Abstract
In this study, the detailed mechanisms for the effects of vitamin A on the expression of polymeric immunoglobulin receptor (pIgR) were examined. Expression of the pIgR by tumour necrosis factor (TNF-α) was enhanced by the addition of all-trans retinoic acid (ATRA) or 9-cis retinoic acid (9CRA). This enhancement was mediated mainly by RARα, and regulated at the transcriptional level. Transcription factor nuclear factor-κB (NF-κB) binding and activation were not influenced by addition of ATRA. These data imply that RA, in combination with TNF-α, could up-regulate the expression of pIgR. In addition, we hypothesize that up-regulation of pIgR by RA is controlled through the RAR-dependent signalling pathway and that it plays a role in enhancement of mucosal immunity.
Keywords: NF-κB, polymeric immunoglobulin receptor, retinoic acid, TNF-α
INTRODUCTION
Vitamin A, or retinol, is a polyisoprenoid compound containing a cyclohexenyl ring, and found in various foods including liver, cod-liver oil, eggs and vegetables. In vegetables, vitamin A exists as a provitamin in the form of the yellow pigment β-carotene, which is cleaved by a deoxygenase to generate two molecules of retinal. Within the intestinal mucosa, retinal is then reduced to retinol by a specific retinaldehyde reductase utilizing NADPH. Approximately 90% of the vitamin A in the body is stored in the liver as retinyl esters. Retinol is released from the liver in a molar combination with retinol binding protein and transthyretin, and enters certain target cells via specific receptors. A small fraction of retinal is oxidized to form retinoic acid (RA) in the cytosol. In the nucleus, RA influences gene activation via specific retinoic acid nuclear receptors, RARα, β, γ for all-trans-RA (ATRA) and 9-cis-RA (9CRA), and retinoid X receptors, RXRα, β, γ for 9CRA [1].
It has been suggested that vitamin A is essential for immunity. For example, vitamin A deficiency is associated with increased morbidity and mortality from infectious diseases, which are preventable or reversible by vitamin A supplementation or fortification [2,3]. In the intestine, disruption of intestinal chondroitin sulphates within the extracellular matrix is responsible for various inflammatory diseases. One of the mediators of this effect is nitric oxide [4], the release of which is enhanced by vitamin A deficiency [5], suggesting an important role of vitamin A in the mucosal inflammatory reaction. Vitamin A deficiency is also associated with exacerbation of immunodeficiency, diminished numbers of circulating lymphocytes, atrophy of lymphoid organs with decreased levels of antibodies and an impaired delayed-type hypersensitivity reaction [6,7]
Within the mucosal immune system, protein-energy malnutrition has been shown to result in decreased secretory Ig concentration due most probably to reduced transport of mucosal Ig onto epithelial surfaces [8,9]. Nikawa et al. have described that the administration of retinyl acetate to mice prevents the decline of IgA levels caused by protein deficiency [10]. Administration of high-dose vitamin A to influenza virus-infected mice enhances the Th-2 mediated immune response and secretory IgA production [11]. It has been reported that S-IgA levels in the intestinal fluid in vitamin A-deficient rats were significantly low resulting from a decrease of secretory component (SC) [12]. pIgR is present on glandular epithelial cells as a type I transmembrane protein of the immunoglobulin superfamily and extracellular domain is cleaved by unknown protease and released as SC.
In addition, ATRA-treated HT-29 cells constitutively expressing pIgR showed a significantly high expression of pIgR in the presence of IL-4 and/or IFN-γ, compared to ATRA-untreated cells [13], suggesting that vitamin A may be required for the proper regulation of IgA transport in response to mucosal infection. It is therefore important to examine the effect of vitamin A on pIgR expression and elucidate the detailed mechanisms by which these effects occur.
In order to determine whether RA absorbed by intestinal epithelial cells can affect the expression of the pIgR gene in vitro, HT-29 cells were treated with TNF-α and ATRA or 9CRA. We report here that the expression of the human pIgR gene by TNF-α is enhanced by ATRA or 9CRA. Furthermore, this up-regulation is mediated through the RARα dependent pathway and controlled at the mRNA level without a redundant activation of NF-κB.
MATERIALS AND METHODS
cDNA
Human pIgR [14] cDNA was kindly provided by Dr P. Brandtzeag (LIIPAT, Institute of Pathology, National Hospital, University of Oslo, Oslo, Norway). As a probe for Northern blot analysis, Pst I [668 base pairs (bp)] fragments were used for the detection of pIgR mRNA.
Reagents
Recombinant human TNF-α (specific activity = 1 × 107 U/mg) was purchased from Genzyme Corporation (Cambridge, MA, USA). ATRA, 9CRA, 4-[E-2-(5 6, 7, 8-tetrahydro-5, 5, 8, 8-tetramethyl-2-naphthalenyl)-1-propenyl] benzoic acid (TTNPB), a RAR agonist [15] (+)-2E, 4E-11-methoxy-3, 7, 11-trimethyl-2, 4-dodecadienoic acid (methoprene acid, MA), a RXR agonist [16], were purchased from Sigma (St Louis, MO, USA). 4-[(5, 6, 7, 8-tetrahydro-5, 5, 8, 8-tetramethyl-2-naphthalenyl) carboxamido]-benzoic acid (AM-580), a RARα selective agonist [17] and N-(4-hydroxyphenyl) retinamide (4-HPR), a RARβ and RARγ selective agonist [18], were purchased from Calbiochem (La Jolla, CA, USA).
Cell culture
Human colonic adenocarcinoma cell line, HT-29 cells were maintained in RPMI-1640 medium (Nissui Pharmaceutical Co., Tokyo, Japan) supplemented with 10% fetal calf serum (FCS) (Cansera International Inc., Ontario, Canada), 1 mm glutamine (Life Technologies Inc., Gaithersburg, MD, USA), 25 mm of glucose, amphotericin B and penicillin/streptomycin (Sigma) at 37°C in an atmosphere of humidified 5% CO2. Another human colon adenocarcinoma cell line, Caco-2 cells were cultured in Eagle's minimum essential medium containing 20% FCS, 2 mm of glutamine and 0·1 mm of nonessential amino acid (Life Technologies Inc., Gaithersburg, MD, USA). Both cell lines were checked the infection of Mycoplasma using a polymerase chain reaction (PCR) Mycoplasma detection kit (Takara). Prior to treatment, both cells were plated at 1 × 106 cells/ml with a fresh medium and then cultured. The next day, human recombinant TNF-α at the concentration of 10 ng/ml and ATRA (1 µm) or 9CRA(1 µm) was added to the medium without FCS and cultivation was allowed to continue for 48 h. Then, cultured cells were harvested and 2 ml of Solution D was added [19] for RNA extraction. The supernatants were also collected and concentrated 20 times using Vivapore 5 (Sartorius AG, Germany) and subjected to enzyme-linked immunosorbent assay (ELISA) as described previously [20,21]. Briefly, polyclonal anti-human SC antibody was coated on microtitre plates at 4°C for 16 h. Then wells were washed three times with PBS(–) and then incubated with 1% bovine serum albumin (BSA)/PBS (–) at 37°C for 1 h. After washing with PBS(–) three times, samples were loaded and incubated at room temperature for 1 h. After wells were washed with PBS-Tween-20 three times, horseradish peroxidase-labelled polyclonal antihuman SC antibody was loaded onto the well and incubated at room temperature for 30 min. Then wells were washed with PBS-Tween-20 three times and colour reaction was developed by incubation with substrate solution containing phenylenediamine/H2O2. The reaction was stopped by adding H2SO4 and measured the absorbance of 495 nm. The concentration of SC in the culture supernatants was calculated using purified SC protein as a standard. All experiments were performed in triplicate and statistical analysis of the results was performed by Student's t-test.
Northern blot analysis
Total RNA from HT-29 cells was prepared by the acid guanidinium thiocyanate–phenol–chloroform (AGPC) extraction method [19]. Ten µg of total RNA was mixed with loading buffer [50% formamide, 6% formalin, 1× Goldberg buffer (40 mm Na-MOPS {pH 7·2}, 5 mm sodium acetate, 0·5 mm EDTA)], denatured at 65°C for 15 min and cooled on ice. Then, RNA was electrophoresed through a 1·0% agarose gel containing 6% formalin. After electrophoresis, the gel was neutralized by incubation with 0·25 m ammonium acetate solution for 5 min and the RNA was transferred onto Hybond-N (Amersham, Buckinghamshire, UK). The RNA was fixed to the membrane by using an UV-crosslinker. Before hybridization, the membrane was incubated with prehybridization solution [50% formamide, 0·65 m NaCl, 5 mm EDTA (pH 7·5), 0·1% sodium dodecyl sulphide (SDS), 0·1 m PIPES (pH 6·8), 5× Denhardt's solution] at 42°C for 6 h. The hybridization probe was prepared by using a random prime DNA labelling kit (Takara Shuzo Co., Shiga, Japan) and purified by Sephadex G-50 (Pharmacia Biotech, Uppsala, Sweden). For hybridization, dextran sulphate (Pharmacia Biotech) was added to the prehybridization solution at a final concentration of 10%. After hybridization, the membrane was washed four times with washing buffer (2× SSC, 0·1% SDS, 0·2% sodium pyrophosphate) at 55°C for 30 min and exposed to X-OMAT film (Kodak, Rochester, NY, USA) at − 80°C.
Preparation of nuclear extracts
Nuclear extracts were prepared by the method of Natarajan et al. [22]. Briefly, 5 × 106 HT-29 cells were stimulated with TNF-α and ATRA or 9CRA. Then, cells were resuspended in 400 µl of lysis buffer [10 mm Hepes (pH 7·9), 10 mm KCl, 0·1 mm EDTA, 0·1 mm EGTA, 0·5 mm DTT, 0·5 mmphenylmethylsulfonyl fluoride (PMSF), 2·0 µg/ml leupeptin, 2·0 µg/ml aprotinin], and placed on ice. After 15 min, 12·5 µl of 10% NP40 was added and mixed for 10 s, and the suspension was centrifuged at 700 g at 4°C for 1 min. Pellets were resuspended in 50 µl of nuclear extraction buffer [20 mm Hepes (pH 7·9), 0·4 m NaCl, 1 mm EDTA, 1 mm EGTA, 0·5 mm DTT, 1 mm PMSF, 2·0 µg/ml leupeptin, 2·0 µg/ml aprotinin] and placed on ice for 30 min with an intermittent mixing. The samples were centrifuged at 12 000 g at 4°C for 30 min and the supernatants were used as nuclear extracts. Protein concentration was measured by use of Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA).
Electrophoretic mobility shift assay (EMSA)
For preparation of the probe, synthetic oligomers [κB2, corresponding to − 461∼ – 440; 5′-GATCGTCGGAGGGGATTCCA GAGCAA-3′, 3′-CAGCCTCCCCTAAGGTCTCGTTCTAG-5′] were annealed and radiolabelled with the Klenow fragment of DNA polymerase I and [α-32P] dCTP (Amersham). For the binding reaction, 20 µg of nuclear extract were incubated with 22 µl of buffer containing 25 mm HEPES (pH 7·9), 2 mm EDTA (pH 8·0), 50 mm KCl, 10% glycerol, 2 µg of poly-dI-dC (Pharmacia Biotech), 1% BSA and 104 counts per minute (cpm) of radiolabelled probe for 30 min at 30°C. After the reaction, 1 µl of loading buffer [5% glycerol, 50 mm EDTA (pH 8·0), 0·05% bromophenol blue, 0·05% xylene cyanol] was added and separated through a 6% native polyacrylamide gel in TAE buffer [67 mm Tris, 33 mm sodium acetate (pH 6·0), 10 mm EDTA] at room temperature. The gel was then dried and exposed to Kodak X-OMAT film at − 80°C for 12 h.
Transfection
HT-29 cells were seeded at a concentration of 6·25 × 104 cells/well of the 48-well microplates in RPMI-1640 containing 10% FCS. Cells were transfected by liposome-mediated transfection method. Briefly, 2·5 µl of lipofectamine (Life Technologies Inc.) and 625 ng of pNF-κB-luc (Promega) were mixed in 25 µl of Opti-MEM (Life Technologies Inc.) at room temperature for 15 min. Cells were washed three times with PBS(–) and 100 µl of Opti-MEM was added. Then, the liposome-DNA complex was added into the medium and cultured at 37°C for 6 h. After transfection, the medium was aspirated and RPMI-1640 medium containing 10% FCS was added into the transfection medium and cultured at 37°C for 24 h. Subsequently, TNF-α (10 ng/ml) and/or ATRA stimulation were performed and cultured for further 24 h. Transfection efficiency was normalized to the Renilla luciferase activity by cotransfection of pRL-TK vector (Promega).
RESULTS
Effect of RA for the release of SC
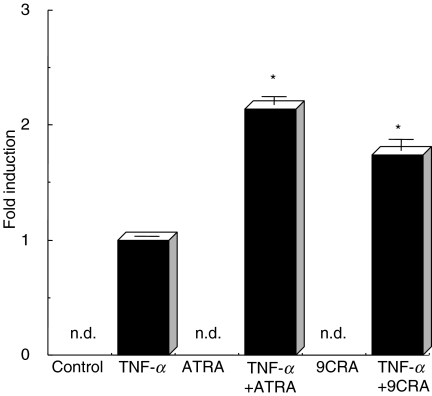
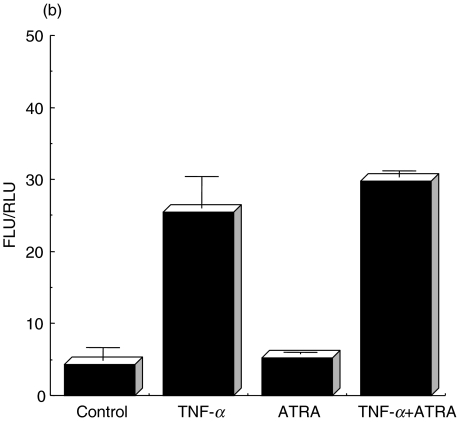
To assess the effect of ATRA or 9CRA on expression of the pIgR, ATRA or 9CRA was added to the culture medium with or without TNF-α and then amounts of SC were measured by ELISA. Figure 1 shows that the amount of SC secreted into the culture medium was increased by addition of ATRA or 9CRA in combination with TNF-α compared to the addition of TNF-α alone. The release of SC was not increased by addition of ATRA or 9CRA alone.
Fig. 1.
Production of SC stimulated with TNF-α and/or RA. HT-29 cells were treated with TNF-α and 1 µm of ATRA (*P < 0·004) or 9CRA (*P < 0·003). Controls represent that HT-29 cells were treated with neither TNF-α nor RA. After 48 h cultivation, the culture supernatants were concentrated and SC amounts were determined by ELISA as described in Materials and methods. Experiments were performed in triplicate.
Enhancement of SC release by addition of RA is regulated at the mRNA level
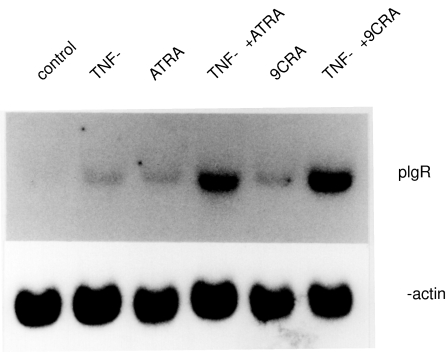
It has been reported that the pIgR gene is up-regulated by TNF-α treatment. To assess the effect of ATRA or 9CRA on expression of the pIgR gene, HT-29 cells were treated with ATRA or 9CRA in combination with or without TNF-α. The expression of pIgR gene was increased by addition of ATRA or 9CRA in the presence of TNF-α(Fig. 2).
Fig. 2.
Expression of the pIgR mRNA by RA and TNF-α or no treatment (control) in HT-29 cells. HT-29 cells were treated in the same manner as Fig. 1. Ten µg of total RNA was separated by 1·0% agarose gel containing formalin, transferred onto nylon membrane and hybridized with Pst I fragment of human pIgR cDNA.
Enhancement of pIgR gene expression by RA is mediated by RAR
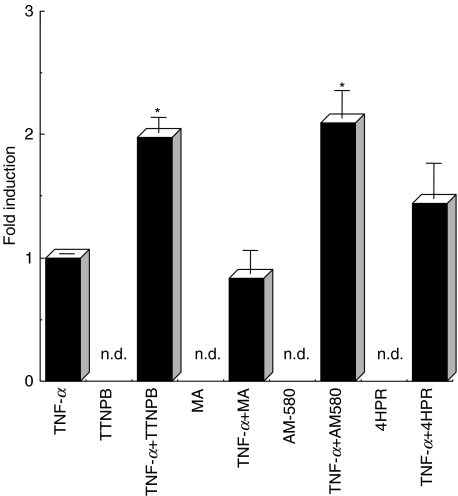
To confirm whether the effect of ATRA or 9CRA is specific for retinoic acid receptor, HT-29 is treated with 1 µm of TTNPB, a specific agonist of RARs, or 1 µm of MA, an agonist of RXRs, with or without TNF-α for 48 h and then the amount of SC was measured. The release of SC was increased by addition of TTNPB. However, the release of SC was not increased by addition of MA (Fig. 3). To test which RAR receptor mediates these effects, HT-29 cells were treated with TNF-α and AM-580, a RARα selective agonist. Co-treatment of HT-29 cells with TNF-α and 1 µm of AM-580 also up-regulated the release of SC. Co-treatment with TNF-α and 4-HPR, a RARβ- and -γ-specific agonist, the expression level of SC was not significantly increased (Fig. 3).
Fig. 3.
The effect of ATRA or 9CRA on HT-29 cells is an RAR specific reaction. HT-29 cells were co-stimulated with TNF-α and 1 µm of TTNPB (*P < 0·01) or 1 µm of MA or AM-580 (*P < 0·04) or 4-HPR for 48 h. After cultivation, culture supernatants were subjected to ELISA as described in Materials and methods. Experiments were performed in triplicate.
Effect of RA on the binding of NF-κB
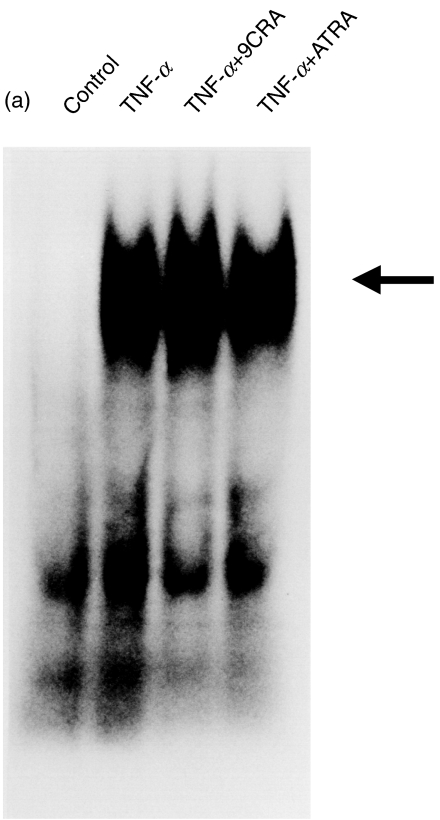
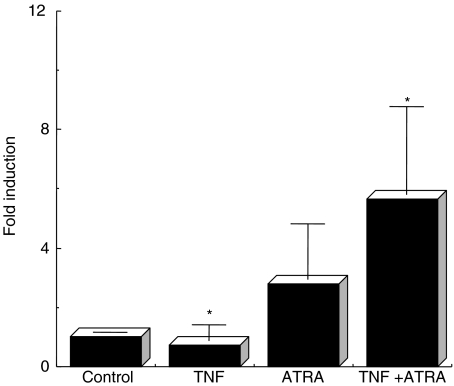
To determine whether NF-κB binding to κB2 is directly involved in pIgR gene expression, HT-29 cells were co-stimulated with TNF-α and 1 µm of ATRA or 9CRA for 90 min, and nuclear extracts were isolated. Analysis of NF-κB binding protein by EMSA using a κB2 probe showed that NF-κB binding to the pIgR 5′-flanking region was not enhanced by ATRA and 9CRA (Fig. 4a). We also checked the activity of NF-κB change in HT-29 cells by ATRA using transfection method (Fig. 4b). The activity of NF-κB did not change by addition of ATRA. Because Schjerven et al. indicated that cooperation with intronic NF-κB/Rel site and proximal promoter is involved in TNF-α-induced pIgR transcription [23], we also tested whether the binding to the intron NF-κB site is up-regulated or not. The binding of intron NF-κB was not affected by addition of ATRA (data not shown).
Fig. 4.
(a) Effect of ATRA or 9CRA on the NF-κB binding to the human pIgR NF-κB binding site. Nuclear extracts were prepared from TNF-α treated (10 ng/ml) HT-29 cells with or without 1 µm of ATRA or 9CRA, or neither TNF-α nor RA (control) as described Materials and methods. Ten µg of nuclear extract was incubated with a κB2 probe for the detection of NF-κB. Arrow indicates the NF-κB. (b) Cellular NF-κB activity by ATRA treatment. Reporter plasmid pNF-κB-luc was treansfected into HT-29 cells, and then TNF-α and/or ATRA were added and incubated for 24 h. Luciferase activity was measured as indicated in Materials and methods.
Enhancement of SC release by RA is also observed in another human colon cancer cell line, Caco-2
To confirm that the effect of RA in combination with TNF-α is specific for intestinal epithelial cell lines, an additional human colon adenocarcinoma cell line was used. It has been described that when Caco-2 cells were treated with IFN-γ, the amount of SC was increased [24], but the effect of TNF-α on SC release is unknown. Treatment with 10 ng/ml TNF-α or ATRA alone did not increase expression levels of SC significantly compared to controls. However, co-treatment of Caco-2 cells with TNF-α and ATRA together enhanced SC secretion significantly (Fig. 5).
Fig. 5.
Production of SC by TNF-α is also enhanced by ATRA in Caco-2 cells. Cells were treated with 10 ng/ml TNF-α and 1 µm ATRA or neither TNF-α nor ATRA (control) for 48 h and then supernatants were subjected to ELISA. Asterisk indicates the significant differences between TNF-α stimulation and TNF-α plus RA stimulation (P < 0·01). Experiments were performed in triplicate.
DISCUSSION
The human pIgR is a ∼100 000 MW glycoprotein present on glandular epithelial cells and functions as a receptor for pIg. It transports polymeric IgA (pIgA) into external secretions as secretory IgA (S-IgA), which is critical for the defence of mucosal tissues [25]. It has been reported that IFN-γ[26], TNF-α[27], IL-4 [28] and IL-1β[20] can up-regulate release of the SC into the culture supernatant of the human colonic adenocarcinoma cell line, HT-29. In addition, IL-4 and IFN-γ increased synergistically the release of SC in HT-29 cells [28]. These data indicate that the pIgR expression is regulated by cytokines, particularly inflammatory cytokines. In the case of IFN-γ stimulation, the interferon-stimulating response element (ISRE) located at intron 1 and 5′-flanking region is involved in up-regulation of pIgR gene expression [29]. Blanch et al. showed that the expression levels of transcription factor IRF-1 and pIgR mRNA by TNF-α, IFN-γ and IL-1β are regulated coordinately in HT29 cells [30]. Recently, it has been suggested that intron 1 is important for the regulation of pIgR gene transcription. In addition, the signal transducer and activator of transcription (STAT)-6 binding site located at intron 1 is important for the up-regulation of pIgR gene expression by IL-4 [31], and this expression is required for cooperation of STAT-6 and tissue specific factor, HNF-1, whose binding site is located upstream of STAT-6 binding site [32]. Because the release of SC was not enhanced by ATRA or 9CRA alone, up-regulation of SC release by TNF-α and RA may not be caused by enhancement of basal expression. pIgR gene expression by TNF-α has also been suggested to be regulated by NF-κB located upstream of the transcription initiation site [33,34].Moreover, NF-κB binding site located at intron 1 is strongly involved in TNF-α induced pIgR gene expression [23]. Local up-regulation of pIgR may result in the promotion of external transport of polymeric IgA, and consequently contribute to mucosal defenses.
In this study, we report that up-regulation of SC release and pIgR gene expression by TNF-α is enhanced further by the addition of 1 µm of ATRA or 9CRA (Fig. 1), respectively. The contribution of ATRA to the up-regulation of the pIgR gene by IL-4 and IFN-γ has been reported [13]. Our results showed that ATRA or 9CRA in combination with TNF-α has a similar effect on up-regulation of the pIgR gene as that reported for IL-4 and IFN-γ.
The effect of ATRA on the growth of human colon cancer cell lines has been reported. In HT-29 cells, treatment with ATRA, 13CRA and 9CRA for 10 days causes anchorage-independent growth inhibition mediated by RARα, without evidence for the induction of cellular differentiation [35]. On the other hand, Lee et al. showed that ATRA did not affect the growth of HT-29 and DLD-1 by using an MTT assay [36]. In other cell lines, such as HCT-15 and colo201, treatment with ATRA induced inhibition of cell growth and apoptosis [36]. This growth inhibition was mediated by RARβ. It has also been shown that treatment of HT-29 cells with 1 µm of ATRA induced elevated levels of the RARα and RARγ, but not RARβ[37].
The expression of RXRα and RXRγ mRNA in HT-29 cells was increased by treatment with 9CRA [37]. In this study, HT-29 cells were co-treated with TNF-α and MA, and no significant enhancement of SC release was observed (Fig. 3). In contrast, our results using RAR agonists such as TTNPB, AM-580 and 4-HPR indicate that the enhancement of pIgR expression by RA is mediated mainly by RARα (Fig. 3). It has been reported that the ICAM-1 expression was induced synergistically by TNF-α and 9CRA mediated by the interaction of retinoid receptor and NF-κB [38]. However, our structural analysis, as well as others [39] of the 5′-flanking region of the human pIgR gene, indicates that putative RA response elements do not exist, at least within 3·5 kb upstream from the transcription initiation site. Enhanced binding of NF-κB and activation of NF-κB by treatment with RA were not observed in this study (Fig. 4). Our data indicated that in intestinal mucosal epithelial cells, RA can increase the pIgR expression without excessive increase of NF-κB. If anything, the possible mechanism by which RA contribute to increase TNF-α-stimulated pIgR expression is mRNA levels, such as increase of mRNA stability. In addition, up-regulation of SC by TNF-α in combination with ATRA could be detected in another colon cell line, Caco-2 (Fig. 5). It has been indicated that free SC is important for enhancing the immune response. For example, degranulation of eosinophils by binding of S-IgA or SC [40,41] is mediated through the 15 000 MW SC receptor expressed in eosinophils [42]. Free SC can also bind to some bacterial protein such as colonization factor antigen 1 [43], Clostridium difficile toxin A [44] and Streptococcus pneumoniae protein SpsA [45,46]. The expression of pIgR is rate-limiting step for transport of pIgA indicating the possibility for the therapeutic value of vitamin A for inflammatory diseases in mucosal tissues by increase the production of SC.
Acknowledgments
This work was supported by grants from Nihon University Research Grant for Assistants and from Uemura Fund, Nihon University School of Dentistry, as well as a grant for promotion of multi-disciplinary research projects and a grant from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (no.11307043 and 50246914).
REFERENCES
- Leid M, Kastner P, Lyons R, et al. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992;68:377–95. doi: 10.1016/0092-8674(92)90478-u. [Published erratum appears in Cell 1992 November 27; 71 (5): following 886.] [DOI] [PubMed] [Google Scholar]
- Semba RD. Vitamin A, immunity, and infection. Clin Infect Dis. 1994;19:489–99. doi: 10.1093/clinids/19.3.489. [DOI] [PubMed] [Google Scholar]
- Semba RD, Lyles CM, Margolick JB, et al. Vitamin A supplementation and human immunodeficiency virus load in injection drug users. J Infect Dis. 1998;177:611–6. doi: 10.1086/514235. [DOI] [PubMed] [Google Scholar]
- Hassan MS, Mileva MM, Dweck HS, Rosenfeld L. Nitric oxide products degrade chondroitin sulfates. Nitric Oxide. 1998;2:360–5. doi: 10.1006/niox.1998.0198. [DOI] [PubMed] [Google Scholar]
- Wiedermann U, Chen XJ, Enerback L, Hanson LA, Kahu H, Dahlgren UI. Vitamin A deficiency increases inflammatory responses. Scand J Immunol. 1996;44:578–84. doi: 10.1046/j.1365-3083.1996.d01-351.x. [DOI] [PubMed] [Google Scholar]
- Edelman R, Suskind R, Olson RE, Sirisinha S. Mechanisms of defective delayed cutaneous hypersensitivity in children with protein-calorie malnutrition. Lancet. 1973;1:506–8. doi: 10.1016/s0140-6736(73)90326-7. [DOI] [PubMed] [Google Scholar]
- Smythe PM, Brereton-Stiles GG, Grace HJ, et al. Thymolymphatic deficiency and depression of cell-mediated immunity in protein-calorie malnutrition. Lancet. 1971;2:939–43. doi: 10.1016/s0140-6736(71)90267-4. [DOI] [PubMed] [Google Scholar]
- Ha CL, Woodward B. Reduction in the quantity of the polymeric immunoglobulin receptor is sufficient to account for the low concentration of intestinal secretory immunoglobulin A in a weanling mouse model of wasting protein-energy malnutrition. J Nutr. 1997;127:427–35. doi: 10.1093/jn/127.3.427. [DOI] [PubMed] [Google Scholar]
- McGee DW, McMurray DN. The effect of protein malnutrition on the IgA immune response in mice. Immunology. 1988;63:25–9. [PMC free article] [PubMed] [Google Scholar]
- Nikawa T, Odahara K, Koizumi H, et al. Vitamin A prevents the decline in immunoglobulin A and Th2 cytokine levels in small intestinal mucosa of protein-malnourished mice. J Nutr. 1999;129:934–41. doi: 10.1093/jn/129.5.934. [DOI] [PubMed] [Google Scholar]
- Cui D, Moldoveanu Z, Stephensen CB. High-level dietary vitamin A enhances T-helper type 2 cytokine production and secretory immunoglobulin A response to influenza A virus infection in BALB/c mice. J Nutr. 2000;130:1132–9. doi: 10.1093/jn/130.5.1132. [DOI] [PubMed] [Google Scholar]
- Sirisinha S, Darip MD, Moongkarndi P, Ongsakul M, Lamb AJ. Impaired local immune response in vitamin A-deficient rats. Clin Exp Immunol. 1980;40:127–35. [PMC free article] [PubMed] [Google Scholar]
- Sarkar J, Gangopadhyay NN, Moldoveanu Z, Mestecky J, Stephensen CB. Vitamin A is required for regulation of polymeric immunoglobulin receptor (pIgR) expression by interleukin-4 and interferon-gamma in a human intestinal epithelial cell line. J Nutr. 1998;128:1063–9. doi: 10.1093/jn/128.7.1063. [DOI] [PubMed] [Google Scholar]
- Krajci P, Grzeschik KH, Geurts van Kessel AH, Olaisen B, Brandtzaeg P. The human transmembrane secretory component (poly-Ig receptor): molecular cloning, restriction fragment length polymorphism and chromosomal sublocalization. Hum Genet. 1991;87:642–8. doi: 10.1007/BF00201717. [DOI] [PubMed] [Google Scholar]
- Boehm MF, Zhang L, Badea BA, et al. Synthesis and structure–activity relationships of novel retinoid X receptor-selective retinoids. J Med Chem. 1994;37:2930–41. doi: 10.1021/jm00044a014. [DOI] [PubMed] [Google Scholar]
- Harmon MA, Boehm MF, Heyman RA, Mangelsdorf DJ. Activation of mammalian retinoid X receptors by the insect growth regulator methoprene. Proc Natl Acad Sci USA. 1995;92:6157–60. doi: 10.1073/pnas.92.13.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Bernardon JM, Cavey MT, et al. Selective synthetic ligands for human nuclear retinoic acid receptors. Skin Pharmacol. 1992;5:57–65. doi: 10.1159/000211018. [DOI] [PubMed] [Google Scholar]
- Fanjul AN, Delia D, Pierotti MA, et al. 4-Hydroxyphenyl retinamide is a highly selective activator of retinoid receptors. J Biol Chem. 1996;271:22441–6. doi: 10.1074/jbc.271.37.22441. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Takenouchi N, Asano M, et al. The polymeric immunoglobulin receptor (secretory component) in a human intestinal epithelial cell line is up-regulated by interleukin-1. Immunology. 1997;92:220–5. doi: 10.1046/j.1365-2567.1997.00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusama K, Takahashi T, Asano M, et al. Murine monoclonal antibody to secretory component. Shika Kiso Igakkai Zasshi. 1989;31:471–3. doi: 10.2330/joralbiosci1965.31.471. [DOI] [PubMed] [Google Scholar]
- Natarajan K, Singh S, Burke TR, Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci US A. 1996;93:9090–5. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schjerven H, Brandtzaeg P, Johansen FE. A novel NF-kappaB/Rel site in intron 1 cooperates with proximal promoter elements to mediate TNF-alpha-induced transcription of the human polymeric Ig receptor. J Immunol. 2001;167:6412–20. doi: 10.4049/jimmunol.167.11.6412. [DOI] [PubMed] [Google Scholar]
- Hirata M, Bamba T, Hosoda S. The human colon cancer cell line CaCo-2 produces secretory components during enterocytic differentiation. Gastroenterol Jpn. 1993;28:528–34. doi: 10.1007/BF02776951. [DOI] [PubMed] [Google Scholar]
- Mestecky J, McGhee JR. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Sollid LM, Kvale D, Brandtzaeg P, Markussen G, Thorsby E. Interferon-gamma enhances expression of secretory component, the epithelial receptor for polymeric immunoglobulins. J Immunol. 1987;138:4303–6. [PubMed] [Google Scholar]
- Kvale D, Lovhaug D, Sollid LM, Brandtzaeg P. Tumor necrosis factor-alpha up-regulates expression of secretory component, the epithelial receptor for polymeric Ig. J Immunol. 1988;140:3086–9. [PubMed] [Google Scholar]
- Phillips JO, Everson MP, Moldoveanu Z, Lue C, Mestecky J. Synergistic effect of IL-4 and IFN-gamma on the expression of polymeric Ig receptor (secretory component) and IgA binding by human epithelial cells. J Immunol. 1990;145:1740–4. [PubMed] [Google Scholar]
- Piskurich JF, Youngman KR, Phillips KM, et al. Transcriptional regulation of the human polymeric immunoglobulin receptor gene by interferon-gamma. Mol Immunol. 1997;34:75–91. doi: 10.1016/s0161-5890(96)00079-x. [DOI] [PubMed] [Google Scholar]
- Blanch VJ, Piskurich JF, Kaetzel CS. Cutting edge. coordinate regulation of IFN regulatory factor-1 and the polymeric Ig receptor by proinflammatory cytokines. J Immunol. 1999;162:1232–5. [PubMed] [Google Scholar]
- Schjerven H, Brandtzaeg P, Johansen FE. Mechanism of IL-4-mediated up-regulation of the polymeric Ig receptor: role of STAT6 in cell type-specific delayed transcriptional response. J Immunol. 2000;165:3898–906. doi: 10.4049/jimmunol.165.7.3898. [DOI] [PubMed] [Google Scholar]
- Schjerven H, Brandtzaeg P, Johansen FE. Hepatocyte NF-1 and STAT6 cooperate with additional DNA-binding factors to activate transcription of the human polymeric Ig receptor gene in response to IL-4. J Immunol. 2003;170:6048–56. doi: 10.4049/jimmunol.170.12.6048. [DOI] [PubMed] [Google Scholar]
- Nilsen EM, Johansen FE, Kvale D, Krajci P, Brandtzaeg P. Different regulatory pathways employed in cytokine-enhanced expression of secretory component and epithelial HLA class I genes. Eur J Immunol. 1999;29:168–79. doi: 10.1002/(SICI)1521-4141(199901)29:01<168::AID-IMMU168>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Takenouchi-Ohkubo N, Takahashi T, Tsuchiya M, Mestecky J, Moldoveanu Z, Moro I. Role of nuclear factor-kappaB in the expression by tumor necrosis factor-alpha of the human polymeric immunoglobulin receptor (plgR) gene. Immunogenetics. 2000;51:289–95. doi: 10.1007/s002510050622. [DOI] [PubMed] [Google Scholar]
- Nicke B, Kaiser A, Wiedenmann B, Riecken EO, Rosewicz S. Retinoic acid receptor alpha mediates growth inhibition by retinoids in human colon carcinoma HT29 cells. Biochem Biophys Res Commun. 1999;261:572–7. doi: 10.1006/bbrc.1999.1086. [DOI] [PubMed] [Google Scholar]
- Lee MO, Han SY, Jiang S, Park JH, Kim SJ. Differential effects of retinoic acid on growth and apoptosis in human colon cancer cell lines associated with the induction of retinoic acid receptor beta. Biochem Pharmacol. 2000;59:485–96. doi: 10.1016/s0006-2952(99)00355-x. [DOI] [PubMed] [Google Scholar]
- Kane KF, Langman MJ, Williams GR. Antiproliferative responses to two human colon cancer cell lines to vitamin D3 are differently modified by 9-cis-retinoic acid. Cancer Res. 1996;56:623–32. [PubMed] [Google Scholar]
- Chadwick CC, Shaw LJ, Winneker RC. TNF-alpha and 9-cis-retinoic acid synergistically induce ICAM-1 expression: evidence for interaction of retinoid receptors with NF-kappa B. Exp Cell Res. 1998;239:423–9. doi: 10.1006/excr.1997.3913. [DOI] [PubMed] [Google Scholar]
- Verrijdt G, Swinnen J, Peeters B, Verhoeven G, Rombauts W, Claessens F. Characterization of the human secretory component gene promoter. Biochim Biophys Acta. 1997;1350:147–54. doi: 10.1016/s0167-4781(96)00214-x. [DOI] [PubMed] [Google Scholar]
- Iikura M, Yamaguchi M, Fujisawa T, et al. Secretory IgA induces degranulation of IL-3-primed basophils. J Immunol. 1998;161:1510–5. [PubMed] [Google Scholar]
- Motegi Y, Kita H. Interaction with secretory component stimulates effector functions of human eosinophils but not of neutrophils. J Immunol. 1998;161:4340–6. [PubMed] [Google Scholar]
- Lamkhioued B, Gounni AS, Gruart V, Pierce A, Capron A, Capron M. Human eosinophils express a receptor for secretory component. Role in secretory IgA-dependent activation. Eur J Immunol. 1995;25:117–25. doi: 10.1002/eji.1830250121. [DOI] [PubMed] [Google Scholar]
- Giugliano LG, Ribeiro ST, Vainstein MH, Ulhoa CJ. Free secretory component and lactoferrin of human milk inhibit the adhesion of enterotoxigenic Escherichia coli. J Med Microbiol. 1995;42:3–9. doi: 10.1099/00222615-42-1-3. [DOI] [PubMed] [Google Scholar]
- Dallas SD, Rolfe RD. Binding of Clostridium difficile toxin A to human milk secretory component. J Med Microbiol. 1998;47:879–88. doi: 10.1099/00222615-47-10-879. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt S, Talay SR, Brandtzaeg P, Chhatwal GS. SpsA, a novel pneumococcal surface protein with specific binding to secretory immunoglobulin A and secretory component. Mol Microbiol. 1997;25:1113–24. doi: 10.1046/j.1365-2958.1997.5391899.x. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt S, Tillig MP, Wolff S, Vaerman JP, Chhatwal GS. Species-specific binding of human secretory component to SpsA protein of Streptococcus pneumoniae via a hexapeptide motif. Mol Microbiol. 2000;36:726–36. doi: 10.1046/j.1365-2958.2000.01897.x. [DOI] [PubMed] [Google Scholar]