Abstract
Pigs were injected intramuscularly (i.m.) twice with human serum albumin (HSA) with or without 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3] with a 5-week interval. The supplementation of 1α,25(OH)2D3 enhanced the HSA-specific IgA serum antibody response but decreased the IgM, IgG, IgG1 and IgG2 responses. Furthermore, higher numbers of HSA-specific IgA antibody-secreting cells were obtained in systemic lymphoid tissues (local draining lymph node, spleen and bone marrow) as well as in Peyer's patches and lamina propria of the gut (GALT). In addition, the in vivo mRNA expression for Th1 [interferon (IFN)-γ, interleukin (IL-2)], Th2 (IL-4, IL-6 and IL-10) and Th3 [transforming growth factor (TGF)-β] cytokines as well as the percentage of different cell subsets (CD2+, CD4+, CD8+, IgM+, MHC II+, CD25+) of monomorphonuclear cells from the local draining lymph node were determined at different time-points after the i.m. immunizations. Cytokine profiles did not resemble a typical Th-cytokine profile using 1α,25(OH)2D3: higher levels of IL-10 and significantly lower levels of IL-2 were observed the first day after the primary immunization. However, significantly higher levels of IL-2 and significantly lower levels of IFN-γ were observed the first day after the second immunization. Furthermore, after the second immunization TGF-β mRNA expression decreased more quickly in the 1α,25(OH)2D3 group. This difference became significant 7 days after the second immunization. One week later a significantly higher percentage of CD25+ cells was observed in this group, indicating more activated T and B cells using the steroid hormone. These results suggest that in pigs the addition of 1α,25(OH)2D3 to an intramuscularly injected antigen can enhance the antigen-specific IgA-response and prime GALT tissues, but the relation with cytokines and cell phenotype in the local draining lymph node needs further clarification.
Keywords: 1α,25(OH)2D3; cytokine mRNA expression; IgA; pigs
INTRODUCTION
1α,25-dihydroxyvitamin D3 [(1α,25(OH)2D3], the active metabolite of vitamin D3, is a liphophilic steroid hormone which exerts its actions through binding to a nuclear receptor, the vitamin D receptor (nVDR) [1]. Traditionally 1α,25(OH)2D3 has been associated with calcium homeostasis but the discovery of the nVDR in most cells of the immune system such as monocytes, macrophages, dendritic cells [2], T and B lymphocytes [3–7] suggested a role of the hormone in the immune system. Indeed, 1α,25(OH)2D3 stimulates the differentiation and maturation of monocytes [8] while it inhibits the differentiation and maturation of dendritic cells [9,10]. The steroid hormone also diminishes T cell proliferation [11,12] as well as production of cytokines required for Th1 differentiation such as interleukin (IL)-12 [2,13], interferon (IFN)-γ [14], IL-2 [15,16] as well as granulocyte-macrophage colony stimulating factor (GM-CSF) [17]. On the other hand, 1α,25(OH)2D3 is able to enhance the production of Th2-cytokines such as IL-4 and IL-10 [2,18–20] as well as of the Th3-cytokine transforming growth factor (TGF)-β [21,22]. The latter cytokine is involved in the induction of a mucosal immune response and/or oral tolerance. Moreover, in man and mice TGF-β is responsible for the switch in B cells towards the IgA isotype [23]. Therefore, the steroid hormone is classified as a Th2 and/or Th3-modulating-adjuvant. In addition, 1α,25(OH)2D3 is able to mimic in the local draining lymph node the cytokine pattern which is produced normally by the intestinal Peyer's patches after oral immunization with replicating antigens. This cytokine pattern is essential for the development of a mucosal protective immune response after oral immunization [24]. In mice, subcutaneous injection of antigen together with 1α,25(OH)2D3 evoked a Th2 pattern in the local draining lymph node, an increased homing of IgA and IgG antibody secreting cells (ASC) to the LP of the intestine and the lungs as well as an enhanced IgA response in serum and mucosal secretions [18,19]. Later it was shown by the same group that induction of the mucosal immune response is also due to the migration of antigen-pulsed dendritic cells from the local draining lymph nodes towards the Peyer's patches, where the activation and differentiation of antigen-specific B cells is initiated [25,26].
In pigs it was shown that supplementation of antigen with 1α,25(OH)2D3 increased the antigen-specific IgA serum antibody response as well as the number of IgA and IgG ASC in the local draining lymph node of an intramuscular induced immune response [27]. This suggested that a similar immunomodulating mechanism for 1α,25(OH)2D3 as described for mice could also occur in the pig. However, in pigs the effect of 1α,25(OH)2D3 on the cytokine profile as well as on homing of antigen-specific ASC towards mucosal lymphoid tissues was not demonstrated. The aim of the present study was to obtain insights into the immunomodulating mechanism of 1α,25(OH)2D3 in pigs. Therefore, the phenotype of and Th1-/Th2-/Th3-like cytokine mRNA expression by monomorphonuclear cells (MC) from the local draining lymph node were examined after intramuscular (i.m.) injection with human serum albumin (HSA) supplemented with 1α,25(OH)2D3. In addition, the antigen-specific serum antibodies as well as the number of ASC in systemic and mucosal lymphoid tissues (Peyer's patches and lamina propria) were analysed to determine the effect of 1α,25(OH)2D3 on homing towards the mucosae.
MATERIALS AND METHODS
Pigs
Conventional pigs (Belgian Landrace × Piétrain) from six different litters were used in the experiments. These pigs were weaned at the age of 4 weeks, transported to the faculty and housed in isolation units where they obtained water and food ad libitum. All pigs were seronegative for antibodies against human serum albumin (HSA) as determined by enzyme-linked immunosorbent assay (ELISA) at the start of the experiment.
Experimental design
Effect of a single i.m. injection on immune parameters in the local draining lymph node ( the popliteal lymph node)
At the age of 7 weeks, pigs were immunized i.m. with 1 mg HSA (Sigma-Aldrich, Bornem, Belgium). Fifteen animals received one i.m. injection in the right musculus gastrocnemius with 1 mg of HSA and 2 µg of 1α,25(OH)2D3 (Sigma-Aldrich, Bornem, Belgium) (D3 group) and one in the left m. gastrocnemius with HSA only (control group). This was conducted to compare immune parameters at the left site in the absence of 1α,25(OH)2D3 with these at the right site with 1α,25(OH)2D3) on the same pig, so excluding interanimal variation.
Analysis of the effect of a booster immunization
After the first i.m. immunization, cells of the local draining lymph node will also home to other lymphoid tissues. Therefore the effect of a booster immunization on immune parameters was analysed in another 24 animals. These animals received two identical i.m. injections with a 5-week interval: 12 animals received the antigen together with 1α,25(OH)2D3 (left and right musculus gastrocnemius, D3 group) while the other 12 animals received HSA only (control group). In these animals not only effects in the local draining lymph node but also on the HSA-specific serum antibody response and the HSA-specific ASC numbers in systemic and mucosal lymphoid tissues were analysed.
The HSA-antigen was dissolved in 0·5 ml phosphate buffered saline (PBS) and suspended in an equal volume of incomplete Freund's adjuvant (IFA, Difco Laboratories, Bierbeek, Belgium). IFA was used to allow a gradual and slow release of the antigen and 1α,25(OH)2D3. The 1α,25(OH)2D3 was dissolved in absolute ethanol at a concentration of 8 µg/ml and stored at −20°C. Only at the moment of immunization the HSA antigen in IFA and the dissolved 1α,25(OH)2D3 were mixed. The control group received the same amount of HSA-antigen and an equal volume of ethanol (250 µl) as the D3 group.
The immune response after the i.m. immunizations was evaluated by (i) determining weekly the HSA-specific antibody titres (IgM, IgA, IgG1, IgG2 and IgG) in serum from the moment of the first immunization until 14 days post-secondary immunization (dpsi); (ii) quantifying and localizing the number of HSA-specific IgM, IgA, IgG1, IgG2 and IgG ASC in different lymphoid tissues via ELIspot assays: 10 and 30 days post-primary immunization (dppi) and at 1, 2, 7 and 15 dpsi; (iii) defining the phenotype of MC of the local draining lymph node by flow-cytometric analysis 1, 2, 10 and 30 dppi and at 1, 2, 7 and 15 dpsi; and (iv) determining the cytokine mRNA expression for IL-2, IL-4, IL-6, IL-10, IFN-γ and TGF-β in the local draining lymph node via real-time reverse transcription–polymerase chain reaction (RT-PCR) at 1, 2 and 10 dppi and at 1, 2 and 7 dpsi. Euthanasia was performed by intravenous injection of an overdose of pentobarbital (24 mg/kg; Nembutal, Sanofi Sant Animale, Brussels, Belgium) followed by exsanguination. The number of animals used for each parameter in both groups is shown in Table 2 and in the legends of Figs 1–4.
Table 2.
Sequences of primers with the fragment length of PCR products for different porcine cytokines
| Target mRNA | Oligonucleotide sequences 5′–3′ | Length PCR-fragment | Reference |
|---|---|---|---|
| Cyclophylin | F14571a | 368 | [36] |
| Sb | TAA CCC CAC CGT CTT CTT | ||
| ASc | TGC CAT CCA ACC ACT CAG | ||
| IL-2 | X56750 | 338 | [37] |
| S | GAT TTA CAG TTG CTT TTG AAG | ||
| AS | GTT GAG TAG ATG CTT TGA CA | ||
| IL-4 | F68330 | 311 | [38] |
| S | TAC CAG CAA CTT CGT CCA | ||
| AS | ATC GTC TTT AGC CTT TCC AA | ||
| IL-6 | M86722 | 310 | [39] |
| S | ATG AGA ATC ACC ACC GGT CTT G | ||
| AS | TGC CCC AGC TAC ATT ATC CGA | ||
| IL-10 | L20001 | 295 | [40] |
| S | CCA TGC CCA GCT CAG CAC TG | ||
| AS | CCC ATC ACT CTC TGC CTT CGG | ||
| IFN-γ | X53085 | 360 | [41] |
| S | A TGT ACC TAA TGG TGG ACC TC | ||
| AS | C TCT CTG GCC TTG GAA CAT AG | ||
| TGF-β | Y00111 | 399 | [42] |
| S | GAC CCG CAG AGA GGC TAT AG | ||
| AS | GAG CCA GGA CCT TGC TGT AC |
GenBank accession number
sense primer
antisense primer.
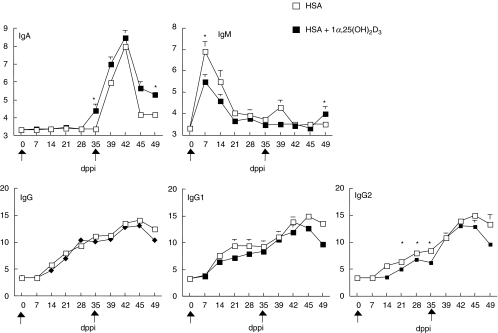
Fig. 1.
Kinetics of the HSA-specific IgM, IgA, IgG1, IgG2 and IgG serum response following two intramuscular (i.m.) immunizations. Pigs (the number of pigs sampled/group is 12 between 0 and 35 dppi, six on 39 and 42 dppi and three on 45 and 49 dppi) had been injected in the m. gastrocnemi with 1 mg HSA in IFA supplemented with either 2 µg of 1α,25(OH)2D3 (▪) or without steroid hormone supplementation (□). The antibody titres are plotted as mean log2 titres ± s.e.m. Significant differences (P < 0·05) between both groups are indicated with an asterisk.
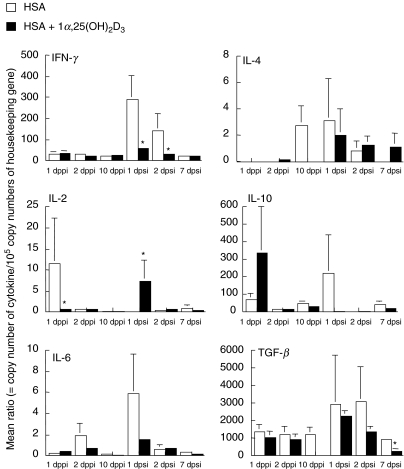
Fig. 4.
Porcine cytokine mRNA expression 1 (n = 5), 2 (n = 5) and 10 (n = 3) dppi, 1 (n = 2), 2 (n = 3) and 7 (n = 3) dpsi of MC from the local draining lymph node (lnn popliteus) of pigs i.m. injected with either 1 mg HSA supplemented with (black bars) or without 2 µg of 1α,25(OH)2D3 (white bars). In vivo cytokine mRNA expression was measured and quantified using real-time PCR and corrected for individual variation by making a ratio to a housekeeping gene. The mean ratios/cytokine for the pigs with the same immunization at the same time-point (copy number of particular cytokin/105 copy numbers of housekeeping gene) were calculated. The cytokine mRNA expression was determined on the same animals after the first i.m. immunization by comparing left (without 1α,25(OH)2D3, control) and right (1α,25(OH)2D3-supplemented) popliteal lymph node while different animals were used after the second immunization. Significant differences (P < 0·05) between both groups are indicated with an asterisk.
Samples
Serum
Blood samples were taken from the jugular vein at the time-points shown in Fig. 1. Serum was collected and inactivated subsequently at 56°C during 30 min. Thereafter, the serum was treated with kaolin (Sigma-Aldrich) to decrease the background reading in ELISA as described by Van den Broeck et al. [28]. Finally the serum was diluted 1/10 (v/v) in ELISA buffer (PBS, 150 mm, pH 7·4) + 0·05% (v/v) Tween®20). The diluted samples were stored at −20°C until tested in ELISA.
MC
The spleen and the lymph node (LN) cells were removed aseptically by teasing the tissues apart gently followed by lysis of erythrocytes with ammonium chloride (0·8% (wt/vol)). After centrifugation (380 g at 4°C for 10 min) the cells were washed and resuspended at 1 × 107 MC/ml in leucocyte medium (RPMI-1640 supplemented with penicillin (100 IU/ml) and streptomycin (100 µg/ml), kanamycin (100 µg/ml), glutamine (200 mm), sodium pyruvate (100 mm), non-essential amino acids (100 mm) and 10% (v/v) fetal bovine serum (FBS; Gibco BRL, Life Technologies, Merelbeke, Belgium).
Bone marrow MC
The sternum was compressed with a forceps and cells from the bone marrow (BM) were collected in centrifugation tubes. Following centrifugation, the cells were washed three times in PBS (150 mm, pH 7·4). The erythrocytes were lysed as described above and the cells were resuspended in leucocyte medium at 1 × 107 MC/ml.
Lamina propria and Peyer's patches MC
Lamina propria (LP) and Peyer's patches (PP) were sampled only 7 and 15 dpsi. The MC of the LP of the jejunum were isolated as described [28,29], with slight modifications. Fifteen to 20-cm-long segments of the mid-jejunum were flushed with PBS (150 mm, pH 7·4) to remove the intestinal content. Subsequently, these segments were opened longitudinally and cut into pieces of 4 cm2. These pieces were rinsed twice with PBS and twice with Ca2+ and Mg2+-free balanced salt solution (CMF-buffer, pH 7·2). This was followed by incubation for 15 min at 37°C in the CMF-buffer containing 0·37 mg/ml ethyline diamine tetra-acetic acid (EDTA) (Sigma) and 0·37 mg/ml dithiothreitol (DTT, Sigma) to remove the epithelial cells and intraepithelial lymphocytes. The remaining tissue fragments were rinsed with RPMI-1640 containing 5% FBS and 20 mm HEPES (Gibco) and incubated thereafter with collagenase and DNAse (RPMI-1640 + 0·1 mg/ml DNAse (Roche Diagnostics) + 300 U/ml collagenase (Sigma) + 100 IU/ml penicillin + 100 µg/ml streptomycin) for 30 min at 37°C and rotating at 250 rounds per minute. A first fraction of cells was collected following filtration through stainless steel sieves (80, 150 and 200 mesh screens; Sigma). A second fraction of MC was collected by mechanical scraping and squeezing the remaining tissue pieces on the sieves. Subsequently, the obtained cell suspension was filtered through a gauze filter. Both cell fractions were combined and washed in RPMI-1640 containing 5% (v/v) FBS, 20 mm HEPES and 0·1 mg/ml DNAse. The MC were isolated by Percoll (Amersham Pharmacia, Uppsala, Sweden) gradient centrifugation. Subsequently, the MC were washed and resuspended in leucocyte medium.
For the isolation of MC from jejunal PP (JPP) and ileal PP (IPP), small intestinal pieces were washed and incubated in CMF–EDTA medium as described for the lamina propria MC isolation. Subsequently, MC were collected by scraping the PPs with glass slides followed by washing, filtration through a gauze filter and resuspending of the MC in leucocyte medium.
ELISA for HSA-specific serum antibody responses
HSA-specific serum IgM, IgA, IgG1, IgG2 and IgG titres were determined in an indirect ELISA as described previously [27]. The antibody titre was determined as the inverse of the highest dilution that still had an OD405 higher than the cut-off value. The cut-off value was determined by calculating the average plus three times the standard deviation of the optical densities of the 1/10 diluted samples measured at day 0. The cut-off values were 0·10, 0·14, 0·11, 0·15 and 0·35 for IgM, IgA, IgG1, IgG2 and IgG, respectively.
ELIspot assay for HSA-specific antibody-secreting cells
The ELIspot test was performed as described previously [27]. For each MC suspension, spots in 10 wells (1 × 106 MC/well) were counted, so that a final amount of isotype-specific ASC per 1 × 107 MC was obtained.
Flow cytometric analysis
Monomorphonuclear cell suspensions of the popliteal lymph node were analysed for expression of cell surface antigens by flow cytometry. MC (1·106/ml) were suspended in ice-cold 100 µl RPMI-1640 supplemented with 2% immunoglobulin-free horse serum and 0·02% sodium azide (staining medium). Subsequently, 100 µl of staining medium containing a swine leucocyte surface-specific MoAb was added for 45 min on ice: MSA-4 = anti-CD2 (IgG2a), 74-12-4 = anti-CD-4 (IgG2b) [30], 76-2-11 = anti-CD8 (IgG2b) [31], anti-IgM = anti IgM+B cells (IgG1) [32], MSA3 = anti-MHC II (SLA-DR, IgG2a) [33] and 231·3B2 = anti-CD25 (IL-2R, IgG1) [34]. Subsequently, the cells were washed twice with staining medium and resuspended in staining medium containing FITC-conjugate [Sheep antimouse IgG (whole molecule) Fab-fragments (Sigma-Aldrich)]. Cells were incubated for 45 min on ice, washed twice with staining medium and once with PBS. Flow cytometric analysis was conducted using a FACScalibur flow cytometer (Becton-Dickinson, San Jose, CA, USA) equipped with a 15 mW air-cooled argon ion laser. At least 10 000 cells were analysed per sample. Data are presented as mean percentage of positive cells ± s.e.m. and as mean fluorescence intensity (MFI) per cell.
RNA extraction and RT-PCR
RNA was extracted from MC of freshly isolated LN of each pig using the acid guanidinium–isothiocyanate phenol–chloroform-based method (RNAgents®, Promega, Leiden, the Netherlands) as described by Verfaillie et al. [35]. Reverse transcription was performed in a 20 µl reaction mixture containing (i) 3 µg of total RNA; (ii) 1 µl of random hexanucleotide primer (50 µm, Perkin Elmer, Oosterhout, the Netherlands); (iii) 4 µl of MgCl2 (25 mm); (iv) 2 µl 10 × PCR II buffer (500 mm KCl, 100 mm Tris-HCl, pH 8·3); (v) 1 mm deoxynucleotide triphosphate mix (dNTP-mix) of the four dNTPs (Roche, Mannheim, Germany); (vi) 200 units of Superscript II Rnase H− reverse transcriptase (Gibco BRL); and (vii) 20 U of RNAsin® ribonuclease inhibitor (Promega) in diethylpyrocarbonate-treated (DEPC) water. Briefly, the template RNA and the random hexanucleotide primer were preincubated for 10 min at 70°C. After chilling on ice, all other components were added. Subsequently, the reaction mix was incubated at room temperature for 10 min and then at 42°C for 45 min followed by heating for 5 min at 95°C to inactivate the reverse transcriptase.
The oligonucleotide primers used for the detection of the porcine IL-2, IL-4, IL-6, IL-10, IFN-γ, TGF-β and of cyclophilin cDNA were designed from the published nucleic acid sequences available from the GenBank/EMBL data bases (Table 2).
Cyclophilin was used as constitutively expressed ‘housekeeping control gene’ to determine the uniformity of the reverse transcription reactions and as a reference for quantification of cytokine mRNA [36]. Cytokine-cDNA was amplified and quantified by real-time PCR using the Light Cycler® (Roche, Mannheim, Germany) and the light-cycler–fast-start DNA Master SYBR Green I kit (Roche, Mannheim, Germany, no. 2239264). The reaction mixture consists of a master mix containing Taq DNA polymerase, dNTP mixture and SYBR Green I, 3–5 mm MgCl2, 20 pmol of sense and antisense primer and 2 µl of template cDNA in a total volume of 20 µl. Subsequent steps were initial denaturation for 10 min at 95°C, followed by 40 cycles of denaturation for 15 s at 94°C, annealing for 5–14 s at 50–60°C depending on the particular cytokine and an extension for 7–15 s at 72°C depending on the length of the product [approximately 1 s/25 base pairs (bp)]. These products were subjected to a melting curve analysis and subsequently agarose gel electrophoresis for confirmation of the specificity of the PCR products. Quantification occurred using external standards of cytokine cDNA. Calculation was performed with the LightCycler® analysis software. The relative amount of cytokine expression was plotted as a ratio [(= copy number of target cytokine/copy number of housekeeping gene) × 105].
Statistical analysis
Statistical analysis was performed using SPSS 7·5 for Windows. Differences in log2 serum antibodies (IgM, IgA, IgG1, IgG2 and IgG) between the 1α,25(OH)2D3 (D3 group) and the control group were tested for statistical significance using the general linear model (repeated-measures analysis of variance). For ELIspot assays differences in the number of HSA-specific ASC between the D3 group and the control group as well as differences in the expression of surface antigens (flow cytometer) were tested for statistical significance using a two-sample t-test. For RT-PCR experiments, the concentrations of the cytokines were corrected for variations between different samples by using cyclophylin as a housekeeping gene and are presented as a ratio (= copy numbers of target cytokine gene/1 × 105 copy numbers of housekeeping gene). Within each group the ratios of the individual pigs were grouped and the differences in the mean ratios between the D3 group and the control group were tested for statistical significance with a two-sample t-test. Statistical significance was assessed at a P-value of < 0·05.
RESULTS
The HSA-specific IgM, IgA, IgG1, IgG2 and IgG serum responses
The HSA-specific IgM titre peaked 7 dppi in both groups. At that time the titre was significantly higher (P < 0·05) in the control group (Fig. 1). The IgM titres declined to baseline values at 35 dppi when the second i.m. immunization was given. This second immunization induced temporarily a slight increase of the IgM titre in the control group 4 dpsi but not in the D3 group. In the D3 group, IgM became significantly higher 14 dpsi.
Serum HSA-specific IgA titres were continuously higher in pigs of the D3 group than in pigs of the control group between 35 and 49 dppi. This difference was significant (P < 0·05) at the moment of second immunization (35 dppi) and 14 days later. In contrast, the HSA-specific IgG1, IgG2 and IgG titres in the D3 group were reduced in comparison with the control group and this reduction was significant for IgG2 (P < 0·05) between 21 and 35 dppi.
HSA-specific IgM, IgA, IgG1, IgG2, IgG and IgM ASC in lymphoid tissues
At different time-points post-primary (10 and 30 dppi) and secondary immunization (1, 2, 7 and 15 dpsi) pigs were euthanized for quantifying and localizing of the HSA-specific IgM, IgA, IgG1, IgG2 and IgG ASC in the LN, the spleen and the BM (systemic lymphoid tissues). The IPP, JPP and LP (gut-associated lymphoid tissues: GALT) were sampled only at 7 and 15 dpsi.
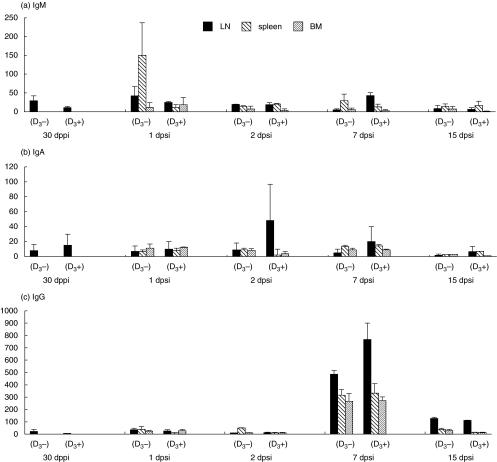
Ten dppi, only HSA-specific IgM ASC could be detected in the LN. The number of IgM ASC was slightly higher in the D3 group (mean of 23 IgM ASC per 1 × 107 MC) than in the control animals (10 IgM ASC per 1 × 107 MC) (data not shown). The highest numbers of HSA-specific IgM were found in the spleen 1 day after the second immunization. However, there were no significant differences in IgM ASC between the groups (Fig. 2a).
Fig. 2.
Mean number of HSA-specific IgM (a), IgA (b) and IgG (c) ASC per 1 × 107 MC in different systemic lymphoid tissues [the popliteal lymph node (LN), spleen and bone marrow (BM)] at 30 dppi and at 1, 2, 7 and 15 dpsi. Animals were i.m. injected with either 1 mg HSA in IFA supplemented with either 2 µg of 1α,25(OH)2D3(D3+) or without steroid hormone (D3–). The numbers of HSA-specific ASC are the mean of three pigs/group ± s.e.m.
The numbers of HSA-specific IgA ASC were always higher for the D3 group in the LN while this was not the case in the spleen and BM, indicating the local effect of the steroid hormone on IgA (Fig. 2b). The numbers of IgA ASC peaked after the second immunization with more than 50 IgA ASC in the D3 group 2 dpsi. However, this was not significantly higher than in the control group (less than 20 IgA ASC 2 dpsi).
HSA-specific IgG ASC were higher in the three systemic lymphoid tissues than in the GALT (Fig. 3) and peaked 7 dpsi with no significant differences between the groups (Fig. 2c). Similar results were obtained for the IgG1 and IgG2 ASC (data not shown).
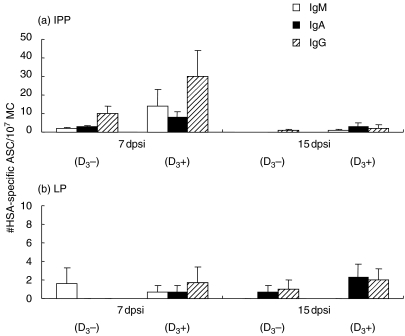
Fig. 3.
Mean number of HSA-specific IgM (open bars), IgA (filled bars) and IgG (shaded bars) ASC per 1 × 107 MC in ileal Peyer's patches (a, IPP) and lamina propria (b, LP) 7 and 15 dpsi. Animals were i.m. injected with 1 mg HSA in IFA supplemented with either 2 µg of 1α,25(OH)2D3(D3+) or without steroid hormone (D3–).The numbers of HSA-specific ASC are the mean of three pigs/group ± s.e.m.
GALT was collected only 7 and 15 dpsi. Seven dpsi higher numbers of HSA-specific IgM, IgA and IgG ASC were found in the IPP of the D3 group (P = 0·268 for IgM, P = 0·242 for IgA and P = 0·238 for IgG) (Fig. 3a). Interestingly, in both groups the number of IgG ASC was higher than the number of IgA ASC. Similar tendencies were seen in the JPP (data not shown). At that moment, low numbers of IgM, IgA and IgG ASC were found in the LP of both groups (Fig. 3b). One week later, HSA-specific ASC were low in both PP and in the LP in both groups.
Phenotype of MC from the local draining lymph node
No significant differences were detected between both groups in the percentages of CD2+, CD4+, CD8+, IgM+ and MHC II+ cells at any moment tested (Table 1). However, 7 and 14 dpsi the mean percentage CD8+ cells was clearly lower in the D3 group. Furthermore, 15 dpsi the percentage CD25+ cells was significantly higher in the D3 group and the degree of MHC II expression was significantly lower (P = 0·019) in this D3 group (MFI 651 ± 38) in comparison with the control group (MFI 917 ± 59). The MHC II expression after the second immunization in both groups (MFI of 653 ± 60) was also significantly higher than after the first immunization (MFI 175 ± 30).
Table 1.
Percentages of positive cells (± s.e.m.) in the local draining lymph node at different times post-primary and -secondary immunization
| Mean percentage of leucocyte subpopulation | |||||||
|---|---|---|---|---|---|---|---|
| 1,25(OH)2D3 | CD2 | CD4 | CD8 | IgM | MHC II | CD25 | |
| 1 dppia | – | 49 ± 12·7 | 34 ± 5·8 | 32 ± 4·5 | 33 ± 8·0 | n.d.d | n.d. |
| (n = 2) | + | 49 ± 8·14 | 33 ± 2·7 | 35 ± 3·4 | 42 ± 4·5 | n.d. | n.d. |
| 2 dppi | – | 69 ± 2·4 | 45 ± 4·1 | 30 ± 5·1 | 26 ± 5·1 | 35 ± 14·6 | 43 ± 3·0 |
| (n = 5) | + | 68 ± 3·7 | 42 ± 2·2 | 28 ± 5·6 | 30 ± 5·0 | 31 ± 11·4 | 41 ± 1·9 |
| 10 dppi | – | 61 ± 7·8 | 33 ± 4·4 | 27 ± 1·6 | 38 ± 7·1 | 52 ± 9·9 | 49 ± 4·8 |
| (n = 5) | + | 65 ± 4·5 | 37 ± 2·8 | 27 ± 1·7 | 34 ± 5·8 | 48 ± 9·6 | 42 ± 3·6 |
| 30 dppi | – | 77 ± 1·6 | 35 ± 1·4 | 30 ± 2·5 | 25 ± 3 | 62 ± 3·2 | 47 ± 5·2 |
| (n = 3) | + | 79 ± 1·9 | 40 ± 3·1 | 29 ± 2·5 | 24 ± 2·2 | 56 ± 6·8 | 50 ± 4·5 |
| 1 dpsic | – | 68a ± 2 | 29 ± 3·2 | 29 ± 3·2 | 36 ± 7·6 | 39 ± 4·5 | 52 ± 1·8 |
| (n = 3) | + | 69 ± 2·1 | 36 ± 6·5 | 30 ± 1·8 | 32 ± 1·3 | 39 ± 3·6 | 43 ± 4·1 |
| 2 dpsi | – | 68 ± 3·7 | 31 ± 2·0 | 34 ± 1·2 | 33 ± 4·7 | 47 ± 7·1 | 57 ± 2·3 |
| (n = 3) | + | 62 ± 5·5 | 31 ± 3·1 | 33 ± 1·6 | 34 ± 2·7 | 51 ± 5·0 | 56 ± 6·6 |
| 7 dpsi | – | 71 ± 2·0 | 40 ± 5·6 | 44 ± 3·6 | 30 ± 2·5 | 46 ± 3·4 | 54 ± 2·3 |
| (n = 3) | + | 65 ± 3·2 | 38 ± 2·4 | 33 ± 2·9 | 27 ± 0·2 | 39 ± 6·4 | 44 ± 6·5 |
| 15 dpsi | – | 67 ± 2·5 | 28 ± 1·5 | 37 ± 3·2 | 23 ± 3·7 | 47 ± 1·6 | |
| (n = 3) | MFIe 917 | 56 ± 2·0 | |||||
| + | 65 ± 4·2 | 32 ± 1·7 | 31 ± 0·6 | 20 ± 3·1 | 56 ± 2·8 | ||
| MFI 651† | 66† ± 1·7 | ||||||
After the first immunization the percentages were determined on the same animals by comparing left (without 1α,25(OH)2D3) and right (with 1α,25(OH)2D3) popliteal lymph node while different animals were used after the second immunization. MC suspensions form popliteal lymph nodes were stained with MoAbs directed against porcine CD2, CD4, CD8, IgM, CD25 and MHC II. bdppi: days post-primary immunization,
dpsi: days post-secondary immunization;
n.d.: not determined.
MFI: mean fluorescence intensity.
P < 0·05.
In vivo cytokine mRNA expression by MC from the local draining lymph node
The in vivo cytokine mRNA expression was measured 1, 2 and 10 dppi and 1, 2 and 7 dpsi. The cytokine mRNA responses peaked 1–2 days after the first (innate response) as well as after the second (memory response) i.m. injections. The cytokine mRNA expression per cytokine revealed the following descending rank, irrespective of the use of 1α,25(OH)2D3: TGF-β (1·67 × 1 × 105± 1·8 × 1 × 105) > IL-10 (1·38 × 104 ± 2·65 × 104) > IFN-γ (7·9 × 103± 1·3 × 104) > IL-2 (2·91 × 102 ± 5·24 × 102) > IL-4 (1·3 × 102 ± 3·17 × 102) > IL-6 (1 × 102 ± 1·2 × 102).
The relative amount of cytokine mRNA expression in both groups is shown in Fig. 4. One day after the first immunization, IL-10 mRNA was higher and IL-2 was significantly lower (P < 0·05) in the D3 group than in the control group. The IFN-γ, TGF-β and IL-6 mRNA expression did not differ statistically between the groups, whereas IL-4 mRNA was not detected. One day later IL-2 and IL-10 mRNA were almost absent in both groups, whereas levels of IFN-γ and TGF-β mRNA expression had not changed. On the other hand, IL-6 mRNA expression was higher in the control group and traces of IL-4 mRNA were detected in the D3 group. However, 10 dppi IL-4 and TGF-β mRNA expression could be detected only in the control group (P = 0·26 and 0·15, respectively) in comparison with the D3 group, while the expression of the other cytokines was similar in both groups.
One day after the booster immunization, the expression of IL-2 and IL-10 mRNA were significantly higher (P = 0·043) and lower (P = 0·14), respectively, in the D3 group than in the control group, which was opposite to the first immunization. The other cytokines showed a higher mRNA expression than after the first immunization, but expression was lower in the D3 group than in the control group. Moreover 1α,25(OH)2D3 seems to inhibit significantly the IFN-γ mRNA expression with at least 50% 1 and 2 dpsi (P = 0·023 and 0·013, respectively), whereas the TGF-β mRNA expression became significantly lower (P = 0·04) in the D3 group 7 dpsi. Interleukin-2, IL-6 and IL-10 mRNA were low to absent 2 and 7 dpsi. Interleukin-4 could be detected only in the D3 group (P = 0·37) 7 dpsi.
DISCUSSION
In the present study, we examined whether addition of 1α,25(OH)2D3 could result in the appearance of antigen-specific ASC in the GALT after i.m. administration of an antigen and whether the steroid hormone polarizes the cytokine response towards a Th2-cytokine profile. As in a previous study [27], co-administration of 1α,25(OH)2D3 enhanced significantly the serum HSA-specific IgA response and increased the numbers of HSA-specific IgA ASC in the local draining lymph node of pigs after i.m. injection. However, in the present study it decreased the antigen-specific serum IgM, IgG1, IgG and IgG2 (P < 0·05) serum response. This was most pronounced after the first i.m. injection. This decreased IgM antibody response was not observed in a previous study [27]. The reason is not clear, but the i.m. injection occurred at a different place (musculus gastrocnemius compared to musculus gluteobiceps) and it has been observed in previous experiments with pigs comparing immunizations in the back versus the neck that the injection site can influence the isotype profile of the antibody response [43]. Therefore, in contrast to the serum IgA response, the steroid hormone does not enhance consistently the serum IgM response in pigs. A decreased as well as an increased serum IgM response using 1α,25(OH)2D3 was also observed in man [3]. In cattle, milk IgM and IgA titres increased and IgG2 decreased [44] and in mice serum IgG2a decreased, whereas IgG1 increased using high doses of 1α,25(OH)2D3 [45]. The situation in the mice led to the interpretation that 1α,25(OH)2D3 stimulates the Th2 or humoral branch of the immune system, as a Th2-cytokine profile stimulates preferentially the formation of IgG1, IgM and IgA [46]. In relation to this we examined if addition of 1α,25(OH)2D3 correlates with type I/type II control of immunoglobulin isotype switching in pigs as well with the presupposition that IgG1 is a type II (IL-10) immunoglobulin subclass and IgG2 is a type I (IFN-γ) subclass. Pig cytokines regulate immunoglobulin isotype expression, because recombinant porcine IFN-γ or recombinant porcine IL-12 up-regulate IgG2 production by porcine B-cells cultured in vitro, while recombinant porcine IL-10 up-regulates IgG1 production [47]. The results obtained do not show that 1α,25(OH)2D3 up-regulates the IgG1 production in pigs as it does in mice.
In order to know whether i.m. immunization with 1α,25(OH)2D3 directed the response towards the GALT, the number of HSA-specific ASC in the jejunal PP and ileal PP as well as in the LP were determined. Intramuscular immunization induced IgA and IgG ASC first in the PP and later, although in very low numbers, in the LP. This seems to indicate that there is no direct homing of the LN cells to the effector sites in the GALT but a preferential homing to the inductive sites. Others found that memory B cells can reside in the GALT inductive sites after a systemic immunization [48–50]. Upon mucosal antigen challenge these memory B cells differentiate and migrate towards the lamina propria, where they produce antigen-specific IgA [51]. The ASC in the present study, however, are not memory cells and no mucosal antigen challenge was performed. Therefore, it is not surprising that only low numbers of ASC were found in the LP. Another reason for these low numbers might be a too-short interval (7 days) between both samplings of GALT. Indeed, the time required for activation, differentiation and homing of antigen-specific ASC from PP towards LP is at least 6 days [52]. Nevertheless, the low number of ASC may already result in a protective effect. Indeed, i.m. injection with F4-fimbriae co-administered with 1α,25(OH)2D3 induced a partial protection upon oral challenge with F4+ enterotoxigenic Escherichia coli which was accompanied with a secondary serum IgA response, indicating priming of the intestinal mucosal immune system [53]. Further studies are necessary in pigs to elucidate if this mucosal priming occurs via migration of dendritic cells towards the GALT, as observed in mice [25,26].
No higher numbers of antigen-specific ASC were found in the ileal PP (Fig. 3) compared to the jejunal PP (3–6 IgM ASC, 6–10 IgA ASC and 34 IgG ASC) of the pigs, as could be expected from the 10-fold excess of B cells in the ileal PP in comparison with the jejunal PP, as observed by Pabst et al. [54]in normal in gnotobiotic pigs. However, Bianchi et al. [55] found no significant differences in B cell populations between ileal PP and jejunal PP in pigs. This is more in agreement with our findings and attributes a similar importance to ileal PP as jejunal PP in the induction of an intestinal antibody response in 12-week-old pigs. Bianchi et al. [55] did observe an increase with age in the number of antibody-secreting cells in jejunal but not in ileal PP. Together with the observation that pig's ileal PP show morphological differences with the jujunal PP, this suggests another specific role such as B cell ontogeny, as has been reported in sheep [56].
As 1α,25(OH)2D3 primarily enhances the antigen-specific ASC in the local draining lymph node and clearly enlarges the local draining lymph node [27], the distribution of various lymphocyte subsets in the local draining lymph node was analysed at different time-points after the i.m. injection. In the present study, however, enlargement of the local draining lymph node due to 1α,25(OH)2D3 was not observed consistently. Moreover, no statistically significant changes were observed in either the percentage of CD2+ (T cells), CD4+ (Th cells), CD8+ (CTL-cells) and IgM+ (B cells) cells or in the degree of expression of these molecules on the cells. In human studies, however, a decreased CD4+/CD8+ ratio, due to a significant increase in CD8+ cells, was observed after treatment with 0·5 µg or 2 µg of 1α,25(OH)2D3 for 60 and 14 days, respectively [57–59]. Although not statistically significant, the CD4+/CD8+ T cell ratio appeared to be increased 7 and 15 dpsi in the present study. The most obvious differences were observed after the second i.m. injection. This booster immunization with 1α,25(OH)2D3 resulted in a significantly higher percentage of CD25+ cells with, however, a lower expression of MHC II molecules. The latter is consistent with human studies in which a decreased expression of MHC class II molecules on human monocytes was observed due to 1α,25(OH)2D3. This decrease was dose- and time-dependent [60–62]. The higher percentage of CD25+ cells suggests at least an activation of more cells or a better persistance of activated cells by the steroid hormone, as the porcine IL-2R (CD25) is expressed on activated T [63] and B cells in lymph nodes [64]. For human B cells it was shown that the degree of cellular activation, rather than the differentiation, is a criterion for the biological receptivity to 1α,25(OH)2D3 [65]. In humans the steroid hormone also favours the induction of CD4+CD25+ regulatory T cells which are able to inhibit Th1 cells [66].
The in vivo mRNA expression of Th1-like (IFN-γ, IL-2), Th2-like (IL-4 and IL-10) and Th3-like (TGF-β) cytokines showed a high variability among the pigs. Following the first immunization with 1α,25(OH)2D3, increased IL-10 and IL-4 mRNA and decreased levels of IL-2 mRNA (Th1-like) were observed, indicating a Th2 modulation by 1α,25(OH)2D3. A Th2-like response could also explain the increased serum IgA and the higher number of IgA ASC in the local draining lymph node. Th2-polarization by 1α,25(OH)2D3 was also observed after in vitro stimulation of mice lymph node cell suspensions [19,67], while others observed inhibition of IL-4 by naive murine T cells [68]. IL-6 and TGF-β, which are involved in the proliferation of IgA+ B cells and in isotype switching towards IgA, respectively [69], were not increased in the present study and therefore seem not to be crucial for the effect of 1α,25(OH)2D3 in pigs. More striking differences were observed after the second immunization. Significant lower levels of IFN-γ mRNA, a Th1 cytokine, were observed in the D3 group compared to the control group. However, lower levels of Th2- (IL-4 and IL-10 mRNA) and Th3-like (TGF-β mRNA) cytokines together with statistically significant higher levels of IL-2 were also observed in the 1α,25(OH)2D3-treated animals. In conclusion, the results of the present study do not allow us to conclude that 1α,25(OH)2D3 induces a strict Th2-like response. Only after the primary inoculation was a Th2-like response seen. The inverse correlation between IL-10 and IL-2 mRNA expression appears consistent in our experiments and suggests that IL-10 is able to down-regulate Th1-mediated immune responses as described for man [70,71] as well as for pigs [40]. However, in man and cattle, IL-10 is expressed by all Th and not selectively by Th2 cells, as in mice [72].
In conclusion, supplementation of 1α,25(OH)2D3 to parenteral immunization enhanced the IgA serum antibody response as well as the number of antigen-specific ASC in different systemic lymphoid tissues. Moreover, 1α,25(OH)2D3 administration resulted in an enhanced priming of the GALT, especially in the Peyer's patches. This was not correlated with major changes in T/B cell subsets and a clear Th2-like (IL-4, IL-6, IL-10) cytokine profile in the local draining lymph node.
REFERENCES
- Bouillon R, Garmyn M, Verstuyft A, Segaert S, Casteels K, Mathieu C. Paracrine role for calcitriol in the immune system and skin creates new therapeutical possibilities for vitamin D analogs. Eur J Endocrinol. 1995;133:7–16. doi: 10.1530/eje.0.1330007. [DOI] [PubMed] [Google Scholar]
- Adorini L, Penna G, Casorati M, Davalli AM, Gregori S. Induction of transplantation tolerance by 1α,25-dihydroxyvitamin D3. Transplant Proc. 2001;33:58–9. doi: 10.1016/s0041-1345(00)02262-4. [DOI] [PubMed] [Google Scholar]
- Lemire JM, Adams JS, Sakai R, Jordan SC. 1-alpha,25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood monomorphonuclear cells. J Clin Invest. 1984;74:657–61. doi: 10.1172/JCI111465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman CM, Cantorna MT, DeLuca HF. Expression of 1α,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys. 2000;374:334–8. doi: 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- Morgan JW, Reddy GS, Uskokovic MR, et al. Functional block for 1 alpha,25-dihydroxyvitamin D3-mediated gene regulation in human B lymphocytes. J Biol Chem. 1994;269:13437–43. [PubMed] [Google Scholar]
- Morgan JW, Morgan DM, Lasky SR, Ford D, Kouttab N, Maizel AL. Requirements for the induction of vitamin D-mediated gene regulation in normal human B lymphocytes. J Immunol. 1996;157:2900–8. [PubMed] [Google Scholar]
- Morgan JW, Sliney DJ, Morgan DM, Maizel AL. Differential regulation of gene transcription in subpopulations of human B lymphocytes by vitamin D3. Endocrinology. 1999;140:381–91. doi: 10.1210/endo.140.1.6395. [DOI] [PubMed] [Google Scholar]
- Abe E, Miyaura C, Sakagami H, et al. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA. 1981;78:4990–4. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna G, Adorini L. 1-alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–11. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci USA. 2001;98:6800–5. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby WF, Stacy T, Fanger MW. Inhibition of T lymphocyte mitogenesis by 1α,25-dihydroxyvitamin D3 (calcitriol) J Clin Invest. 1984;74:1451–5. doi: 10.1172/JCI111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla AK, Amento EP, Serog B, Glimcher LH. 1α,25-Dihydroxyvitamin D3 inhibits antigen-induced T cell activation. J Immunol. 1984;133:1748–54. [PubMed] [Google Scholar]
- D’Ambrosio D, Cippitelli M, Coccilo GM, et al. Inhibition of IL-12 production by 1α,25-dihydroxyvitamin D3. J Clin Invest. 1998;101:252–62. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli M, Santoni A. Vitamin D3: a transcriptional modulator of the interferon-γ gene. Eur J Immunol. 1998;28:3017–30. doi: 10.1002/(SICI)1521-4141(199810)28:10<3017::AID-IMMU3017>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Alroy I, Towers T, Freedman L. Transcriptional repression of the interleukin-2 gene by vitamin D3. Direct inhibition of NFATp/AP-1 complex formation by a nuclear hormone receptor. Mol Cell Biol. 1995;15:5789–99. doi: 10.1128/mcb.15.10.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A, Reddy GS, Kobayashi T, Okano T, Park J, Sharma S. Nuclear factor of activated T-cells (NFAT) as a molecular target for 1α,25-dihydroxyvitamin D3-mediated effects. J Immunol. 1998;160:209–18. [PubMed] [Google Scholar]
- Towers TL, Freedman LP. Granulocyte-macrophage colony stimulating factor gene transcription is directly repressed by the vitamin D3 receptor. J Biol Chem. 1998;273:8483–91. doi: 10.1074/jbc.273.17.10338. [DOI] [PubMed] [Google Scholar]
- Daynes RA, Araneo BA. The development of effective vaccine adjuvants employing natural regulators of T-cell lymphokine production in vivo. In: Ades EW, Rest RF, Morse SA, editors. Annals of the New York Academy of Sciences: microbial pathogenesis and immune response. New York: New York Academy of Science; 1994. pp. 144–61. [DOI] [PubMed] [Google Scholar]
- Daynes A, Elena Y, Enioutina SB, et al. The induction of common mucosal immunity by hormonally immunomodulated peripheral immunisation. Infect Immun. 1996;64:1100–9. doi: 10.1128/iai.64.4.1100-1109.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna TM, Woodward WD, Hayes CE, DeLuca HF. 1α,25-dihydroxyvitamin D3 is a positive regulator for the two anti-encephalitogenic cytokines TGF-β1 and IL-4. J Immunol. 1998;160:5314–9. [PubMed] [Google Scholar]
- Fukaura H, Kent SC, Pietrusewicz MJ, Khy SJ, Weiner HL, Hafler DA. Induction of circulating myelin basic protein and proteolipid protein-specific transforming growth factor-beta 1-secreting Th3 T cells by oral administration of myelin in multiple sclerosis patients. J Clin Invest. 1996;98:70–7. doi: 10.1172/JCI118779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich T, Landolt M, Booy C, Wuthrich R, Binswanger U. 1α,25-dihydroxyvitamin D3 stimulates transforming growth factor-beta 1 synthesis by mouse renal proximal tubular cells. Kidney Blood Press Res. 1999;22:99–105. doi: 10.1159/000025914. [DOI] [PubMed] [Google Scholar]
- Letterio JJ, Roberts AB. Regulation of immune responses by TGF-β. Annu Rev Immunol. 1998;16:137–61. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- McGhee JR, Mestecky J, Dertzbaugh MT, Eldridge JH, Hirasawa M, Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10:75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- Enioutina EY, Visic D, McGee A, Daynes RA. The induction of systemic and mucosal immune responses following the subcuteneous immunisation of mature adult mice: characterisation of the antibodies in mucosal secretions of animals immunized with antigen formulations containing a vitamin D3 adjuvant. Vaccine. 1999;17:3050–64. doi: 10.1016/s0264-410x(99)00147-4. [DOI] [PubMed] [Google Scholar]
- Enioutina EY, Visic D, Daynes RA. The induction of systemic and mucosal immune responses to antigen–adjuvant compositions administered into the skin: alterations in the migratory properties of dendritic cells appears to be important for stimulating mucosal immunity. Vaccine. 2000;18:2753–67. doi: 10.1016/s0264-410x(00)00059-1. [DOI] [PubMed] [Google Scholar]
- Van der Stede Y, Cox E, Van den Broeck W, Goddeeris BM. Enhanced induction of the IgA response in pigs by calcitriol after intramuscular immunisation. Vaccine. 2001;19:1870–8. doi: 10.1016/s0264-410x(00)00440-0. [DOI] [PubMed] [Google Scholar]
- Van den Broeck W, Cox E, Goddeeris BM. Induction of immune responses in pigs following oral administration of purified F4 fimbriae. Vaccine. 1999;17:2020–9. doi: 10.1016/s0264-410x(98)00406-x. [DOI] [PubMed] [Google Scholar]
- Van der Heijden PJ, Stok W, Bianchi ATJ. Contribution of immunoglobulins secreting cells in the murine small intestine to the total ‘bacground’ immunoglobulin production. Immunology. 1987;62:551–5. [PMC free article] [PubMed] [Google Scholar]
- Pescovitz MD, Lunney JK, Sachs DH. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J Immunol. 1984;133:368. [PubMed] [Google Scholar]
- Zuckermann FA, Gaskins HR. distribution of porcine CD4/CD8 double positive T lymphocytes in mucosa-associated lymphoid tissues. Immunology. 1996;87:493–9. [PMC free article] [PubMed] [Google Scholar]
- Van Zaane D, Hulst MM. Monoclonal antibodies against porcine immunoglobulin isotypes. Vet Immunol Immunopathol. 1987;16:23–36. doi: 10.1016/0165-2427(87)90171-1. [DOI] [PubMed] [Google Scholar]
- Hammerberg C, Shurig GG. Characterization of monoclonal antibodies directed against swine leukocytes. Vet Immunol Immunopathol. 1986;11:107. doi: 10.1016/0165-2427(86)90092-9. [DOI] [PubMed] [Google Scholar]
- Bailey M, Stevens K, Bland PW, Stokes CR. A monoclonal antibody recognizing an epitope associated with pig interleukin-2 receptors. J Immunol Meth. 1992;153:85–91. doi: 10.1016/0022-1759(92)90309-h. [DOI] [PubMed] [Google Scholar]
- Verfaillie T, Cox E, To LT, et al. Comparative analysis of porcine cytokine production by mRNA and protein detection. Vet Immunol Immunopathol. 2001;81:97–112. doi: 10.1016/s0165-2427(01)00339-7. [DOI] [PubMed] [Google Scholar]
- Dozois CM, Oswald E, Gautier N, Serthelon J-P, Fairbrother JM, Oswald IP. Reverse transcription-polymerase chain reaction method to analyze the porcine cytokin gene expression. Vet Immunol Immunpathol. 1997;58:287–300. doi: 10.1016/s0165-2427(97)00039-1. [DOI] [PubMed] [Google Scholar]
- Goodall JC, Emery DC, Bailey M, English LS, Hall L. cDNA cloning of porcine interleukin 2 by polymerase chain reaction. Biochem Biophys Acta. 1991;1089:257–8. doi: 10.1016/0167-4781(91)90019-i. [DOI] [PubMed] [Google Scholar]
- Bailey M, Perry AC, Bland PW, Stokes CR, Hall L. Nucleotide and deduced amino acid sequence of porcine interleukin 4 cDNA derived from lamina propria lymphocytes. Biochim Biophys Acta. 1993;1171:328–30. doi: 10.1016/0167-4781(93)90077-q. [DOI] [PubMed] [Google Scholar]
- Richards CD, Saklatvala J. Molecular cloning and sequence of porcine interleukin 6 cDNA and expression of mRNA in synovial fibroblasts in vitro. Cytokine. 1991;3:269–76. doi: 10.1016/1043-4666(91)90494-x. [DOI] [PubMed] [Google Scholar]
- Blancho G, Gianello P, Germana S, Baetscher M, Sachs DH, LeGuern C. Molecular identification of porcine interleukin 10: regulation of expression in a kidney allograft model. Proc Natl Acad Sci USA. 1995;92:2800–4. doi: 10.1073/pnas.92.7.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkmans R, Vandenbroeck K, Beuken E, Billiau A. Sequence of the porcine interferon-gamma (IFN-γ) gene. Nucl Acids Res. 1990;18:4259. doi: 10.1093/nar/18.14.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Goeddel DV, Ullrich A, et al. Synthesis of messenger RNAs for transforming growth factors alpha and beta and the epidermal growth factor receptor by human tumors. Cancer Res. 1987;47:707–12. [PubMed] [Google Scholar]
- Vanderpooten A, Goddeeris BM, De Roose P, Hedrickx L, Biront P, Desmettre P. Evaluation of parenteral vaccination methods with glycoproteins against Aujeszky's disease in pigs. Vet Microbiol. 1997;55:81–9. doi: 10.1016/s0378-1135(96)01300-4. [DOI] [PubMed] [Google Scholar]
- Reinhardt TA, Stabel JR, Goff JP. 1α,25-Dihydroxyvitamin D3 enhances milk antibody titers to Escherichia coli J5 vaccine. J Dairy Sci. 1999;82:1904–9. doi: 10.3168/jds.S0022-0302(99)75425-1. [DOI] [PubMed] [Google Scholar]
- Lemire JM, Archer DC, Beck L, Spiegelberg HL. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 function. J Nutr. 1995;125(Suppl.):1704S–8. doi: 10.1093/jn/125.suppl_6.1704S. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- Crawley A, Raymond C, Wilkie BN. Differential control of immunoglobulin isotype production by porcine B-cells cultured with cytokines. Vet Immunol Immunopathol. 2002;91:141–54. doi: 10.1016/s0165-2427(02)00293-3. [DOI] [PubMed] [Google Scholar]
- Pierce NF, Gowans JL. Cellular kinetics of the intestinal immune response to cholera toxoid in rats. J Exp Med. 1975;142:1550–63. doi: 10.1084/jem.142.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman JA, Cebra JJ. Special features of the priming process for a secretory IgA response. B cell priming with cholera toxin. J Exp Med. 1981;153:534–44. doi: 10.1084/jem.153.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Geyer A, Saif L. Short-term immunoglobulin A B-cell memory resides in intestinal lymphoid tissues but not in bone marrow of gnotbiotic pigs inoculated with Wa human rotavirus. Immunology. 2001;103:188–98. doi: 10.1046/j.1365-2567.2001.01229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin SE, Offit PA. Induction of mucosal B-cell memory by intramuscular inoculation of mice with rotavirus. J Virol. 1998;72:3479–83. doi: 10.1128/jvi.72.4.3479-3483.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin SE, Moser CA, Cohen MS, Clark HF, Offit AP. Immunologic correlates of protection against rotavirus challenge after intramuscular immunisation of mice. J Virol. 1997;71:7851–6. doi: 10.1128/jvi.71.10.7851-7856.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Stede Y, Cox E, Verdonck F, Vancaeneghem S, Goddeeris BM. Reduced faecal excretion of F4+-E. coli by intramuscular immunisation of suckling piglets by addition of 1α,25-dihydroxyvitamin D3 of CpG-oligodeoxynucleotides. Vaccine. 2003;21:1023–32. doi: 10.1016/s0264-410x(02)00553-4. [DOI] [PubMed] [Google Scholar]
- Pabst R, Geist M, Röthkötter HJ, Fritz FJ. Postnatal development and lymphocyte production of jejunal and ileal Peyer's patches in normal and gnotobiotic pigs. Immunology. 1988;64:539–44. [PMC free article] [PubMed] [Google Scholar]
- Bianchi ATJ, Scholten JW, Moonen-Leusen HWM, Boersma WJ. Development of the natural response of immunoglobulin secreting cells in the pig as function of organ, age and housing. Dev Comp Immunol. 1999;23:511–20. doi: 10.1016/s0145-305x(99)00026-9. [DOI] [PubMed] [Google Scholar]
- Barman NN, Bianchi ATJ, Zwart RJ, Pabst R, Röthkötter HJ. Jejunal and ileal Peyer's patches in pigs differ in their postnatal development. Anat Embryol. 1996;195:41–50. doi: 10.1007/s004290050023. [DOI] [PubMed] [Google Scholar]
- Fujita T, Matsui T, Nakao Y, Watanabe S. T lymphocyte subsets in osteoporosis. Effect of 1-alpha hydroxyvitamin D3. Miner Electrolyte Metab. 1984;10:375–8. [PubMed] [Google Scholar]
- Matsui T, Nakao Y, Koizumi T, Nakagawa T, Fujita T. 1,25-Dihydroxyvitamin D3 regulates proliferation of activated T-lymphocyte subsets. Life Sci. 1985;37:95–101. doi: 10.1016/0024-3205(85)90630-7. [DOI] [PubMed] [Google Scholar]
- Zofkova I, Kancheva RL. The effect of 1α,25 (OH)2 vitamin D3 on CD4+/CD8+ subsets of T lymphocytes in postmenopausal women. Life Sci. 1997;61:147–52. doi: 10.1016/s0024-3205(97)00369-x. [DOI] [PubMed] [Google Scholar]
- Rigby WFC, Waugh MG. Decreased accessory cell function and costimulatory activity by 1α,25-dihydroxyvitamin D3-treated monocytes. Arthritis Rheum. 1992;35:110–9. doi: 10.1002/art.1780350117. [DOI] [PubMed] [Google Scholar]
- Xu H, Soruri A, Gieseler RK, Peters JH. 1,25-Dihydroxyvitamin D3 exerts opposing effects to IL-4 on MHC class-II antigen expression, accessory activity, and phagocytosis of human monocytes. Scand J Immunol. 1993;38:535–40. doi: 10.1111/j.1365-3083.1993.tb03237.x. [DOI] [PubMed] [Google Scholar]
- Splitter A, Willheim MG, Leutmezer F. Effect of 1α,25-dihydroxyvitamin D3 and cytokines on the expression of MHC antigens, complement receptors and other antigens on human blood monocytes and U 937 cells: role in cell differentiation, activation and phagocytosis. Immunology. 1997;90:286. doi: 10.1046/j.1365-2567.1997.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunney JK, Walker K, Goldman T, et al. Overview of the first international workshop to define swine leukocyte cluster of differentiation (CD) antigens. Vet Immunol Immunopathol. 1994;43:193–206. doi: 10.1016/0165-2427(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Denham S, Shimizu M, Bianchi ATJ, Zwart RJ, Carr MM, Parkhouse RME. Monoclonal antibodies recognising differentiation antigens on B-cells. Vet Immunol Immunopathol. 1994;43:259–67. doi: 10.1016/0165-2427(94)90146-5. [DOI] [PubMed] [Google Scholar]
- Morgan JW, Kouttab N, Ford D, Maizel AL. Vitamin D-mediated gene regulation in phenotypically defined human B cell subpopulations. Endoricnology. 2000;141:3225–34. doi: 10.1210/endo.141.9.7666. [DOI] [PubMed] [Google Scholar]
- Mathieu C, Adorini L. The coming of age of 1,25-dihydroxyvitamin D3 analogs as immunomodulatory agents. Trends Mol Med. 2002;8:174–9. doi: 10.1016/s1471-4914(02)02294-3. [DOI] [PubMed] [Google Scholar]
- Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25-Dihydroxyvitamin D3 has a direct effect on naive CD4 (+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–80. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- Staeva-Vieira TP, Freedman LP. 1,25-dihydroxyvitamin D3 inhibits IFN-gamma and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. J Immunol. 2002;168:1181–9. doi: 10.4049/jimmunol.168.3.1181. [DOI] [PubMed] [Google Scholar]
- Lebman DA, Lee FD, Doffman RL. Mechanism for transforming growth factor β and IL-2 enhancement of IgA expression in lipopolysaccharide-stimulated B cell cultures. J Immunol. 1990;144:942–59. [PubMed] [Google Scholar]
- Wanidworanun C, Strober W. Predominant role of tumor necrosis factor-alpha in human monocytes IL-10 synthesis. J Immunol. 1993;151:6853–61. [PubMed] [Google Scholar]
- Randow F, Syrbe U, Meisel C, et al. Mechanism of endotoxin desensitisation: involvement of interleukin 10 and transforming growth factor beta. J Exp Med. 1995;181:1887–92. doi: 10.1084/jem.181.5.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WC, Woods VM, Chitko-McKown CG, Hash SM, Rice-Ficht AC. Interleukin-10 is expressed by bovine type 1, type 2 helper, and unrestricted parasite-specific T-cell clones and inhibits proliferation of all three subsets in an accessory-dependent manner. J Virol. 1994;62:4697–708. doi: 10.1128/iai.62.11.4697-4708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]