Abstract
Recent studies suggest that pre-eclampsia is associated with a Th1 predominant state and may be considered a failure of tolerance. Granulysin is a cytotoxic granule protein of natural killer (NK) cells and cytotoxic T lymphocytes (CTLs). Recently, we developed an enzyme-linked immunosorbent assay (ELISA) system for detecting serum granulysin, and reported that serum granulysin is a useful marker to evaluate the cell-mediated immunity. In this study, we show that the serum levels of granulysin were significantly elevated in pre-eclamptic patients compared with those in normal pregnancy subjects. In addition, the serum granulysin levels in pre-eclamptic patients were well associated with mean blood pressure, percentage of peripheral blood Th1 cells and Th1/Th2 ratios. The present results suggest that the serum granulysin levels would be a useful and novel serum marker to evaluate the Th1/Th2 balance, especially Th1 type immunity in pre-eclampsia.
Keywords: cell-mediated immunity, granulysin, pre-eclampsia, pregnancy, Th1/Th2
Introduction
Pregnancy represents the growth of an allograft where fetal trophoblast cells evade immune rejection and invade maternal tissue [1,2]. There should be a balance between fetal trophoblast and maternal immune responsive cells and alterations in the proportion of these cells may relate to pregnancy disorders such as pre-eclampsia. Indeed, histological changes in the placental beds of women with pre-eclampsia were noted to resemble those of acute graft rejection [3–6]. Natural killer (NK) activity and CD8+ cytotoxic T cells are activated in pre-eclampsia [7–9], and decidual CD56+ NK cells and CD8+ T cells increase in pre-eclampsia [10].
Adaptive immune systems can be classified as cell-mediated immunity and humoral immunity. CD4+ T cells are classified as Th1 cells, which synthesize interleukin (IL)-2, interferon (IFN)-γ and tumour necrosis factor (TNF)-α, and induce cellular immunity, or Th2 cells, which synthesize IL-4, IL-5, IL-6, IL-10 and IL-13, and induce antibody production [11]. Recent studies suggested that pre-eclampsia is associated with a Th1 predominant profile and may be considered a failure of the tolerance system, allowing the second physiological trophoblast invasion [8, 12–19]. To study fresh ex-vivo cytokine production at a single-cell level, flow cytometry for detecting intracellular cytokine was developed [20]. This method has the capacity to analyse large numbers of cells, although much skill is required to obtain consistent results. Detection of serum cytokines does not reflect the Th1/Th2 balance correctly because the half-life of serum cytokines is very short and we could not detect these trace cytokines. Based on these conditions, the present study sought to find a reliable serum marker for detecting Th1/Th2 balance.
Recently, we developed an enzymed-linked immunosorbent assay (ELISA) for detecting serum granulysin and reported that the serum granulysin levels are associated well with the activities of NK cells and cytotoxic T lymphocytes (CTLs) and could be a useful novel serum marker to evaluate the overall status of host cellular immunity [21]. In the present study, we measured the serum granulysin in pre-eclamptic patients and normal pregnancy subjects. Serum granulysin levels were elevated in pre-eclampsia and these levels appeared to correlate with clinical status such as mean blood pressure and immunological status such as Th1/Th2 balance, especially Th1-type immunity. The present results suggest that serum granulysin could be used as a marker for Th1-type immunity in pre-eclampsia.
Materials And Methods
Subjects
Peripheral blood samples were collected from 17 non-pregnant women, 50 normal pregnant women (gestational age at sampling: 28·7 ± 3·1 weeks, mean ± s.d.), seven mild pre-eclampsia (gestational age at sampling: 28·1 ± 3·7 weeks) and 14 severe pre-eclampsia (gestational age at sampling: 29·1 ± 3·6 weeks) (Table 1). Collected serum samples were stored in polypropylene tubes at −70°C.
Table 1.
Subject characteristics
| Pre-eclampsia | ||||
|---|---|---|---|---|
| Not pregnant (n = 17) | Normal pregnancy (n = 50) | mild (n = 7) | severe (n = 14) | |
| No. of primigravidas (%) | – | 26 (52·0) | 4 (57·1) | 8 (61·5) |
| Age (years) | 28·1 ± 3·7 | 28·7 ± 3·1 | 27·2 ± 3·8 | 26·7 ± 3·4 |
| Gestational age at blood sampling (weeks) | – | 34·1 ± 1·3 | 34·4 ± 3·8 | 34·3 ± 3·3 |
| Gestational age at delivery (weeks) | – | 39·3 ± 1·4 | 36·2 ± 7·0* | 34·9 ± 6·8* |
| Birth weight (g) | – | 3032 ± 290 | 2221 ± 318* | 1692 ± 438** |
| Blood pressure | ||||
| Systolic (mmHg) | 110 ± 9 | 111 ± 7 | 146 ± 9** | 179 ± 13** |
| Diastolic (mmHg) | 66 ± 8 | 63 ± 7 | 95 ± 7* | 118 ± 9** |
| Proteinuria (+ + or more) at time of blood sampling | 0 | 0 | 7** | 14** |
| Oedema | 0 | 0 | 2* | 8** |
Values are presented as means ± standard deviation.
P < 0·001
P < 0·0001 compared with the normal pregnancy.
All pre-eclamptic patients had persistently elevated blood pressure ≥140/90 mmHg in conjugation with proteinuria. Pre-eclampsia was characterized as severe because of blood pressure ≥160/110 mmHg in all patients. Non-pregnant subjects and normal pregnancy subjects were matched individually with pre-eclamptic patients of similar age and gestational age at sampling (Table 1). None had histories or showed signs of autoimmune disease. This study was approved by the human-subjects review board at Toyama Medical and Pharmaceutical University Hospital. Informed consent was obtained from all patients and subjects before sampling.
Elisa
An ELISA method for granulysin has been reported elsewhere [21]. In brief, antigranulysin monoclonal antibody (MoAb) RB1 was coated on microtitre plates at 5 µg/ml in 100 mm carbonate buffer at 40°C overnight. The plates were washed with phosphate-buffered saline (PBS) containing 0·1% Tween-20 and blocked with 10% fetal bovine serum in washing buffer (blocking buffer) at 37°C for 1–2 h. Then, the plates were reacted at room temperature with serum samples in blocking buffer for 2 h and reacted with 0·1 µg/ml of biotinylated RC8 MoAb in blocking buffer for 1 h. After washing, 0·05 U/ml of α-galactosidase-conjugated streptavidine (Roche Diagnostics, Basel, Switzerland) were reacted. After washing, the plates were finally incubated with 0·4 mm of 4-methylumbelliferyl-α-D-galactosidase (Sigma, St Louis, MO, USA) in 10 mm sodium phosphate buffer (pH 7·0) containing 0·02% bovine serum albumin, 100 mm NaCl and 1 mm MgCl2 at 37°C for 17 h. The enzyme reaction was then stopped with 100 mm glycine-NaOH (pH 10·3) and the fluorescence intensity was measured with a Cyto Fluor 4000 fluorescence multi-well plate reader (Applied Biosystems, Foster City, CA, USA) with excitation and emission wavelength of 360 nm and 460 nm, respectively. Details of the specificity of the RB1 and RC8 monoclonal antibodies for granulysin were described in our previous paper [21]. The detection limit for granulysin was 20 pg/ml.
Flow cytometric analysis staining of intracellular and surface antigens
Percentages of Th1 cells and Th2 cells, and the Th1/Th2 ratios, were determined by flow cytometry, as reported previously [12]. Briefly, cells in heparinized peripheral blood diluted 1 : 2 with RPMI-1640 medium were stimulated for 4 h with phorbol 12-myristate 13-acetate (PMA; 50 ng/ml; Sigma) and calcium ionophore A23187 (250 ng/ml; Sigma) in the presence of 10 µm brefeldin A (Sigma). Cells were stained with peridium chlorophyll protein (Per-CP)-conjugated MoAb for CD4 (Becton Dickinson, San Jose, CA, USA) and washed twice with PBS. After erythrocyte lysis by adding FACS lysing solution (Becton Dickinson) for 5 min, cells were centrifuged and supernatant was removed. FACS permeabilizing solution (Becton Dickinson) was added for 10 min, and cells were washed twice with PBS. The permeabilized cells were stained with fluorescein isothiocyanate (FITC)-labelled anti-human IFN-γ MoAb (Becton Dickinson) and phycoerythrin (PE)-labelled anti-human IL-4 MoAb (Becton Dickinson). Fluorochrome-conjugated, isotype-matched IgG1 and IgG2a were used as controls. Data from 50 000 cells were analysed with CellQuest software (Becton Dickinson). Analysis gates were set for CD4+ cells, and correlated fluorescence from PE (IL-4) and FITC (IFN-γ) were displayed. CD4+ IFN-γ+ IL-4– cells examined as Th1 cells, and CD4+IFN-γ–IL-4+ cells were examined as Th2 cells and then ratios of Th1 : Th2 cells were calculated. Cell viability was checked by confirming the activation marker CD69 on the CD4+ cells. Greater than 90% of the CD4+ T cells expressed CD69 in this study.
Statistical analysis
Values are presented as means ± s.d. Differences between pre-eclamptic patients, healthy pregnant subjects and non-pregnant women were analysed by the Mann–Whitney U-test and Fisher's Z-transformation test. A value of P < 0·05 was considered to indicate statistical significance.
Results
Serum granulysin levels in non-pregnant women, normal pregnant subjects and pre-eclamptic patients
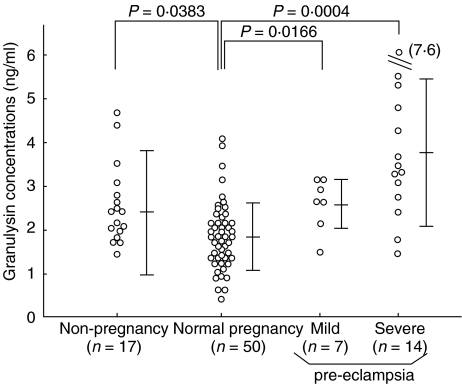
Serum concentration of granulysin in non-pregnant women, normal pregnant subjects, mild pre-eclamptic patients and severe pre-eclamptic patients were 2·3 ± 1·4 ng/ml (range 1·2–4·5); 1·9 ± 0·8 ng/ml (range 0·4–3·4); 2·6 ± 0·6 ng/ml (range 1·4–3·2); and 3·8 ± 1·8 ng/ml (range 1·4–7·6), respectively. Serum granulysin levels in normal pregnancy subjects were significantly lower than those in non-pregnant subjects (P = 0.0383; Fig. 1). Serum granulysin levels in mild pre-eclamptic patients were significantly higher than those in normal pregnancy subjects, and were elevated further in severe pre-eclamptic patients (Fig. 1).
Fig. 1.
Serum granulysin levels in non-pregnant women, normal pregnant women and mild or severe pre-eclamptic patients. Vertical bars indicate means ± s.d. of each group.
Correlation between clinical severity and serum granulysin concentrations in pre-eclamptic patients
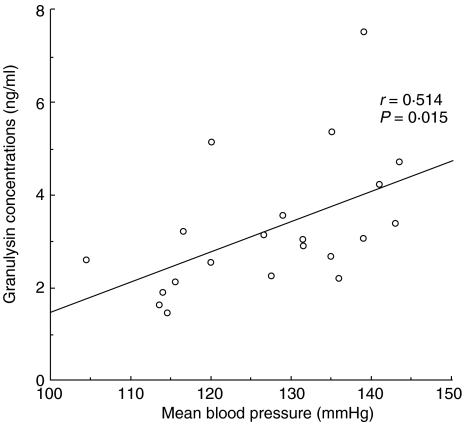
We compared the mean blood pressure and serum granulysin levels in 21 pre-eclamptic patients. A significant, positive correlation was observed between mean blood pressure and serum granulysin levels (r = 0·514, P = 0·015) (Fig. 2).
Fig. 2.
Relation between mean blood pressure and serum granulysin concentration in pre-eclamptic patients (n = 21).
Correlation between serum granulysin levels and the percentages of Th1 cells, Th2 cells, and the ratios of Th1/Th2
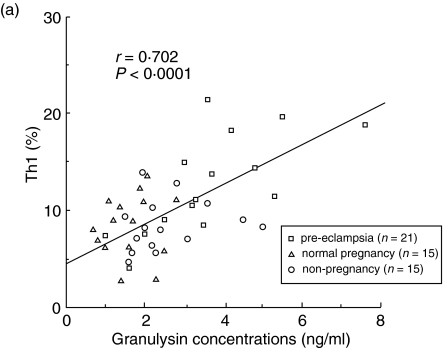
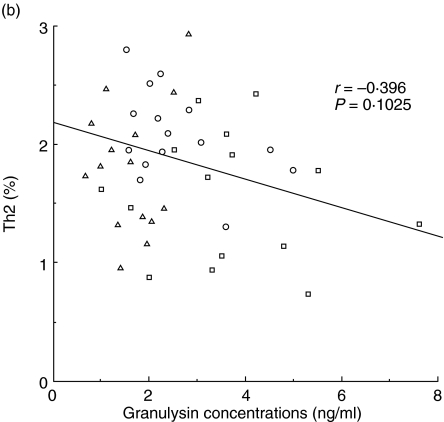
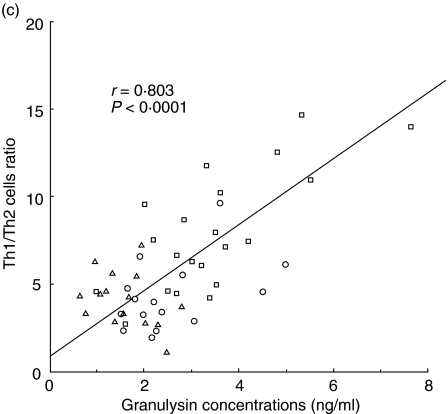
The percentages of Th1 and Th2 cells and the ratios of Th1/Th2 were analysed by flow cytometry in 15 non-pregnant women, 15 normal pregnancy subjects and 21 pre-eclamptic patients. Significant positive correlations were observed between serum granulysin levels and the percentage of Th1 cells (r = 0.702, P < 0·0001; Fig. 3a). However, there were no significant correlations between serum granulysin concentrations and the percentages of Th2 cells (Fig. 3b). Significant positive correlations were also observed between serum granulysin levels and the Th1/Th2 cell ratios (r = 0.803, P < 0.0001; Fig. 3c).
Fig. 3.
Relation between serum granulyin levels and the percentage of Th1 cells (a), percentage of Th2 cells (b) and the ratio of Th1/Th2 (c). □, pre-eclamptic patients (n = 21); ▵, normal pregnancy subjects (n = 15); ○, non-pregnant women (n = 15). The percentage of Th1 cells and the ratio of Th1/Th2 increased with the increase of the serum granulysin concentrations.
Discussion
In this study we have revealed for the first time that serum granulysin levels were elevated in pre-eclampsia, while these levels were decreased in normal pregnancy. We have reported previously that serum granulysin levels were significantly elevated during the active viral infections such as primary parvovirus B19 infections and infectious mononucleosis [21]. Granulysin is a cytolytic granule protein of NK cells, CTLs, NK T cells and γδT cells and also a novel antimicrobial protein [22–26]. It is active against a broad range of microbes, including Gram-positive and Gram-negative bacteria, Mycobacterium tuberculosis, fungi and parasites [24]. In addition, granulysin has been shown to possess tumoricidal [22, 23, 27, 28] and antiviral activity [29]. We reported that unlike granzyme B and perforin, granulysin was produced constitutively in every NK cells and a small population of T cells, and 15-kDa granulysin was detected in the supernatant of unstimulated peripheral blood mononuclear cells (PBMC) culture [21]. The serum granulysin levels among healthy individuals might reflect the spontaneous release of granulysin from NK cells, because NK cells are the major granulysin source in normal PBMC [30]. In addition to this, elevation of the serum granulysin levels might reflect the increased secretion of granulysin from activated CTL. Indeed, granulysin is expressed after T cell activation by either alloantigen, mitogen (PHA) or cytokines such as IL-2 and IL-15 [31–34], or after NK cell activation [21, 23, 32]. Of T cells, both CD4+ CTL and CD8+ CTL produce granulysin [33,35]. In pre-eclampsia both T cells and NK cells are activated [8,9]; elevated serum granulysin could be derived from these activated T cells and NK cells. Indeed, fetal erythroblasts, syncytiotrophoblast microvillous membrane vesicles and fetal DNA are increased in pre-eclampsia, suggesting that fetal cells stimulate maternal immunocompetent cells [35–37].
Pre-eclampsia, which is unique to pregnant women, is one of the most difficult complications of human pregnancy. Histological examination in the placental bed demonstrated that the immunological environment in pre-eclampsia resembles graft rejection [3–6]. An elevation of granulysin mRNA levels in transplanted patients with acute allograft rejection has been reported [36,37]. The present findings showed elevated serum granulysin levels in pre-eclampsia, especially in severe cases. These findings suggest that maternal immune cells might reject the fetus in pre-eclampsia. Th1-type immunity plays a pivotal role in acute graft rejection [11]. In this study, we first demonstrated that serum granulysin levels were well associated with the percentages of peripheral blood Th1 cells and the Th1/Th2 ratios. Recent studies suggest that normal human pregnancy is associated with a Th2 predominant profile [8, 12, 16] and pre-eclampsia is associated with a Th1 predominant profile [8, 12, 13–18]. These findings are consistent with present data that serum granulysin levels were decreased in normal pregnancy, while these levels were elevated in pre-eclampsia. Although serum granulysin levels in pre-eclampsia were the same as those in non-pregnant subjects, these levels might be problematic during pregnancy because it has been proposed that suppressed Th1 type immunity might prevent maternal rejection against the fetus [1,2]. Therefore, the process of pregnancy may include a mechanism preventing allograft rejection. Suppressed Th1-type immunity might be one of the main mechanisms that is responsible for maintaining pregnancy. Increased Th1-type immunity in pre-eclampsia compared to that in normal pregnancy might induce pregnancy disorders such as pre-eclampsia, although serum granulysin levels and Th1/Th2 ratios in pre-eclamptic cases were the same as those in non-pregnant women. Interestingly, the serum granulysin levels in pre-eclamptic patients were also well associated with mean blood pressure, a marker of severity of disease. We have reported previously that significant positive correlations were observed between mean blood pressure and concentrations of the Th1-type cytokines, IL-2 and IFN-γ produced by PBMC [8]. These findings suggest that increased Th1-type immunity occurs in women with pre-eclampsia compared to that in normal pregnancy subjects and is associated with the development of pre-eclampsia. Hayakawa et al. reported that transfer of IL-12-stimulated splenocytes into pregnant mice resulted in pre-eclampsia-like symptoms, glomerular nephritis associated with hypertension and proteinuria [38], suggesting that excessive Th1-type immunity could induce pre-eclampsia. Serum granulysin levels might be used as a clinical marker for pre-eclampsia as well as LDH, platelet counts, aspartate aminotransferase, alanine aminotransferase, antithrombin III, etc. The serum granulysin levels could be used as a reliable marker to evaluate Th1-type immunity in pre-eclampsia. Serum granulysin might be used as a predictive marker for pre-eclampsia because elevation in serum IL-2 and TNF-α is observed before the clinical manifestations of pre-eclampsia [39]. Early prophylactic treatment for pregnant women with a risk of pre-eclampsia might improve their prognosis. In addition, serum granulysin could be used as a Th1-type immunity in other diseases in which Th1-predominant immunity is present, such as rheumatoid arthritis and psoriasis. Serum cytokine levels are very low (∼pg/ml) because of their short half-life. However, serum levels of granulysin are high (2·3 ± 1·4 ng/ml in control subjects). Serum granulysin might be steady and their half-life might be longer than that in cytokines, although we did not check the half-life of serum granulysin.
Granulysin is synthesized as a 15-kDa precursor form, which is then sorted into cytolytic granules where it is processed into a 9-kDa effector form [22]. We reported that only 15-kDa granulysin was found in the sera and culture medium [21]. It is possible that the 9-kDa granulysin molecules that leaked from the intercellular space between effector and target cells may be adsorbed rapidly to membrane lipids or extracellular matrix [22,40] or degraded rapidly to avoid its bystander killing effect. It has been reported that sera in pre-eclamptic patients had an anti-proliferative effect of human umbilical vein endothelial cell (HUVEC) [41]. This endothelial dysfunction was suggested to be one of the aetiology and pathophysiology factors or signs of pre-eclampsia [42]. This preliminary study demonstrated that recombinant 15-kDa granulysin had no effect on the proliferation of HUVEC. There is no available information about the biological activities of the 15-kDa granulysin other than being a precursor protein, while the 9-kDa granulysin was reported to be multi-functional. However, serum 15-kDa granulysin levels are highly elevated in pathological conditions such as viral infections and pre-eclampsia, suggesting that 15-kDa granulysin reflects the intracellular 9-kDa granulysin in activated NK cells and CTLs. Additional studies are needed to clarify the biological function of 15-kDa granulysin.
The findings in the present study support a role for Th1-type immunity in the development of pre-eclamptic syndrome. Serum granulysin levels would be a useful and novel serum marker to evaluate the Th1/Th2 balance, especially Th1-type immunity in pre-eclampsia.
References
- 1.Medawar PB. Some immunological and endocrinological problems raised by the evolution of viviperity in vertebrates. Symp Soc Exp Biol. 1953;7:320–6. [Google Scholar]
- 2.Thellin O, Coumans B, Zarzi W, Igout A, Heinen E. Tolerance to foeto-maternal ‘graft’: ten ways to support a child for nine months. Curr Opin Immunol. 2000;12:731–7. doi: 10.1016/s0952-7915(00)00170-9. [DOI] [PubMed] [Google Scholar]
- 3.Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of pre-eclampsia. Obstet Gynecol Annu. 1972;1:177–91. [PubMed] [Google Scholar]
- 4.Kitzmiller IL, Benirschke K. Immunofluorescent study of placental bed vessels in pre-eclampsia of pregnancy. Am J Obstet Gynecol. 1973;115:248–51. doi: 10.1016/0002-9378(73)90293-7. [DOI] [PubMed] [Google Scholar]
- 5.Labarrere CA. Acute atherosis. A histopathological hallmark of immune aggression? Placenta. 1998;9:95–108. doi: 10.1016/0143-4004(88)90076-8. [DOI] [PubMed] [Google Scholar]
- 6.Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–18. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- 7.Toder V, Blank M, Gleicher N, Voljovich I, Mashiah S, Nebel L. Activity of natural killer cells in normal pregnancy and edema–proteinuria–hypertension gestosis. Am J Obstet Gynecol. 1983;145:7–10. doi: 10.1016/0002-9378(83)90332-0. [DOI] [PubMed] [Google Scholar]
- 8.Saito S, Umekage H, Sakamoto Y, et al. Increased Th1-type immunity and decreased Th2-type immunity in patients with pre-eclampsia. Am J Reprod Immunol. 1999;41:297–306. doi: 10.1111/j.1600-0897.1999.tb00442.x. [DOI] [PubMed] [Google Scholar]
- 9.Darmochwal-Kolarz D, Leszynska-Gorzelak B, Rolinski J, Oleszczuk J. The expression and concentrations of Fas/Apo-1 (CD95) antigen in patients with severe pre-eclampsia. J Reprod Immunol. 2001;49:153–64. doi: 10.1016/s0165-0378(00)00086-3. [DOI] [PubMed] [Google Scholar]
- 10.Stallmach T, Hebisch G, Orben P, Lu X. Aberrant positioning of trophoblast and lymphocytes in the feto–maternal interface with pre-eclampsia. Virchows Arch. 1999;434:207–11. doi: 10.1007/s004280050329. [DOI] [PubMed] [Google Scholar]
- 11.Mosman TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Ann Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 12.Saito S, Sakai M, Sasaki Y, Tanebe K, Tsuda H, Michimata T. Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1 : Th2 cell ratio during normal human pregnancy and pre-eclampsia. Clin Exp Immunol. 1999;117:550–5. doi: 10.1046/j.1365-2249.1999.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darmochwal-Kolarz D, Leszcynska-Gorzelark B, Robinski J, Oleszczuk J. T helper 1- and T helper 2-type cytokine imbalance in pregnant women with pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 1999;86:165–70. doi: 10.1016/s0301-2115(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 14.Ohkuchi A, Minakami H, Aoya Y, Haga T, Kimura H, Sato I. Expansion of the fraction of Th1 cells in women with pre-eclampsia: inverse correlation between the percentage of Th1 cells and the plasma level of PAI-2. Am J Reprod Immunol. 2001;46:252–9. doi: 10.1034/j.1600-0897.2001.d01-10.x. [DOI] [PubMed] [Google Scholar]
- 15.Yoneyama Y, Suzuki S, Sawa R, Yoneyama K, Power GG, Araki T. Relation between adenosine and T-helper 1/T-helper 2 imbalance in women with pre-eclampsia. Obstet Gynecol. 2002;99:641–6. doi: 10.1016/s0029-7844(02)01657-5. [DOI] [PubMed] [Google Scholar]
- 16.Sakai M, Tsuda H, Tanebe K, Sasaki Y, Saito S. Interleukin 12 secretion by peripheral blood mononuclear cells is decreased in normal pregnant subjects and increased in pre-eclamptic patients. Am J Reprod Immunol. 2002;47:91–7. doi: 10.1034/j.1600-0897.2002.1o020.x. [DOI] [PubMed] [Google Scholar]
- 17.Wilczynski JR, Tchorzewski H, Glowacka E, et al. Cytokine secretion by decidual lymphocytes in transient hypertension of pregnancy and pre-eclampsia. Med Inflamm. 2002;11:105–11. doi: 10.1080/09629350220131962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rein DT, Schondorf T, Gohring U-J, et al. Cytokine expression in peripheral blood lymphocytes indicates a switch to T helper cells in patients with pre-eclampsia. J Reprod Immunol. 2002;54:133–42. doi: 10.1016/s0165-0378(01)00128-0. [DOI] [PubMed] [Google Scholar]
- 19.Darmochwal-Kolarz D, Robinski J, Lesznska-Gorzelak B, Oleszczuk J. The expression of intracellular cytokines in the lymphocytes of pre-eclamptic patients. Am J Reprod Immunol. 2002;48:381–6. [Google Scholar]
- 20.Picker LJ, Singh MK, Zdraveski S, et al. Direct demonstration of cytokine synthesis heterogeneity among human memory-effector T cells by flow cytometry. Blood. 1995;86:1408–19. [PubMed] [Google Scholar]
- 21.Ogawa K, Takamori Y, Suzuki K, et al. Granulysin in human serum as a marker of cell-mediated immunity. Eur J Immunol. 2003;33:1925–33. doi: 10.1002/eji.200323977. [DOI] [PubMed] [Google Scholar]
- 22.Pena SV, Hanson DA, Carr BA, Goralski TJ, Krensky AM. Processing subcellular localization, and function of 519 (granulysin), a human late T cell activation molecule with homology to small, lytie, granute proteins. J. Immunol. 1997;158:2680–8. [PubMed] [Google Scholar]
- 23.Pena SV, Krensky AM. Granulysin, a new human cytolytic granule-associated protein with possible involvement in cell-mediated cytotoxicity. Sem Immunol. 1997;9:117–25. doi: 10.1006/smim.1997.0061. [DOI] [PubMed] [Google Scholar]
- 24.Stenger S, Rosat JP, Bloom BR, Krensky AM, Modlin RL. Granulysin: a lethal weapon of cytolytic T cell. Immunol Today. 1999;20:390–4. doi: 10.1016/s0167-5699(99)01449-8. [DOI] [PubMed] [Google Scholar]
- 25.Mincheva-Nilsson L, Nagaeva O, Sundqvist KG, Hammarstrom ML, Hammarstrom S, Baranov V. γδ T cells of human early pregnancy deciduas: evidence for cytotoxic potency. Int Immunol. 2000;12:585–96. doi: 10.1093/intimm/12.5.585. [DOI] [PubMed] [Google Scholar]
- 26.Gansert JL, Kiebler V, Engele M, et al. Human NKT cells express granulysin and exhibit antimycobacterial activity. J Immunol. 2003;170:3154–61. doi: 10.4049/jimmunol.170.6.3154. [DOI] [PubMed] [Google Scholar]
- 27.Gamen S, Hanson DA, Kaspar A, Naval J, Krensky AM, Anel A. Granulysin-induced apoptosis. I. Involvement of at least two distinct pathways. J Immunol. 1998;161:1758–64. [PubMed] [Google Scholar]
- 28.Wang Z, Choice E, Kaspar A, et al. Bactericidal and tumoricidal activities of synthetic peptides derived from granulysin. J Immunol. 2000;165:1486–90. doi: 10.4049/jimmunol.165.3.1486. [DOI] [PubMed] [Google Scholar]
- 29.Hata A, Zerboni L, Sommer M, et al. Granulysin blocks replication of varicella-zoster virus and triggers apoptosis of infected cells. Viral Immunol. 2001;14:125–33. doi: 10.1089/088282401750234501. [DOI] [PubMed] [Google Scholar]
- 30.Obata-Onai A, Hashimoto S, Onai N, et al. Comprehensive gene expression analysis of human NK cells and CD8+ T lymphocytes. Int Immunol. 2002;14:1085–98. doi: 10.1093/intimm/dxf086. [DOI] [PubMed] [Google Scholar]
- 31.Jongstra J, Schall TJ, Dyer BJ, et al. The isolation and sequence of a novel gene from a human functional T cell line. J Exp Med. 1987;165:601–14. doi: 10.1084/jem.165.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houchins JP, Yabe T, McSherry C, Miyokawa N, Bach FH. Isolation and characterization of NK cell and NK/T cell-specific cDNA clones. J Mol Cell Immunol. 1990;4:295–306. [PubMed] [Google Scholar]
- 33.Sun Q, Burton RL, Lucas KG. Cytokine production and cytokine mechanism of CD4+ cytotoxic T lymphocytes in ex vivo expanded therapeutic Epstein–Barr virus-specific T-cell cultures. Blood. 2002;99:3302–9. doi: 10.1182/blood.v99.9.3302. [DOI] [PubMed] [Google Scholar]
- 34.Ma LL, Spurrell JCL, Wang JF, et al. CD8 T cell-mediated killing of Cryptococcus neoformans requires granulysin and is dependent on CD4 T cells and IL-15. J Immunol. 2002;169:5787–95. doi: 10.4049/jimmunol.169.10.5787. [DOI] [PubMed] [Google Scholar]
- 35.Ochoa MT, Stenger S, Sieling PA, et al. T-cell release of graulysin contributes to host defense in leprosy. Net Med. 2001;7:174–9. doi: 10.1038/84620. [DOI] [PubMed] [Google Scholar]
- 36.Sarwal MM, Jani A, Chang S, et al. Granulysin expression is a marker for acute rejection and steroid resistance in human renal transplantation. Hum Immunol. 2001;62:21–31. doi: 10.1016/s0198-8859(00)00228-7. [DOI] [PubMed] [Google Scholar]
- 37.Soccal PM, Doyle RL, Jani A, et al. Quantification of cytotoxic T-cell gene transcripts in human lung transplantation. Transplantation. 2000;69:1923–7. doi: 10.1097/00007890-200005150-00030. [DOI] [PubMed] [Google Scholar]
- 38.Hayakawa S, Fujikawa T, Fukuoka H, et al. Murine fetal resorption and experimental pre-eclampsia are induced by both excessive Th1 and Th2 activation. J Reprod Immunol. 2000;47:121–38. doi: 10.1016/s0165-0378(00)00053-x. [DOI] [PubMed] [Google Scholar]
- 39.Hamai Y, Fujii T, Nishina H, Kozuma S, Mikami Y. Evidence for an elevation in serum interleukin-2 and tumor necrosis factor-α levels before the clinical manifestations of pre-eclampsia. Am J Reprod Immunol. 1997;38:89–93. doi: 10.1111/j.1600-0897.1997.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 40.Kaspar AA, Okada S, Kumar J, et al. A distinct pathway of cell-mediated apoptosis initiated by granulysin. J Immunol. 2001;167:350–6. doi: 10.4049/jimmunol.167.1.350. [DOI] [PubMed] [Google Scholar]
- 41.Tsukimori K, Maeda H, Shingu M, Koyanagi T, Nobunaga M, Nakano H. The possible role of endothelial cell in hypertensive disorders during pregnancy. Obstet Gynecol. 1993;80:229–33. [PubMed] [Google Scholar]
- 42.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–4. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]