Abstract
It is well documented that patients with cystic fibrosis (CF) are unable to clear persistent airway infections in spite of strong local inflammation, suggesting a dysregulation of immunity in CF. We and others have reported previously that T lymphocytes may play a prominent role in this immune imbalance. In the present work, we compared the reactivity of CD3+ T cells obtained from young CF patients in stable clinical conditions (n = 10, aged 9–16·5 years) to age-matched healthy subjects (n = 6, aged 9–13·5 years). Intracellular levels of interferon (IFN)-γ, interleukin (IL)-2, IL-8 and IL-10 were determined by flow cytometry after whole blood culture. The data identified T lymphocyte subsets producing either low levels (M1) or high levels (M2) of cytokine under steady-state conditions. We found that the production of IFN-γ and IL-10 by T lymphocytes was similar between young CF patients and healthy subjects. In contrast, after 4 h of activation with PMA and ionomycin, the percentage of T cells producing high levels of IL-2 (M2) was greater in CF patients (P = 0·02). Moreover, T cells from CF patients produced lower levels of IL-8, before and after activation (P = 0·007). We conclude that a systemic immune imbalance is present in young CF patients, even when clinically stable. This disorder is characterized by the capability of circulating T lymphocytes to produce low levels of IL-8 and by the emergence of more numerous T cells producing high levels of IL-2. This imbalance may contribute to immune dysregulation in CF.
Keywords: cystic fibrosis, T lymphocytes, flow cytometry, IL-2, IL-8
INTRODUCTION
Cystic fibrosis (CF) is the most common lethal genetic disorder in populations with a Caucasian origin [1]. Mutations in the CF gene alter the chloride channel function of the cystic fibrosis transmembrane conductance regulator (CFTR) protein that is expressed mainly by epithelial cells. This basic defect is associated with dehydrated and hyper-viscous secretions. Depending on the severity of the CF disease, the inefficient mucociliary clearance associated with airway obstruction may favour recurrent airway infections with opportunistic pathogens such as Staphylococcus aureus, Haemophilus influenzae, Pseudomonas aeruginosa and Aspergillus fumigatus [2].
Progressive destruction of the lung tissue [3], influx of polymorphonuclear neutrophils in the airway lumen and elevated levels of interleukin (IL)-8 in bronchoalveolar lavages (BAL) are hallmarks of CF [4]. Although infectious factors are clearly involved in the airway disease [5], the sustained, yet inefficient, lung inflammation observed in CF patients strongly suggests a primary dysregulation of immunity before any evidence of infection [5–9]. Whether defective expression of CFTR results in a proinflammatory microenvironment in CF airways [10] or mutations in the CF gene cause a general dysregulation of inflammation/immunity [5] is still under debate and the focus of our present study.
We have reported previously an abnormal pattern of macrophage and mast cell populations during the airway development in human CF fetuses [7], indicating that an early proinflammatory state exists prior to infection in the CF lung [8]. We have also shown that inflammation in CF airways features an influx of neutrophils in the lumen, as well as an increased number of inflammatory cells that infiltrate the respiratory mucosa at the time of lung transplantation [11]. A similar increase has also been identified in the submucosa of cftrm1HGU/cftrm1HGU transgenic mice prior to infection [9].
The hypothesis that T lymphocytes could play a major role in the inflammatory response observed in CF patients is supported by data in chronic obstructive pulmonary disease [12], asthma [13] and sarcoidosis [14]. Mutations in the CF gene alter biological function of human T lymphocytes. Dong and co-workers [15] have shown that the nitric oxide-dependent transduction pathway involved in the regulation of inflammation by human T lymphocytes is defective in human CF-derived cloned T cells. More importantly, Moss and co-workers have reported that CF CD4+ T cell clones secrete abnormally low levels of IL-10 after polyclonal activation [16], while CF peripheral blood mononuclear cells (PBMC) and CF CD4+ T cells show reduced interferon (IFN)-γ production after various stimuli [17].
In the present study we hypothesized that, if a basic immune disorder involves T lymphocytes in CF, peripheral blood T cells collected from young CF patients in stable clinical condition would exhibit an imbalanced production of cytokines. We addressed the question by performing cytometric analysis after whole blood culture. Because the history of infection is a major issue in determining the origin of inflammation in CF, we chose to include only CF patients with no evidence of acute infection and no anti-inflammatory or antibiotic treatment that might have influenced their immune response. We report here that in young CF patients with no evidence of acute infection or inflammation, peripheral blood CD3+ T lymphocytes produce IFN-γ and IL-10 at similar levels to healthy controls but produce low levels of IL-8. In addition, the subset of T lymphocytes producing high levels of IL-2 is higher in CF patients than in healthy subjects.
MATERIALS AND METHODS
CF patients’ characteristics
Ten young CF patients (age: 9–16·5 years) were included in the study and compared to six age-matched healthy subjects (age: 9–13·5 years). Most of the CF patients bore the mutation ΔF 508, either as homozygotes (n = 4) or heterozygotes (n = 4). Seven CF patients showed a pancreatic deficiency. All the CF patients were in stable clinical conditions (Table 1), and only two of them showed positive bronchopulmonary cultures for Pseudomonas aeruginosa (treated previously by intravenous antibiotherapy). The CF patients did not show any recent history of acute respiratory exacerbation. They did not receive any oral or intravenous antibiotic therapy, systemic glucocorticoid treatment, non-steroid anti-inflammatory drugs or azithromycin medication for more than 3 months before blood collection. Moreover, we found no difference in terms of blood cell counts, fibrinogen and C-reactive protein levels in CF patients compared to healthy subjects (data not shown).
Table 1.
Clinical characteristics of the CF young patients included in the study; identification of bacteria (Pseudomonas aeruginosa and Staphylococcus aureus) in airway secretions was performed in parallel to the respiratory function analysis
| Patients | Age (years) | Sex | Genotype | Pancreatic insufficiency | P. aeruginosa | Precipitating P. aeruginosa antibody | S. aureus | FCV% | FEV1% | PaO2 mmHg | PaCO2 mmHg |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 9 | M | ΔF508/R553X | + | − | − | − | 107 | 100 | 87 | 38·4 |
| 2 | 12 | M | ΔF508/ΔF508 | + | + | 7 | − | 52 | 35 | 68 | 30·8 |
| 3 | 10 | M | R1162X/3272–26 A/G | − | − | − | + | 88 | 89 | 87 | 36·8 |
| 4 | 10 | M | ΔF508/G85E | − | − | − | − | 90 | 80 | 80 | 32 |
| 5 | 16·5 | M | ΔF508/ΔF508 | + | − | − | − | 99 | 96 | 87 | 37·8 |
| 6 | 15 | F | ΔF508/R1066C | + | + | 2 | + | 69 | 50 | 70 | 43 |
| 7 | 11·5 | F | R553X/306 del TAGA | + | − | − | + | 84 | 83 | 90 | 35 |
| 8 | 14 | M | ΔF508/ΔF508 | + | − | − | + | 70 | 35 | 63 | 40·7 |
| 9 | 10·5 | M | ΔF508/ΔF508 | + | − | − | − | 91 | 80 | 103 | 32·9 |
| 10 | 11 | M | ΔF508/R117H | − | − | − | − | 96 | 98 | 105 | 37·7 |
FVC%: forced vital capacity (percentage of predicted values); FEV1%: forced expiratory volume in 1 s (percentage of predicted values); PaO2: arterial O2 pressure (in mmHg); PaCO2: arterial CO2 pressure (in mmHg).
The healthy subjects included in the study were free from any detectable inflammation, infection or allergic disease. Three of them were evaluated for small stature with a negative screening, and two were examined with retrograde cystography because of enuresis or past urinary tract infection. No healthy subject had recently received medication.
CF patients and healthy subjects were all examined at the American Memorial Hospital in Reims, France. Blood samples were collected by venipuncture into sodium heparinized endotoxin-free tubes (Chromogenix-Biogenic, Maurin, France). The total volume of blood sample was less than 3 ml.
The Ethical Committee of the CHU de Reims had approved the protocol and the parents of each CF patient/healthy subject provided informed consent.
Whole blood culture and polyclonal activation
Each blood sample was diluted at 1 : 2 in RPMI-1640 culture medium (Gibco BRL-Life Technologies, Eragny, France). Cells from whole blood cultures were subdivided into two groups and analysed either before or after polyclonal activation with 25 ng/ml phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich, Saint Quentin Fallavier, France) and 1 µg/ml ionomycin (Sigma-Aldrich) for 4 h at 37°C in humidified atmosphere and 5% CO2, as described elsewhere [18]. An optimal 4-h incubation period was determined to maximize production of cytokines while minimizing the number of dead cells and debris. To detect intracytoplasmic cytokines, the secretion inhibitor Brefeldin A (BFA, 10 µg/ml, Sigma-Aldrich) was present in the culture medium during cell stimulation.
Detection of surface markers and intracytoplasmic cytokines
All the monoclonal antibodies were purchased from Becton-Dickinson Pharmingen (Le Pont de Claix, France): fluorescein isothiocyanate (FITC)-conjugated mouse antihuman CD3 (clone UCHT1, IgG1), allophycocyanin (APC) anti-CD69 (clone FN50, IgG1), rhodamin phycoerythrin (RPE) anti-IFN-γ (clone 4S.B3, IgG1), RPE anti-IL-2 (clone MQ1–17H12, IgG2a), RPE anti-IL-8 (clone G265-8, IgG2b) and RPE anti-IL-10 (clone JES3–19F1, IgG2a, Pharmingen).
In each flow cytometry tube (Becton Dickinson Labware, Le Pont de Claix, France), 50 µl of blood were incubated with 1 µg/ml anti-CD3 antibody, with or without anti-CD69, for 30 min in the dark to prevent fluorescence quenching. Red cells were then eliminated by incubation for 10 min with lysing buffer purchased from Becton-Dickinson (15% formaldehyde, 50% diethylene glycol). After centrifugation (500 g for 5 min), cell pellets were resuspended and incubated in 500 µl permeabilization buffer (Becton-Dickinson) for 10 min and washed with 3 ml phosphate buffer saline (PBS pH = 7·4; 0·5% BSA; 0·1% NaN3). After washing with PBS, cell pellets were incubated with anticytokine antibodies for 30 min. Finally, washed cell pellets were resuspended in 500 µl PBS containing 1% paraformaldehyde (PFA) and stored in the dark at 4°C until analysis.
For detecting CD69, cells in whole blood cultures were not exposed to BFA. Optimal antibody concentrations were determined by preliminary experiments. Isotype-matched controls (IgG1-FITC, IgG1-RPE, IgG2a-RPE and IgG2b-RPE) were used to determine nonspecific background. Cells labelled with only one fluorescent antibody were used to determine the separation threshold of the cytometer channels.
Flow cytometry
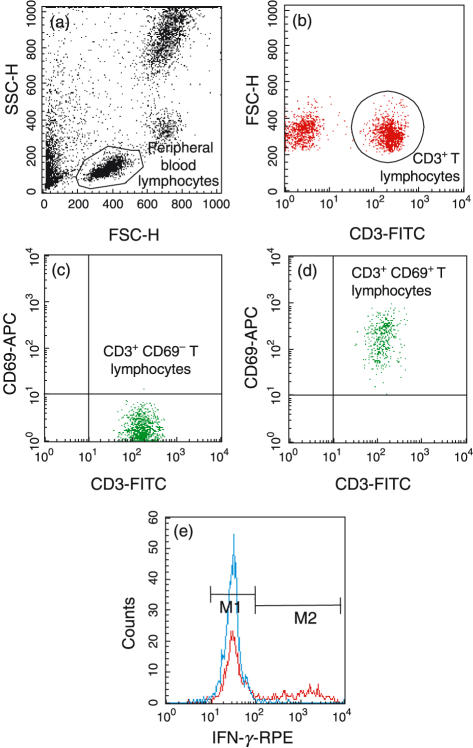
A two-colour flow cytometric analysis was performed with a Becton-Dickinson FACScalibur™ (Becton-Dickinson Instruments, Le Pont de Claix, France). Blood leucocytes from CF patients and healthy subjects were gated initially on the T lymphocyte population based on forward and side light-scattering properties (Fig. 1a), and thereafter on the presence of the marker CD3 (Fig. 1b). A minimum of 10 000 events was acquired in this population of CD3+ T cells. Analysis was performed on a logarithmic scale using cellquest™ software (Software Becton-Dickinson Instruments).
Fig. 1.
Consecutive steps in the analysis of single-cell cytokine production in the CD3+ T lymphocyte population by flow cytometry after whole blood culture. (a) The complete lymphocyte population was identified on the basis of morphological characteristics. (b) The T cell population was identified by the expression of CD3. (c,d) Verification of T cell activation by expression of CD69 before and after 4 h of activation (respectively) with PMA and ionomycin. (e) Example of intracytoplasmic cytokine (IFN-γ) production by CD3+ T cells (blue: before activation, red: after activation), and separation in two cell subsets expressing either low levels (M1) or high levels (M2) of cytokine.
Statistical analysis
The expression level of each cytokine is presented as a mean fluorescence index (MFI) for each T cell subset (M1/M2). The proportion of T cells producing each cytokine is presented as a percentage of the CD3+ T cell population before and after activation. Differences between CF patients and healthy subjects were compared using a two-way analysis of variance (anova), with genotype and activation (PMA and ionomycin) as factors, along with a protected least significant differences (PLSD) Fisher's test. A P < 0·05 was considered significant.
RESULTS
T cell activation and definition of T cell subsets
T cell activation was confirmed by the presence of the glycoprotein CD69 that is rapidly expressed on the surface of T lymphocytes after stimulation with PMA and ionomycin [19]. Non-activated T cells did not express CD69 (Fig. 1c), whereas more than 95% of activated CD3+ T cells did (Fig. 1d). Expression of CD69 was similar between CF patients and healthy subjects (95 ± 2·2%, and 96 ± 3·2%, respectively).
Intracytoplasmic staining for IFN-γ, IL-2, IL-8 and IL-10 revealed two distinct T cell subsets producing either low levels (designated as ‘M1’) or high levels (designated as ‘M2’) of each cytokine, before (CD3+CD69− T cells) or after (CD3+CD69+ T cells) activation. We thus used two positive cut-offs (defined by the horizontal bars M1 and M2 in Fig. 1e) to determine the mean fluorescence intensity for each region and compare the cytokine levels as well as the percentage of CD3+ T cells expressing each cytokine.
Intracytoplasmic expression of IFN-γ, IL-2, IL-8 and IL-10 by CD3+CD69− and CD3+CD69+ T lymphocytes
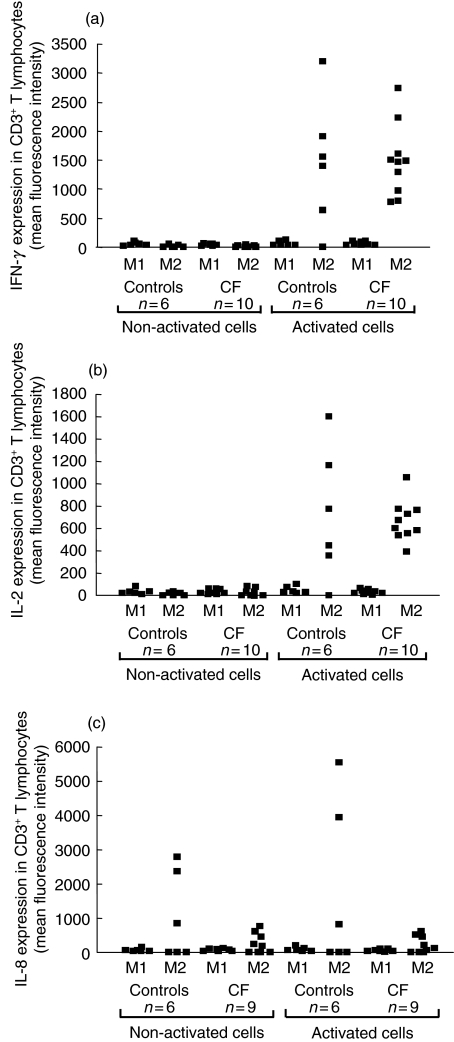
Figure 2 shows the production of IFN-γ, IL-2 and IL-8 by CD3+ T cells in whole blood culture before and after 4 h of polyclonal activation with PMA and ionomycin. Before activation, IFN-γ (Fig. 2a) and IL-2 (Fig. 2b) were already detectable and produced at similar levels by CD3+ T lymphocytes from either healthy subjects or CF patients. After activation, IFN-γ and IL-2 productions were significantly increased in the M2 region for both CF patients and healthy subjects (P < 0·0001), whereas no changes were detected in the M1 region. In spite of this increased expression of cytokines by T cells in the M2 region, there was no significant difference in the production of IFN-γ and IL-2 by T cells from CF patients compared to healthy subjects. Moreover, CD3+ T lymphocytes expressed detectable levels of IL-8 in the M2 region before activation (Fig. 2c). Interestingly, the production of IL-8 was significantly lower in non-activated T lymphocytes from CF patients compared with healthy subjects (P = 0·02). Activation did not increase significantly the basal IL-8 production in either CF or healthy T lymphocytes. The difference between the CF group and the control group remained significant after activation (P = 0·02).
Fig. 2.
Flow cytometric analysis of the intracellular expression of IFN-γ (a), IL-2 (b) and IL-8 (c) by the whole CD3+ T lymphocyte population. T cells were analysed before and after 4 h of activation with PMA and ionomycin. Results are presented for each CF patient and healthy subject, and show the reactivity of T lymphocytes in regions M1 and M2.
Similar levels of IL-10 were also detected in non-activated CD3+ T lymphocytes from CF patients and healthy subjects. Activation of CD3+ T lymphocytes did not increase IL-10 production in either the CF or healthy groups (data not shown).
These results show that T lymphocytes from young CF patients produce normal levels of IFN-γ, IL-2 and IL-10, but have impaired production of IL-8 at the single-cell level.
Percentage of CD3+ T cells producing IFN-γ, IL-2 and IL-8
The percentage of T cells producing IFN-γ, IL-2 and IL-8 showed additional data regarding the capability of CF T lymphocytes to produce cytokines. IFN-γ was produced by a similar number of T cells in the blood of healthy subjects and CF patients (Table 2). After activation, the increased production of IFN-γ observed in the M2 region (Fig. 2a) was associated with an increased percentage of T cells located in the M2 region, with no significant difference between CF patients and healthy subjects (healthy subjects: 21·1 ± 11·2% after activation versus 2·6 ± 2·3% before activation, P = 0·05 and CF patients: 20·3 ± 9·6% after activation versus 2·1 ± 1·7% before activation, P = 0·05).
Table 2.
Percentages of cytokine-producing CD3+ T cells. Values indicate the proportion of T lymphocytes located in regions M1 and M2 in CF patients and healthy subjects before and after activation with PMA and ionomycin in whole blood culture
| % of CD3+ cells | ||||||||
|---|---|---|---|---|---|---|---|---|
| Non-activated cells | Activated cells | |||||||
| Controls | CF | Controls | CF | |||||
| Cytokines | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 |
| IFN-γ | 98·9 ± 1·7 | 2·6 ± 2·3 | 99·2 ± 0·7 | 2·1 ± 1·7 | 83·3 ± 8·8 | 21·1 ± 11·2 | 79·2 ± 9·7 | 20·3 ± 9·6 |
| IL-2 | 87·9 ± 7·2 | 2·1 ± 0·7 | 85·7 ± 8·1 | 1·8 ± 1·2 | 79·7 ± 15·2 | 11·3 ± 3·8 | 68·0 ± 8·7 | 20·2 ± 1·5 |
| IL-8 | 93·5 ± 2·9 | 2·8 ± 2·4 | 91·6 ± 6·4 | 5·3 ± 5·1 | 78·8 ± 21·3 | 11·7 ± 9·3 | 90·6 ± 5·7 | 6·5 ± 5·9 |
The level of IL-2 detected after activation was also associated with an increased number of CD3+ T cells in the M2 region (healthy subjects: 11·3 ± 3·8% after activation versus 2·1 ± 0·7% before stimulation, P = 0·05 and CF patients: 20·2 ± 1·5% after activation versus 1·8 ± 1·2% before activation, P = 0·05). In this case, the percentage of activated T lymphocytes located in the M2 region (thus producing high levels of IL-2) was significantly higher in samples from CF patients compared to healthy subjects (20·2 ± 1·5% in CF patients versus 11·3 ± 3·8% in healthy subjects, P = 0·007).
Before activation, only a few CD3+ T cells producing IL-8 were located in the M2 region (healthy subjects: 2·8 ± 2·4% and CF patients: 5·3 ± 5·1%), while more than 90% of CD3+ T cells were located in the M1 region (healthy subjects: 93·5 ± 2·9% and CF patients: 91·6 ± 6·4%). After activation, no significant difference was found in the percentage of IL-8-producing T cells located in the M2 region in CF patients compared to healthy subjects.
These results show that only a subset of circulating CD3+ T cells is responsive to a strong polyclonal stimulus such as PMA and ionomycin. In our hands, the percentage of CD3+ T cells producing either IFN-γ or IL-8 was similar in young CF patients compared with healthy subjects. In contrast, a larger proportion of T cells produced high levels of IL-2 (M2 region) in patients with CF.
DISCUSSION
In the present work, we investigated the reactivity of the entire T cell population (CD3+) in blood samples from young CF patients compared to age-matched healthy subjects. In order to study T cell responses under conditions as close as possible to physiology, we performed analysis using whole blood cultures. This protocol offers the unique advantage to culture leucocytes in the presence of autologous cellular and serum components that may be physiologically relevant [20]. Results reported here demonstrate that circulating T lymphocytes present in the blood of clinically stable young CF patients exhibit a pattern of cytokine production different from that of normal T cells.
It is noteworthy that the CF patients included in the present study did not show any recent infectious/inflammatory exacerbation, and did not receive any treatment during the 3 months before blood collection. Of importance, T cells obtained from the two CF patients with P. aeruginosa positive cultures did not show any specific pattern of cytokine production compared with T cells from noninfected CF patients.
In this study, we focused only on clinically stable CF patients. We tested the production of several cytokines produced by T lymphocytes and reported previously in the literature as expressed abnormally by CF respiratory cells and CF leucocytes. This imbalance was described usually as a direct effect of the disease for IL-8 [10] and IL-10 [16], and/or as a consequence of bacterial infection for IFN-γ [21]. We also measured the IL-2 production as an essential growth factor for T lymphocyte proliferation and activation in inflammation [22].
Produced by activated T cells, IFN-γ plays a crucial role in the antimicrobial defense of the lung, most notably by further activation of alveolar macrophages [23]. After stimulation with PMA and ionomycin in whole blood culture, CD3+ T lymphocytes from young CF patients produced levels of IFN-γ similar to those from healthy subjects. These data contrast with a recent report by Moss and co-workers [17], who described a lower expression of IFN-γ in CF leucocytes. One possible explanation for our conflicting findings may be that we focused on CD3+ T lymphocytes in whole blood conditions, whereas Moss and co-workers showed data obtained from either the whole population of blood mononuclear cells or isolated CD4+ T cells. Moreover, the CF patients included in this study were mainly adults (age: 27 ± 11 years) and were all on suppressive antibiotic therapy for chronic bacterial endobronchitis. Thus, it is likely that a history of bacterial colonization may result in the low secretion of IFN-γ reported. Indeed, PBMC collected from CF patients with chronic P. aeruginosa lung infection produce lower levels of IFN-γ and higher levels of IL-4 compared to CF patients without chronic infection [21]. Conversely, Epelman and co-workers [24] have shown that virulence factors from P. aeruginosa lead to the decreased expression of IFN-γ by normal PBMC. These studies support the hypothesis that reduced production of IFN-γ by CF leucocytes is a consequence of infection. In contrast, T lymphocytes from young CF patients in stable clinical conditions as well as CD4+ T cell clones expressing mutant CFTR [16] secrete normal amounts of IFN-γ.
Although activated T lymphocytes from young CF patients produced levels of IL-2 similar to those of healthy subjects, the proportion of T cells producing large amounts of IL-2 (M2 region) was higher in CF patients. Moss and co-workers [16,17] have also reported normal levels of IL-2 secreted by either CF CD4+ T cell clones or PBMC, but they did not investigate the number of IL-2-producing cells. IL-2 is well known as a growth/survival factor for T lymphocytes [22], and therefore as a determinant cytokine in the inflammatory process. In the present study, we demonstrate that enhanced IL-2 production in CF patients is not associated with an over-production at the single-cell level, but with the selection of a larger population of IL-2-producing T cells compared to healthy subjects. However, we observed that the number of circulating T lymphocytes and their capability to be activated (expression of CD69) were similar in CF patients and healthy subjects (data not shown). In a recent report by Hausler and co-workers [25] changes of peripheral blood lymphocyte subsets were described in CF patients, with low absolute counts of memory CD4+CD45RO+ T cells and activated (CD25+, the IL-2 low affinity receptor) T cells in peripheral blood samples. Although such decreases in ‘memory’ and ‘activated’ T cells were due probably to infection, they may explain the emergence of a large number of T cells producing high levels of IL-2 after activation. In our study, preliminary experiments showed that there was no difference of CD25 expression between CF patients and controls (data not shown). Moreover, the absence of recurrent infections in our young CF patients argues against a different naive/memory (CD45RA+/CD45RO+) cell ratio as an explanation for the number of T lymphocytes producing high IL-2 levels. Thus, if a larger part of the T cell population is readily able to produce high IL-2 levels in CF patients, its peculiar phenotype needs to be further characterized.
In our study, T lymphocytes from young CF patients compared to healthy subjects appeared to produce lower levels of IL-8 either before or after polyclonal activation. A large number of authors has reported increased levels of the neutrophil chemoattractant IL-8 in different types of specimens collected from CF lungs and airway epithelium. High levels of IL-8 along with neutrophilia are usually observed in the BAL of patients with CF [4,26,27], produced mainly by respiratory epithelial cells [28]. Recently, Corvol and co-workers [29] showed that BAL neutrophils from CF children produce higher levels of IL-8 compared to control subjects. The fact that peripheral blood T cells from young CF patients produced low levels of IL-8 showed that, in contrast to respiratory epithelial cells and neutrophils, circulating T lymphocytes are not a predominant source of IL-8 in CF pathology. Elevated IL-8 levels in CF airways appear to depend on the local environment. Bacterial exoproducts and proinflammatory cytokines released in the airway lumen activate macrophages and neutrophils, and may stimulate further the production of IL-8. The abnormal ionic concentration in CF airways may also modulate IL-8 production [10]. However, the cause for decreased IL-8 production by CF T cells in the present study is unclear. Whether it is related to a decreased number of IL-8 receptors, as reported for CF blood neutrophils in patients with CF [30], remains to be determined. Nevertheless, this low expression of IL-8 suggests that CF T lymphocytes may fail to participate in the antibacterial response, supporting the hypothesis of an imbalanced immunity in CF patients.
Spontaneous production of the regulatory cytokine IL-10 was also detected in circulating CD3+ T lymphocytes at similar levels in CF patients and in healthy subjects. This result agrees with clinical studies of young CF patients showing normal levels of IL-10 in BAL and nasal lavages [26,27]. Collectively, our data and these previous studies indicate that young patients with CF are probably able to produce normal levels of IL-10, both locally (airways) and systemically (blood). In contrast, older CF patients show a decreased production of IL-10 [31], suggesting that infection and malnutrition occurring in the course of the disease diminish the expression of IL-10 in CF [32].
In conclusion, we show here that the pattern of cytokines produced by circulating T lymphocytes from young clinically stable CF patients is different from that of aged-matched healthy subjects. Whereas the production of IFN-γ and IL-10 was similar in CF patients and healthy subjects, the CF T lymphocyte population showed an abnormal capacity to produce high levels of IL-2 and low levels of IL-8. This suggests that a proinflammatory imbalance exists at the systemic level in CF patients, and supports the emerging theory of a basal inflammation and/or immune disorder in CF subjects.
Acknowledgments
This work was supported by INSERM and Association Vaincre la Mucoviscidose (AVLM), and presented in part at the NACFF Congress in October 2002. We would like to acknowledge Professor G. Potron and Mrs V. Creuzat for helpful suggestions about whole blood cultures, Mr S. Prouvost for technical assistance and Dr Jean Marie Zahm for statistical analysis.
REFERENCES
- Davis PB, Drumm M, Konstan MW. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154:1229–56. doi: 10.1164/ajrccm.154.5.8912731. [DOI] [PubMed] [Google Scholar]
- Rastogi D, Ratner AJ, Prince A. Host–bacterial interactions in the initiation of inflammation. Paediatr Respir Rev. 2001;2:245–52. doi: 10.1053/prrv.2001.0147. [DOI] [PubMed] [Google Scholar]
- Sobonya RE, Taussig LM. Quantitative aspects of lung pathology in cystic fibrosis. Am Rev Respir Dis. 1986;134:290–5. doi: 10.1164/arrd.1986.134.2.290. [DOI] [PubMed] [Google Scholar]
- Muhlebach MS, Noah TL. Endotoxin activity and inflammatory markers in the airways of young patients with cystic fibrosis. Am J Respir Crit Care Med. 2002;165:911–5. doi: 10.1164/ajrccm.165.7.2107114. [DOI] [PubMed] [Google Scholar]
- Berger M. Lung inflammation early in cystic fibrosis: bugs are indicted, but the defense is guilty. Am J Respir Crit Care Med. 2002;165:857–8. doi: 10.1164/ajrccm.165.7.2202030a. [DOI] [PubMed] [Google Scholar]
- Bals R, Weiner DJ, Wilson JM. The innate immune system in cystic fibrosis lung disease. J Clin Invest. 1999;103:303–7. doi: 10.1172/JCI6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubeau C, Puchelle E, Gaillard D. Distinct pattern of immune cell population in the lung of human fetuses with cystic fibrosis. J Allergy Clin Immunol. 2001;108:524–9. doi: 10.1067/mai.2001.118516. [DOI] [PubMed] [Google Scholar]
- Tirouvanziam R, de Bentzmann S, Hubeau C, et al. Inflammation and infection in naive human cystic fibrosis airway grafts. Am J Respir Cell Mol Biol. 2000;23:121–7. doi: 10.1165/ajrcmb.23.2.4214. [DOI] [PubMed] [Google Scholar]
- Zahm JM, Gaillard D, Dupuit F, et al. Early alterations in airway mucociliary clearance and inflammation of the lamina propria in CF mice. Am J Physiol. 1997;272:C853–9. doi: 10.1152/ajpcell.1997.272.3.C853. [DOI] [PubMed] [Google Scholar]
- Tabary O, Escotte S, Couetil JP, et al. High susceptibility for cystic fibrosis human airway gland cells to produce IL-8 through the I kappa B kinase alpha pathway in response to extracellular NaCl content. J Immunol. 2000;164:3377–84. doi: 10.4049/jimmunol.164.6.3377. [DOI] [PubMed] [Google Scholar]
- Hubeau C, Lorenzato M, Couetil JP, et al. Quantitative analysis of inflammatory cells infiltrating the cystic fibrosis airway mucosa. Clin Exp Immunol. 2001;124:69–76. doi: 10.1046/j.1365-2249.2001.01456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saetta M, Turato G, Maestrelli P, et al. Cellular and structural bases of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:1304–9. doi: 10.1164/ajrccm.163.6.2009116. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Jeffery PK, Busse WW, et al. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–45. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- Agostini C, Facco M, Chilosi M, et al. Alveolar macrophage–T cell interactions during Th1-type sarcoid inflammation. Microsc Res Tech. 2001;53:278–87. doi: 10.1002/jemt.1094. [DOI] [PubMed] [Google Scholar]
- Dong YJ, Chao AC, Kouyama K, et al. Activation of CFTR chloride current by nitric oxide in human T lymphocytes. EMBO J. 1995;14:2700–7. doi: 10.1002/j.1460-2075.1995.tb07270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss RB, Bocian RC, Hsu YP, et al. Reduced IL-10 secretion by CD4+ T lymphocytes expressing mutant cystic fibrosis transmembrane conductance regulator (CFTR) Clin Exp Immunol. 1996;106:374–88. doi: 10.1046/j.1365-2249.1996.d01-826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss RB, Hsu YP, Olds L. Cytokine dysregulation in activated cystic fibrosis (CF) peripheral lymphocytes. Clin Exp Immunol. 2000;120:518–25. doi: 10.1046/j.1365-2249.2000.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trushin SA, Pennington KN, Algeciras-Schimnich A, et al. Protein kinase C and calcineurin synergize to activate IkappaB kinase and NF-kappaB in T lymphocytes. J Biol Chem. 1999;274:22923–31. doi: 10.1074/jbc.274.33.22923. [DOI] [PubMed] [Google Scholar]
- Cebrian M, Yague E, Rincon M, et al. Triggering of T cell proliferation through AIM, an activation inducer molecule expressed on activated human lymphocytes. J Exp Med. 1988;168:1621–37. doi: 10.1084/jem.168.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suni MA, Picker LJ, Maino VC. Detection of antigen-specific T cell cytokine expression in whole blood by flow cytometry. J Immunol Meth. 1998;212:89–98. doi: 10.1016/s0022-1759(98)00004-0. [DOI] [PubMed] [Google Scholar]
- Moser C, Kjaergaard S, Pressler T, et al. The immune response to chronic Pseudomonas aeruginosa lung infection in cystic fibrosis patients is predominantly of the Th2 type. Apmis. 2000;108:329–35. doi: 10.1034/j.1600-0463.2000.d01-64.x. [DOI] [PubMed] [Google Scholar]
- Marrack P, Bender J, Hildeman D, et al. Homeostasis of alpha beta TCR+ T cells. Nat Immunol. 2000;1:107–11. doi: 10.1038/77778. [DOI] [PubMed] [Google Scholar]
- Munk ME, Emoto M. Functions of T-cell subsets and cytokines in mycobacterial infections. Eur Respir J Suppl. 1995;20:668s–675s. [PubMed] [Google Scholar]
- Epelman S, Bruno TF, Neely GG, et al. Pseudomonas aeruginosa exoenzyme S induces transcriptional expression of proinflammatory cytokines and chemokines. Infect Immun. 2000;68:4811–4. doi: 10.1128/iai.68.8.4811-4814.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausler M, Schweizer K, Biesterfel S, et al. Peripheral decrease and pulmonary homing of CD4+ CD45RO+ helper memory T cells in cystic fibrosis. Respir Med. 2002;96:87–94. doi: 10.1053/rmed.2001.1217. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M, Gibson RL, McNamara S, et al. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatr Pulmonol. 2001;32:356–66. doi: 10.1002/ppul.1144. [DOI] [PubMed] [Google Scholar]
- Noah TL, Black HR, Cheng PW, et al. Nasal and bronchoalveolar lavage fluid cytokines in early cystic fibrosis. J Infect Dis. 1997;175:638–47. doi: 10.1093/infdis/175.3.638. [DOI] [PubMed] [Google Scholar]
- Bonfield TL, Konstan MW, Berger M. Altered respiratory epithelial cell cytokine production in cystic fibrosis. J Allergy Clin Immunol. 1999;104:72–8. doi: 10.1016/s0091-6749(99)70116-8. [DOI] [PubMed] [Google Scholar]
- Corvol H, Fitting C, Chadelat K, et al. Distinct cytokine production by lung and blood neutrophils from children with cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2003;284:L997–1003. doi: 10.1152/ajplung.00156.2002. [DOI] [PubMed] [Google Scholar]
- Dai Y, Dean TP, Church MK, et al. Desensitisation of neutrophil responses by systemic interleukin 8 in cystic fibrosis. Thorax. 1994;49:867–71. doi: 10.1136/thx.49.9.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfield TL, Panuska JR, Konstan MW, et al. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med. 1995;152:2111–8. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- Yu H, Nasr SZ, Deretic V. Innate lung defenses and compromised Pseudomonas aeruginosa clearance in the malnourished mouse model of respiratory infections in cystic fibrosis. Infect Immun. 2000;68:2142–7. doi: 10.1128/iai.68.4.2142-2147.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]