg/ml IgG from BP patients (BP-1, BP-2, BP-3; □), healthy controls (normal human-1, normal human-2;  ), and with medium alone (▪) for 9 h. Levels of (a) tPA and (b) uPA in the culture supernatants were analysed in quadruplicate by ELISA. Whereas tPA levels in response to BP IgG were about 3-fold higher compared to treatment with normal human IgG, uPA levels, although about 10-fold higher than tPA levels, were not different between the 2 groups. Bars indicate mean ± SD (ng/ml). Asterisks indicate a statistically significant difference between BP IgG- and normal IgG-treated cells (P < 0·001). This pattern is representative of the pattern seen in 2 separate experiments with keratinocytes from different donors.
), and with medium alone (▪) for 9 h. Levels of (a) tPA and (b) uPA in the culture supernatants were analysed in quadruplicate by ELISA. Whereas tPA levels in response to BP IgG were about 3-fold higher compared to treatment with normal human IgG, uPA levels, although about 10-fold higher than tPA levels, were not different between the 2 groups. Bars indicate mean ± SD (ng/ml). Asterisks indicate a statistically significant difference between BP IgG- and normal IgG-treated cells (P < 0·001). This pattern is representative of the pattern seen in 2 separate experiments with keratinocytes from different donors.
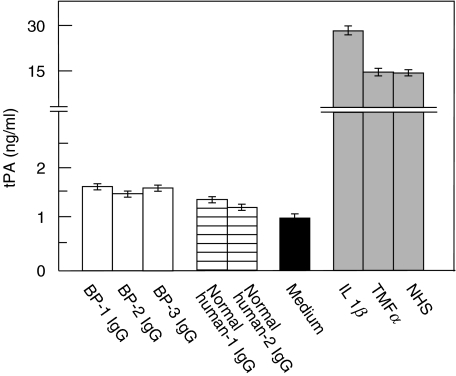
The tPA release from NHEK, upon stimulation with BP IgG, was mediated by antibodies to BP180. Keratinocytes from a patient with GABEB, that lack BP180 expression, were grown to 70–80% confluence. After treatment with 4 mg/ml IgG affinity-purified from our 3 bp patients, tPA levels in the culture supernatant were not increased compared to incubation with normal human IgG. In contrast, stimulation of GABEB cells with the known PA stimulators IL-1β, TNFα, or diluted normal human serum resulted in the release of high tPA levels into the culture medium (Fig. 4).
Fig. 4.
The tPA release from cultured human keratinocytes, upon incubation with BP IgG, was mediated by antibodies to BP180. BP180-deficient GABEB keratinocytes were treated for 9 h with 4 mg/ml IgG affinity-purified from 3 bp patients (BP-1, BP-2, BP-3; □), 2 healthy volunteers (controls (normal human-1, normal human-2)  ), medium alone (▪), and known inducers of tPA, including IL-1β, TNFα, and normal human serum (NHS;
), medium alone (▪), and known inducers of tPA, including IL-1β, TNFα, and normal human serum (NHS;  ). tPA levels were analysed in quadruplicate by ELISA. Bars show mean ± SD (ng/ml).
). tPA levels were analysed in quadruplicate by ELISA. Bars show mean ± SD (ng/ml).
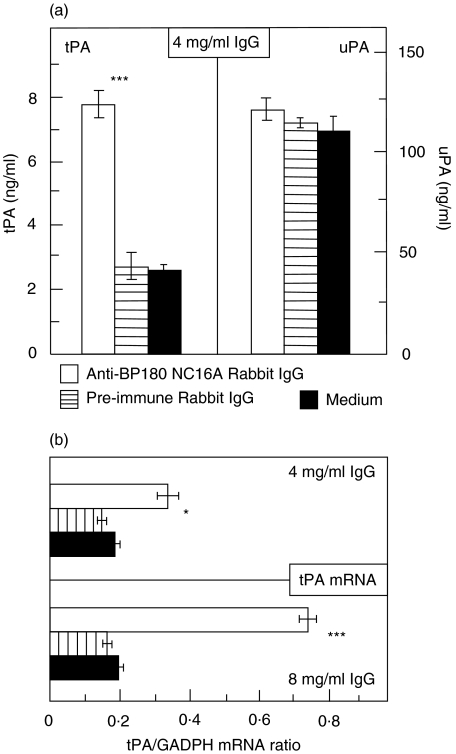
Treatment of NHEK with BP180 NC16A-specific antibodies resulted in increased levels of tPA, but not of uPA, at both protein and mRNA levels. When NHEK were incubated with R594 IgG for a period of 9 h, levels of tPA in the culture supernatant were 3·1-fold higher compared to levels in supernatants of NHEK treated with preimmune rabbit IgG. In contrast, under these conditions, uPA levels were not increased (Fig. 5a). When, after a 9 h incubation period, tPA and uPA mRNA levels were analysed by RT-PCR, tPA mRNA levels were found to be increased by 2-fold (P = 0·03); when NHEK were treated with 8 mg/ml R594 IgG, this difference was 4·2-fold (P < 0·001; Fig. 5b). Similar to the protein data, uPA mRNA levels were not elevated in R594 IgG-treated NHEK compared to NHEK incubated with preimmune rabbit IgG (P = 0·15 for 8 mg/ml IgG; P = 0·89 for 4 mg/ml IgG; data not shown). Similar data were obtained when R2296 and R8009 IgG and NHEK from a different donor were used (data not shown). Furthermore, a 9 h incubation of NHEK with 1 mg/ml human IgG that was affinity purified against BP180 NC16A2-4 resulted in a tPA release of 26·8 ± 1·0 ng/ml compared to 14·5 ± 4·1 ng/m tPA secreted after incubation of NHEK with IgG generated from serum of a healthy volunteer (1·9-fold difference, P < 0·001, data not shown). In contrast, no significantly different secretion of uPA was observed when NHEK were treated with 1 mg/ml BP180 NC16A2-4-specific human IgG for an incubation period of 9 h (92·0 ± 11·3 pg/ml) compared with incubation of NHEK with normal human IgG (102·4 ± 2·7 pg/ml, P = 0·12, data not shown).
Fig. 5.
Antibodies to BP180 induced both elevated tPA protein and mRNA levels in cultured normal human epidermal keratinocytes (NHEK). Cultured NHEK were treated for a 9 h incubation time with 4 and 8 mg/ml IgG from rabbit R594 (immunized against recombinant human BP180 NC16A; □), with preimmune rabbit IgG ( ), and with medium alone (▪). (a) Culture supernatants were assayed for tPA and uPA reactivity in quadruplicate by ELISA. Bars indicate mean ± SD (ng/ml). (b) tPA mRNA was detected by RT-PCR and expressed as ratio to GAPDH. Asterisks indicate statistical significant difference between R594 IgG- and preimmune IgG-treated cells (*P < 0·05, ***P < 0·001). This pattern is representative of the pattern seen in 2 separate experiments with keratinocytes from different donors.
), and with medium alone (▪). (a) Culture supernatants were assayed for tPA and uPA reactivity in quadruplicate by ELISA. Bars indicate mean ± SD (ng/ml). (b) tPA mRNA was detected by RT-PCR and expressed as ratio to GAPDH. Asterisks indicate statistical significant difference between R594 IgG- and preimmune IgG-treated cells (*P < 0·05, ***P < 0·001). This pattern is representative of the pattern seen in 2 separate experiments with keratinocytes from different donors.
DISCUSSION
In BP, the binding of autoantibodies to their target antigens is not sufficient to induce blisters, but must be accompanied by the activation of complement and the infiltration of inflammatory cells into the skin. Particularly, the subsequent release of proteases has been shown to be crucial for the blister formation [15–18]. Plasminogen activators have been implicated in the disease process of BP by several lines of evidence: (i) elevated plasmin activity has been found in the blister fluid of BP patients [35] (ii) tPA, but not uPA expression, has been detected in lesional epidermis of BP patients by immunohistochemistry, while uninvolved epidermis or epidermis of healthy controls did not express tPA [36,37] (iii) increased tPA, but not uPA activity, was found zymographically compared to both noninvolved BP epidermis and epidermis of healthy controls [38] (iv) elevated mRNA levels of tPA were present in keratinocytes of BP lesions but not in keratinocytes of uninvolved BP skin or skin of normal individuals [39], and (v) PA knock-out mice are no longer susceptible for the blister-inducing capacity of anti-BP180 antibodies in the experimental mouse model of BP [18].
In the first set of our experiments, elevated levels of tPA, uPA, and plasmin were detected in BP blisters compared to suction blisters in healthy volunteers. In contrast, only levels of tPA and plasmin, but not of uPA, were significantly increased in BP blisters compared to blisters in TEN patients. TEN blisters were chosen as an example for a noninfectious immunologically mediated subepidermal blistering disease that is not associated with circulating autoantibodies to the dermal-epidermal junction. These data indicate that tPA and plasmin are present at the site of blister formation and may be associated with the process of blister formation in BP. Since human keratinocytes produce both uPA and tPA [20], we were then interested whether the PA system is directly activated by the binding of anti-BP180 antibodies to their cell-surface receptor. For this purpose, cultured normal human epidermal keratinocytes (NHEK) were treated with IgG that had been affinity-purified from the serum of BP patients or rabbits, immunized against recombinant fragments of human BP180. In this system, we had previously observed that autoantibodies directed against the NC16A domain of BP180 lead to the expression and release of IL-6 and IL-8 [25].
When we treated NHEK with (i) BP patient total IgG (ii) BP180 NC16A-specific IgG generated from rabbits, or (iii) BP180 NC16A2-4-specific IgG affinity purified from a BP patient, elevated levels of tPA, but not of uPA, were detected in the culture supernatants. The specificity of this finding was demonstrated when BP180-deficient GABEB keratinocytes were found unable to secrete elevated tPA levels in response to anti-BP180 antibodies although they released high amounts of tPA after treatment with known inducers of tPA. PA levels detected in supernatants of untreated cells reflect the physiological secretion of both tPA and uPA by NHEK as previously described [20]. Interestingly, both background levels of uPA, as observed by treating NHEK with normal IgG or medium alone, and uPA levels in response to treatment with BP IgG were much higher than levels of tPA. This finding corresponds to the much greater expression of uPA in both NHEK, cultured in low Ca++ medium, and normal human epidermis [20,37,39].
In a further experiment, the induction of tPA, but not uPA, mRNA levels by BP180-specific antibodies in NHEK demonstrated that the release of tPA, in response to anti-BP180 antibodies, is at least in part due to a signal transducing event that leads to an increased tPA synthesis. Since the release of proteinases has been attributed to inflammatory cells recruited into BP lesions [10,11,15–18], our findings indicate a second, earlier event, involving the release of keratinocyte-derived tPA within a few hours after the binding of autoantibodies. Hence, keratinocytes appear to play an active role in the blister forming process of BP.
Pemphigus vulgaris (PV) is another blistering autoimmune disease characterized by intraepidermal blisters and autoantibodies against two desmosomal components, desmoglein 1 and desmoglein 3 [reviewed in 40,41]. Elevated mRNA levels and increased activity of tPA have also been found in lesional epidermis of PV patients [38,39]. This shows that tPA secretion from keratinocytes is not restricted to the binding of anti-BP180 antibodies. In contrast to our findings in BP, however, elevated uPA, but not tPA levels were detected in the culture supernatants of NHEK in response to PV IgG [42]. Furthermore, in contrast to experimental murine BP, PA knock-out mice are still susceptible to the acantholytic effect of anti-desmoglein antibodies speaking against a pathogenic relevance of the PA system in pemphigus [43].
In summary, our data show high levels of tPA at the site of blister formation in BP patients. The binding of anti-BP180 antibodies to their cell-surface antigen was demonstrated to result in an increased secretion of tPA, but not of uPA, from cultured NHEK and to mediate a signal-transducing event that leads to elevated tPA, but not uPA, mRNA levels in these cells. Our findings suggest both an active role of keratinocytes and the involvement of the PA system in blister formation of BP.
Acknowledgments
This work was supported by grant Zi 439/4–1 from the Deutsche Forschungsgemeinschaft (D.Z). Dr Boris Bastian, Würzburg, contributed in the initial phase of this study. We are grateful to Dr M. P. Marinkovich, Stanford, for kindly providing us with GABEB keratinocytes and to Dr George J. Giudice, Milwaukee, for rabbit serum 594. We thank Andrea Knopp and Katrin Müller-Blech, Würzburg, for excellent technical assistance.
REFERENCES
- Stanley JR, Hawley-Nelson P, Yuspa SH, Shevach EM, Katz SI. Characterisation of bullous pemphigoid antigen: a unique basement membrane protein of stratified squamous epithelia. Cell. 1981;24:987–1003. doi: 10.1016/0092-8674(81)90115-x. [DOI] [PubMed] [Google Scholar]
- Stanley JR, Tanaka T, Mueller S, Klaus-Kortun V, Roop D. Isolation of complementary DNA for bullous pemphigoid antigen by use of patients’ autoantibodies. J Clin Invest. 1988;82:1864–70. doi: 10.1172/JCI113803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz LA, Ratrie H, Saunders WS, Futamura S, Squiquera HL, Anhalt GJ, Giudice GJ. Isolation of human epidermal cDNA corresponding to the 180-kDa autoantigen recognized by bullous pemphigoid and herpes gestationis sera. J Clin Invest. 1990;86:1088–94. doi: 10.1172/JCI114812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol. 1992;99:243–50. doi: 10.1111/1523-1747.ep12616580. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Korman NJ, Shimizu H, Eady RAJ, Klaus-Kovtun V, Cehrs K, Stanley JR. Production of rabbit antibodies against carboxy-terminal epitopes encoded by bullous pemphigoid cDNA. J Invest Dermatol. 1990;94:617–23. doi: 10.1111/1523-1747.ep12876200. [DOI] [PubMed] [Google Scholar]
- Zillikens D, Giudice GJ. BP180/type XVII collagen: its role in acquired and inherited disorders or the dermal-epidermal junction. Arch Dermatol Res. 1999;291:187–94. doi: 10.1007/s004030050392. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Amagai M, Nishikawa T, Hashimoto T. The majority of bullous pemphigoid and herpes gestationis serum samples react with the NC16a domain of the 180-kDa bullous pemphigoid antigen. Arch Dermatol Res. 1996;288:507–9. doi: 10.1007/BF02505245. [DOI] [PubMed] [Google Scholar]
- Zillikens D, Mascaro JM, Rose PR, Liu Z, Ewing SM, Olague-Marchan M, Diaz LA, Giudice GJ. A highly sensitive enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Invest Dermatol. 1997;109:679–83. doi: 10.1111/1523-1747.ep12338088. [DOI] [PubMed] [Google Scholar]
- Perriard J, Jaunin F, Favre B, Büdinger L, Hertl M, Saurat JH, Borradori L. IgG autoantibodies from bullous pemphigoid (BP) patients bind antigenic sites on both the extracellular and the intracellular domains of the BP antigen 180. J Invest Dermatol. 1999;112:141–7. doi: 10.1046/j.1523-1747.1999.00497.x. [DOI] [PubMed] [Google Scholar]
- Naito K, Morioka S, Ogawa H. The pathogenic mechanisms of blister formation in bullous pemphigoid. J Invest Dermatol. 1982;79:303–6. doi: 10.1111/1523-1747.ep12500082. [DOI] [PubMed] [Google Scholar]
- Gammon WR, Merrit CC, Lewis DM, Sams WM, Jr, Carlo JR, Wheeler CE., Jr An in vitro model of immune complex-mediated basement membrane zone separation caused by pemphigoid antibodies, leukocytes, and complement. J Invest Dermatol. 1982;78:285–90. doi: 10.1111/1523-1747.ep12507222. [DOI] [PubMed] [Google Scholar]
- Liu Z, Diaz LA, Troy JL, Taylor AF, Emery DJ, Fairley JA, Giudice GJ. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J Clin Invest. 1993;92:2480–8. doi: 10.1172/JCI116856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaru C, Schmidt E, Petermann S, Munteanu LS, Bröcker EB, Zillikens D. Autoantibodies to BP180 induce dermal-epidermal separation in cryosections of human skin. J Invest Dermatol. 2002;118:664–71. doi: 10.1046/j.1523-1747.2002.01720.x. [DOI] [PubMed] [Google Scholar]
- Schmidt E, Obe K, Bröcker E-B, Zillikens D. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000;136:174–8. doi: 10.1001/archderm.136.2.174. [DOI] [PubMed] [Google Scholar]
- Liu Z, Shipley JM, Vu TH, Zhou X, Diaz LA, Werb Z, Senior RM. Gelatinase B-deficient mice are resistant to experimental bullous pemphigoid. J Exp Med. 1998;188:475–82. doi: 10.1084/jem.188.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Shapiro SD, Zhou X, Twining SS, Senior RM, Giudice GJ, Fairley JA, Diaz LA. A critical role for neutrophil elastase in experimental bullous pemphigoid. J Clin Invest. 2000;105:113–23. doi: 10.1172/JCI3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhou X, Shapiro SD, Shipley JM, Twining SS, Diaz LA, Senior RM, Werb Z. The serpin alpha1-proteinase inhibitor is a critical substrate for gelatinase B/MMP-9 in vivo. Cell. 2000;102:647–55. doi: 10.1016/s0092-8674(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Liu Z, Chen R, Diaz LA, Senior RM, Werb Z. Plasminogen/plasmin system in experimental bullous pemphigoid. J Invest Dermatol. 2000;114:778. (Abstract) [Google Scholar]
- Andreasen PA, Egelund R, Petersen HH. The plasminogen activation system in tumor growth invasion, and metastasis. Cell Mol Life Sci. 2000;57:25–40. doi: 10.1007/s000180050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PJ, John M, Baird J. Urokinase and tissue type plasminogen activators in human keratinocyte culture. Exp Cell Res. 1990;187:162–9. doi: 10.1016/0014-4827(90)90131-s. [DOI] [PubMed] [Google Scholar]
- Zillikens D, Kawahara Y, Ishiko A, et al. A novel subepidermal blistering disease with autoantibodies to a 200-kDa antigen of the basement membrane zone. J Invest Dermatol. 1996;106:1333–8. doi: 10.1111/1523-1747.ep12349283. [DOI] [PubMed] [Google Scholar]
- Balding SD, Diaz LA, Giudice GJ. A recombinant form of the human BP180 ectodomain forms a collagen-like, homotrimeric complex. Biochemistry. 1997;36:8821–30. doi: 10.1021/bi970675n. [DOI] [PubMed] [Google Scholar]
- Kiistala U. Suction blister device for separation of viable epidermis from dermis. J Invest Dermatol. 1968;50:129–37. doi: 10.1038/jid.1968.15. [DOI] [PubMed] [Google Scholar]
- Schmidt E, Bastian B, Dummer R, Tony HP, Bröcker E-B, Zillikens D. Detection of elevated levels of IL-4, IL-6, and IL-10 in blister fluid of bullous pemphigoid. Arch Dermatol Res. 1996;288:353–7. doi: 10.1007/BF02507102. [DOI] [PubMed] [Google Scholar]
- Schmidt E, Reimer S, Kruse N, Jainta S, Bröcker EB, Marinkovich MP, Giudice GJ, Zillikens D. Autoantibodies to BP180 associated with bullous pemphigoid release IL-6 and IL-8 from cultured human keratinocytes. J Invest Dermatol. 2000;115:842–8. doi: 10.1046/j.1523-1747.2000.00141.x. [DOI] [PubMed] [Google Scholar]
- Seitz CS, Giudice GJ, Balding SD, Marinkovich MP, Khavari PA. BP180 gene delivery in junctional epidermolysis bullosa. Gene Ther. 1999;6:42–7. doi: 10.1038/sj.gt.3300809. [DOI] [PubMed] [Google Scholar]
- Marinkovich MP, Tran HH, Rao SK, et al. LAD-1 is absent in a subset of junctional epidermolysis bullosa patients. J Invest Dermatol. 1997;109:356–9. doi: 10.1111/1523-1747.ep12336033. [DOI] [PubMed] [Google Scholar]
- Jones JM, Cohen RL, Chambers DA. Collagen modulates gene activation of plasminogen activator system molecules. Exp Cell Res. 2002;280:244–54. doi: 10.1006/excr.2002.5644. [DOI] [PubMed] [Google Scholar]
- Bator JM, Cohen RL. Chambers DA. Hydrocortisone regulates the dynamics of plasminogen activator and plasminogen activator inhibitor expression in cultured murine keratinocytes. Exp Cell Res. 1988;242:110–9. doi: 10.1006/excr.1998.4065. [DOI] [PubMed] [Google Scholar]
- Chen CS, Jensen PJ. Serum is a potent stimulator of keratinocyte tissue plasminogen activator expression. J Invest Dermatol. 1996;106:238–42. doi: 10.1111/1523-1747.ep12340609. [DOI] [PubMed] [Google Scholar]
- Rox JM, Reinartz J, Kramer MD. Interleukin-1 beta upregulates tissue-type plasminogen activator in a keratinocyte cell line (HaCaT) Arch Dermatol Res. 1996;288:554–8. doi: 10.1007/BF02505254. [DOI] [PubMed] [Google Scholar]
- Friberger P. Chromogenic peptide substrates. Their use for the assay of factors in the fibrinolytic and the plasma kallikrein-kinin systems. Scand J Clin Laboratory Invest. 1982;162:1–298. [PubMed] [Google Scholar]
- Aikens ML, Grenett HE, Benza RL, Tabengwa EM, Davis GC, Booyse FM. Alcohol-induced upregulation of plasminogen activators and fibrinolytic activity in cultured human endothelial cells. Alcohol Clin Exp Res. 1998;22:375–81. [PubMed] [Google Scholar]
- Tabengwa EM, Benza RL, Grenett HE, Booyse FM. Hypertriglyceridemic VLDL downregulates tissue plasminogen activator gene transcription through cis-repressive region (s) in the tissue plasminogen activator promoter in cultured human endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:1675–81. doi: 10.1161/01.atv.20.6.1675. [DOI] [PubMed] [Google Scholar]
- Kramer MD, Reinartz J. The autoimmune blistering skin disease bullous pemphigoid. The presence of plasmin/alpha 2-antiplasmin complexes in skin blister fluid indicates plasmin generation in lesional skin. J Clin Invest. 1993;92:978–83. doi: 10.1172/JCI116674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gissler HM, Simon MM, Kramer MD. Enhanced association of plasminogen/plasmin with lesional epidermis of bullous pemphigoid. Br J Dermatol. 1992;127:272–7. doi: 10.1111/j.1365-2133.1992.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Venning VA, Wojnarowska F, Cederholm-Williams S. An immunohistochemical study of the distribution of plasminogen and plasminogen activators in bullous pemphigoid. Clin Exp Dermatol. 1993;18:119–23. doi: 10.1111/j.1365-2230.1993.tb00990.x. [DOI] [PubMed] [Google Scholar]
- Jensen PJ, Baird J, Morioka S, Lessin S, Lazarus GS. Epidermal plasminogen activator is abnormal in cutaneous lesions. J Invest Dermatol. 1988;90:777–82. doi: 10.1111/1523-1747.ep12461494. [DOI] [PubMed] [Google Scholar]
- Baird J, Lazarus GS, Belin D, Vassalli JD, Busso N, Gubler P. Jensen. mRNA for tissue-type plasminogen activator is present in lesional epidermis from patients with psoriasis, pemphigus, or bullous pemphigoid, but is not detected in normal epidermis. J Invest Dermatol. 1990;95:548–52. doi: 10.1111/1523-1747.ep12504901. [DOI] [PubMed] [Google Scholar]
- Stanley JR. Bullous Pemphigoid. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, Fitzpatrick TB, editors. Dermatology in General Medicine. 5. New York: McGraw-Hill, Inc; 1999. pp. 666–73. [Google Scholar]
- Anhalt GJ, Diaz LA. Research advances in pemphigus. J Am Med Assoc. 2001;285:652–4. doi: 10.1001/jama.285.5.652. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Shafran KM, Webber PA, Lazarus GS, Singer KH. Anti-cell surface pemphigus autoantibody stimulates plasminogen activator activity of human epidermal cells. A mechanism for the loss of epidermal cohesion and blister formation. J Exp Med. 1983;157:259–72. doi: 10.1084/jem.157.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney MG, Wang ZH, Stanley JR. Pemphigus vulgaris and pemphigus foliaceus antibodies are pathogenic in plasminogen activator knockout mice. J Invest Dermatol. 1999;113:22–5. doi: 10.1046/j.1523-1747.1999.00632.x. [DOI] [PubMed] [Google Scholar]