Abstract
The chemokine stromal cell-derived factor 1α (SDF-1α) is a potent stimulator of T cell infiltration into three-dimensional type I collagen matrices as demonstrated using T cells freshly isolated from blood and an activated T cell clone. The neuropeptide somatostatin selectively inhibits SDF-1α induced T cell infiltration by the same T cells including CD4 as well as CD8 positive cells, while somatostatin does not inhibit ‘spontaneous’ T cell infiltration. A number of other neuropeptides and opioids do not inhibit SDF-1α-induced T cell infiltration, indicating that the inhibitory effect is somatostatin-specific. The neuropeptide antagonist cyclosomatostatin abrogated the inhibitory effect of somatostatin on T cell infiltration, indicating that the effect of somatostatin is mediated via specific somatostatin receptors. Somatostatin does not inhibit SDF-1α-induced T cell attachment to the collagen substrate, which indicates that this neuropeptide specifically inhibits the process of chemokine-induced T cell penetration and migration through the collagen.
Keywords: extracellular matrix, neuropeptides, somatostatin, SDF-1α, T cell infiltration
INTRODUCTION
Lymphocytes can migrate to specific sites in tissues by passage of endothelial cells and subsequent infiltration of perivascular extracellular matrix (ECM) [1,2]. The capacity to migrate and localize in tissues is of vital importance for the protective function of lymphocytes against infectious agents. However, the capacity to migrate and infiltrate tissues is also a major reason why lymphocytes can cause autoimmunity, allergy and graft rejection or why neoplastic lymphocytes accumulate in tissues.
T lymphocytes can migrate into/infiltrate three-dimensional (3D) matrices of collagen type I or matrigel [3–6]. The capacity to infiltrate 3D substrata is distinguishable from migration on two-dimensional (2D) substrates, such as plastic or lymphocyte migration through the holes of Boyden chamber filters. Thus, T cell infiltration seems to be a specific property distinguishable from migration per se, as illustrated by the fact that virtually all leukaemic T cell lines can migrate in Boyden assays but only some cell lines can penetrate a 3D-matrix [7]. Infiltrative capacity correlates with the expression of matrix metalloproteinases, indicating that degradation of ECM may be a prerequisite for the cells to penetrate [6].
Chemokines are secreted basic proteins of 8–10 kDa that represent the largest family of cytokines encoded in the human genome with close to 50 members [8]. The chemokine system has emerged as the critical regulator of lymphocyte trafficking in vivo. Chemokines deliver their activity by interacting with cell surface receptors that belong to the family of seven transmembrane domain G-protein coupled rhodopsin-like receptors [9,10]. Chemokines and their receptors are critical elements for controlling lymphocyte migration and localization [11–13].
Chemokines have been studied extensively to determine their role in T lymphocyte migration [14–16]. Given the critically important role of chemokines in regulating T cell migration, it is important to find ways to interfere with chemokine-induced T cell infiltration of tissues in inflammatory diseases of autoimmune and allergic origin and in rejection of transplants. Therefore, in the present study we have examined whether it is possible to interfere with chemokine-induced T cell infiltration using a collagen type I matrix as an in vitro model of the ECM and with the chemokine SDF-1α, which has a single receptor on T cells (CXCR4), as an attractant [17]. We were particularly interested in neurotransmitters as possible regulators of the infiltrative capacity of T cells. T cells express specific receptors for neurotransmitters on their surface [18,19] and all primary and secondary lymphoid organs are massively innervated [20]. Therefore, T cells are likely to be exposed to a variety of neurotransmitters. Neurotransmitters are also released from nerve terminals at various extravasal sites to which T cells are attracted under inflammatory conditions. Here we show that the neuropeptide somatostatin, which has multiple receptors on T lymphocytes [21,22], specifically inhibits chemokine-induced but not spontaneous T cell infiltration of a collagen type I matrix, while a number of other neuropeptides and opioids do not interfere with chemokine-induced T cell infiltration.
MATERIALS AND METHODS
Chemicals
Somatostatin-14, neuropeptide Y (NPY), substance P, vasoactive intestinal peptide (VIP), β-endorphin and metenkephalin were purchased from Sigma Chemical Co., St Louis, MO, USA. For flow cytometry we used antibodies to SDF-1α and CXCR4 from R&D Systems Europe Ltd, Abington, UK; antibodies to β1-integrins α1-α6 and the β1-subunit from Immunotech, Marseille, France; goat gamma globulin from Jackson Immunoreserch Lb. Inc., West Grove, PA, USA; and FITC-GAM secondary antibodies from Daco A/S, Glostrup, Denmark. The human T cell clone AF 24 was a gift from R. J. J. van Neerven, ALK Research Laboratories, Hoersholm, Denmark.
Cell preparations and culture conditions
Mononuclear cells were isolated from healthy blood donors by centrifuging heparinized venous blood on a Ficoll gradient (Lymphoprep, Nycomed, Oslo, Norway). Recovered cells in the interface were washed carefully in phosphate buffered saline (PBS) and remaining erythrocytes were lysed with a buffer containing 0·15 m NH4Cl, 0·01 m KHCO3 and 0·1 m EDTA. The remaining mononuclear cells were then treated with carbonyl iron and phagocytic cells removed by a magnet. Recovered cells were resuspended in RPMI-1640 (Life Technologies Inc., Paisley, Scotland, UK) supplemented with 2 mm l-glutamine, 0·16% sodium bicarbonate, 100 IU/ml benzyl penicillin, 100 g/ml streptomycin and 10% fetal calf serum (FCS). The cells were cultured in Nunclon Bottles (Nunc, Delta, Denmark) in a humidified CO2 incubator at 37°C and the cell infiltration assays were performed the following day. We also performed experiments with the human T cell clone AF 24. The cell lines were cultured in complete RPMI supplemented with 10% FCS as described above.
Cell infiltration assays
Collagen type I was prepared as described previously [5], diluted in serum free RPMI and H2O (8 : 1 : 1) and 1 ml was applied in plastic Petri dishes (30 mm, Becton Dickinson, Mountain View, CA, USA). The gel was allowed to polymerize at room temperature for 30–40 min. The chemokine SDF-1α (50 ng/ml), one of the peptides somatostatin-14, NPY, substance P, VIP, β-endorphin and metenkephalin (10–8−10–12m) or SDF-1α and one of the peptides were present in the gel. Infiltration into a collagen type I gel without the addition of SDF-1α or peptides served as a negative control. Cells (1·0 × 106) were added to each well and incubated for 4 h in a humidified CO2 incubator at 37°C. Plates were fixed with 2·5% glutaraldehyde and washed twice with PBS. Infiltration depth and cell number was evaluated in five fixed positions in each well by the use of an inverted microscope (Nikon Eclipse TE300) and a digital depth meter (Heidenheim ND221) The results are given as mean number of infiltrated cells/microscopic field (100×)/100 µm infiltration depth.
Flow cytometry
The cells were incubated in the absence and presence of somatostatin 10–6−10−10m for 2 h before flow cytometry, which was performed in microtitre plates with U-shaped wells and with 250 000 cells in 25 µl FACS buffer (Hanks’s TRIS + 0·2% HSA + 0·02% NaN3) per well. After blocking with goat gamma globulin (3 µl/106 cells) for 30 min at room temperature and centrifugation at 1500 r.p.m. the supernatant was removed. The cells were diluted in FACS buffer mixed with 2 µl RPE conjugated monoclonal antibodies (MoAb) against CXCR4, the β1-integrins α1–α6 or the β1-subunit to a final concentration of 0·37 µg/ml and the samples were incubated for 30 min on ice with slow shaking. After this incubation the cells were washed with 100 µl FACS buffer/well with 50 µl FCS at the bottom of each well. The plates were centrifuged at 2000 r.p.m. for 10 min at 4°C and the supernatant was removed. The cells were then mixed with 2 µl FITC-conjugated MoAb against CD4 and incubated for 30 min on ice with slow shaking. Subsequently 100 µl FACS buffer and 50 µl FCS were added to the bottom of each well, the plates centrifuged at 2000 r.p.m. for 10 min at 4°C and the supernatant was removed. The samples were diluted in FACS buffer and the cell-bound fluorescence was determined in a FACScan (Becton Dickinson, Mountain View, CA, USA).
Statistical analysis
Each experiment was repeated at least three times. Student's t-test was used to asses the influence of neuropeptides and endorphins on the depth of T cell infiltration.
RESULTS
The influence of neuropeptides and opioids on spontaneous and chemokine-induced T cell infiltration
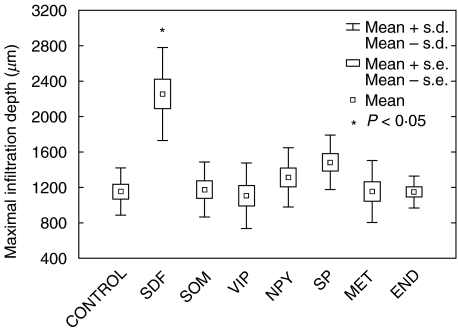
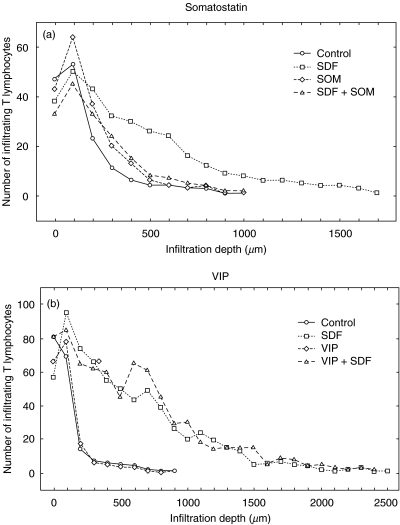
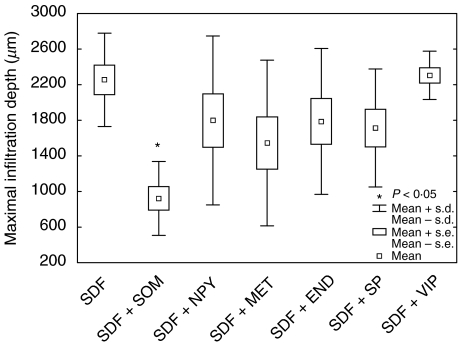
To analyse the influence of neuropeptides and opioids on T cell infiltration, blood T cells and a T cell clone (AF24) were allowed to migrate into a type I collagen gel containing SDF-1α. The neuropeptides and opioids were also present inside the collagen gel. The number of cells at different levels of the collagen was determined and the results summarized as described in Figs 1–3 for blood T cells. It can be seen in Figs 1 and 3 that SDF-1α significantly stimulated T cell migration/infiltration into the collagen matrix (P < 0·05). Blood T cells and the T cell clone (AF24) also exhibited spontaneous infiltration in the absence of chemokines but to a lower extent, indicating that SDF-1α has an important role as a stimulator of infiltration (Figs 1–3). The results in Figs 2 and 3 show further that somatostatin significantly inhibited the SDF-1α induced T cell infiltration (P < 0·05). In contrast, somatostatin did not inhibit the ‘spontaneous’ infiltration of T cells into the collagen but in the absence of SDF-1α seemed, rather, to augment the attachment of T cells to the upper layers of the collagen. The inhibitory effect of somatostatin on T cell infiltration in the different cell types tested was maximal at concentrations 10–8−10–12m.
Fig. 1.
Somatostatin does not influence spontaneous T cell infiltration. The figure shows the effect of neuropeptides, endorphins and the chemokine stromal cell-derived factor 1α (SDF-1α) on the depth of infiltration of blood T lymphocytes into a collagen type I gel (>90% of the infiltrating cells were CD3+). The chemokine SDF-1α (50 ng/ml) in the gel increased significantly the infiltration depth of T lymphocytes, while somatostatin (SOM, 10−8m), vasoactive intestinal peptide (VIP, 10−8m), neuropeptide Y (NPY, 10−8m), substance P (SP, 10−8m) metenkephalin (MET 10−8m) and β-endorphin (END, 10−8m) in the gel did not influence T cell infiltration. The results show mean maximal infiltration depth of three independent experiments and of 10 microscopic fields in each experiment (×100).
Fig. 3.
(a,b) Somatostatin (Som) inhibits chemokine (SDF-1α)-induced infiltration of T cells. Blood T lymphocytes were allowed to migrate into a collagen type I gel (> 90% of the infiltrating cells were CD3+). It can be seen that SDF-1α (50 ng/ml) in the gel increased the number of infiltrating T lymphocytes and the infiltration depth (a, b), while this infiltration was inhibited in the presence of somatostatin (10−8m) in the gel (a). VIP (10−8m) in the gel did not influence this SDF-induced infiltration of T lymphocytes (b). The figures show the number of infiltrating cells at different levels from the top of the gel as a mean of five microscopic fields (×100) in a single representative experiment.
Fig. 2.
Somatostatin (SOM) inhibits chemokine (SDF-1α)-induced infiltration of T cells. Blood T lymphocytes were allowed to migrate into a collagen type I gel (>90% of the infiltrating cells were CD3+). SDF-1α (50 ng/ml) in the gel increased the infiltration depth of T lymphocytes (Fig. 1). This increase was inhibited significantly in the presence of somatostatin (10−8m) in the gel, while neuropeptide Y (NPY, 10−8m), metenkephalin (MET, 10−8m), β-endorphin (END, 10−8m), substance P (SP, 10−8m) and vasoactive intestinal peptide (VIP, 10−8m) in the gel had no effect on SDF-1α-induced infiltration. The figure shows mean maximal infiltration depth determined from three independent experiments and of 10 microscopic fields in each experiment (×100).
Preincubation of the lymphocytes with the somatostatin antagonist cyclosomatostatin abrogated the inhibitory effect of somatostatin on chemokine-induced T cell infiltration. This indicates that somatostatin inhibits SDF-1α-induced T cell infiltration via specific somatostatin receptors SSTR1-5. Figures 2 and 3 demonstrate that the neuropeptide vasoactive intestinal peptide (VIP) did not inhibit SDF-1α-induced T cell infiltration, nor did the neuropeptides substance P, neuropeptide Y and the opioids β-endorphin and metenkephalin affect SDF-1α-induced T cell infiltration into the collagen (Fig. 2). It follows from the results in Figs 1–3 that somatostatin is a specific inhibitor of SDF-1α-induced T cell infiltration.
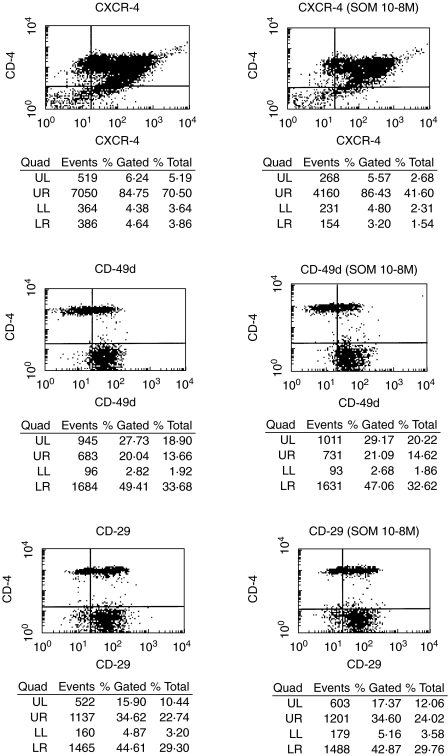
Somatostatin does not influence the expression of chemokine receptors or β1-integrins on T cells
To elucidate the mechanism of the somatostatin-induced inhibition of T cell infiltration we examined the possibility that somatostatin interfered with the expression of relevant chemokine receptors or integrins. Flow cytometry analysis before and after incubation with somatostatin detected no differences with respect to expression of the chemokine receptor for SDF-1α CXCR4 and β1-integrin subunits, as shown for CD29 and CD49d in Fig. 4; α1, α2, α3, α4 and α5 were tested with similar results (not shown). The flow cytometry results probably exclude that the inhibitory effect of somatostatin on SDF-1α-induced T cell infiltration reflects an alteration of the expression of integrins or chemokine receptors for SDF-1α.
Fig. 4.
Somatostatin does not influence the expression of CXCR4 and β1-integrins. Flow cytometry analysis was performed using RPE-conjugated monoclonal antibodies to CXCR4 (the receptor for SDF-1α), the β1-integrin α-subunits α4 (CD49d), the β1-subunit (CD29) and FITC-conjugated monoclonal antibody to CD4. In addition, the expression of the other α-chains and somatostatin 10−6 and 10−10m has been analysed. The results detected no differences with respect to the expression of these cell surface receptors in the absence and presence of somatostatin 10−6−10−10m. The cells were incubated with somatostatin for 2 h before flow cytometry.
DISCUSSION
This study presents new information regarding the cross-talk between the nervous and immune systems. Thus, the neuropeptide somatostatin appears to be a specific inhibitor of chemokine-dependent T cell infiltration while the other neuropeptides analysed did not affect chemokine-dependent T cell infiltration. The mechanism of the somatostatin-induced inhibition of T cell infiltration is not understood at present. It may be relevant in this context that somatostatin is an established inhibitor of two key cellular processes, secretion and cell proliferation [23,24]. Thus, somatostatin has been shown to inhibit the release of a number of hormones and has a generalized inhibitory effect on gut exocrine secretion [23,25]. Somatostatin further inhibits proliferation and induces apoptosis of neoplastic cells [26,27]. However, it is highly unlikely that the inhibitory effect of somatostatin on lymphocyte infiltration reflects inhibition of proliferation. In contrast, inhibitors of secretion have been shown to inhibit T cell infiltration [5] and therefore it is possible that somatostatin induces its inhibitory effect on T cell infiltration via inhibition of secretion or intercompartmental transport of molecules which play a role for the infiltration process, such as matrix metalloproteinases (MMPs). Accordingly, MMPs have been proposed to play a role for infiltrative T cell behaviour and chemokines stimulate MMP release [28,29]. However, recent evidence has demonstrated that the T cell infiltration into 3D collagen gels, the substrate used for the present migration studies, is independent of MMPs [30]. Another possibility is that somatostatin affected the expression of the chemokine receptor CXCR4 (the receptor for SDF-1α) and that this led to inhibition of T cell infiltration. However, flow cytometry detected no somatostatin-induced alteration of the cell surface expression of CXCR4 and therefore this explanation seems unlikely. Other mechanisms to be considered include somatostatin-inhibition of chemokine binding to its receptor or desensitization of the chemokine receptor [31]. Functional inactivation of chemokine receptor-mediated responses through up-regulation of suppressors of cytokine signalling (SOCS) proteins have also been shown to impair the response to chemokines [32]. Further studies are needed to elucidate the mechanism of the inhibitory action of somatostatin on chemokine-induced T cell infiltration.
The finding that somatostatin inhibits chemokine-induced T cell infiltration is relevant to the understanding of the functional role of neuropeptides for the immune system. Somatostatin-producing cells are distributed widely in the central and peripheral nervous systems, in the endocrine pancreas and gut, but they also occur in the thyroid, prostate, placenta, adrenals, kidneys and skin [23,25]. Somatostatin can be released from nerve endings and secreting cells in lymphoid tissues, which implies that this neuropeptide is normally present in close proximity to lymphocytes. The present finding that somatostatin inhibits chemokine-induced T cell infiltration and is distributed widely throughout the organism suggest that this neuropeptide prevents cells from penetrating into and accumulating in tissues, particularly the ECM when attracted by chemokines, such as SDF-1α. This is consistent with a counter-inflammatory role of somatostatin, while this neuropeptide does not affect basic chemokine-independent T cell migration. In light of the inhibitory effect of somatostatin on chemokine-induced T cell infiltration, it seems reasonable to speculate that somatostatin renders potentially chemokine-responsive infiltrative T cells to choose a non-infiltrative ‘basic’ migration pattern, rather than a chemokine-dependent migration pattern with penetration of the ECM, which may cause T cell infiltration of tissues. It follows that somatostatin modulation of the pattern of T cell migration may constitute a suppressive mechanism against potentially harmful T cell infiltration leading to autoimmunity or allergy. More information is required to elucidate the fact that somatostatin inhibits selectively chemokine-induced infiltration but not basic T cell migration. Thus, there is growing evidence indicating that the control of T cell migration is complex, involving multiple regulatory mechanisms that modulate in a delicate manner the motile behaviour of the cells within the organism. One important question in this context is to define clearly the factors that distinguish T cell migration in a 2D manner from T cell infiltration/migration into 3D substrate. The present findings indicate that infiltration is a controlled T cell function, which is stimulated by chemokines and suppressed by the neuropeptide somatostatin.
Acknowledgments
This study was supported by grants from the Swedish Cancer Society (1940), the Children's Cancer Foundation, the Edvard Welander Foundation and the Finsen Foundation.
REFERENCES
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–6. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Wilkinson PC, Lackie JM, Forrester J, et al. Chemokinetic accumulation of human neutrophils on immune complex-coated substrata: analysis at a boundary. J Cell Biol. 1984;99:1761–8. doi: 10.1083/jcb.99.5.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor SL, Allen TD, Winn B. Lymphocyte migration into three-dimensional collagen matrices: a quantitative study. J Cell Biol. 1983;96:1089–96. doi: 10.1083/jcb.96.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist KG, Hauzenberger D, Hultenby K, et al. T lymphocyte infiltration of two- and three-dimensional collagen substrata by an adhesive mechanism. Exp Cell Res. 1993;206:100–10. doi: 10.1006/excr.1993.1125. [DOI] [PubMed] [Google Scholar]
- Ivanoff A, Ivanoff J, Hultenby K, et al. Infiltrative capacity of T leukemia cell lines: a distinct functional property coupled to expression of matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of metalloproteinases-1 (TIMP-1) Clin Exp Metastasis. 1999;17:695–711. doi: 10.1023/a:1006749304315. [DOI] [PubMed] [Google Scholar]
- Ivanoff J, Ivanoff A, Sundqvist KG. Defective chemokine production in T leukemia cell lines and its possible functional role. Dev Immunol. 2000;7:67–75. doi: 10.1155/2000/28085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster AD. Chemokines − chemotactic cytokines that mediate inflammation. New Engl J Med. 1998;338:436–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- Cantrell D. G proteins in lymphocyte signalling. Curr Opin Immunol. 1994;6:380–4. doi: 10.1016/0952-7915(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Böhm SK, Grady EF, Bunnett NW. Regulatory mechanisms that modulate signalling by G-protein-coupled receptors. J Biochem. 1997;322:1–18. doi: 10.1042/bj3220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AG. Proteins and small GTPases: distant relatives keep in touch. Science. 1998;280:2074–5. doi: 10.1126/science.280.5372.2074. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F, del Pozo MA. Leukocyte polarization in cell migration and immune interactions. EMBO J. 1999;18:501–11. doi: 10.1093/emboj/18.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voermans C, Anthony EC, Mul E, et al. SDF-1-induced actin polymerization and migration in human hematopoietic progenitor cells. Exp Hematol. 2001;29:1456–64. doi: 10.1016/s0301-472x(01)00740-8. [DOI] [PubMed] [Google Scholar]
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–8. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–34. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber JM. Mig and IP-10: CXC chemokines that targets lymphocytes. J Leukoc Biol. 1997;61:246–57. [PubMed] [Google Scholar]
- Murdoch C. CXCR4: chemokine receptor extraordinaire. Immunol Rev. 2000;177:175–84. doi: 10.1034/j.1600-065x.2000.17715.x. [DOI] [PubMed] [Google Scholar]
- Reubi JC, Horisberger U, Kappeler A, et al. Localization of receptors for vasoactive intestinal peptide, somatostatin, and substance P in distinct compartments of human lymphoid organs. Blood. 1998;92:191–7. [PubMed] [Google Scholar]
- Van Hagen PM. Somatostatin receptor expression in clinical immunology. Metabolism. 1996;45:86–7. doi: 10.1016/s0026-0495(96)90092-x. [DOI] [PubMed] [Google Scholar]
- Stevens-Felten SY, Bellinger DL. Noradrenergic and peptidergic innervation of lymphoid organs. Chem Immunol. 1997;69:99–131. doi: 10.1159/000058655. [DOI] [PubMed] [Google Scholar]
- Talme T, Ivanoff J, Hägglund M, et al. Somatostatin receptor (SSTR) expression and function in normal and leukemic T cells. Evidence for selective effects on adhesion to extra-cellular matrix components via SSTR2 and/or 3. Clin Exp Immunol. 2001;123:1–10. doi: 10.1046/j.1365-2249.2001.01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi A, Takano H, Ichikawa K, et al. Expression of somatostatin receptor subtype 2 mRNA in human lymphoid cells. Cell Immunol. 1997;181:44–9. doi: 10.1006/cimm.1997.1193. [DOI] [PubMed] [Google Scholar]
- Reichlin S. Somatostatin. N Engl J Med. 1983;309:1556–63. doi: 10.1056/NEJM198312223092506. [DOI] [PubMed] [Google Scholar]
- Payan DG, Hess CA, Goetzl EJ. Inhibition by somatostatin of the proliferation of T lymphocytes and molt-4 lymphoblasts. Cell Immunol. 1984;84:433–8. doi: 10.1016/0008-8749(84)90117-5. [DOI] [PubMed] [Google Scholar]
- Patel YC. General aspects of the biology and function of somatostatin. In: Weil C, Muller EE, Thorner MO, editors. Basic and clinical aspects of neuroscience. Vol. 4. Berlin: Springer-Verlag; 1992. pp. 1–16. [Google Scholar]
- Lamberts SW, Krenning EP, Reubi JC. The role of somatostatin and its analogs in the diagnosis and treatment of tumors. Endocr Rev. 1991;12:450–82. doi: 10.1210/edrv-12-4-450. [DOI] [PubMed] [Google Scholar]
- Pollak MN, Scally AV. Mechanisms of antineoplastic action of somatostatin analogs. Proc Soc Exp Biol Med. 1998;217:143–52. doi: 10.3181/00379727-217-44216. [DOI] [PubMed] [Google Scholar]
- Xia M, Leppert D, Hauser SL, et al. Stimulus specificity of matrix metalloproteinase dependence of human T cell migration through a model basement membrane. J Immunol. 1996;156:160–7. [PubMed] [Google Scholar]
- Johnatty RN, Taub DD, Reeder SP, et al. Cytokine and chemokine regulation of proMMP-9 and TIMP-1 production by human peripheral blood lymphocytes. J Immunol. 1997;158:2327–33. [PubMed] [Google Scholar]
- Wolf K, Mueller R, Borgmann S, Broecker EB, Friedl P. Amoeboid shape change and contact guidance. T lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood. 2003;102:3262–9. doi: 10.1182/blood-2002-12-3791. [DOI] [PubMed] [Google Scholar]
- Szabo I, Chen XH, Xin L, et al. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proc Natl Acad Sci USA. 2002;99:10276–81. doi: 10.1073/pnas.102327699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano SF, Hernanz-Falcon P, Rodriguez-Frade JM, et al. Functional inactivation of CXC chemokine receptor 4-mediated responses through SOCS3 up-regulation. J Exp Med. 2002;196:311–21. doi: 10.1084/jem.20012041. [DOI] [PMC free article] [PubMed] [Google Scholar]