Abstract
Safer and more effective human rotavirus (HRV) vaccines are needed. We evaluated oral priming with attenuated WaHRV (AttHRV) followed by boosting with two intranasal (IN) doses of VP2/6 virus-like particles (2/6 VLP) with immunostimulating complexes (ISCOM) to determine if this regimen induces protection against diarrhoea and viral shedding in the gnotobiotic pig model. IgM, IgA and IgG antibody titres in serum and intestinal contents were quantified by enzyme-linked immunosorbent assay (ELISA) and serum neutralizing antibody titres were measured by a virus neutralization (VN) test. Seven groups of neonatal gnotobiotic pigs were vaccinated at post-inoculation days (PID) 0, 10 and 21 and challenged with virulent WaHRV at PID 28. The vaccine groups included: (1, 2) oral priming with AttHRV and boosting with two IN immunizations with 2/6 VLP–ISCOM (Att + 2/6 VLP–ISCOM) at VLP concentrations of 250 µg or 25 µg; (3, 4) three IN immunizations with 2/6 VLP–ISCOM at VLP concentrations of 250 µg or 25 µg (2/6 VLP–ISCOM); (5) three oral immunizations with AttHRV (3×AttHRV); (6) one oral immunization with AttHRV (1×AttHRV); (7) controls (ISCOM matrix and/or diluent). The pigs that received 3×AttHRV or Att + 2/6 VLP250–ISCOM had the highest protection rates against diarrhoea upon challenge at PID 28 with virulent WaHRV. The IgA antibody titres to HRV in intestinal contents were significantly higher in the Att + 2/6 VLP250–ISCOM group than in all other groups prechallenge (PID 28). Serum VN antibody titres were statistically similar after the first inoculation among the groups given AttHRV, but at PID 28 VN antibody titres were significantly higher for the 3×AttHRV and Att + 2/6 VLP250–ISCOM groups than for the 1×AttHRV group suggesting that boosting with 2/6 VLP also boosted VN antibody responses. In humans, intestinal IgA antibodies have been correlated with protection against symptomatic reinfection. Thus the vaccine regimen of one oral dose of AttHRV and two IN immunizations with 2/6 VLP250–ISCOM may be an alternative to multiple-dose live oral vaccines in humans.
Keywords: gnotobiotic pig model, intestinal immunity, 2/6 rotavirus-like particles, rotavirus antibody responses, rotavirus intranasal vaccine
INTRODUCTION
Rotavirus gastroenteritis is responsible for the deaths of 600 000–870 000 children worldwide, with the highest impact in developing countries [1]. Recently, the first licensed rotavirus vaccine, a tetravalent reassortant rhesus rotavirus, was associated with an increased risk of intussusception and was withdrawn in October 1999 [2,3].
The gnotobiotic pig model of human rotavirus (HRV)-induced diarrhoea has the advantage of susceptibility of pigs to HRV-induced disease, a lack of maternal antibodies and similarity to infants in development of mucosal immunity [4]. We studied a new prime/boost strategy for rotavirus vaccination using oral priming with attenuated HRV (Wa strain) followed by boosting with two intranasal (IN) doses of recombinant VP2 (from RF bovine rotavirus)/VP6 (from Wa HRV) virus-like particles (2/6 VLP). This same regimen induced partial protection and intestinal antibody secreting cell (ASC) responses in gnotobiotic pigs using 2/6 VLPs with mutant Esherichia coli heat labile-toxin (mLT) as adjuvant (58% and 44% protection rates against virus shedding and diarrhoea, respectively) [5]. In the same study priming with 2/6 VLP + mLT followed by boosting with oral AttHRV was also examined, but this vaccine regimen induced only low protection rates, so it was not repeated in the present study. Although we have studied ASC responses previously in systemic and intestinal tissues after oral AttHRV priming and oral 2/6 VLP boosting [6], neutralizing and isotype antibody responses in serum and intestinal contents following the use of 2/6 VLP vaccines with ISCOM adjuvant-administered IN have not been examined. Analysis of such antibody responses is important for comparison with the corresponding serum and faecal antibody responses in human infants given rotavirus vaccines. Immune stimulating complex (ISCOM) are cage-like structures composed of cholesterol and Quillaja saponins [7,8]. They stimulate activation of lymphocytes through the production of proinflammatory cytokines and subsequent leucocyte migration [9,10]. ISCOM have been used previously as adjuvants and delivery vehicles with appropriate antigens against a variety of pathogens in different animal models and humans [6,8,11,12]. Only in our previous studies have ISCOM been used with VLPs to elicit intestinal immunity to rotavirus [6]
Double-shelled VLPs were generated using recombinant baculoviruses expressing the individual rotavirus proteins VP2 and VP6 [13]. The rotavirus inner capsid is composed of the VP2 core and surrounded by VP6, the major inner capsid protein [14,15]. In the murine model, the generation of non-neutralizing IgA monoclonal antibodies to VP6 using a back-pack tumour was sufficient to protect adult mice against primary rotavirus infection and induce viral clearance in chronically infected mice [16]. In contrast, in sucking mice, only IgA VN antibodies to the VP8 subunit of VP4, but not IgA antibodies to VP6, were protective against diarrhoea [17]. Because it accounts for more than 50% of the virion mass, VP6 is a dominant antigenic target for HRV-specific IgA antibodies detected in faecal specimens [15,18,19], but its role in eliciting protective immunity is controversial.
Intestinal (or faecal) and, in some studies, serum rotavirus-specific IgA antibody titres correlate with protection against reinfection in humans and in different animal models. In children with acute rotavirus infection, higher serum titres of rotavirus-specific IgA antibodies were correlated with less severe symptoms [20,21]. Other researchers have demonstrated that children with higher serum rotavirus-specific IgA antibody geometric mean titres (GMT) were better protected against reinfection [21,22]. Children that had persisting high titres of rotavirus-specific IgA antibodies in stools showed lower rates of reinfection [23]. Vaccination studies in pig and mouse models, revealed intestinal rotavirus-specific IgA antibody titres and ASC were also associated with protection after challenge with virulent rotavirus [5,24–28]. Serum neutralizing antibodies have also been correlated with protection against secondary HRV infection in children [29,30], but there is still controversy about their role in protection [20,29,31]. Higher serum HRV-specific IgG antibody GMT were also correlated with protection in some studies [21,22] but not in others [24].
In this study we determined rotavirus-specific IgA, IgM and IgG antibody titres in serum and small and large intestinal contents by enzyme-linked immunosorbent assay (ELISA) and serum neutralizing antibody titres by a VN assay. Rates of protection induced by oral immunizations with AttHRV, IN immunizations with recombinant (bovine–human) 2/6 VLP with ISCOM matrix as adjuvant or the two vaccines combined (Att + 2/6 VLP-ISCOM) in a prime/boost strategy were compared. Prior rotavirus vaccines were based on three doses of AttHRV, so the responses detected in the Att + 2/6 VLP-ISCOM group were compared with those obtained from pigs that received three oral doses of AttHRV (3×AttHRV). A group that received three doses of 2/6 VLP (2/6 VLP–ISCOM), a vaccination regimen shown previously to be unprotective in pigs when used with E. coli mLT adjuvant [25], was also studied to determine if ISCOM would stimulate higher and protective immune responses. Finally, to determine the booster effect of the 2/6 VLP-ISCOM on the responses elicited by the Att + 2/6 VLP–ISCOM vaccine, we vaccinated a group of pigs with AttHRV alone (1×AttHRV).
MATERIALS AND METHODS
Virulent rotavirus
Virulent Wa HRV (P1A, G1) was pooled from intestinal contents of gnotobiotic pigs after the 18th pig passage. Pigs were challenged with virulent HRV at a dose of 106 ID50 in order to assure that 100% of the primary mock-inoculated pigs developed diarrhoea after challenge [32].
Attenuated virus
The attenuated cell-culture adapted HRV Wa strain (P1A,G1) was used at the 27th passage level in monkey kidney cells (MA104). It was used for oral inoculation of gnotobiotic pigs at a dose of 5 × 107 fluorescent focus-forming units and for the immunoassays [28].
Recombinant 2/6 virus-like particles (VLP)
The 2/6 VLP containing bovine rotavirus VP2 (RF) and human rotavirus VP6 (Wa) were produced in Spodoptera frugiperda 9 insect cells, as described previously [6]. The assembled 2/6 VLPs were purified by CsCl density gradient ultracentrifugation. Characterization of the VLPs was performed by immune electron microscopy (IEM), Western blot, protein and endotoxin assays [6].
VLP–ISCOM preparation
To increase the capacity of the 2/6 VLPs to bind to ISCOM matrix, they were mixed with 2 m LiCl for 30 min at room temperature and then incubated overnight at − 70° [6]. The LiCl-treated 2/6 VLPs (positively charged by the Li + ions) were mixed with lyophilized ISCOM matrix (1 mg of 2/6 VLP per 5 mg of ISCOM matrix) and then dialysed in 0·09% NaCl solution for 72 h. The association of 2/6VLPs with ISCOM matrix was confirmed by IEM.
Gnotobiotic pigs
Near-term pigs were derived aseptically by hysterectomy and kept under sterile conditions in isolation units as described previously [32]. The gnotobiotic pigs were assigned to one of the following seven groups: (1, 2) one oral immunization with Wa AttHRV and two IN immunizations with recombinant 2/6 VLP at a VLP dose of 250 µg + ISCOM dose of 1250 µg or at a VLP dose of 25 µg + ISCOM dose of 125 µg per dose (Att + 2/6 VLP–ISCOM); (3, 4) three IN immunizations with 2/6 VLP at a VLP dose of 250 µg + ISCOM dose of 1250 µg or at a VLP dose of 25 µg + ISCOM dose of 125 µg per dose (2/6 VLP–ISCOM); (5) three oral immunizations with AttHRV (3×AttHRV); (6) one oral immunization with AttHRV (1×AttHRV); and (7) controls: three IN immunizations with ISCOM matrix or one oral immunization with diluent and two IN immunizations with ISCOM matrix. The first inoculation was performed at 3–5 days after derivation (post-inoculation day 0, PID 0). Subsequent inoculations were at 10 (PID 10) and 21 days (PID 21). All procedures were conducted in accordance with protocols reviewed by the Ohio State University's Institutional Laboratory Animal Care and Use Committee.
Assessment of protection
At PID 28, subsets of pigs from each group were challenged with virulent HRV (post-challenge day 0, PCD 0) and observed for 6 days for signs of diarrhoea. Faecal consistency was scored as follows: 0: normal; 1: pasty; 2: semiliquid; 3: liquid. Faecal scores equal to or greater than 2 were considered diarrhoeic. The mean cumulative faecal score was calculated as the sum of daily faecal scores from PCD 1–6 divided by the total number of pigs in each of the groups. Rectal swabs were collected daily and virus shedding was determined in rectal swabs fluids by antigen capture ELISA and cell-culture immunofluorescence assay (CCIF) as described previously [33].
Blood and intestinal contents
Blood samples were collected at PID 0, 10, 21, 28/PCD 0 and PID 35/PCD 7. Serum was collected and complement inactivated at 56°C for 30 min, then stored at − 20°C until tested. Small intestinal contents (SIC) and large intestinal contents (LIC) were collected only at euthanasia at PID 28/PCD 0 and PID 35/PCD 7. The LIC or SIC were diluted 1 : 2 in diluent containing protease inhibitors (25 µg trypsin inhibitor and 5 µg leupeptin; Sigma, St Louis, MO, USA). The intestinal contents were stored at − 20°C until tested.
ELISA for antibody isotypes
Rotavirus-specific IgA, IgM and IgG antibody titres were determined as described previously [34]. Briefly, the reagents were added to 96-well microtitre plates coating initially with guinea pig antibovine rotavirus hyperimmune serum (incubated overnight at 4°C or 2 h at 37°C). Washes were performed four times with phosphate buffered saline (PBS)-Tween 0·5% between each step and most of the incubations, unless otherwise stated, lasted 1 for h at 37°C. The plates were blocked with 2% non-fat dry milk (incubated overnight at 4°C) followed by the addition of a 1 : 3 dilution in PBS (pH 7·4) of semipurified rotavirus or mock-infected MA104 supernatants. Serial fourfold dilutions of serum or intestinal contents in 2% non-fat dry milk were followed by the addition of biotin-labelled monoclonal antibodies to porcine IgM (KPL Laboratories, Inc., Gaithersburg, MD, USA), IgG (clone 3H7D7) or IgA (clone 6A11). Streptavidin-horseradish peroxidase was added (Roche, Indianapolis, IN, USA) and 2–2′-azino-bis(3-ethylbenz-thiazoline-6-sulphonic acid (ABTS- Sigma, St Louis, MO, USA) was used as a chromagenic substrate. The antibody titres were calculated as the reciprocal of the sample dilution which had a mean absorbance greater than the mean of the negative controls (three negative controls per plate) plus three standard deviations, after subtracting mock-coated well absorbances from antigen-coated well absorbances for each sample. Serum samples were considered positive for IgA or IgG if the value was positive at a dilution of >1 : 4. Some serum negative samples (controls and PID 0 samples) had background reactions at 1 : 4 for IgM antibodies; for this reason the initial test dilution was 1 : 16. For intestinal contents, all three isotypes were considered positive above a 1 : 4 dilution. Each plate had a positive control with a known antibody titre that was used to estimate reproducibility of the individual assays.
Virus neutralization assay
To determine rotavirus VN antibody titres in serum samples, fourfold dilutions of the sera were mixed with equal volumes of Wa HRV (initial dilution 1 : 4) as described previously [28,33]. Briefly, after incubation at 37°C for 1 h, the virus-antibody mixtures were added to MA104 monolayers, incubated 1 h at 37° and the agar overlay added. The plates were incubated at 37°C and plaques were counted at 4–5 days. Titres were expressed as the reciprocal of the serum dilution that reduced the number of plaques by 80% compared to the control.
Statistical analyses
Antibody responses were compared using one-way analysis of variance followed by Duncan's multiple-range test (SAS Inc., Cary NC, USA) using log-transformed data. Percentage of pigs with diarrhoea and shedding, intestinal content conversion and seroconversion rates were compared using Fisher's exact test. A P-value of <0·05 was considered significant.
RESULTS
The Att + 2/6 VLP250–ISCOM and 3×AttHRV groups had the highest protection rates against diarrhoea and shedding after challenge with virulent Wa HRV
Pigs in the Att + 2/6 VLP250–ISCOM and 3×AttHRV groups had the highest protection rates against diarrhoea (71% and 44%, respectively) and shedding (71% and 67%, respectively) (Table 1), and these rates did not differ statistically for the two groups. All the pigs from the 2/6 VLP250–ISCOM and control groups developed shedding and diarrhoea following challenge. A 2/6 VLP vaccine dose effect with respect to virus shedding was detected among the Att + 2/6 VLP250–ISCOM, Att + 2/6 VLP25–ISCOM and 1×AttHRV groups with 71%, 34% and 14% protection against viral shedding, respectively, but only shedding in the 1×AttHRV group differed statistically from the other two groups.
Table 1.
Protection rates against virus shedding and diarrhoea in gnotobiotic pigs receiving the various vaccination regimensa
| Percentage of pigs with | |||||
|---|---|---|---|---|---|
| Vaccination groupa | na | Virus sheddingb | Diarrhoeac | Protection rate (%) against sheddingd | Protection rate (%) against diarrhoead |
| Att+2/6 VLP250–ISCOM | 7 | 29B | 29B | 71 | 71 |
| Att+2/6 VLP25 –ISCOM | 3 | 66B | 100A | 34 | 0 |
| 2/6 VLP250–ISCOM | 5 | 100A | 100A | 0 | 0 |
| 3×AttHRV | 9 | 33B | 56B | 67 | 44 |
| 1×AttHRV | 7 | 86A | 100A | 14 | 0 |
| Controls | 12 | 100A | 100A | 0 | 0 |
Gnotobiotic pigs receiving the various vaccination regimens at PID 0, 10 and 21 were challenged with virulent WaHRV at PID28. Diarrhoea scores and virus shedding were determined at PCD 0–6. n = Number of pigs. Proportions in the same column with different superscript letters differ significantly (Fisher's exact test). A portion of this protection data was published previously by Iosef et al. [6].
Determined by ELlSA and cell-culture immunofluorescence infectivity assay (CCIF).
Diarrhoea determined by faecal scores greater or equal to 2: faeces were scored as follows: 0 = normal; 1 = pasty; 2 = semiliquid; 3 = liquid.
Protection rate = [1-(% of vaccinated pigs in each group with diarrhoea-shedding/% of control pigs with diarrhoea-shedding)]×100.
The Att + 2/6 VLP250–ISCOM group had the highest percentage of intestinal content conversion for IgA and IgM antibodies at PID 28/PCD 0
At prechallenge (PID 28/PCD 0), the Att + 2/6 VLP250–ISCOM group had the highest percentage (100%) of pigs with IgM and IgA antibody titres to HRV in LIC and SIC, but no significant differences were detected compared to the 3×AttHRV and 1×AttHRV groups in IgA antibody titres in SIC (Table 2). Post-challenge (PID 35/PCD 7), all pigs had IgA and IgM antibody titres in LIC and SIC, except for the 3×AttHRV group in which 90% of pigs had IgM antibody titres to HRV and the control group that failed to develop IgA antibody titres by PID 35/PCD 7, but had IgM antibodies consistent with a primary immune response.
Table 2.
Percentage of conversion in intestinal contents for IgM, IgA and IgG antibodies in gnotobiotic pigs receiving the various regimensa
| SICb | SICb | SICb | SICb | SICb | SICb | |
|---|---|---|---|---|---|---|
| Att + 2/6 VLP250–ISCOM | 100A | 100A | 100A | 100A | 16B | 71AB |
| 2/6 VLP250–ISCOM | 33·3B | 100A | 66·6B | 100A | 66·6A | 80A |
| 3×AttHRV | 66·6B | 90A | 93B | 100A | 46A | 36B |
| 1×AttHRV | 75B | 100A | 75B | 100A | 25A | 42B |
| Controls | 0C | 100A | 0C | 0B | 0C | 0C |
Gnotobiotic pigs receiving the various vaccination regimens were euthanized at PID 28/PCD 0 or PID 35/PCD 7 and LIC and SIC were collected the day of the euthanasia. IgM, IgA and IgG antibody titres to HRV were quantified by ELISA.
Proportions in the same column with different letters differ significantly (Fisher's exact test).
At PID 28/PCD 0, the 2/6 VLP250–ISCOM group had the highest IgG conversion rate in intestinal contents. The percentage of pigs with IgG antibody titres to HRV was variable between SIC and LIC; higher percentage positives were detected in SIC for the 3×AttHRV and 1×AttHRV groups. At PID 35/PCD 7, the percentage of pigs positive for IgG antibodies to HRV was highest in the Att + 2/6 VLP250–ISCOM and 2/6 VLP250–ISCOM groups. IgG antibody titres were not detected in the control group by PID 35/PCD 7.
The Att + 2/6 VLP250–ISCOM group had the highest intestinal IgA antibody titres detected prechallenge in small and large intestinal contents
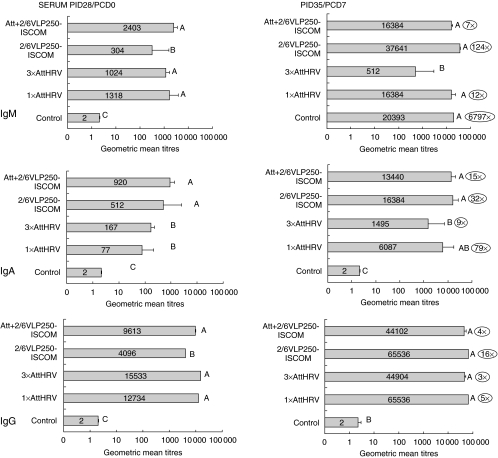
Antibody titres in LIC and SIC were measured by ELISA prechallenge (PID 28/PCD 0) and post-challenge (PID 35/PCD 7). Antibody titres in LIC were analysed and were similar to SIC antibody titres; for this reason the LIC data were omitted. Prechallenge in SIC, the Att + 2/6 VLP250–ISCOM group had the highest rotavirus-specific IgA and IgM antibody titres (Fig. 1). At post-challenge, all groups had increased IgM and IgA antibody titres (2–737-fold); the 3×AttHRV group had the lowest IgM antibody titre and the control group did not have IgA antibodies pre- or post-challenge. The rotavirus-specific IgG antibody titres were low pre- and post-challenge in the vaccine groups and the Att + 2/6 VLP250–ISCOM and 2/6 VLP250–ISCOM groups had the highest titres post-challenge. The groups with the highest protection rates, the 3×AttHRV and Att + 2/6 VLP250–ISCOM groups, had the lowest antibody increases (0–26-fold) post-challenge compared to prechallenge titres (PID 28/PCD 0) for IgA and IgM (Fig. 1).
Fig. 1.
Small intestinal contents (SIC) geometric mean antibody titres (GMT, bars) at PID 28/PCD 0 and PID 35/PCD 7 of gnotobiotic pigs receiving the various vaccination regimens. Pigs were inoculated with vaccines at PID 0, 10 and 21 and challenged at PID 28/PCD 0. Approximately half the pigs were euthanized prechallenge at PID 28 and the rest of the group was euthanized post-challenge at PID 35/PCD 7 and SIC/LIC was collected. Error bars represent standard error of the mean. Antibody titres that differ significantly are marked with different letters (one-way anova and Duncan's multiple range test on log10-transformed titres).  Numbers to the right of the bars indicate fold increases of the PID 35/PCD 7 GMT over the PID 28/PCD 0 GMT.
Numbers to the right of the bars indicate fold increases of the PID 35/PCD 7 GMT over the PID 28/PCD 0 GMT.
The first group to achieve 100% IgA serconversion was the Att + 2/6 VLP250–ISCOM group
All pigs from each of the vaccine groups had IgM antibody titres to HRV from PID 10 until PID 35/PCD 7. The control group developed IgM antibody titres only post-challenge at PID 35/PCD 7 (Fig. 2). Low titres of IgA antibodies to HRV in serum were first detected in the Att + 2/6 VLP250–ISCOM group at PID 10, but the percentage of positive pigs did not differ significantly compared to the other groups. At PID 21, 100% of the Att + 2/6 VLP250–ISCOM group had IgA antibody titres; the highest seroconversion rate detected followed by the 3×AttHRV and the 1×AttHRV groups (74% and 72%, respectively). At PID 28/PCD 0, all the groups except for the 1×AttHRV (81%) and control groups (0%) had 100% IgA seroconversion and at PID 35/PCD 7 all groups except for the control group had 100% IgA seroconversion.
Fig. 2.
Serum IgM, IgA and IgG antibody seroconversion at PID 0, PID 10, PID 21, PID 28/PCD 0 and PID 35/PCD 7 of gnotobiotic pigs receiving the various vaccination regimens. Pigs were inoculated with vaccines at PID 0, PID 10 and PID 21 and challenged at PID 28/PCD 0. Approximately half the pigs were euthanized prechallenge at PID 28 and the rest of the group was euthanized post-challenge at PID 35/PCD 7 and blood samples were collected at each of the different inoculation days. For each vaccination regimen, the number of pigs that were positive for IgM, IgA and IgG antibodies to HRV at each inoculation day were divided by the total number of animals per group and expressed as a percentage. Groups at the same PID with different letters differ significantly (Fisher's exact test).
At PID 10, the groups that received oral AttHRV had the highest rate of IgG seroconversion to HRV (18–51% compared to 0% of the 2/6 VLP250–ISCOM and control groups). After PID 21 all groups had 100% IgG seroconversion, except for the control group.
Neutralizing antibody seroconversion was evident at PID 10 only in the groups that received oral AttHRV and 100% of the pigs in these groups had VN antibody titres at PID 21 to PID 35/PCD 7 (data not shown). After challenge, 80% of the pigs from the 2/6 VLP250–ISCOM group developed neutralizing antibodies.
All groups that received AttHRV as a first dose had higher serum IgM and IgG antibody titres at PID 28/PCD 0 and PID 35/PCD 7
Antibody titres to the cumulative three doses (PID 28/PCD 0) and three doses plus challenge (PID 35/PCD 7) were measured (Fig. 3). At PID 28/PCD 0, IgM and IgG antibody titres to HRV were significantly higher in all the groups that received oral AttHRV. The IgA antibody titres to HRV were higher in the Att + 2/6 VLP250–ISCOM group, but these were statistically similar to the 2/6 VLP250–ISCOM group.
Fig. 3.
Serum IgM, IgA and IgG geometric mean antibody titres (GMT, bars) at PID 28/PCD 0 and PID 35/PCD 7 of gnotobiotic pigs receiving the various vaccination regimens. Pigs were inoculated with vaccines at PID 0, PID 10 and PID 21 and challenged at PID 28/PCD 0. Approximately half the pigs were euthanized prechallenge at PID 28 and the rest of the group was euthanized post-challenge at PID 35/PCD 7 and blood samples were collected at each of the different inoculation days. Lines represent standard error of the mean. Antibody titres that differ significantly are marked with different letters (one-way ANOVA and Duncan's multiple range test on log10-transformed titres).  Numbers to the right of the bars indicate fold increases of the PID 35/PCD 7 GMT over the PID 28/PCD 0 GMT.
Numbers to the right of the bars indicate fold increases of the PID 35/PCD 7 GMT over the PID 28/PCD 0 GMT.
At PID 35/PCD 7 IgM antibody titres were high, and increased in all vaccine groups (7–6797-fold), but the 3×AttHRV group had the lowest titres and no increase compared to prechallenge (Fig. 3). The IgA antibody titres to HRV increased in all groups post-challenge (9–79-fold) but the 3×AttHRV group also had the lowest titres and lowest increase (ninefold) and the control group did not show any increase. The IgG antibody titres to HRV were high in all the groups except for the control group. The groups with the highest protection rates (3×AttHRV and Att + 2/6 VLP250–ISCOM) had the lowest antibody titre increases post-challenge (0–15-fold) compared to prechallenge titres (PID 28/PCD 0).
Only groups that received AttHRV had neutralizing antibodies to HRV prechallenge
From PID 10 to PID 28/PCD 0, only the three groups that received oral AttHRV as a first dose developed neutralizing antibodies (Fig. 4). However, by PID 28/PCD 0, both the Att + 2/6 VLP250–ISCOM and 3×AttHRV groups had significantly higher VN antibody titres than the 1×AttHRV group. At PID 35/PCD 7, all vaccine groups had VN antibodies, but the ones that received AttHRV had the highest VN antibody titres (Fig. 4).
Fig. 4.
Serum VN geometric mean antibody titres (GMT) at PID 0, PID 10, PID 21, PID 28/PCD 0 and PID 35/PCD 7 of gnotobiotic pigs receiving the various vaccination regimens. Pigs were inoculated with vaccines at PID 0, PID 10 and PID 21 and challenged at PID 28/PCD 0. Approximately half the pigs were euthanized prechallenge at PID 28 and the rest were euthanized post-challenge at PID 35/PCD 7. Blood samples were collected at each of the different inoculation days. Error bars represent standard error of the mean. VN antibody titres at the same PID with different letters differ significantly (one-way anova and Duncan's multiple range test on log10-transformed titres).
Serum antibody titres to HRV were consistently lower in the groups that received 25 µg of 2/6 VLP compared to the 250 µg 2/6 VLP dose
Groups that received Att + 2/6 VLP250–ISCOM, Att + 2/6 VLP25–ISCOM, 2/6 VLP250–ISCOM and 2/6 VLP25–ISCOM were tested to measure the effect of VLP–ISCOM dose on antibody titres (Fig. 5) and protection rates (Table 1). The IgA, IgM and IgG antibody titres to HRV were consistently lower in the groups that received 25 µg of 2/6 VLP compared to the 250 µg 2/6 VLP dose, but statistically lower responses were not detected at all time-points (Fig. 5). Lower protection rates were detected in the Att + 2/6 VLP25–ISCOM vaccination group (34% and 0% protection against shedding and diarrhoea) than in the Att + 2/6 VLP250–ISCOM group. The 2/6 VLP–ISCOM 250 µg and 25 µg vaccination regimes did not confer any protection.
Fig. 5.
Effect of dose of 2/6 VLP and ISCOM on IgM, IgA and IgG antibody titres to HRV in serum of Att+2/6 VLP–ISCOM (250 µg/1250 µg or 25 µg/125 µg) and 2/6 VLP–ISCOM (250 µg/1250 µg or 25 µg/125 µg) pigs. Pigs were inoculated with vaccines at PID 0, 10 and 21 and challenged at PID 28/PCD 0. Approximately half the pigs were euthanized prechallenge at PID 28 and the rest of the group was euthanized post-challenge at PID 35/PCD 7 and blood samples were collected at each of the different inoculation days. Error bars represent standard error of the mean. Statistical analysis was performed between groups as follows: Att+2/6 VLP250–ISCOM versus Att+2/6 VLP25–ISCOM and 2/6 VLP250–ISCOM versus 2/6 VLP25–ISCOM and significantly lower titres are marked with an asterisk (*) (one-way anova and Duncan's multiple range test on log10-transformed titres).
DISCUSSION
We studied a new vaccination regimen consisting of priming with oral AttHRV and boosting with 2/6 VLP IN. Intestinal IgA antibodies were significantly higher at PID 28/PCD 0 in the pigs that received this vaccine regimen and the protection rates were similar to those pigs that received three doses of AttHRV. Intranasal vaccination has been proposed to be more effective than per oral because of the low exposure of the immunogen to the adverse conditions found in the gastrointestinal tract such as proteolytic enzymes, extremes of pH and the abundance of different commensals and non-commensal pathogens [35]. Responses in nasal-associated lymphoid tissue (NALT) after IN inoculation and gut-associated lymphoid tissue (GALT) after oral inoculation were compared in mice inoculated with Streptococcus mutans surface protein AgI/AgII coupled to subunit B of cholera toxin. After IN inoculation, more AgI/AgII-specific IgA ASC were located in NALT compared to the number of IgA ASC located in the Peyer's patches (PP) after oral inoculation with twice the amount of antigen as was given by the nasal dose [1005 versus 6 IgA ASC in NALT and Peyer's patches (PP), respectively] [35].
Intranasal inoculation of adult mice with inactivated rotavirus gave better protection rates and higher intestinal and systemic IgA antibody titres to rotavirus than oral inoculation [36]. Adult mice inoculated IN with 2/6 VLPs and E. coli mLT had higher levels of interleukin (IL)-2 and IL-5 in supernatants of stimulated cells from spleen, mesenteric lymph nodes (MLN), cervical LN and PP compared to the responses generated by the oral route [37].
In our previous studies of pigs, we used ISCOM as adjuvant and gave oral AttHRV followed by oral 2/6 VLP250–ISCOM, achieving 50% protection against diarrhoea and 75% protection against shedding [6]. In the current study, oral inoculation of AttHRV and IN inoculation of 2/6 VLP250–ISCOM gave higher protection rates against diarrhoea (71%). We showed further that the oral AttHRV priming, IN 2/6 VLP-boosting regimen was better than oral priming and oral boosting. It was highly immunogenic and induced protection rates similar to the actual vaccine regimen of three oral doses of AttHRV. In a previous publication from our laboratory we showed that the converse regimen, IN priming with 2/6 VLP and oral boosting with AttHRV was much less protective (17% and 25% protection against shedding and diarrhoea, respectively) [5].
The mechanism of protection from an enteric pathogen induced by IN vaccines has been examined in studies using porcine and murine models [5,25,36,38]. In the gnotobiotic pig model, antibody responses after inoculation of oral Att + 2/6 VLP250–ISCOM [39] or IN in the present study were similar except for slightly higher intestinal IgA antibodies and a significant boosting effect of the 2/6 VLP (in the Att + 2/6 VLP250–ISCOM compared to the 1×AttHRV) on VN antibodies by IN route in the present report. The IN inoculation of the 2/6 VLPs alone induced partial to complete protection (86–100%) against rotavirus shedding in adult mice [40]. Adult mice knock-outs for the polymeric immunoglobulin receptor (pIgR) (with low levels of intestinal IgA and IgM) and previously inoculated with 2/6 VLP IN were not protected against shedding after challenge with virulent wild-type murine ECw rotavirus, suggesting that transcytosis of IgA and/or IgM is necessary to achieve the protection conferred by IN inoculation with 2/6 VLP [38]. On the other hand, IN and not oral inoculation of adult mice with inactivated rotavirus induced higher IgA antibody titres in serum and intestinal contents and complete or near-complete protection against rotavirus shedding upon challenge [36]. In order to determine the origin of the antibodies, the authors measured IgA antibodies in the supernatants of different lymphoid tissue cultures. Interestingly, quantities of IgA antibodies were higher in the supernatant from RALT (bronchial lymphoid tissue, 10·3 ng/ml) than in the supernatant from GALT (PP, 1 ng/ml), suggesting that the major source of virus-specific IgA came from RALT. However, the authors did not determine IgA antibody titres to rotavirus in intestinal contents at challenge, so it is unknown if IgA was transudated from serum and, by this mechanism, conferred protection against shedding. Adult mice lacking pIgR and the molecule for intestinal trafficking, α4β7 still had IgA in intestinal contents suggesting that transport of IgA from serum to the intestine may occur but the function of this transudated immunoglobulin remains to be determined [38,41]. In the study by Fromantin et al. [37], IL-2 and IL-5 responses to IN 2/6 VLP were higher than the responses generated by the oral 2/6 VLP inoculation suggesting that T cells might play a greater role in the protection generated after IN 2/6 VLP vaccination of mice.
Intestinal IgA has been suggested as a marker of protection against reinfection after natural rotavirus infection in children and after virus or viral antigen inoculation and challenge in pigs and sucking mice [5,23–28,32,42]. Also in adult mice, IgA monoclonal antibodies to VP6 secreted by hybridoma cell backpack tumours conferred protection against shedding [10], but contrary findings with lack of protection were reported using a similar approach in infant mice in which only neutralizing IgA monoclonal antibodies to VP8 were protective [11]. In the current report, we showed that the Att + 2/6 VLP–ISCOM vaccine approach greatly increases IgA antibodies to HRV in large and small intestinal contents, which was associated with higher protection rates detected in this group of pigs. Future studies will assess the levels of protection conferred by a single dose of triple-layered (2/6/4/7) VLPs + two doses of 2/6 VLPs in order to avoid a first oral dose of AttHRV. The primary oral dose of the live rhesus human rotavirus reassortment vaccine was correlated with most of the cases of intussusception after vaccination of infants [43].
Intestinal IgM was also high at PID 28/PCD 0 in the Att + 2/6 VLP250–ISCOM group. In previous reports from our laboratory, gnotobiotic pigs inoculated with Wa HRV oral and Wa 2/6 VLPs IN with mutant E. coli mLT also had the highest rotavirus-specific IgM ASC in duodenum and ileum and intestinal IgM antibody titres to HRV [5,44]. Although IgM has been considered as a primary immunoglobulin, previous studies have shown that human intestinal IgM secreting plasma cells had 8·5% V region gene mutation frequency compared to germline sequences and that almost all the intestinal plasma cells had V region genes mutated, suggesting that most of them come from germinal centres [45]. Human rearranged VH gene IgM (+) memory B cells from blood, tonsils and spleen showed mutation frequencies of only 2–6% [43,46]. These data suggest that intestinal IgM responses could be more than simply an expression of the unprimed state of the host and might also be playing an important role in protection against reinfection.
The antibody responses in serum clearly show that the groups that received oral AttHRV as a first dose had higher HRV-specific IgM and VN titres from PID 10 until PID 28/PCD 0 (also PID 35/PCD 7 for VN antibody titres) and IgA and IgG titres at PID 21 and PID 28/PCD 0. The VN antibody titres to HRV were statistically higher in the 3×AttHRV and Att + 2/6 VLP250–ISCOM groups compared to the 1×AttHRV, 2/6 VLP250–ISCOM and control groups at PID 28/PCD 0 suggesting that the second and third doses of 2/6 VLP could boost the antibody responses for the generation of VN antibodies without necessarily expressing the proteins (VP4 and VP7) containing the neutralization epitopes and that this strategy was as effective as giving three doses of oral AttHRV. This trend was described previously by our laboratory, and higher, but not statistically significant responses were detected after the oral AttHRV dose followed by 2/6 VLP-E.coli mLT IN [5]. No VN antibodies were detected in the 2/6 VLP250–ISCOM or 2/6 VLP25–ISCOM groups. The serum IgA antibody titres to HRV were highest in the Att + 2/6 VLP250–ISCOM group at PID 21 (which was also the group with the highest percentage of pigs that seroconverted to IgA) and at PID 28/PCD 0, but statistically similar to the 2/6 VLP250–ISCOM group at PID 28/PCD 0. Post-challenge, the groups with the highest protection rates had the lowest antibody titre increases as described previously in mice [47] and in our previous studies of pigs [39,46]
Investigators previously used a variety of viruses, including rotavirus, influenza virus and human immunodeficiency virus with ISCOM adjuvant for vaccine studies in humans and animals [6,18,19]. Studies with influenza vaccine have shown that ISCOM can enhance either the production of mucosal (virus-specific IgA) or systemic (lymphocyte proliferation and cytotoxic activity) responses measured in vitro [17]. Previous studies from our laboratory showed that AttHRV + 2/6 VLP-induced moderate protection rates and intestinal antibody secreting cell (ASC) responses in gnotobiotic pigs using mutant E. coli heat-labile toxin (mLT) as adjuvant. Somewhat lower protection rates against virus shedding (58%) and diarrhoea (44%) were noted [5]. In the current study we demonstrated that the use of ISCOMs increased protection rates, they did not induce toxicity in the animals and they may be promising for future use in humans [15].
In summary, 2/6 VLP IN increased systemic IgA antibody titres to HRV but only low titres of intestinal IgA antibodies were induced. Three doses of oral AttHRV stimulated intermediate titres of systemic IgA antibodies compared to the Att + 2/6 VLP250–ISCOM and 2/6 VLP250–ISCOM group responses and lower titres of intestinal IgA antibodies than the Att + 2/6 VLP250–ISCOM group. Priming with oral AttHRV followed by boosting with 2/6 VLP250 IN increased systemic and intestinal IgA antibody responses. Based on these results, even though Att + 2/6 VLP250–ISCOM and 3×AttHRV vaccine regimes stimulated the highest protection rates, it seems that each regimen may stimulate different types of immune responses since IgA and IgM intestinal antibody responses differed between these groups. There is evidence that adult mice inoculated with live rotavirus depend more on B cell immunity compared to mice inoculated IN with the chimeric protein VP6 +E. coli-labile toxin depend more on CD4 T cells, suggesting that successful protection can be achieved by different immune mechanisms [48]. It is possible that protection induced by 3×AttHRV is more dependent on VN antibodies than that induced by the Att + 2/6 VLP250–ISCOM vaccine; however, both groups had similar levels of serum VN antibody titres. The VN antibody responses in intestinal contents are difficult to assess, but a comparison of T cell responses in the Att + 2/6 VLP250–ISCOM and 3×AttHRV groups is feasible to explore further the mechanisms of protection stimulated by each vaccine regimen.
Acknowledgments
We thank Dr Juliette Hanson for the clinical care of the gnotobiotic pigs, Paul Nielsen for technical assistance and Bert Bishop for advice regarding the statistical analysis data. This work was supported by grants from the National Institutes of Health, NIAID (RO1AI33561 and RO1AI37111). Marli Azevedo is a fellow of National Council of Scientific and technological development (CNPq),Brazil.
REFERENCES
- Cohen J. Medicine. Rethinking a vaccine's risk. Science. 2001;293:1576–7. doi: 10.1126/science.293.5535.1576. [DOI] [PubMed] [Google Scholar]
- Kombo LA, Gerber MA, Pickering LK, Atreya CD, Breiman RF. Intussusception, infection, and immunization: summary of a workshop on rotavirus. Pediatrics. 2001;108:37. doi: 10.1542/peds.108.2.e37. [DOI] [PubMed] [Google Scholar]
- Tucker AW, Haddix AC, Bresee JS, Holman RC, Parashar UD, Glass RI. Cost–effectiveness analysis of a rotavirus immunization program for the United States. JAMA. 1998;279:1371–6. doi: 10.1001/jama.279.17.1371. [DOI] [PubMed] [Google Scholar]
- Saif LJ, Ward LA, Yuan L, Rosen BI, To TL. The gnotobiotic piglet as a model for studies of disease pathogenesis and immunity to human rotaviruses. Arch Virol Suppl. 1996;12:153–61. doi: 10.1007/978-3-7091-6553-9_17. [DOI] [PubMed] [Google Scholar]
- Yuan L, Iosef C, Azevedo MS, et al. Protective immunity and antibody-secreting cell responses elicited by combined oral attenuated Wa human rotavirus and intranasal Wa 2/6-VLPs with mutant Escherichia coli heat-labile toxin in gnotobiotic pigs. J Virol. 2001;75:9229–38. doi: 10.1128/JVI.75.19.9229-9238.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iosef C, Nguyen TV, Jeong K, et al. Systemic and intestinal antibody secreting cell responses and protection in gnotobiotic pigs immunized orally with attenuated Wa human rotavirus and Wa 2/6-rotavirus-like-particles associated with immunostimulating complexes. Vaccine. 2002;20:1741–53. doi: 10.1016/s0264-410x(02)00031-2. [DOI] [PubMed] [Google Scholar]
- Morein B, Bengtsson KL. Immunomodulation by ISCOMS, immune stimulating complexes. Methods (Duluth) 1999;19:94–102. doi: 10.1006/meth.1999.0833. [DOI] [PubMed] [Google Scholar]
- Rimmelzwaan GF, Nieuwkoop N, Brandenburg A, et al. A randomized, double blind study in young healthy adults comparing cell mediated and humoral immune responses induced by influenza ISCOM vaccines and conventional vaccines. Vaccine. 2000;19:1180–7. doi: 10.1016/s0264-410x(00)00310-8. [DOI] [PubMed] [Google Scholar]
- Behboudi S, Morein B, Villacres-Eriksson MC. In vitro activation of antigen-presenting cells (APC) by defined composition of Quillaja saponaria Molina triterpenoids. Clin Ex Immunol. 1996;105:26–30. doi: 10.1046/j.1365-2249.1996.d01-730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behboudi S, Morein B, Villacres-Eriksson MC. Quillaja saponin formulations that stimulate proinflammatory cytokines elicit a potent acquired cell-mediated immunity. Scand J Immunol. 1999;50:371–7. doi: 10.1046/j.1365-3083.1999.00603.x. [DOI] [PubMed] [Google Scholar]
- Ennis FA, Cruz J, Jameson J, Klein M, Burt D, Thipphawong J. Augmentation of human influenza A virus-specific cytotoxic T lymphocyte memory by influenza vaccine and adjuvanted carriers (ISCOMS) Virology. 1999;259:256–61. doi: 10.1006/viro.1999.9765. [DOI] [PubMed] [Google Scholar]
- Verschoor EJ, Mooij P, Oostermeijer H, et al. Comparison of immunity generated by nucleic acid-, MF59-, and ISCOM-formulated human immunodeficiency virus type 1 vaccines in Rhesus macaques: evidence for viral clearance. J Virol. 1999;73:3292–300. doi: 10.1128/jvi.73.4.3292-3300.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore HP, Estes MK, Zarley CD, et al. Biochemical and immunologic comparison of virus-like particles for a rotavirus subunit vaccine. Vaccine. 1999;17:2461–71. doi: 10.1016/s0264-410x(98)00319-3. [DOI] [PubMed] [Google Scholar]
- Charpilienne A, Nejmeddine M, Berois M, et al. Individual rotavirus-like particles containing 120 molecules of fluorescent protein are visible in living cells. J Biol Chem. 2001;276:29361–7. doi: 10.1074/jbc.M101935200. [DOI] [PubMed] [Google Scholar]
- Estes M. Rotavirus and their replication. In: Knipe DM, Holey PM, editors. Field's virology. 4. Philadelphia: Lippincott. Williams & Wilkins; 2001. p. 1747. [Google Scholar]
- Burns JW, Siadat-Pajouh M, Krishnaney AA, Greenberg HB. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science. 1996;272:104–7. doi: 10.1126/science.272.5258.104. [DOI] [PubMed] [Google Scholar]
- Ruggeri FM, Johansen K, Basile G, Kraehenbuhl JP, Svensson L. Antirotavirus immunoglobulin A neutralizes virus in vitro after transcytosis through epithelial cells and protects infant mice from diarrhea. J Virol. 1998;72:2708–14. doi: 10.1128/jvi.72.4.2708-2714.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomina J, Gil MT, Codoner P, Buesa J. Viral proteins VP2, VP6, and NSP2 are strongly precipitated by serum and fecal antibodies from children with rotavirus symptomatic infection. J Med Virol. 1998;56:58–65. doi: 10.1002/(sici)1096-9071(199809)56:1<58::aid-jmv10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Johansen K, Granqvist L, Karlen K, Stintzing G, Uhnoo I, Svensson L. Serum IgA immune response to individual rotavirus polypeptides in young children with rotavirus infection. Arch Virol. 1994;138:247–59. doi: 10.1007/BF01379129. [DOI] [PubMed] [Google Scholar]
- Clemens JD, Ward RL, Rao MR, et al. Seroepidemiologic evaluation of antibodies to rotavirus as correlates of the risk of clinically significant rotavirus diarrhea in rural Bangladesh. J Infect Dis. 1992;165:161–5. doi: 10.1093/infdis/165.1.161. [DOI] [PubMed] [Google Scholar]
- Jiang B, Gentsch JR, Glass RI. The role of serum antibodies in the protection against rotavirus disease: an overview. Clin Infect Dis. 2002;34:1351–61. doi: 10.1086/340103. [DOI] [PubMed] [Google Scholar]
- Velazquez FR, Matson DO, Guerrero ML, et al. Serum antibody as a marker of protection against natural rotavirus infection and disease. J Infect Dis. 2000;182:1602–9. doi: 10.1086/317619. [DOI] [PubMed] [Google Scholar]
- Coulson BS, Grimwood K, Hudson IL, Barnes GL, Bishop RF. Role of coproantibody in clinical protection of children during reinfection with rotavirus. J Clin Microbiol. 1992;30:1678–84. doi: 10.1128/jcm.30.7.1678-1684.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng N, Burns JW, Bracy L, Greenberg HB. Comparison of mucosal and systemic humoral immune responses and subsequent protection in mice orally inoculated with a homologous or a heterologous rotavirus. J Virol. 1994;68:7766–73. doi: 10.1128/jvi.68.12.7766-7773.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Geyer A, Hodgins DC, et al. Intranasal administration of 2/6-rotavirus-like particles with mutant Escherichia coli heat-labile toxin (LT-R192G) induces antibody-secreting cell responses but not protective immunity in gnotobiotic pigs. J Virol. 2000;74:8843–53. doi: 10.1128/jvi.74.19.8843-8853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Geyer A, Saif LJ. Short-term immunoglobulin A B-cell memory resides in intestinal lymphoid tissues but not in bone marrow of gnotobiotic pigs inoculated with Wa human rotavirus. Immunology. 2001;103:188–98. doi: 10.1046/j.1365-2567.2001.01229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Kang SY, Ward LA, To TL, Saif LJ. Antibody-secreting cell responses and protective immunity assessed in gnotobiotic pigs inoculated orally or intramuscularly with inactivated human rotavirus. J Virol. 1998;72:330–8. doi: 10.1128/jvi.72.1.330-338.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Ward LA, Rosen BI, To TL, Saif LJ. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Virol. 1996;70:3075–83.3. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S, Nakata S, Ukae S, Adashi N. Virological and serological aspects of immune resistance to rotavirus gastroenteritis. Clin Infect Dis. 1993;16(Suppl. 2):S117–21. doi: 10.1093/clinids/16.supplement_2.s117. [DOI] [PubMed] [Google Scholar]
- Chiba S, Yokoyama T, Nakata S, et al. Protective effect of naturally acquired homotypic and heterotypic rotavirus antibodies. Lancet. 1986;2:417–21. doi: 10.1016/s0140-6736(86)92133-1. [DOI] [PubMed] [Google Scholar]
- Ward RL, Clemens JD, Knowlton DR, et al. Evidence that protection against rotavirus diarrhea after natural infection is not dependent on serotype-specific neutralizing antibody. J Infect Dis. 1992;166:1251–7. doi: 10.1093/infdis/166.6.1251. [DOI] [PubMed] [Google Scholar]
- Ward LA, Rosen BI, Yuan L, Saif LJ. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J Gen Virol. 1996;77:1431–41. doi: 10.1099/0022-1317-77-7-1431. [DOI] [PubMed] [Google Scholar]
- Saif LJ, Redman DR, Smith KL, Theil KW. Passive immunity to bovine rotavirus in newborn calves fed colostrum supplements from immunized or non immunized cows. Infect Immun. 1983;41:1118–31. doi: 10.1128/iai.41.3.1118-1131.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parreno V, Hodgins DC, de Arriba L, et al. Serum and intestinal isotype antibody responses to Wa human rotavirus in gnotobiotic pigs are modulated by maternal antibodies. J Gen Virol. 1999;80:1417–28. doi: 10.1099/0022-1317-80-6-1417. [DOI] [PubMed] [Google Scholar]
- Wu HY, Nikolova EB, Beagley KW, Russell MW. Induction of antibody-secreting cells and T-helper and memory cells in murine nasal lymphoid tissue. Immunology. 1996;88:493–500. doi: 10.1046/j.1365-2567.1996.d01-690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin SE, Clark SL. Induction of intestinal rotavirus-specific antibodies in respiratory, but not gut, lymphoid tissues following mucosal immunization of mice with inactivated rotavirus. Virology. 2001;291:235–40. doi: 10.1006/viro.2001.1180. [DOI] [PubMed] [Google Scholar]
- Fromantin C, Jamot B, Cohen J, Piroth L, Pothier P, Kohli E. Rotavirus 2/6 virus-like particles administered intranasally in mice, with or without the mucosal adjuvants cholera toxin and Escherichia coli heat-labile toxin, induce a Th1/Th2-like immune response. J Virol. 2001;75:11010–6. doi: 10.1128/JVI.75.22.11010-11016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz-Cornil I, Benureau Y, Greenberg H, Hendrickson BA, Cohen J. Heterologous protection induced by the inner capsid proteins of rotavirus requires transcytosis of mucosal immunoglobulins. J Virol. 2002;76:8110–7. doi: 10.1128/JVI.76.16.8110-8117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, Iosef C, Jeong K, et al. Protection and antibody responses to oral priming by attenuated human rotavirus followed by oral boosting with 2/6-rotavirus-like particles with immunostimulating complexes in gnotobiotic pigs. Vaccine. 2003;21:4059–70. doi: 10.1016/s0264-410x(03)00267-6. [DOI] [PubMed] [Google Scholar]
- O'Neal CM, Crawford SE, Estes MK, Conner ME. Rotavirus virus-like particles administered mucosally induce protective immunity. J Virol. 1997;71:8707–17. doi: 10.1128/jvi.71.11.8707-8717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuklin NA, Rott L, Feng N, et al. Protective intestinal anti-rotavirus B cell immunity is dependent on alpha 4 beta 7 integrin expression but does not require IgA antibody production. J Immunol. 2001;166:1894–902. doi: 10.4049/jimmunol.166.3.1894. [DOI] [PubMed] [Google Scholar]
- Matson DO, O'Ryan ML, Herrera I, Pickering LK, Estes MK. Fecal antibody responses to symptomatic and asymptomatic rotavirus infections. J Infect Dis. 1993;167:577–83. doi: 10.1093/infdis/167.3.577. [DOI] [PubMed] [Google Scholar]
- Bresee JS, Glass RI, Ivanoff B, Gentsch JR. Current status and future priorities for rotavirus vaccine development, evaluation and implementation in developing countries. Vaccine. 1999;17:2207–22. doi: 10.1016/s0264-410x(98)00376-4. [DOI] [PubMed] [Google Scholar]
- Azevedo MSP, Yuan L, Josef C, et al. Magnitude of serum and intestinal antibody responses induced by sequential replicating and nonreplicating rotavirus vaccines in gnotobiotic pigs and correlation with protection. Clin Oiagn Lab Immunol. 2004;11:12–20. doi: 10.1128/CDLI.11.1.12-20.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Kuppers R. Human IgA- and IgM-secreting intestinaplasma cells carry heavily mutated VH region genes. Eur J Immunol. 1998;28:2971–7. doi: 10.1002/(SICI)1521-4141(199809)28:09<2971::AID-IMMU2971>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Klein U, Kuppers R, Rajewsky K. Variable region gene analysis of B cell subsets derived from a 4-year-old child: somatically mutated memory B cells accumulate in the peripheral blood already at young age. J Exp Med. 1994;180:1383–93. doi: 10.1084/jem.180.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal MM, Rae MN, Bean JA, Ward RL. Antibody-dependent and -independent protection following intranasal immunization of mice with rotavirus particles. J Virol. 1999;73:7565–73. doi: 10.1128/jvi.73.9.7565-7573.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal MM, VanCott JL, Choi AH, et al. CD4 T cells are the only lymphocytes needed to protect mice against rotavirus shedding after intranasal immunization with a chimeric VP6 protein and the adjuvant LT (R192G) J Virol. 2002;76:560–8. doi: 10.1128/JVI.76.2.560-568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]