Abstract
Orofacial granulomatosis (OFG) is a condition of unknown aetiology with histological and, in some cases, clinical association with Crohn's disease (CD). However, the exact relationship between OFG and CD remains uncertain. The aim of this study was to determine whether OFG could be distinguished immunologically from CD by comparing non-specific and specific aspects of humoral immunity in serum, whole saliva and parotid saliva in three groups of patients: (a) OFG only (n = 14), (b) those with both oral and gut CD (OFG + CD) (n = 12) and (c) CD without oral involvement (n = 22) and in healthy controls (n = 29). Non-specific immunoglobulin (IgA, SigA, IgA subclasses and IgG) levels and antibodies to whole cells of Saccharomyces cerevisiae, Candida albicans and Streptococcus mutans were assayed by enzyme-linked immunosorbent assay (ELISA) in serum, whole saliva and parotid saliva. Serum IgA and IgA1 and IgA2 subclasses were raised in all patient groups (P < 0·01). Salivary IgA (and IgG) levels were raised in OFG and OFG + CD (P < 0·01) but not in the CD group. Parotid IgA was also raised in OFG and OFG + CD but not in CD. The findings suggest that serum IgA changes reflect mucosal inflammation anywhere in the GI tract but that salivary IgA changes reflect involvement of the oral cavity. Furthermore, the elevated levels of IgA in parotid saliva suggest involvement of the salivary glands in OFG. Serum IgA antibodies to S. cerevisiae were raised markedly in the two groups with gut disease while serum IgA (or IgG) antibodies to C. albicans were elevated significantly in all three patient groups (P < 0·02). No differences were found with antibodies to S. mutans. Whole saliva IgA antibodies to S. cerevisiae (and C. albicans) were raised in the groups with oral involvement. These findings suggest that raised serum IgA antibodies to S. cerevisiae may reflect gut inflammation while raised SIgA antibodies to S. cerevisiae or raised IgA or IgA2 levels in saliva reflect oral but not gut disease. Analysis of salivary IgA and IgA antibodies to S. cerevisiae as well as serum antibodies in patients presenting with OFG may allow prediction of gut involvement.
Keywords: Candida albicans, Crohn's disease, mucosal immunity, orofacial granulomatosis, secretory IgA, Saccharomyces cerevisiae
INTRODUCTION
The term orofacial granulomatosis (OFG) describes a group of conditions characterized by typical features of non-caseating granulomata, lymphocytic infiltration and oedema at various sites in the oral cavity [1]. Patients characteristically present with lip swelling but gingivae, buccal mucosa, floor of mouth and other sites in the oral cavity can be involved. Most cases of OFG occur as a separate clinical entity, but a proportion present in association with established Crohn's disease of the small or large intestine [2]. A smaller number of cases of OFG may be a manifestation of sarcoidosis or occur as part of the Melkersson–Rosenthal syndrome (MRS) [3,4].
A significant proportion of OFG cases have chronic inflammation of the gut detected by endoscopy [5] or subsequently develop CD [1,6,7] despite the absence of gastrointestinal symptoms. It remains unclear, however, whether such cases represent one presentation of CD or a separate disease entity. The cellular and humoral immunology of CD has been studied extensively [8], but no previous studies have addressed the immunological aspects of oral CD or OFG. Recent interest has focused on the demonstration of increased responses to Saccharomyces cerevisiae antigens in CD [9–12]. The significance of this finding remains uncertain but the presence of IgA anti-S. cerevisiae antibodies has been proposed as a means of distinguishing between CD and other types of inflammatory bowel diseases [13].
The cause of OFG is unknown in most patients. Thus agents suggested as being possibly associated with CD are of particular interest. These include antigens from Mycobacterium paratuberculosis [14–17] and from a common dietary component, S. cerevisiae [12,13]. Studies have shown elevated serum titres of both IgG and IgA to S. cerevisiae in CD patients compared with ulcerative colitis patients and healthy controls [11–13]. It had been suggested that serum IgA anti-S. cerevisiae may be a highly specific serological marker for CD [11,12]. An unconfirmed case report [18] has also implicated Candida albicans as a possible aetiological agent in MRS.
The aim of this study was to provide further insight into the relationship between OFG and CD and to determine whether OFG could be distinguished immunologically from CD. Non-specific and specific aspects of humoral immunity (serum and salivary antibodies to S. cerevisiae, C. albicans and Streptococcus mutans) from patients with OFG only, with oral and gut Crohn's disease (OFG ± CD) and with CD without oral involvement were compared with healthy controls.
MATERIALS AND METHODS
Patients
The patient group consisted of 48 individuals with clinically and histologically diagnosed OFG and/or CD presenting to either the oral medicine or gastroenterology departments at Guy's Hospital, London (Guy's and St Thomas's Hospital NHS Trust). The age range was 10–72 years with a mean of 38·4 ± 4·2 (s.e.m.). The project was approved by the Institutional Research Ethics Committee and informed consent following explanation of the project was obtained from all subjects/guardians.
All patients underwent standardized detailed examination of their oral cavities and all underwent ileo-colonoscopy. Biopsies were taken whenever there was clinical evidence of pathology. Patients were then divided into three subgroups: (a) those with OFG only (OFG group) with no evidence on colonoscopy of significant pathology (n = 14, age range 12–73 years, mean 38·6 ± 6·9); (b) those with CD and with OFG signs (OFG ± CD group) including patients presenting with OFG and in whom evidence of intestinal pathology consistent with CD was found on colonoscopy (n = 12, age range 10–55 years, mean 28·3 ± 7); and (c) CD patients with no detectable oral signs (CD group) (n = 22, age range 21–70 years, mean 46·7 ± 7·4. With the exception of the CD group, in which there were 14 males and eight females, the other groups showed an even male/female distribution.
Serum samples
Venous blood samples were collected during routine patient consultations and following centrifugation of the clotted blood, several aliquots were taken and stored at −70°C. Control serum samples were obtained from staff and patients referred to the Department of Oral Medicine and Pathology, Guy's Hospital. None had been referred for assessment of conditions likely to be related to increased carriage of C. albicans or other fungal organisms, or for ulcerative conditions. As far as possible they were age- and sex-matched to the entire patient group, which had an age range from 10 to 72 years with a mean of 38·4 ± 4·2 (s.e.m.). A total of 29 sera were selected from controls with an age range of 10–73 years, mean 37·9 ± 3·6 (s.e.m.).
Saliva samples
Unstimulated whole saliva was collected in sterile universal containers, and 50 µl cultured on standard Sabouraud's dextrose agar at 37°C for 48 h. The remainder was centrifuged and several aliquots stored at −70°C until required. Stimulated parotid saliva was collected over 10 min in all patients and controls from the right parotid duct as described previously [19]. Ten of the control subjects and 12 of the patient group showed salivary carriage of C. albicans but all had less than 1500 colony-forming units (cfu)/ml of saliva [20], which was regarded as normal carriage and were therefore not excluded.
Antigen preparation
An oral isolate of C. albicans, NCPF 2272, was subcultured on Sabouraud's dextrose agar, harvested at 48 h, washed and stored in sterile isotonic saline/sodium azide (0·02%), heat-treated at 56°C for 2 h (and non-viability confirmed by subculture for 48 h) and then stored at 4°C until required. An aliquot was washed in distilled water and brought to a final working dilution of 4 × 108 cells/ml in 0·3% methyl glyoxal. A suspension of S. cerevisiae was prepared in a similar manner from an oral isolate identified using the AP1 20C AUX test system (Bio Merieux, Marcy L’Etoile, France). Subcultures were incubated at 37°C for 96 h and the heat-treated suspensions were also brought to 4 × 108 cells/ml in 0·3% methyl glyoxal. Strep. mutans NCTC 10449 was included as a control and was grown in Todd Hewitt broth and after washing made to a suspension of 4 × 109 cells/ml in 0·3% glyoxal.
Antibody detection
Serum and salivary IgG and IgA antibodies to whole cells of C. albicans, S. cerevisiae and S. mutans were assayed by enzyme-linked immunosorbent assay (ELISA) according to the method described previously [20]. Standard sera were constructed by pooling 10 normal samples found to have high antibody titres against the three antigens and used at doubling dilutions from 1 : 1000. The standard serum was given an arbitrary value of 100 000 units. The mean of the test sample values falling within the range of the standard curve (a minimum of three of the four dilutions was used) was then compared against the standard curve and an antibody concentration determined [20]. A coefficient of variation was calculated for each sample and values in excess of 15% were re-assayed.
Antibody specificity
Cross-reactivity of patient sera between the antigens of C. albicans and S. cerevisiae was screened for by a non-competitive absorption protocol. Six high titre samples were absorbed with a one-tenth volume of washed suspension of formalin-fixed C. albicans, S. cerevisiae and S. mutans, respectively, for 1 h at 37°C and overnight at 4°C before centrifugation [20]. The serum samples were then assayed in parallel with identical non-absorbed samples for anti-C. albicans, anti-S. cerevisiae or anti-S. mutans activity as described previously and the percentage inhibition calculated.
Statistical analyses
All data analyses were undertaken using the Minitab 10·5 statistics package. The raw data were transformed and analysed using Student's t-test. The validity of outlying values was assessed using Dixon's Q-value. Relationships between groups were assessed using the Pearson correlation coefficient.
RESULTS
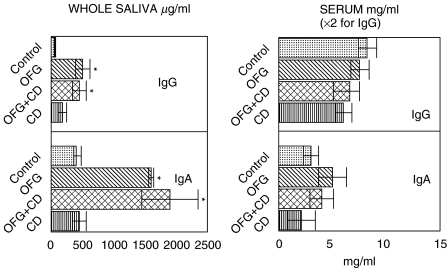
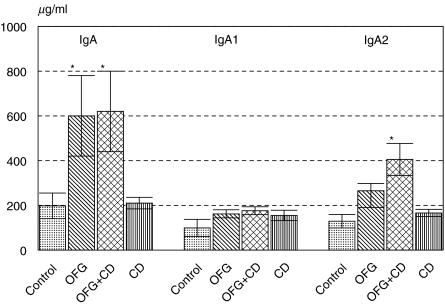
Serum and salivary immunoglobulin concentrations are shown in Fig. 1. In whole saliva the mean IgG and IgA concentrations were raised significantly (P < 0·02) in the OFG group and in the OFG + CD (oral and gut Crohn’s) group but not in the CD (Crohn's disease) group. Similar trends were found in parotid saliva (Fig. 2), where the mean IgA concentration was raised significantly in the OFG and in the OFG + CD groups (P < 0·01). This appeared to be due mainly to an increase in IgA2 concentrations in this group (Fig. 2). Serum IgG and serum IgA concentrations were not significantly altered in any of the disease groups (Fig. 1).
Fig. 1.
Serum and salivary immunoglobulin IgG and IgA concentrations in three patient groups and controls. Data are mean ELISA values ± s.e.m. expressed as mg/ml (serum) or µg/ml (whole saliva), *P < 0·01. OFG = oral signs only, OFG + CD = oral and gut involvement, CD = Crohn's disease gut only group.
Fig. 2.
Immunoglobulin IgA, IgA1 and IgA2 concentrations in parotid saliva in patient and control groups. Data are mean ELISA values ± s.e.m. expressed as µg/ml. OFG = oral signs only, OFG + CD = oral and gut involvement, CD = Crohn's disease gut only group.
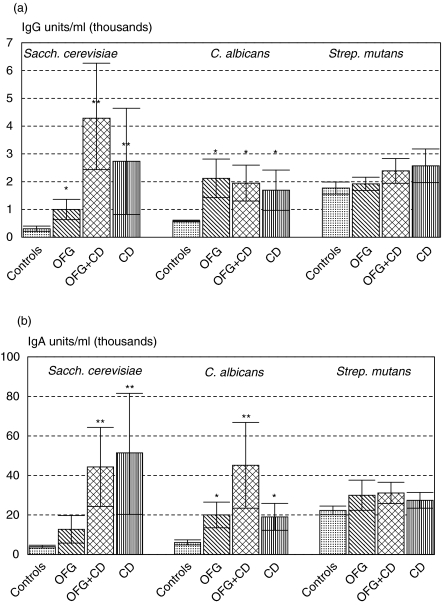
Serum IgA antibodies to S. cerevisiae were raised significantly in the two groups with gut involvement (P < 0·01, Fig. 3b), whereas serum IgG antibodies were raised in all three disease groups, but more pronounced in those groups with gut involvement (Fig. 3a). Serum IgG and IgA antibodies to C. albicans were raised in all three patient groups in comparison with the controls (Fig. 3a,b), although considerable variation was seen with individual patients. S. mutans was used as a non-fungal control and serum antibodies to S. mutans were not raised significantly in any group compared with controls.
Fig. 3.
(a,b) Serum IgG and IgA antibodies to S. cerevisiae, C. albicans and Strep. mutans in three patient groups and controls. Data are mean ELISA values ± s.e.m. and expressed as units/ml (×1000), *P < 0·05, **P < 0·01. OFG = oral signs only, OFG + CD = oral and gut involvement, CD = Crohn's disease gut only group.
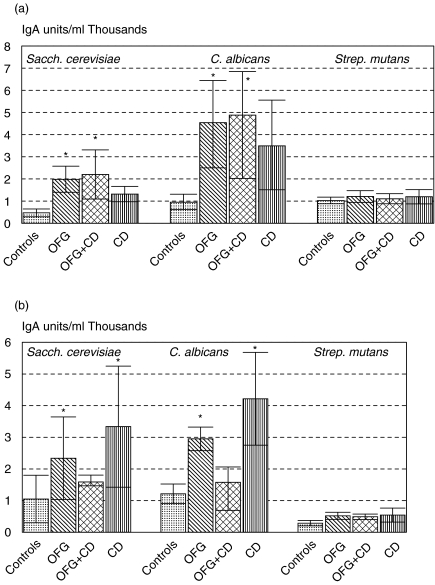
Salivary IgA antibodies to S. cerevisiae in whole saliva (Fig. 4a) were raised significantly in OFG and OFG + CD groups while antibodies to C. albicans were raised in all three groups. Salivary IgG antibodies were examined in whole saliva but mean titres were essentially similar in all groups (data not shown). IgA antibodies to S. mutans in whole saliva did not differ in disease groups from controls (Fig. 4a).
Fig. 4.
(a,b) Salivary IgA antibodies to S. cerevisiae, C. albicans and Strep. mutans in three patient groups and controls. (a) whole saliva, (b) parotid saliva. Data are mean ELISA values ± s.e.m. and expressed as units/ml (×1000), *P < 0·01. (a) or <0·05 (b), OFG = oral signs only, OFG + CD = oral and gut involvement, CD = Crohn's disease gut only group.
IgA antibodies were also analysed in parotid saliva (Fig. 4b). There was considerable variation between patients and significant differences only found with antibodies to C. albicans in the OFG and CD groups (P < 0·05). This pattern was different from that seen with immunoglobulin levels (Figs 1 and 2).
Pearson correlation coefficients revealed positive correlations between serum IgA and serum IgG antibodies to C. albicans in controls (r = 0·876, n = 29, P < 0·001) but not in patients taken as the three groups combined (r = 0·182, n = 48). Serum IgA antibodies to S. cerevisiae and C. albicans showed a positive correlation in controls (r = 0·677, P < 0·01, n = 29) and in patients (r = 0·408, n = 48, P < 0·05). A strong relationship between salivary IgA anti-C. albicans and anti-S. cerevisiae antibodies was observed in patients (r = 0·572, n = 48, P < 0·01) but not in controls.
Absorption of serum samples with C. albicans reduced the IgA anti-C. albicans by over 90% and the anti-S. cerevisiae by 58% (Table 1). Similar absorption with S. cerevisiae produced a homologous inhibition of 80% and reduced the IgA antibody to C. albicans by approximately 30%. Serum IgG titres showed a similar pattern, except that no significant absorption of candida antibodies was found with S.cerevisiae. Absorption with S. mutans reduced the homologous titre by over 80% but the titres to C. albicans and S. cerevisiae were reduced by less than 25%.
Table 1.
Antibody specificity: mean percentage serum IgG, serum IgA and salivary IgA antibodies remaining after absorption of six samples with S.cerevisiae, C. albicans, and S. mutans (see Methods)
| Absorbed with: | ||||||||
|---|---|---|---|---|---|---|---|---|
| Saline | S. cerevisiae | C. albicans | S. mutans | |||||
| Serum IgG | ||||||||
| S. cerevisiae | 100 | 19 | 46 | 78 | ||||
| C. albicans | 100 | 95 | 7 | 96 | ||||
| S. mutans | 100 | 94 | 96 | 18 | ||||
| Serum IgA | ||||||||
| S. cerevisiae | 100 | 22 | 42 | 88 | ||||
| C. albicans | 100 | 72 | 1 | 90 | ||||
| S. mutans | 100 | 80 | 85 | 15 | ||||
| Salivary IgA | ||||||||
| S. cerevisiae | 100 | 15 | 45 | 85 | ||||
| C. albicans | 100 | 85 | 6 | 62 | ||||
| S. mutans | 100 | 81 | 88 | 12 | ||||
DISCUSSION
One of the main findings of this study was that non-specific immunoglobulin levels in both whole saliva and parotid saliva are raised in patients with OFG, with or without gut inflammation, suggesting that salivary gland involvement may occur in this disorder. While there have been occasional case reports of salivary gland involvement and acceptance that sarcoid may involve the glands, it has not been suggested previously that there may be subclinical involvement of the parotid glands in OFG. We are not aware of any previous studies comparing OFG with CD in this way.
The findings in this study show clearly that there are elevated serum antibody titres of the IgA isotype to S. cerevisiae in the patients with CD or OFG + CD compared with healthy control subjects. This is in agreement with the previously cited studies showing elevated serum IgA titres to S. cerevisiae in CD patients [10,13]. Serum IgG antibodies to S. cerevisiae and serum IgG or IgA antibodies to C. albicans were not different in the three patient groups. (Fig. 3a,b) and thus did not discriminate between the groups, as found with serum or salivary IgA antibodies to S. cerevisiae.
The differences in anti-S. cerevisiae IgA antibody titres between OFG with and without CD suggests that it may be possible that to use such antibody titres as both a marker of coincident gut disease in those presenting with OFG, and as a progress screen to alert clinicians to the possible development of CD. That OFG may be an early manifestation of CD receives some support from reports of the development of CD in a significant percentage of OFG patients [1,6,7]. In addition, while antibody levels to an oral control bacterium were not raised, it is not excluded that serum responses to other members of the gut flora might be elevated and it is possible that CD patients have altered responses to specific antigens.
A recent study [21] examined serum antibodies to dietary antigens in monozygotic twins with inflammatory bowel disease (IBD) and confirmed high titres of IgA, IgM and IgG to yeast cell wall mannan and IgA to whole cells of S. cerevisiae in CD patients. The increase in antibodies of all three major isotypes supports involvement of both mucosal and systemic immune systems. Other studies have shown an antigen specific increase in lymphocyte proliferation in CD in response to S. cerevisiae [22]. An investigation of this in OFG patients is clearly worthwhile. Darroch et al. [23] extended the initial observations of raised lymphoproliferative responses of peripheral blood mononuclear cells (PBMC) in CD patients by using the immunodominant 200 kDa S. cerevisiae antigen reactive with anti-S. cerevisiae antibodies from CD patients identified by Heelan et al. [24] as a mitogen. PBMC from CD showed a dose-dependent response to S. cerevisiae antigen. The kinetics of this response are similar to that of purified protein derivative (PPD) of M. tuberculosis, prompting the suggestion that the S. cerevisiae antigen is causing blastogenesis in an antigen-dependent manner, rather than non-specifically as is the case with mitogens [24]. The mannans, which form the dominant yeast cell wall antigen, are also present in mycobacteria and the latter have been implicated in CD [25]. It is therefore necessary to focus on a group of potential antigen sources rather than specific organisms only and the use of this antigen in OFG may be helpful in discrimination.
Significantly raised titres of C. albicans antibody were found in all three patient groups. Patients with OFG alone had similar mean serum and salivary antibody titres to C. albicans as the CD group (Figs 3 and 4), although both were raised in comparison with the control group. This is contrast to the previously reported absence of elevated serum titres of anti-C. albicans antibodies in CD patients [26]. A more recent study [27] examined the degree of cross-reactivity between strains of S. cerevisiae and C. albicans and showed that anti-S. cerevisiae antibodies can be absorbed by C. albicans but with no apparent significant absorption of anti-C. albicans antibodies by S. cerevisiae. Similarly in this study, the candida strain used was able to absorb out both homologous and a significant proportion of S. cerevisiae antibodies, indicating antigenic cross-reactivity between the strains used, whereas antibodies to C. albicans were absorbed strongly by C. albicans but not by S. cerevisiae (table).
In summary, non-specific IgA and IgA2 immunoglobulin levels in whole and parotid saliva are raised in patients with OFG, with or without gut inflammation, suggesting that salivary gland involvement may occur in this disorder. Although a previous study has reported raised salivary IgA in whole saliva in CD [28], no previous studies have compared OFG with and without gut involvment. Serum IgA responses to S. cerevisiae were raised only in those with existing gut disease and thus assay of serum IgA antibody responses may prove useful in indicating concurrent gut disease and possibly progression of OFG to gut involvment. The absence of any differences in responses to the control organism S. mutans supports the view that raised responses to saccharomyces and candida species may be a particular feature of CD-like gut inflammation. Analysis of salivary IgA subclass levels and IgA antibodies to S. cerevisiae as well as serum IgA antibodies in patients presenting with OFG may allow prediction of gut involvement.
Acknowledgments
The authors acknowledge with appreciation the assistance of Mrs L. Fernandes-Naglik and Dr S. Sweet.
REFERENCES
- Wiesenfeld D, Ferguson MM, Mitchell DN, et al. Orofacial granulomatosis − a clinical and pathological analysis. Q J Med. 1985;54:101. [PubMed] [Google Scholar]
- Williams AJK, Wray D, Ferguson A. The clinical entity of orofacial Crohn's disease. Q J Med. 1991;79:451–8. [PubMed] [Google Scholar]
- Zimmer WM, Rogers RS, III, Reeve CM, Sheridan PJ. Orofacial manifestations of Melkersson–Rosenthal syndrome. A study of 42 patients and review of 220 cases from the literature. Oral Surg Oral Med Oral Pathol. 1992;74:610–9. doi: 10.1016/0030-4220(92)90354-s. [DOI] [PubMed] [Google Scholar]
- Worssae N, Christensen KC, Schioedt M, Reibel J. Melkersson–Rosenthal syndrome and cheilitis granulomatosa. Oral Surg Oral Med Oral Pathol. 1982;54:404–13. doi: 10.1016/0030-4220(82)90387-5. [DOI] [PubMed] [Google Scholar]
- Tyldesley WR. Oral Crohn's disease and related conditions. Br J Oral Surg. 1979–80;17:1–9. doi: 10.1016/0007-117x(79)90001-5. [DOI] [PubMed] [Google Scholar]
- Field EA, Tyldesley WR. Oral Crohn's disease revisited − a ten year review. Br J Oral Surg. 1989;27:1114–23. doi: 10.1016/0266-4356(89)90058-2. [DOI] [PubMed] [Google Scholar]
- Scully C, Cochrane KM, Russell RI, et al. Crohn's disease of the mouth: an indicator of intestinal involvement. Gut. 1982;23:198–201. doi: 10.1136/gut.23.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deventer SJ. Immunomodulation of Crohn's disease [Review] Curr Direct Autoimmun. 2000;2:150–66. doi: 10.1159/000060502. [DOI] [PubMed] [Google Scholar]
- Bartunkova J, Kolarova I, Sediva A, Holzelova E. Antineutrophil cytoplasmic antibodies, anti-Saccharomyces cerevisiae antibodies, and specific IgE to food allergens in children with inflammatory bowel diseases. Clin Immunol. 2002;102:162–8. doi: 10.1006/clim.2001.5145. [DOI] [PubMed] [Google Scholar]
- Main J, McKenzie H, Yeaman GR, et al. Antibody to Saccharomyces cerevisiae (baker's yeast) in Crohn's disease. Br Med J. 1988;297:1005–6. doi: 10.1136/bmj.297.6656.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein CN, Orr K, Blanchard JF, Sargent M, Workman D. Development of an assay for antibodies to Saccharomyces cerevisiae: easy, cheap and specific for Crohn's disease. Can J Gastroenterol. 2001;15:499–504. doi: 10.1155/2001/605470. [DOI] [PubMed] [Google Scholar]
- Peeters M, Joossens S, Vermeire S, Vlietinck R, Bossuyt, Rutgeerts P. Diagnostic value of anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease. Am J Gastroenterol. 2001;96:730–4. doi: 10.1111/j.1572-0241.2001.03613.x. [DOI] [PubMed] [Google Scholar]
- Barnes RMR, Allan S, Taylor-Robinson CH, Finn R, Johnson PM. Serum antibodies reactive with Saccharomyces cerevisiae in inflammatory bowel disease: is IgA antibody a marker for Crohn's disease? Int Arch Allergy Appl Immunol. 1990;92:9–15. doi: 10.1159/000235217. [DOI] [PubMed] [Google Scholar]
- Ivanyi L, Kirby A, Zakrzewska JM. Antibodies to mycobacterial stress proteins in patients with orofacial granulomatosis. J Oral Pathol Med. 1993;22:320–2. doi: 10.1111/j.1600-0714.1993.tb01081.x. [DOI] [PubMed] [Google Scholar]
- Ibbotson JP, Lowes JKR, Chahal H, et al. Mucosal cell-mediated immunity to mycobacterial, enterobacterial and other microbial antigens in inflammatory bowel disease. Clin Exp Immunol. 1992;87:224–30. doi: 10.1111/j.1365-2249.1992.tb02979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen I, Wiker HG, Johnson E, Langeggen H, Reitan LJ. Elevated antibody responses in patients with Crohn's disease against a 14-kDa secreted protein purified from Mycobacterium avium subsp. paratuberculosis. Scand J Immunol. 2001;53:198–203. doi: 10.1046/j.1365-3083.2001.00857.x. [DOI] [PubMed] [Google Scholar]
- Naser SA, Hulten K, Shafran I, Graham DY, El-Zaatari FA. Specific seroreactivity of Crohn's disease patients against p35 and p36 antigens of M. avium subsp. paratuberculosis. Vet Microbiol. 2000;77:497–504. doi: 10.1016/s0378-1135(00)00334-5. [DOI] [PubMed] [Google Scholar]
- Miyashita M, Baba S, Suzuki H. Role of recurrent oral candidiasis associated with lingua plicata in the Melkersson–Rosenthal syndrome [Letter] Br J Dermatol. 1995;132:311–12. doi: 10.1111/j.1365-2133.1995.tb05035.x. [DOI] [PubMed] [Google Scholar]
- Challacombe SJ, Lehner T. Immunoglobulins in parotid saliva and serum in relation to dental caries in man. Caries Res. 1976;10:165–77. doi: 10.1159/000260199. [DOI] [PubMed] [Google Scholar]
- Coogan MM, Sweet SP, Challacombe SJ. Immunoglobulin A (IgA), IgA1 and IgA2 antibodies to Candida albicans in whole and parotid saliva in human immunodeficiency virus infection and AIDS. Infect Immun. 1994;62:892–6. doi: 10.1128/iai.62.3.892-896.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg E, Magnusson K-E, Tysk C, Jarnerot G. Antibody (IgG, IgA and IgM) to baker's yeast, yeast mannan, gliadin, ovalbumin and betalactogobulin in monozygotic twins with inflammatory bowel disease. Gut. 1992;33:909–13. doi: 10.1136/gut.33.7.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CA, Sonnenberg A, Burns EA. Lymphocyte proliferation response to baker's yeast in Crohn's disease. Digestion. 1994;55:40–3. doi: 10.1159/000201121. [DOI] [PubMed] [Google Scholar]
- Darroch CJ, Christmas SE, Barnes RMR. In vitro human lymphocyte proliferative responses to a glycoprotein of the yeast Saccharomyces cerevisiae. Immunology. 1994;81:247–52. [PMC free article] [PubMed] [Google Scholar]
- Heelan BT, Allan S, Barnes RMR. Identification of a 200kDa glycoprotein antigen of Saccharomyces cerevisiae. Immunol Lett. 1991;28:181–6. doi: 10.1016/0165-2478(91)90001-q. [DOI] [PubMed] [Google Scholar]
- Hampson SJ, McFadden JJ, Hermon-Taylor J. Mycobacteria and Crohn's disease. Gut. 1988;29:1017–9. doi: 10.1136/gut.29.8.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie H, Main J, Pennington CR, Parratt D. Antibody to selected strains of Saccharomyces cerevisiae (baker's and brewer's yeast) and Candida albicans in Crohn's disease. Gut. 1990;31:536–8. doi: 10.1136/gut.31.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie H, Parratt D, Main J, Pennington CR. Antigenic heterogeneity of strains of Saccharomyces cerevisiae and Candida albicans recognized by serum antibodies from patients with Crohn's disease. FEMS Microbiol Immunol. 1992;89:219–24. doi: 10.1111/j.1574-6968.1992.tb04997.x. [DOI] [PubMed] [Google Scholar]
- Crama-Bohbouth G, Lems-van Kan P, Weterman IT, Biemond I, Pena AS. Immunological findings in whole and parotid saliva of patients with Crohn's disease and healthy controls. Dig Dis Sci. 1984;29:1089–92. doi: 10.1007/BF01317081. [DOI] [PubMed] [Google Scholar]