Introduction
Many of the organ-specific autoimmune diseases affect endocrine organs. This is due to the fact that the organ-specific autoantigens are often hormones or enzymes specifically engaged in the synthetic pathways resulting in hormone production. Autoimmune endocrine diseases include type 1 diabetes, hypo- and hyperthyroidism, Addison's disease, hypoparathyroidism, and gonadal failure. These diseases may occur as a solitary immunological abnormality but often coincide as polyendocrine autoimmune diseases, indicating a common aetiology in the pathogenesis. Autoimmune endocrine diseases have complex genetic predispositions and they are closely associated to certain HLA haplotypes. In addition to HLA, other genes are known to regulate the disposition to autoimmunity. Only a few autoimmune diseases with a monogenic background are known. An example is a syndrome regulated by a single gene, namely APECED, an acronym for autoimmune polyendocrinopathy candidiasis ectodermal dystrophy, also known as autoimmune polyendocrinopathy syndrome type 1 (APS1). In this autosomal recessive disease, mutations in the AIRE (autoimmune regulator) gene cause organ-specific autoimmune destruction of several, mostly endocrine, tissues. This article reviews current knowledge of APECED and the AIRE gene behind this syndrome.
Apeced or autoimmune polyendocrinopathy syndrome type 1 (aps1)
APS1 (hereafter APECED) was first described in the 60s and 70s by Neufeld et al. [1] and Blizzard et al. [2] who described a rare syndrome affecting children in their early age. Later on, the group of Prof Perheentupa has significantly contributed to the clinical characterization of the syndrome [3,4]. Most of known autoimmune endocrine diseases may occur in patients with APECED, which they usually develop within their childhood and teenage years. Usually the first sign of the syndrome is chronic Candida infection, followed by autoimmune hypoparathyroidism and Addison's disease. At least two of these three major components need to be present for diagnosis. However, although the defect in APECED is inherited in an autosomal recessive manner, the clinical features are very heterogenous (Table 1). APECED is a rare disease but is more prevalent among some populations such as Finns (1/25 000) [5], Sardinians (1/14 400) [6] and Iranian Jews (1/9000) [7]. In Norway, the prevalence of APECED is reported as 1/80 000 [8].
Table 1.
The prevalence of the most common disease components of APECED, in Finnish patients. Adapted from [3]
| Manifestation | Prevalence (%) |
|---|---|
| Endocrine | |
| Hypoparathyroidism | 79 |
| Addison's disease | 72 |
| Ovarian failure | 60† |
| IDDM | 12 |
| Hypothyroidism | 4 |
| Nonendocrine | |
| Candidiasis | 100 |
| Enamel hypoplasia | 77 |
| Alopecia | 72 |
| Nail dystrophy | 52 |
| Keratopathy | 35 |
| Malabsorption | 18 |
| Vitiligo | 13 |
| Pernicious anaemia | 13 |
| Autoimmune hepatitis | 12 |
of postpubertal patients.
Hypoparathyroidism, appearing within the first decade of life, is the most frequent, and sometimes the only, endocrine disease seen in APECED patients. For example, in the majority of Iranian Jews, and in approximately 20% of Finnish patients, hypoparathyroidism remained the only endocrinopathy [4]. Hypoparathyroidism is often followed by adrenocortical insufficiency, with an age at onset of about 4–12 years [4,9] but in several cases it may appear at 20 years of age [4]. Premature ovarian failure in females is far more common than primary testicular failure in males. Type 1 diabetes, hypophyseal failure, and autoimmune thyroid disease deserve to be mentioned as less common disease components of the syndrome. Interestingly, recent studies have indicated that autoimmune gastrointestinal disorders, which are relatively common among APECED patients, are, in fact, due to the immune reaction to the endocrine cells of the stomach and intestine (see below).
The mucocutaneous candidiasis, appearing soon after birth, and the ectodermal dystrophy, affecting mainly nails and the tooth enamel, cannot be readily linked to autoimmunity. While the chronic candidiasis is most likely a part of the immunological dysregulation, the association of ectodermal dystrophy with autoimmunity is still open. Candida infection usually starts already within the first two years of life and appears in more chronic cases as oral thrush. Several cases of oral carcinoma have been reported, suggesting that oral candidiasis might be carcinogenic [10]. Of ectodermal skin diseases, alopecia, as patchy loss of hair, and vitiligo, as pigment-free skin areas, have been reported in approximately 40% and 25% of patients. In addition, urticaria-like erythema with fever was found in 9% of Finnish patients [4].
Splenic atrophy has been suspected to be relatively common among APECED patients. The exact reason for hypo- or asplenism is still open and it has been hypothesized to occur due to autoimmune-mediated destruction [11], or to depend on local AIRE gene dysfunction in the spleen [12].
Self antigens as targets of autoimmune attack
The main immunological finding in the endocrine disorders of APECED is the existence of high levels of serum antibodies reacting specifically with components of the affected organs (Table 2). The nature of these autoantigens became clarified mainly in the early 90 s. Earlier work had demonstrated the presence of antibodies to 3–4 specific antigens present either in the cytosomal, mitochondrial, or ribosomal fraction in Addison's disease [13–15]. In 1992, the first adrenal autoantigen, steroid 17α-hydroxylase (P450c17), was recognized by expressional library screening [16]. Later on, two other enzymes linked to the steroidogenic synthetic pathway, namely steroid 21-hydroxylase (P450c21) and side-chain cleavage enzyme (P450scc), were identified [17–19]. The three enzymes, P450c17, P450c21 and P450scc, belong to the cytochrome P450 superfamily and have significant similarities at the protein level. P450c21 is adrenal cortex specific; P450c17 and P450scc are also expressed in gonads, and the presence of autoantibodies to the latter two enzymes is also associated with hypogonadism in patients with APECED. The autoantibodies inhibit the steroidogenic enzyme activities in vitro [20], but a pathological role for the autoantibodies in vivo has not yet been proved [21].
Table 2.
Autoantigens in APECED. Only those autoimmune entities in APECED where the autoantigens are known are listed. Adapted from Heino et al. [56], Meriluoto et al. [90] and Immunobiology, 5th edition [91]
| APECED components | Tissue | Antigens |
|---|---|---|
| Addison's disease | Adrenals (cortex) | P450c21, P450c17a,P450scc |
| Hypoparathyroidism | Parathyroid glands | Ca++ sensing receptor* |
| Hypothyroidism | Thyroid gland | Thyroid peroxidase |
| Thyroglobulin | ||
| Type 1 diabetes | Endocrine pancreas | GAD65, GAD67, ICA,IA-2 tyrosine phosphatase like protein |
| Autoimmune hepatitis | Liver | P450 CYP1A2, P450 CYP2A6,P450 CYP1A1, P450 CYP2B6 AADC |
| Vitiligo | Skin | SOX9, SOX10 |
| Alopecia | Scalp | Tyrosine hydroxylase |
| Malabsorption | Gastrointestinal tract | Tryptophan hydroxylase |
| Autoimmune gastritis | Stomach | H+K+ ATPase |
| Pernicious anaemia | Gastric mucosa, red blood cells | Intrinsic factor |
This autoantigen in APECED has not been unequivocally proven.
Many other self-antigen targeting autoantibodies have been reported in APECED, often associated with a particular clinical manifestation (reviewed in [22,23]). In APECED, patient sera have specific autoantibodies to parathyroid glands as demonstrated by indirect immunofluorescence, and autoantibodies reacting with the calcium-sensing receptor, a protein specific to parathyroid glands, were initially reported [24], but this result was not supported by a more recent study by another group [25]. Autoantibodies to islet-cell specific autoantigens: GAD65, insulin, and IA2 are found in those with type 1 diabetes, and antithyroid peroxidase and thyroglobulin antibodies in APECED patients with thyroiditis (reviewed in [22]). Antibodies to another pancreatic islet cell expressed protein, aromatic l-amino acid decarboxylase, are described in APECED patients in association with chronic active hepatitis, vitiligo, or type 1 diabetes [26].
Interestingly, malabsorption appears to be a result of destruction of intestinal endocrine cells. Gastrointestinal dysfunction is associated with an autoimmune reaction to tryptophan hydroxylase in serotonin-producing enterochromaffin cells (EC) in the gastric antrum [27], histidine decarboxylase in histamine-producing enterochromaffin-like cells (ECL) in the gastric fundus [28], and to cholecystokinin-producing cells in the proximal portion of the small intestine [29].
It remains unknown why autoimmunity in APECED patients is predominantly targeted to proteins expressed in endocrine tissues and how the immune system specifically selects these antigens. Characteristically, many of the autoantibodies detected in the APECED syndrome are also found in corresponding sporadic clinical entities. The autoantibodies are often considered to arise as a consequence of tissue destruction, whereas the pathogenetic effect is mediated by T-cells. However, information about the cell-mediated immunity in APECED is still lacking. Despite this, the presence of autoantibodies can be used as a diagnostic marker as there generally is a good correlation between the autoantibodies and clinical disease and the appearance of autoantibodies often precedes the clinical manifestations.
Autoimmune regulator (aire)
Characteristics of AIRE
The AIRE gene, identified by positional cloning in 1997 by two independent groups, lies in chromosome 21q22.3 [30,31]. The gene, approximately 13 kb in length, contains 14 exons that encode a polypeptide of 545 amino acids [30,31]. Initial characterization of the AIRE protein, based on the amino acid sequence, revealed a conserved nuclear localization signal (NLS) in the N terminus; two plant homeodomain or PHD type zinc fingers and a proline rich region lying between these, in the C terminus; a SAND domain; and four LXXLL motifs, typical of nuclear receptor binding proteins [30–32]. It was soon discovered that the N terminus of AIRE also harbours an HSR domain, also found in Sp100 and Sp140, mediating Sp100-Sp100 homodimerization [33,34] (Fig. 1).
Fig. 1.
Schematic of the AIRE protein showing the functional protein domains, and the distribution of the APECED-causing mutations for which functional and/or localization data is available (see also Table 3). HSR: homogenously staining region; SAND: Sp100, AIRE, NucP41/75 and DEAF-1; PHD: plant homeodomain zinc finger; PRR, proline rich region; L, LXXLL nuclear receptor interaction motif.
The PHD zinc fingers are characteristically found in proteins involved in the regulation of transcription, typically at the chromatin level [35]. The structure is usually ascribed the function of mediating protein–protein interaction; at present more than 400 PHD finger-containing proteins are known [36]. The solution structures of the PHD fingers from KAP-1 and WSTF have been resolved, with results that support their role in mediating protein interactions [36,37]. The exact structure of the AIRE PHD zinc fingers has not been addressed, nor is there any data to corroborate the hypothesis that they mediate protein–protein interaction for AIRE. We and others have shown, however, that AIRE is a strong activator of transcription, and that the PHD fingers mediate this effect (see below and [38–40]).
The SAND domain is found in a number of proteins with various functions (Sp100, AIRE, NucP41/75 and DEAF-1) [41]. It was originally suggested to function as a DNA binding domain, and recent data following the resolution of its structure confirmed the assumption [41,42]. A signature motif in the SAND domain, KDWK, seems to mediate the DNA binding of nuclear deaf-1-related (NUDR) [42]. Interestingly, the SAND domain seems to coexist almost invariably, in other nuclear proteins as well as in AIRE, with other functional protein domains, including chromatin–associated and protein interaction-mediating motifs [42]. The KDWK motif, however, is not found in the AIRE protein. Also, there are no unequivocal data to date to show that the SAND domain mediates DNA binding for AIRE, although Kumar et al. have reported in a recent paper [43] that AIRE is able to bind DNA oligonucleotides as homodimers or tetramers. The AIRE protein as a monomer was unable to bind DNA [43]. Our unpublished experiments showed that AIRE might actually not directly associate with DNA, but rather do so indirectly via some, at present unknown, intermediary protein (J. Pitkänen and P. Peterson, unpublished observation).
The LXXLL motifs, four of which are found in the AIRE protein [30,31], mediate the binding of various proteins to nuclear receptors, mainly in a ligand-dependent manner [44]. These proteins then function as coactivators to nuclear receptors. Whether AIRE is somehow involved in mediating or regulating the effects of nuclear receptors remains to be seen, as no studies so far have addressed this interesting issue.
Expression pattern of AIRE
Human AIRE expression is found in several tissues; the most prominent of these being the thymus. Other sites of AIRE expression include the lymph nodes, the spleen, and fetal liver [30,31,45]. AIRE expression in human peripheral blood monocytes and differentiated dendritic cells has also been demonstrated [46]. No significant expression is seen in the target organs of autoimmune destruction [45,47]. In the thymus, AIRE expression is seen in a subpopulation of medullary epithelial cells (MEC) [47]. The expression of AIRE in thymic epithelial cells has been further confirmed by isolation of thymocytes, epithelial, and dendritic cells from mouse thymi, which were then analysed by RT-PCR. AIRE expression was mainly seen in epithelial cells and, to a lesser degree, in dendritic cells, but not in thymocytes [48].
In mouse, a similar AIRE expression pattern emerges, with the notable exception that more tissues have been found positive for mouse AIRE than its human counterpart [46,49–52]. In addition to the sites of expression of human AIRE discussed above, mouse AIRE is also found in the bone marrow, the urinary tract, the genitals, the alimentary tract, the respiratory tract, the brain, and in endocrine organs including the adrenals and the thyroid gland [46,49–52].
Disease-causing mutations
To date, at least 49 APECED-causing patient mutations have been identified (Table 3) [6,8,30,31,40,53–67]. The mutations are distributed throughout the coding region of the gene (Fig. 1). The main types of mutations consist of either nonsense or frame shift mutations resulting in a truncated polypeptide, or single amino acid-changing missense mutations. Most of the mutations occur in the functional protein domains of AIRE described above. Sixteen (33%) of these affect the HSR domain in the N terminus. With a single exception (a 36 bp deletion [57]), all are missense mutations. Seven (14%) mutations are found in the PHD fingers, four (8%) in the SAND domain, six (12%) in the PRR domain and three (6%) affect the LXXLL motifs.
Table 3.
The effect of APECED-causing mutations on the function and subcellular localization of the AIRE protein. In addition, the effect of mutations designed to disrupt the structure of the PHD zinc fingers are shown. C1/2G and C3/4G denote changing the first and second, or third and fourth, cysteines of the conserved PHD fingers to glycines, respectively. Adapted from Björses et al. Pitkänen et al. and Halonen (M. Halonen, 2003. Ph.D. thesis. University of Helsinki) [38,66]
| Mutation | Domain | Activation | Fibrils | Nuclear dots |
|---|---|---|---|---|
| Wild type | + + | + + | + + | |
| R15L | HSR | + | + + | + + |
| T16M | HSR | + | – | + |
| A21V | HSR | + | – | + |
| L28P | HSR | – | ++/–‡ | – |
| L29P | HSR | – | – | – |
| W78R | HSR | – | + + | + |
| V80L | HSR | + | + + | + + |
| K83E | HSR | + | + + | ++/–‡ |
| Y85C | HSR | + + | – | + + |
| Y90C | HSR | + | + + | + + |
| L93R | HSR | – | – | – |
| G228W | SAND | – | – | + |
| R257X | SAND | – | + + | – |
| C302P† | PHD1 | – | + + | + |
| C1/2G PHD1† | PHD1 | – | N.D. | N.D. |
| C3/4G PHD1† | PHD1 | – | N.D. | N.D. |
| C311Y | PHD1 | + | – | + |
| P326Q | PHD1 | + | + + | + + |
| L397fsX478 | PHD1 | – | – | – |
| C437P† | PHD2 | – | + + | + |
| C302P/C437P† | PHD1/2 | – | + + | – |
| C1/2G PHD2† | PHD2 | – | N.D. | N.D. |
not patientmutations
difference in results between authors; N.D. no data available.
In populations where APECED exists with a relatively high frequency, typical mutations for each population can be distinguished. The R257X nonsense mutation is the most prevalent APECED-causing mutation in Finnish patients, accounting for 83% of cases [30,31,66]. It is also frequently found in Eastern European and Northern Italian populations [53,60]. The Y85C mutation is typical for Iranian Jews [7,66], R139X among Sardinian patients [6], and the 967–979del13bp is the most common mutation in North American and British APECED patients [55,57,59].
Unlike several multifactorial autoimmune endocrine diseases, APECED does not have a clear HLA association, although some components of APECED seem to correlate with certain HLA haplotypes [40,68]. Furthermore, no definite genotype-phenotype correlation has been described, despite the significant variation in the phenotype and combinations of disease components. Thus, other genetic or environmental factors are likely to affect the manifestations of clinical disease. It should be noted, though, that candidiasis is exceedingly rare in Iranian Jews, all of whom share the Y85C mutation, and that candidiasis has not been seen in Finnish patients with the K83E mutation [7,31,66].
Subcellular localization and function of aire
AIRE is located in nuclear dots
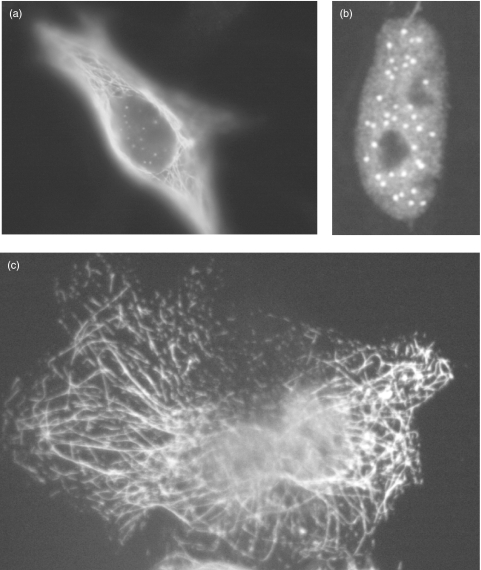
In both human and mouse thymi, AIRE is located in nuclear dots, as revealed by immunofluorescence staining by specific antibodies. This pattern has also been found in peripheral blood monocytes [45,47,66]. In cultured cells transfected with an AIRE-expressing plasmid, a fibrillar cytoplasmic staining is also seen, in addition to nuclear dots. Under these conditions, a diffuse nuclear staining is evident in a minority of cells [38,45,47,66,69] (Fig. 2). Our results have confirmed that the AIRE nuclear localization signal is functional [38].
Fig. 2.
Subcellular localization of the AIRE protein, as illustrated by immunofluorescence staining of AIRE-expressing cells with a monoclonal anti-AIRE antibody. Three typical staining patterns are demonstrated. (a) in this cell AIRE is seen both in the cytoplasm and nucleus. (b) AIRE is seen only in the nucleus, as a diffuse staining, and in nuclear dots. (c) AIRE is seen only in the cytoplasm in typical filaments.
The nuclear dot staining of AIRE is somewhat similar to that seen with the promyelocytic leukaemia protein (PML) in PML bodies [33,45,47,69,70]. Despite the similarities in the staining patterns of AIRE and PML, no colocalization was seen in COS or HeLa cells, either with PML or Sp100, another PML body-occupying protein [45,69].
AIRE is a transcriptional activator
The structural features of the AIRE protein strongly hint at a function in transcriptional regulation, as many of the proteins sharing these protein domains are involved in the control or modulation of transcription, including activation and chromatin modifying complexes. There is evidence that AIRE itself can be found in larger, presumably multiprotein, complexes (M. Halonen, 2003. PhD thesis. University of Helsinki). These other proteins, however, remain unidentified. The only protein interaction partner described for AIRE is the CREB-binding protein CBP that directly binds human AIRE [39]. CBP is thought to be an integrator of multiple signalling pathways (see [71] for review). It has been shown to function as coactivator to several proteins, including the STAT proteins, Jun, Fos, NFκB, and nuclear receptors [44,72–75]. Based on our results, CBP and AIRE also colocalize in nuclear dots in mammalian cells (J. Pitkänen and P. Peterson, unpublished observation).
Both human and mouse AIRE have been shown to be strong activators of transcription [38,39,66]. Two model systems have been used with similar results. One employed the GAL4 one hybrid assay, where AIRE is fused with a GAL4 DNA binding domain. In this system, the reporter gene is preceded by a minimal promoter and a GAL4 binding site [66]. In the other system, we have used the interferon beta minimal promoter (nucleotides −55 to + 19) upstream of a luciferase reporter gene, with no artificial binding sites [38]. The relative activation of reporter genes under these different promoters ranged from 30 to 250 fold. It was then shown that the PHD finger region of AIRE is sufficient for the activation function, although other regions of the protein may modulate this effect [38] and (M. Halonen, 2003. Ph.D. thesis University of Helsinki). The results were confirmed by the fact that missense mutations affecting the PHD fingers (APECED-causing mutations and others designed to disrupt the structure of the PHD fingers) resulted in significantly reduced activation (Table 3) [38,66]. The PHD fingers are also important for the localization of AIRE in nuclear dots, and in nuclear entry [38,66,76].
The HSR domain determines cytoplasmic localization
The fibrillar structures where AIRE resides in cultured cells resemble intermediate filaments, and colocalization with vimentin and α tubulin have been reported [45,47,69]. The N terminal HSR domain of AIRE is responsible for the targeting to these cytoplasmic filaments. Several patient mutations in this region lead to the dissolution of fibrillar staining (Table 3) [38,76]. In addition to localization into cytoplasmic filaments, the HSR domain, which forms a four-helix bundle structure (based on homology modelling [39]), mediates also AIRE-AIRE homodimerization [38,66,76]. Some APECED-causing missense mutations in the HSR domain also disrupt the transcriptional activation imposed by AIRE, but interestingly some mutations have no effect on the activation [38,39,66]. A good example is the Y85C mutation which shows activation and nuclear dot staining indistinguishable from the wild type protein, the only difference being the lack of cytoplasmic filament binding by the mutant protein (Table 3) [38,66]. Thus, no clear correlation between the filamentous staining and transactivation can be drawn.
AIRE IS CONTROLLED BY LYMPHOTOXIN – RelB AXIS
The limited expression of AIRE in specific antigen presenting cells, like thymic medullary epithelium and dendritic cells, suggests tight control over its expression. The human AIRE promoter was recently characterized by our group [77]. The minimal promoter region contains functional binding sites for common transcription factors such as AP-1, NF-Y and Sp-1, as well as a functional TATA-box. In contrast, the silenced status of the AIRE promoter in other tissues than thymic epithelium and dendritic cells is most likely achieved by epigenetic control mechanisms at the chromatin level, regulated by CpG island methylation within the promoter, and histone deacetylation [77].
Recent data has also shed light on the mechanisms how AIRE is regulated in thymic medullary epithelium, pointing to the involvement of the lymphotoxin pathway. Previous analyses of lymphotoxin pathway members in mutant mice have established its importance in proper organogenesis of secondary lymphoid tissues as well as in the maintenance of their microarchitecture (reviewed in [78]). Although both LTβ and LTβR are highly expressed in the thymus [79], the thymus development and microarchitecture appear normal in defective mice [80]. In contrast, the lymphotoxin pathway in the thymus has a critical role in negative selection as blockage of newly described LTβR ligand LIGHT signalling prevents thymocyte apoptosis, and overexpression in a transgenic mouse results in increased deletion of CD4 CD8 double positive cells [81].
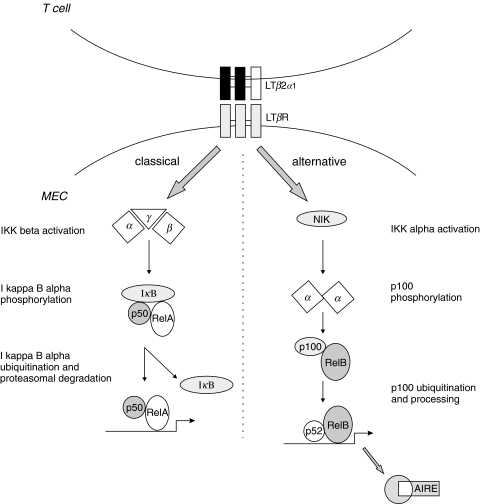
Critical evidence was recently added to this mosaic by showing that the lymphotoxin pathway indeed regulates the expression of AIRE and that of an ectopic peripheral tissue antigen (insulin) in the thymus [82] (Fig. 3). In both LTα- and LTRβ-deficient mice, the expression of AIRE is reduced to 20–25% of that observed in the wild type, and by reconstituting the lymphotoxin pathway, AIRE expression could be induced several fold. Activation through LTβR induces the recently described alternative NF-κB pathway, which in contrast to the classical p50-RelA NF-κB pathway, leads to the specific activation and nuclear accumulation of p52-RelB heterodimers (reviewed in [83]). There is no doubt that both pathways share significant cross-talk because LTβR is also able to induce formation of p50-RelA complexes. Despite that, the subtle differences between NF-κB family member complexes are likely to result in the activation of distinct or only partially overlapping gene sets. The hypothesis that AIRE expression is controlled by the lymphotoxin – RelB axis is further enhanced by results of an analysis of thymic AIRE expression in the RelB deficient mouse, which showed absence of AIRE transcript and protein [48,50]. It can be assumed that other members of the NF-κB alternative pathway are also important in the regulation of AIRE expression.
Fig. 3.
AIRE expression is controlled by the lymphotoxin pathway. The classical (a) and alternative (b) paths of activation through the lymphotoxin beta receptor are shown. LTα and LTβ: lymphotoxin alpha and beta, respectively; LTβR, lymphotoxin beta receptor; IKK alpha and beta, I kappa B kinase alpha and beta, respectively. Adapted from Derudder et al., Muller et al. and Mordmuller et al. [92–94].
Thymocytes in both cortical and medullary regions of the thymus strongly express LTβ, whereas in the adult thymus, LTβ is expressed mainly by medullary thymocytes [79]. In turn, LTβR in the thymus is predominantly expressed by epithelium, indicating that the presence of surrounding thymocytes is needed for AIRE expression in medullary epithelium. The notion that AIRE is dependent on the presence of thymic haematopoietic cells and on normal thymic microarchitecture is supported by the finding that AIRE expression was absent in transgenic Tge26 mice (overexpression of the human CD3 e-chain), in which thymocyte development is blocked before the double negative CD44+ CD25+ stage at E14·5. This also correlates with the start of AIRE expression in a late organogenesis stage of the thymus at E14·5 [48,49]. Another model with abnormal AIRE expression in the thymus and thymic ultrastructural anomalies is the diabetes prone NOD mouse [50,84,85].
Aire regulates thymic expression of self-antigens
Evidence about the essential role of AIRE in central tolerance has emerged recently by the disruption of the AIRE gene in mice. The first AIRE deficient mouse was reported by Ramsey et al. [86], who introduced a stop codon in exon 6, which mimics the prevalent mutation (R257X) found in humans. The overall development of T and B cells appeared normal but the mice had autoantibodies against several peripheral tissues such as the adrenal cortex, exocrine and endocrine pancreas, spermatogonia in testis, and hepatocytes. Although no apparent autoimmune tissue destruction was observed, tissues of several organs had lymphocytic infiltration. In particular, periportal accumulation of lymphocytes in the liver was found in half of the mice, resembling the situation in the RelB deficient mouse. The mice were also hyperresponsive to foreign antigen immunization, exemplified by the fact that when challenged with HEL antigen, the peripheral T cells had a 3–5-fold increased proliferation response.
Similar levels of autoantibodies and lymphocyte infiltrates in several organs were seen in another AIRE targeted mouse, created by deleting exon 2 of the gene [87]. In accordance with the first AIRE deficient mouse, no major changes were observed in the standard cytofluorimetric, functional and histological studies of the immune system apart from a two-fold increase in the number of thymic medullary epithelial cells and a near-doubling in the frequency of activated or memory CD44hiCD62Llow T cells in the peripheral lymphoid tissue. In a radiation bone marrow chimera experiment the autoimmune phenotype was seen only in mice lacking AIRE expression in radioresistant cells. This was followed by a grafting experiment where thymi harvested from both AIRE–/– and wild type animals were depleted of haematopoietic cells and grafted into athymic nude mice. The AIRE–/– thymi transferred the disease to the recipients, supporting the idea that AIRE would exert its effect mainly in the thymic epithelial cells. This was further corroborated by the results of a lymphocyte compartment reconstitution experiment: lymphocytes from AIRE–/– mice were able to transfer the disease to alymphoid Rag°/° AIRE+/+ recipients; peripheral AIRE expression could not protect from the disease.
In order to address the mechanism how AIRE protects from autoimmunity, Anderson et al. [87] isolated RNA from thymic stromal cells and studied the relative abundance of 12 000 expressed transcripts with cDNA microarrays. They hypothesized that AIRE would control the expression of tissue specific genes in the thymus. In the thymus, developing thymocytes with reactivity to self-antigens undergo negative selection by clonal deletion in the thymic medulla. It has been shown that many tissue-specific self antigens are, in fact, expressed in the thymus and that the higher expression levels usually lead to negative selection whereas low level expression allows autoreactive thymocytes to escape clonal deletion. This is in accordance with the ‘promiscuous (ectopic) gene expression’–model of autoimmunity proposed by Derbinski et al. [88]. It indeed turned out that the expression of multiple tissue specific genes is decreased or abolished in samples obtained from AIRE–/– mice. These included salivary proteins 1 and 2, preproinsulin, cytochrome P4501A2, casein alpha, and zona pellucida glycoprotein 3. These proteins are specifically expressed in the tissues where autoantibody stainings and lymphocytic infiltrations were seen.
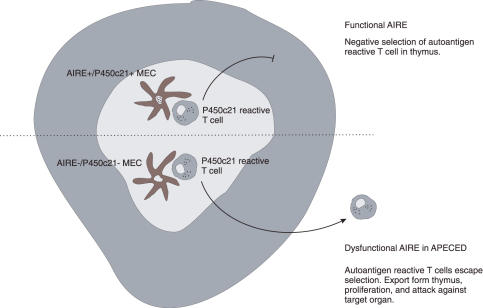
Taken together, findings from the AIRE deficient mice demonstrate a role for AIRE in the induction of central tolerance through the regulation of self-antigen gene expression in the antigen presenting cells of the thymic medulla (Fig. 4). This is also supported by the failure of AIRE–/– mice to negatively select high-avidity, organ-specific T-cells in a transgenic TCR-InsHEL system [89]. The authors further verified the thymic involvement by using chimeric mice with defective AIRE in either haematopoietic or nonhaematopoietic compartments. Following reconstitution, AIRE–/– thymi failed to negatively select the donor T-cells. Any significant role for the CD4+CD25+ regulatory T cells was also ruled out. However, the door is left open for an additional peripheral mechanism mediated by the radioresistant cells of the immune system.
Fig. 4.
AIRE and negative selection in the thymus. One of the APECED autoantigens, P450c21, is used as an example. Under normal circumstances (upper portion), AIRE drives the expression of autoantigens in thymic medullary epithelial cells (MECs), resulting finally in the negative selection of T cells reactive to these autoantigens. In APECED, with dysfunctional AIRE, the autoreactive T cells escape selection with the inevitable consequence of autoimmune attack against the target organs expressing the autoantigens.
Conclusions
Although rare, APECED proves to be an exciting model to study autoimmunity. Starting from the first descriptions of the APECED patients in the seventies, research on this immunodeficiency syndrome has developed to the understanding of molecular tolerance defects behind the disease. Recent findings showing that AIRE controls thymic expression of peripheral self-antigens open further directions and questions. It is still unclear how AIRE at the molecular level, as a transcriptional regulator, directs the transcription of self-antigens, and what the other proteins involved in this process are. Considering that AIRE is regulated by the lymphotoxin–RelB pathway in thymic epithelial cells, other members of this pathway may influence the ectopic expression of self-antigens in thymus. In addition to central tolerance, the possible role of AIRE in peripheral tolerance, presumably by functioning in dendritic cells, needs further studies. Significant achievements during the recent years in this field promise further exciting advances in understanding the induction and maintenance of immune tolerance.
Acknowledgments
The authors are supported by grants from: the Tampere University Hospital Medical Research Fund (J.P., N.S., P.P), the Pirkanmaa Regional Fund of the Finnish Cultural Foundation (J.P), the Emil Aaltonen Foundation (J.P), The Finnish Medical Foundation (J.P), the Finnish Academy (P.P), and the Sigrid Juselius Foundation (P.P) and Wellcome Trust (P.P).
References
- Neufeld M, Maclaren NK, Blizzard RM. Two types of autoimmune Addison's disease associated with differen polyglandular autoimmune (PGA) syndromes. Medicine (Baltimore) 1981;60:355–62. doi: 10.1097/00005792-198109000-00003. [DOI] [PubMed] [Google Scholar]
- Blizzard RM, Kyle M. Studies of the adrenal antigens and antibodies in Addison's disease. J Clin Invest. 1963;42:1653–60. doi: 10.1172/JCI104851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahonen P, Myllärniemi S, Sipila I, et al. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med. 1990;322:1829–36. doi: 10.1056/NEJM199006283222601. [DOI] [PubMed] [Google Scholar]
- Perheentupa JAPS-I APECED. the clinical disease and therapy. Endocrinol Metabol Clin N Am. 2002;31:295–320. doi: 10.1016/s0889-8529(01)00013-5. [DOI] [PubMed] [Google Scholar]
- Björses P, Aaltonen J, Vikman A, et al. Genetic homogeneity of autoimmune polyglandular disease type I. Am J Human Genet. 1996;59:879–86. [PMC free article] [PubMed] [Google Scholar]
- Rosatelli MC, Meloni A, Devoto M, et al. A common mutation in Sardinian autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy patients. Hum Genet. 1998;103:428–34. doi: 10.1007/s004390050846. [DOI] [PubMed] [Google Scholar]
- Zlotogora J, Shapiro MS. Polyglandular autoimmune syndrome type I among Iranian Jews. J Med Genet. 1992;29:824–6. doi: 10.1136/jmg.29.11.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhre AG, Halonen M, Eskelin P, et al. Autoimmune polyendocrine syndrome type 1 (APS I) in Norway. Clin Endocrinol. 2001;54:211–7. doi: 10.1046/j.1365-2265.2001.01201.x. [DOI] [PubMed] [Google Scholar]
- Pearce SH, Cheetham TD. Autoimmune polyendocrinopathy syndrome type 1: treat with kid gloves. Clin Endocrinol. 2001;54:433–5. doi: 10.1046/j.1365-2265.2001.01250.x. [DOI] [PubMed] [Google Scholar]
- Richman RA, Rosenthal IM, Solomon LM, et al. Candidiasis and multiple endocrinopathy. With oral squamous cell carcinoma complications. Arch Dermatol. 1975;111:625–7. [PubMed] [Google Scholar]
- Friedman TC, Thomas PM, Fleisher TA, et al. Frequent occurrence of asplenism and cholelithiasis in patients with autoimmune polyglandular disease type I. Am J Med. 1991;91:625–30. doi: 10.1016/0002-9343(91)90215-j. [DOI] [PubMed] [Google Scholar]
- Starzyk J, Kumorowicz-Kopiec M, Kowalczyk M, et al. Natural history of asplenism in APECED – patient report. J Pediatr Endocrinol Metab. 2001;14:443–9. doi: 10.1515/jpem.2001.14.4.443. [DOI] [PubMed] [Google Scholar]
- Krohn K, Perheentupa J, Heinonen E. Precipitating anti-adrenal antibodies in Addison's disease. Clin Immunol Immunopathol. 1974;3:59–68. doi: 10.1016/0090-1229(74)90023-3. [DOI] [PubMed] [Google Scholar]
- Irvine WJ, Barnes EW. Addison's disease, ovarian failure and hypoparathyroidism. Clin Endocrinol Metab. 1975;4:379–434. [Google Scholar]
- Goudie RB, McDonald E, Anderson JR, et al. Immunological features of idiopathic Addison's disease: characterization of the adrenocortical antigens. Clin Exp Immunol. 1968;3:119–31. [PMC free article] [PubMed] [Google Scholar]
- Krohn K, Uibo R, Aavik E, et al. Identification by molecular cloning of an autoantigen associated with Addison's disease as steroid 17 alpha-hydroxylase. Lancet. 1992;339:770–3. doi: 10.1016/0140-6736(92)91894-e. [DOI] [PubMed] [Google Scholar]
- Winqvist O, Gustafsson J, Rorsman F, et al. Two different cytochrome P450 enzymes are the adrenal antigens in autoimmune polyendocrine syndrome type I and Addison's disease. J Clin Invest. 1993;92:2377–85. doi: 10.1172/JCI116843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winqvist O, Karlsson FA, Kampe O. 21-Hydroxylase, a major autoantigen in idiopathic Addison's disease. Lancet. 1992;339:1559–62. doi: 10.1016/0140-6736(92)91829-w. [DOI] [PubMed] [Google Scholar]
- Uibo R, Aavik E, Peterson P, et al. Autoantibodies to cytochrome P450 enzymes P450scc, P450c17, and P450c21 in autoimmune polyglandular disease types I and II and in isolated Addison's disease. J Clin Endocrinol Metab. 1994;78:323–8. doi: 10.1210/jcem.78.2.8106620. [DOI] [PubMed] [Google Scholar]
- Furmaniak J, Kominami S, Asawa T, et al. Autoimmune Addison's disease – evidence for a role of steroid 21-hydroxylase autoantibodies in adrenal insufficiency. J Clin Endocrinol Metabolism. 1994;79:1517–21. doi: 10.1210/jcem.79.5.7962352. [DOI] [PubMed] [Google Scholar]
- Boscaro M, Betterle C, Volpato M, et al. Hormonal responses during various phases of autoimmune adrenal failure: no evidence for 21-hydroxylase enzyme activity inhibition in vivo. J Clin Endocrinol Metabolism. 1996;81:2801–4. doi: 10.1210/jcem.81.8.8768833. [DOI] [PubMed] [Google Scholar]
- Betterle C, Dal Pra C, Mantero F, et al. Autoimmune adrenal insufficiency and autoimmune polyendocrine syndromes: autoantibodies, autoantigens, and their applicability in diagnosis and disease prediction. Endocr Rev. 2002;23:327–64. doi: 10.1210/edrv.23.3.0466. [DOI] [PubMed] [Google Scholar]
- Weetman AP. Autoimmunity to steroid-producing cells and familial polyendocrine autoimmunity. Baillieres Clin Endocrinol Metabolism. 1995;9:157–74. doi: 10.1016/s0950-351x(95)80899-x. [DOI] [PubMed] [Google Scholar]
- Li Y, Song YH, Rais N, et al. Autoantibodies to the extracellular domain of the calcium sensing receptor in patients with acquired hypoparathyroidism. J Clin Invest. 1996;97:910–4. doi: 10.1172/JCI118513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gylling M, Kaariainen E, Vaisanen R, et al. The hypoparathyroidism of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy protective effect of male sex. J Clin Endocrinol Metab. 2003;88:4602–8. doi: 10.1210/jc.2003-030700. [DOI] [PubMed] [Google Scholar]
- Husebye ES, Gebre-Medhin G, Tuomi T, et al. Autoantibodies against aromatic 1-amino acid decarboxylase in autoimmune polyendocrine syndrome type I. J Clin Endocrinol Metabolism. 1997;82:147–50. doi: 10.1210/jcem.82.1.3647. [DOI] [PubMed] [Google Scholar]
- Ekwall O, Hedstrand H, Grimelius L, et al. Identification of tryptophan hydroxylase as an intestinal autoantigen. Lancet. 1998;352:279–83. doi: 10.1016/S0140-6736(97)11050-9. [DOI] [PubMed] [Google Scholar]
- Sköldberg F, Portela-Gomes GM, Grimelius L, et al. Histidine decarboxylase, a pyridoxal phosphate-dependent enzyme, is an autoantigen of gastric enterochromaffin-like cells. J Clin Endocrinol Metabolism. 2003;88:1445–52. doi: 10.1210/jc.2002-021761. [DOI] [PubMed] [Google Scholar]
- Hogenauer C, Meyer RL, Netto GJ, et al. Malabsorption due to cholecystokinin deficiency in a patient with autoimmune polyglandular syndrome type I. N Engl J Med. 2001;344:270–4. doi: 10.1056/NEJM200101253440405. [DOI] [PubMed] [Google Scholar]
- The Finnish-German APECED Consortium. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy. Nat Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- Nagamine K, Peterson P, Scott HS, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–8. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- Mittaz L, Rossier C, Heino M, et al. Isolation and characterization of the mouse Aire gene. Biochem Biophys Res Commun. 1999;255:483–90. doi: 10.1006/bbrc.1999.0223. [DOI] [PubMed] [Google Scholar]
- Sternsdorf T, Grotzinger T, Jensen K, et al. Nuclear dots: actors on many stages. Immunobiology. 1997;198:307–31. doi: 10.1016/S0171-2985(97)80051-4. [DOI] [PubMed] [Google Scholar]
- Sternsdorf T, Jensen K, Reich B, et al. The nuclear dot protein sp100, characterization of domains necessary for dimerization, subcellular localization, and modification by small ubiquitin-like modifiers. J Biol Chem. 1999;274:12555–66. doi: 10.1074/jbc.274.18.12555. [DOI] [PubMed] [Google Scholar]
- Aasland R, Gibson TJ, Stewart FA. The PHD finger: implications for chromatin-mediated transcriptional regulation. Trends Biochem Sci. 1995;20:56–9. doi: 10.1016/s0968-0004(00)88957-4. [DOI] [PubMed] [Google Scholar]
- Capili AD, Schultz DC, Rauscher FJ, III, et al. Solution structure of the PHD domain from the KAP-1 corepressor: structural determinants for PHD, RING and LIM zinc-binding domains. EMBO J. 2001;20:165–77. doi: 10.1093/emboj/20.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual J, Martinez-Yamout M, Dyson HJ, et al. Structure of the PHD zinc finger from human Williams–Beuren syndrome transcription factor. J Mol Biol. 2000;304:723–9. doi: 10.1006/jmbi.2000.4308. [DOI] [PubMed] [Google Scholar]
- Pitkänen J, Vähämurto P, Krohn K, et al. Subcellular localization of the autoimmune regulator protein. characterization of nuclear targeting and transcriptional activation domain. J Biol Chem. 2001;276:19597–602. doi: 10.1074/jbc.M008322200. [DOI] [PubMed] [Google Scholar]
- Pitkänen J, Doucas V, Sternsdorf T, et al. The autoimmune regulator protein has transcriptional transactivating properties and interacts with the common coactivator CREB-binding protein. J Biol Chem. 2000;275:16802–9. doi: 10.1074/jbc.M908944199. [DOI] [PubMed] [Google Scholar]
- Halonen M, Eskelin P, Myhre AG, et al. AIRE mutations and human leukocyte antigen genotypes as determinants of the autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy phenotype. J Clin Endocrinol Metab. 2002;87:2568–74. doi: 10.1210/jcem.87.6.8564. [DOI] [PubMed] [Google Scholar]
- Gibson TJ, Ramu C, Gemund C, et al. The APECED polyglandular autoimmune syndrome protein, AIRE-1, contains the SAND domain and is probably a transcription factor. Trends Biochem Sci. 1998;23:242–4. doi: 10.1016/s0968-0004(98)01231-6. [DOI] [PubMed] [Google Scholar]
- Bottomley MJ, Collard MW, Huggenvik JI, et al. The SAND domain structure defines a novel DNA-binding fold in transcriptional regulation. Nat Struct Biol. 2001;8:626–33. doi: 10.1038/89675. [DOI] [PubMed] [Google Scholar]
- Kumar PG, Laloraya M, Wang CY, et al. The Autoimmune Regulator (AIRE) Is a DNA-binding Protein. J Biol Chem. 2001;276:41357–64. doi: 10.1074/jbc.M104898200. [DOI] [PubMed] [Google Scholar]
- Heery DM, Kalkhoven E, Hoare S, et al. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–6. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- Björses P, Pelto-Huikko M, Kaukonen J, et al. Localization of the APECED protein in distinct nuclear structures. Hum Mol Genet. 1999;8:259–66. doi: 10.1093/hmg/8.2.259. [DOI] [PubMed] [Google Scholar]
- Kogawa K, Nagafuchi S, Katsuta H, et al. Expression of AIRE gene in peripheral monocyte/dendritic cell lineage. Immunol Lett. 2002;80:195–8. doi: 10.1016/s0165-2478(01)00314-5. [DOI] [PubMed] [Google Scholar]
- Heino M, Peterson P, Kudoh J, et al. Autoimmune regulator is expressed in the cells regulating immune tolerance in thymus medulla. Biochem Biophys Res Commun. 1999;257:821–5. doi: 10.1006/bbrc.1999.0308. [DOI] [PubMed] [Google Scholar]
- Zuklys S, Balciunaite G, Agarwal A, et al. Normal thymic architecture and negative selection are associated with Aire expression, the gene defective in the autoimmune-polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) J Immunol. 2000;165:1976–83. doi: 10.4049/jimmunol.165.4.1976. [DOI] [PubMed] [Google Scholar]
- Blechschmidt K, Schweiger M, Wertz K, et al. The mouse Aire gene: comparative genomic sequencing, gene organization, and expression. Genome Res. 1999;9:158–66. [PMC free article] [PubMed] [Google Scholar]
- Heino M, Peterson P, Sillanpaa N, et al. RNA and protein expression of the murine autoimmune regulator gene (Aire) in normal, RelB-deficient and in NOD mouse. Eur J Immunol. 2000;30:1884–93. doi: 10.1002/1521-4141(200007)30:7<1884::AID-IMMU1884>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Ruan QG, Wang CY, Shi JD, et al. Expression and alternative splicing of the mouse autoimmune regulator gene (Aire) J Autoimmun. 1999;13:307–13. doi: 10.1006/jaut.1999.0326. [DOI] [PubMed] [Google Scholar]
- Halonen M, Pelto-Huikko M, Eskelin P, et al. Subcellular location and expression pattern of autoimmune regulator (Aire), the mouse orthologue for human gene defective in autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED) J Histochem Cytochem. 2001;49:197–208. doi: 10.1177/002215540104900207. [DOI] [PubMed] [Google Scholar]
- Cihakova D, Trebusak K, Heino M, et al. Novel AIRE mutations and P450 cytochrome autoantibodies in Central and Eastern European patients with APECED. Hum Mutat. 2001;18:225–32. doi: 10.1002/humu.1178. [DOI] [PubMed] [Google Scholar]
- Sato K, Nakajima K, Imamura H, et al. A novel missense mutation of AIRE gene in a patient with autoimmune polyendocrinopathy, candidiasis and ectodermal dystrophy (APECED), accompanied with progressive muscular atrophy: case report and review of the literature in Japan. Endocrine J. 2002;49:625–33. doi: 10.1507/endocrj.49.625. [DOI] [PubMed] [Google Scholar]
- Pearce SH, Cheetham T, Imrie H, et al. A common and recurrent 13-bp deletion in the autoimmune regulator gene in British kindreds with autoimmune polyendocrinopathy type 1. Am J Hum Genet. 1998;63:1675–84. doi: 10.1086/302145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heino M, Peterson P, Kudoh J, et al. APECED mutations in the autoimmune regulator (AIRE) gene. Hum Mutat. 2001;18:205–11. doi: 10.1002/humu.1176. [DOI] [PubMed] [Google Scholar]
- Heino M, Scott HS, Chen Q, et al. Mutation analyses of North American APS-1 patients. Hum Mutat. 1999;13:69–74. doi: 10.1002/(SICI)1098-1004(1999)13:1<69::AID-HUMU8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Ward L, Paquette J, Seidman E, et al. Severe autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy in an adolescent girl with a novel AIRE mutation: response to immunosuppressive therapy. J Clin Endocrinol Metab. 1999;84:844–52. doi: 10.1210/jcem.84.3.5580. [DOI] [PubMed] [Google Scholar]
- Wang CY, Davoodi-Semiromi A, Huang W, et al. Characterization of mutations in patients with autoimmune polyglandular syndrome type 1 (APS1) Hum Genet. 1998;103:681–5. doi: 10.1007/s004390050891. [DOI] [PubMed] [Google Scholar]
- Scott HS, Heino M, Peterson P, et al. Common mutations in autoimmune polyendocrinopathy-candidiasis- ectodermal dystrophy patients of different origins. Mol Endocrinol. 1998;12:1112–9. doi: 10.1210/mend.12.8.0143. [DOI] [PubMed] [Google Scholar]
- Meloni A, Perniola R, Faa V, et al. Delineation of the molecular defects in the AIRE gene in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy patients from Southern Italy. J Clin Endocrinol Metab. 2002;87:841–6. doi: 10.1210/jcem.87.2.8209. [DOI] [PubMed] [Google Scholar]
- Cetani F, Barbesino G, Borsari S, et al. A novel mutation of the autoimmune regulator gene in an Italian kindred with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, acting in a dominant fashion and strongly cosegregating with hypothyroid autoimmune thyroiditis. J Clin Endocrinol Metab. 2001;86:4747–52. doi: 10.1210/jcem.86.10.7884. [DOI] [PubMed] [Google Scholar]
- Soderbergh A, Rorsman F, Halonen M, et al. Autoantibodies against aromatic 1-amino acid decarboxylase identifies a subgroup of patients with Addison's disease. J Clin Endocrinol Metab. 2000;85:460–3. doi: 10.1210/jcem.85.1.6266. [DOI] [PubMed] [Google Scholar]
- Saugier-Veber P, Drouot N, Wolf LM, et al. Identification of a novel mutation in the autoimmune regulator (AIRE-1) gene in a French family with autoimmune polyendocrinopathy-candidiasis- ectodermal dystrophy. Eur J Endocrinol. 2001;144:347–51. doi: 10.1530/eje.0.1440347. [DOI] [PubMed] [Google Scholar]
- Ishii T, Suzuki Y, Ando N, et al. Novel mutations of the autoimmune regulator gene in two siblings with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Clin Endocrinol Metab. 2000;85:2922–6. doi: 10.1210/jcem.85.8.6726. [DOI] [PubMed] [Google Scholar]
- Björses P, Halonen M, Palvimo JJ, et al. Mutations in the AIRE gene: effects on subcellular location and transactivation function of the autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy protein. Am J Hum Genet. 2000;66:378–92. doi: 10.1086/302765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A, Strassburg CP, Deiss D, et al. A Novel AIRE Mutation in an APECED Patient with Candidiasis, Adrenal Failure, Hepatitis, Diabetes Mellitus and Osteosclerosis. Exp Clin Endocrinol Diabetes. 2003;111:174–6. doi: 10.1055/s-2003-39790. [DOI] [PubMed] [Google Scholar]
- Gylling M, Tuomi T, Bjorses P, et al. ss-cell autoantibodies, human leukocyte antigen II alleles, and type 1 diabetes in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Clin Endocrinol Metab. 2000;85:4434–40. doi: 10.1210/jcem.85.12.7120. [DOI] [PubMed] [Google Scholar]
- Rinderle C, Christensen HM, Schweiger S, et al. AIRE encodes a nuclear protein co-localizing with cytoskeletal filaments: altered sub-cellular distribution of mutants lacking the PHD zinc fingers. Hum Mol Genet. 1999;8:277–90. doi: 10.1093/hmg/8.2.277. [DOI] [PubMed] [Google Scholar]
- Doucas V, Evans RM. The PML nuclear compartment and cancer. Biochim Biophys Acta. 1996;1288:M25–9. doi: 10.1016/s0304-419x(96)00028-5. [DOI] [PubMed] [Google Scholar]
- Chan HM, La Thangue NB. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J Cell Sci. 2001;114:2363–73. doi: 10.1242/jcs.114.13.2363. [DOI] [PubMed] [Google Scholar]
- Janknecht R, Hunter T. Versatile molecular glue. Transcriptional control. Curr Biol. 1996;6:951–4. doi: 10.1016/s0960-9822(02)00636-x. [DOI] [PubMed] [Google Scholar]
- Kamei Y, Xu L, Heinzel T, et al. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–14. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Tran VK, Goodman RH. The multifunctional role of the co-activator CBP in transcriptional regulation. Recent Prog Horm Res. 1997;52:103–19. [PubMed] [Google Scholar]
- Chakravarti D, LaMorte VJ, Nelson MC, et al. Role of CBP/P300 in nuclear receptor signalling. Nature. 1996;383:99–103. doi: 10.1038/383099a0. [DOI] [PubMed] [Google Scholar]
- Ramsey C, Bukrinsky A, Peltonen L. Systematic mutagenesis of the functional domains of AIRE reveals their role in intracellular targeting. Human Mol Genet. 2002;11:3299–308. doi: 10.1093/hmg/11.26.3299. [DOI] [PubMed] [Google Scholar]
- Murumägi A, Vähämurto P, Peterson P. Characterization of regulatory elements and methylation pattern of the autoimmune regulator (AIRE) promoter. J Biol Chem. 2003;278:19784–90. doi: 10.1074/jbc.M210437200. [DOI] [PubMed] [Google Scholar]
- Gommerman JL, Browning JL. Lymphotoxin/light, lymphoid microenvironments and autoimmune disease. Nature Rev Immunol. 2003;3:642–55. doi: 10.1038/nri1151. [DOI] [PubMed] [Google Scholar]
- Browning JL, French LE. Visualization of lymphotoxin-beta and lymphotoxin-beta receptor expression in mouse embryos. J Immunol. 2002;168:5079–87. doi: 10.4049/jimmunol.168.10.5079. [DOI] [PubMed] [Google Scholar]
- De Togni P, Goellner J, Ruddle NH, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–7. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- Wang J, Fu YX. LIGHT (a cellular ligand for herpes virus entry mediator and lymphotoxin receptor)-mediated thymocyte deletion is dependent on the interaction between TCR and MHC/self-peptide. J Immunol. 2003;170:3986–93. doi: 10.4049/jimmunol.170.8.3986. [DOI] [PubMed] [Google Scholar]
- Chin RK, Lo JC, Kim O, et al. Lymphotoxin pathway directs thymic Aire expression. Nat Immunol. 2003;4:1121–7. doi: 10.1038/ni982. [DOI] [PubMed] [Google Scholar]
- Weih F, Caamano J. Regulation of secondary lymphoid organ development by the nuclear factor-kappaB signal transduction pathway. Immunol Rev. 2003;195:91–105. doi: 10.1034/j.1600-065x.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- Nabarra B, Andrianarison I. Thymus reticulum of autoimmune mice. 3. Ultrastructural study of NOD (non-obese diabetic) mouse thymus. Int J Exp Pathol. 1991;72:275–87. [PMC free article] [PubMed] [Google Scholar]
- Atlan-Gepner C, Naspetti M, Valero R, et al. Disorganization of thymic medulla precedes evolution towards diabetes in female NOD mice. Autoimmunity. 1999;31:249–60. doi: 10.3109/08916939908994070. [DOI] [PubMed] [Google Scholar]
- Ramsey C, Winqvist O, Puhakka L, et al. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet. 2002;11:397–409. doi: 10.1093/hmg/11.4.397. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Klein L, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- Derbinski J, Schulte A, Kyewski B, et al. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–9. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- Liston A, Lesage S, Wilson J, et al. Aire regulates negative selection of organ-specific T cells. Nature Immunol. 2003;4:350–4. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- Meriluoto T, Halonen M, Pelto-Huikko M, et al. The autoimmune regulator: a key toward understanding the molecular pathogenesis of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. Keio J Med. 2001;4:225–39. doi: 10.2302/kjm.50.225. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Travers P, Walport M, et al. Immunobiology. 5. New York: Garland Publishing; 2001. [Google Scholar]
- Derudder E, Dejardin E, Pritchard LL, et al. RelB/p50 dimers are differentially regulated by tumor necrosis factor-alpha and lymphotoxin-beta receptor activation: critical roles for p100. J Biol Chem. 2003;278:23278–84. doi: 10.1074/jbc.M300106200. [DOI] [PubMed] [Google Scholar]
- Muller JR, Siebenlist U. Lymphotoxin beta receptor induces sequential activation of distinct NF-kappa B factors via separate signaling pathways. J Biol Chem. 2003;278:12006–12. doi: 10.1074/jbc.M210768200. [DOI] [PubMed] [Google Scholar]
- Mordmuller B, Krappmann D, Esen M, et al. Lymphotoxin and lipopolysaccharide induce NF-kappaB-p52 generation by a co-translational mechanism. EMBO Reports. 2003;4:82–7. doi: 10.1038/sj.embor.embor710. [DOI] [PMC free article] [PubMed] [Google Scholar]