Abstract
Evidence suggesting a direct role for proteinuria in the pathogenesis of renal tubulointerstitial fibrosis is accumulating. However the mechanism by which proteinuria leads to injury is unknown. In proteinuric states complement proteins are filtered through the glomerulus and could contribute to the tubular damage. The aim of this study was to investigate the role of complement activation in the progression of interstitial fibrosis. To determine whether complement activation may be responsible for the pro-fibrotic response that occurs in the tubulointerstitial compartment we stimulated primary cultures of proximal tubular epithelial cells with membrane attack complex, C5b-9. This led to increased mRNA concentrations of both collagen type IV and its intracellular chaperone, Heat Shock Protein 47 (HSP47). To determine whether this occurred in vivo Adriamycin was used to induce proteinuria in female Balb/c mice. The expression of collagen type IV and HSP47 was increased in proteinuric mice compared to control mice. In proteinuric mouse kidney, C3 was deposited at sites of tubulointerstitial injury and there was a relationship between C3 deposition and immunochemical staining for collagen type IV and HSP47. In situ hybridization suggested that the renal tubular epithelium was actively expressing HSP47 mRNA and, by implication, excess collagen. These observations support the hypothesis that complement activation on tubular epithelial cells can directly increase the pro-fibrotic process associated with tubulointerstitial damage.
Keywords: complement, proteinuria, tubulointerstitial fibrosis, renal failure, heat shock protein 47
Introduction
Many renal diseases of diverse origin disrupt the glomerular filtration barrier and cause a leak of protein into the glomerular filtrate. Our understanding of the pathophysiology of these diseases has increased but there are still limited treatment options available to prevent the progressive renal dysfunction that characterizes many them. One feature that predicts progressive disease is the presence of tubulointerstitial fibrosis on renal biopsy. Tubulointerstitial fibrosis appears to represent a common pathway in the progressive phase of many renal diseases, therefore raising the possibility that therapies directed at this pathway could be applied to a broad range of patients.
There are several strands of clinical evidence linking glomerular proteinuria, tubulointerstitial fibrosis and progressive renal impairment [1–3]. Therapeutic interventions that reduce the level of proteinuria improve the prognosis in patients [4,5]. This is supported by evidence from animal models of proteinuria [6]. This has led to the hypothesis that proteinuria is, at least in part, responsible for the damage within the tubulointerstitial compartment. However, the mechanism by which proteinuria induces tubulointerstitial damage in this setting remains unclear.
One theory is that delivery of excess filtered protein to the proximal tubular epithelial cells (PTEC) leads to cell activation and damage, the release of inflammatory and pro-fibrotic cytokines [7] into the interstitium and induces changes in epithelial cell phenotype [8]. These effects may be due an excessive amount of protein in the tubular lumen, overloading the protein re-absorbtive mechanism, with subsequent cell damage. Alternatively, there may be specific proteins present in the filtrate that damage the epithelial cells, for example transferrin or fatty acid conjugated albumin.
Another possible mediator of epithelial damage is complement activation. The complement system, part of the innate immune system, has a primary role in the removal of pathogens and acts as a link to adaptive immunity. Inappropriate or excessive activation of the complement system can result in damage to host tissues, particularly in autoimmune disease [9,10]. There is evidence to suggest that leak of complement proteins into the urine may damage PTECs [11]. The lumenal border of PTECs activates complement and possesses a relative paucity of complement inhibitors [12]. Human biopsy studies have shown complement activation products deposited in areas of tubulointerstitial damage [13]. More recent studies using complement inhibitors in proteinuric rats showed a reduction in tubulointerstitial injury [14–16]. Similarly rats deficient in C6 are protected from progressive renal injury in the remnant kidney model [17].
Excessive deposition of collagen typifies the tubulointerstitial damage associated with proteinuria. The abnormal quantities of collagen could be derived from native epithelial cells [18], from expansion of fibroblast numbers within the interstitium [19] or a combination of both. Heat shock protein 47 (HSP47) is an intracellular collagen chaperone involved in the assembly of the trimeric collagen structure [20]. Expression of HSP47 is increased in pathological conditions where increased fibrosis is seen, including nonimmunological renal disorders [21,22]. In this study we have investigated whether complement activation has the capacity to increase PTEC collagen IV and HSP47 gene expression and therefore potentially accelerate the onset of tubulointerstial fibrosis. This relevance of these in vitro observations to progressive proteinuria-related tubulointerstitial nephritis was then assessed in a mouse model of proteinuria induced by the injection of Adriamycin.
Materials and Methods
Animals
Female Balb/c mice, aged 6 weeks, weighting 18–20 g, were purchased from Harlan UK Ltd, Bicester UK. All animal procedures were performed in accordance with UK Home Office regulations. General chemicals were purchased from Sigma Chemical Co. (Poole, UK).
Complement-mediated stimulation of mouse tubular epithelial cells
Mouse PTECs were isolated as previously described [23]. Briefly Cortex from normal mouse kidney was collagenase digested (collagenase type II, Worthington Biochemical Corporation, NJ, USA) for 10 min at 37°C and vortexed (repeated twice) and passed through a series of graded sieves. The material collected on the 40 µm sieve was plated onto collagen coated plates in DMEM-F12 supplemented with 2% FCS, 100 U/ml penicillin, 100 µg/ml streptomycin, 5 µg/ml insulin, 5 µg/ml transferrin, 5 ng/ml selenium, 5 × 10−12m triiodtheronine and 40 ng/ml hydrocortisone. Cells were characterized by morphology, the presence of brush border alkaline phosphatase and the presence of microvilli. Cells were grown on collagen coated 6 well plates and used before the first passage.
Isolated human complement components C5b-6 to C9 (Calbiochem, Germany) were used to assemble C5b-9 according to suppliers recommended protocol. The capacity of this complex to lyse erythrocytes was confirmed prior to use. A sublytic concentration, as confirmed by LDH release assay (data not shown), of preassembled C5b-9 was added to the mPTEC and incubated for 30 min, 60min and 240 min at 37°C (C7 1 µg, C8 2 µg, C9 1 µg, C5b-6 2 µg). Cells were lysed and extracted mRNA analysed by semiquantitative RT-PCR.
Analysis of Col IV and HSP47 gene expression by semiquantitative PCR
Messenger RNA was extracted by standard phenol-chloroform methods from cultured epithelial cells or renal cortex and reverse transcribed. Semi-quantitative RT-PCR, using sequence specific primers for Col IV and HSP47 (Table 1) was performed to determine whether gene expression was increased. β-actin was amplified as a control. The PCR products were separated on 1% agarose gels containing ethidium bromide, the gels were photographed and the band intensity measured. The ratio of specific gene PCR product to β-actin PCR product was used as a measure of specific gene expression.
Table 1.
PCR primer sequences used for semiquantitative PCR analysis
| Primer 1 | Primer 2 | Size (bp) | |
|---|---|---|---|
| Col IV | 5′-ATGAGTCCTGAAACATCATG-3′ | 5′-ACAGCTTGCCACAGAATATC-3′ | 320 |
| HSP47 | 5′-ATCAACTTCCGAGACAAGCG-3′ | 5′-TCTGATTATCTCGCACCAGG-3′ | 708 |
| β-actin | 5′GAGCAAGAGAGGTATCCTGACC-3′ | 5′-GGATGCCACAGGATTCCATACC-3′ | 646 |
Induction of adriamycin nephropathy
Six week old female Balb/c mice (n = 8) were given a single intravenous injection of adriamycin, 12 mg per kg body weight (Pharmacia Upjohn, UK). Control mice (n = 8) were injected with the equivalent volume of normal saline. Mice were housed in metabolic cages for 24 h prior to injection, 4 days after injection and at weekly intervals thereafter for urine collection. Serum samples were taken prior to injection, 14 days after injection and at the time of sacrifice 6 weeks after injection. At sacrifice the kidneys were harvested for histological analysis.
Functional assessment of injury
Urinary albumin concentration was measured by ELISA. Briefly, 96 wells plates were coated with 5 µg/ml goat anti-mouse albumin (Nordic Immunological Laboratories, Tilburg, Netherlands) in carbonate buffer, pH 9·6 overnight at 4°C. After blocking with 2% bovine serum albumin at 37°C for 2 h samples or control dilutions of mouse albumin (Sigma) in blocking buffer were added to the plates for 2 h at 37°C. A secondary horseradish peroxidase conjugated goat anti-mouse albumin (Nordic) in blocking buffer was added for 2 h at 37°C. Absorbance was measured after incubation with O-Phenylene diamine. Serum urea and albumin concentrations were measured using Sigma kits according to manufacturer's protocols.
Histological assessment of injury
Kidneys were removed, fixed in formalin and embedded in paraffin. Paraffin sections (2 µm thick) were stained with PAS for histological analysis. Cryostat sections were fixed with acetone and stained. The antibodies used: for C3 staining were rabbit anti-human C3d (Dako Ltd, UK) and horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG; and for collagen type IV staining, rabbit anti-mouse collagen type IV (Chemicon Int., CA, USA) and HRP-conjugated swine anti-rabbit IgG. For HSP47 staining, biotin-conjugated mouse anti-mouse HSP47 (Stressgen, Victoria Canada) and avidin-biotin-HRP complex (Dako) were used. Staining was detected with diaminobenzidine (DAB). Sections were counterstained with methyl green and mounted.
Localization of HSP47 gene expression by in situ hybridization
A 39-base antisense oligonucleotide probe, corresponding to base 248–286 of mouse HSP47 mRNA, was used for in situ hybridization. The probe was labelled using digoxigenin (DIG) oligonucleotide tailing kit according to the manufacturer's instructions (Boehringer Mannheim, Lewes, UK). In situ hybridization was performed according to a modified method as described previously [24]. 4 µm frozen sections were fixed with 4% paraformaldehyde in phosphate-buffered saline. The sections were deproteinized by using HCl and proteinase K (Sigma), prehybridized and then hybridized with DIG-labelled oligonucleotide probe in prehybridization buffer at 37°C overnight. After washing with 2 × standard saline citrate the DIG-labelled probe was visualized using HRP-conjugated sheep polyclonal anti-DIG antibody (Boehringer Mannheim, Lewes, UK) and DAB. Control studies with a sense probe and competitive binding studies confirmed specificity.
Statistical analysis
Results were represented as mean ± SD. Differences between groups were analysed using one way analysis of variance with Bonferroni's test. P-values < 0·05 were considered statistically significant.
Results
In vitro effect of C5b-9 on PTEC Col IV and HSP47 gene expression
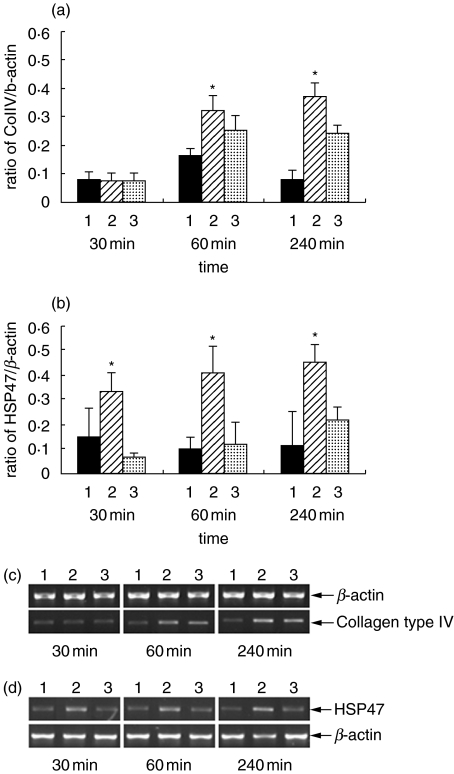
Primary cultures of mouse PTEC were incubated with preassembled C5b-9 for various time periods up to 4 h. Col IV and HSP47 gene expression was analysed by semiquantitative RT-PCR. Incubation of PTEC with C5b-9 significantly increased the expression of Col IV mRNA after 60 min in comparison to unstimulated cells (Fig. 1a,c). In addition within the first 30 min a two to threefold increase in HSP47 mRNA was observed when compared to unstimulated PTEC (Fig. 1b,d). There was only a small increase in quantity of Col IV and HSP47 mRNA between 1 and 4 h suggesting a rapid response to stimulation with C5b-9. When the PTEC were incubated with C5b6, C8 and C9 in the absence of C7 an increase in Col IV gene expression was seen. This cannot be explained by insertion of a membrane-spanning complex and raises the possibility of a direct effect of individual complement components.
Fig. 1.
The effect of C5b-9 on PTEC gene expression of Col IV (a, c) and HSP47 (b, d). Detection of Col IV mRNA and HSP47 mRNA by semiquantitative RT-PCR is shown. Mouse PTEC were incubated in the medium alone (lane 1), C5b-9 (lane 2) and C5b6C8C9 (lane 3) for the times indicated. Increase in Col IV mRNA was observed from 60 min after incubation with C5b-9 (a, c). Incubation of C5b-9 on PTEC increased the HSP47 mRNA after 30 min (b, d). *P < 0·01 compared to medium alone, n = 4 for all experiments, c and d are representative gels.
Proteinuria-induced alterations in Col IV and HSP47 gene expression
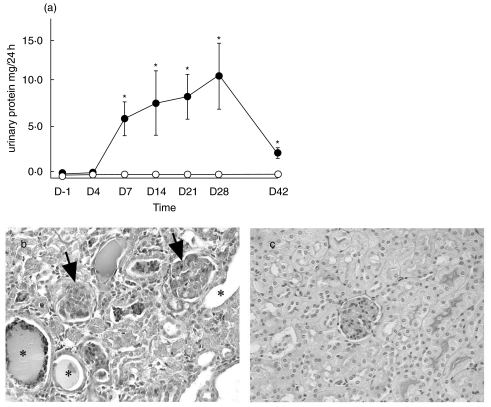
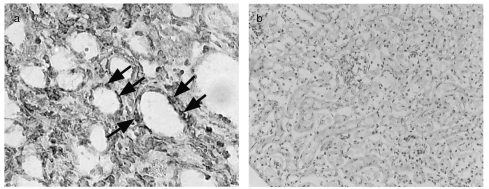
All mice remained healthy throughout the 6 week experimental period. All mice injected with adriamycin developed significant levels of albuminuria 7 days after injection and this continued throughout the 6-week period, peaking 28 days after injection (Fig. 2a). Control, saline-injected mice had persistently low levels of albuminuria. There was a corresponding reduction in serum albumin in the adriamycin-treated group, evident by day 14 after injection (Table 2). Renal function, as assessed by serum urea, declined in the adriamycin-treated group with approximately a 2-fold increase in serum urea at day 14. Glomeruli in the adriamycin-treated group showed expansion of the mesangial matrix and focal sclerosis. Evidence of tubulointerstitial damage included tubular dilatation, cast formation and tubular epithelial cell flattening (Fig. 2b,c). These changes were focal, with some areas showing severe injury and others showing preservation of the tubulointerstitial compartment.
Fig. 2.
(a) Albuminuria in adriamycin-treated mice. Twenty-four-hour albumin excretion, measured by ELISA, was significantly increased 7 days after disease induction and persisted for 6 weeks after injection in adriamycin-treated mice (n = 8). Values are expressed as means ± SD. *P < 0·01 compared to control mice. • adriamycin-treated group; ○ control group. (b) Renal histology. PAS stained section of mice sacrificed 6 weeks after adriamycin injection. Glomeruli of adriamycin-treated mice showed mesangial matrix expansion and glomerular sclerosis (arrows) and tubular dilatation and cast formation (*). Renal morphology of saline-injected, control mice is shown for comparison (c). Magnification (b, c × 200).
Table 2.
Functional analysis of renal injury after adriamycin injection. Serum albumin concentration was reduced 14 days after injection of adriamycin and this persisted to day 42. Serum albumin remained unchanged in saline-injected mice. Serum urea increased by approximately 2 fold at day 42 in adriamycin group in comparison with at day −1.
| Day −1 | Day 14 | Day 42 | |
|---|---|---|---|
| Serum albumin (g/dl) | |||
| Adriamycin-treated | 3·5 ± 0·2 | 2·4 ± 0·4*† | 2·9 ± 0·6*† |
| Saline-treated | 3·4 ± 0·7 | 3·2 ± 0·4 | 3·3 ± 0·9 |
| Serum urea (mmol/l) | |||
| Adriamycin-treated | 7·8 ± 0·5 | 11·5 ± 0·9† | 18·3 ± 2·8*† |
| Saline-treated | 6·3 ± 1·2 | 7·7 ± 0·9 | 9·8 ± 0·7 |
P < 0·01 versus day −1 in adriamycin mice
P < 0·01 versus control mice
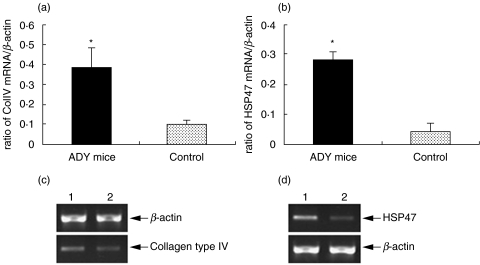
Semi-quantitative RT-PCR was used to assess the level of Col IV and HSP47 gene expression in renal cortex 6 weeks after adriamycin injection. A significant increase in both Col IV and HSP47 PCR products were seen, in comparison to β-actin as a constitutively expressed control (Fig. 3).
Fig. 3.
Semi-quantitative RT-PCR of mRNA extracted from the cortex of adriamycin and saline-treated mice 6 weeks after disease induction. There was a significant increase in the ratio of the PCR product for both Col IV (a) and HSP47 (b) compared to β-actin (*P < 0·01 for both, n = 8). (c, d) show PCR gels demonstrating greater intensity in the Col IV (c) and HSP47 (d) PCR products from cortical mRNA derived from adriamycin-treated mice (lane 1) and control mice (lane 2).
Localization of Col IV and HSP47 protein and gene expression in diseased kidney
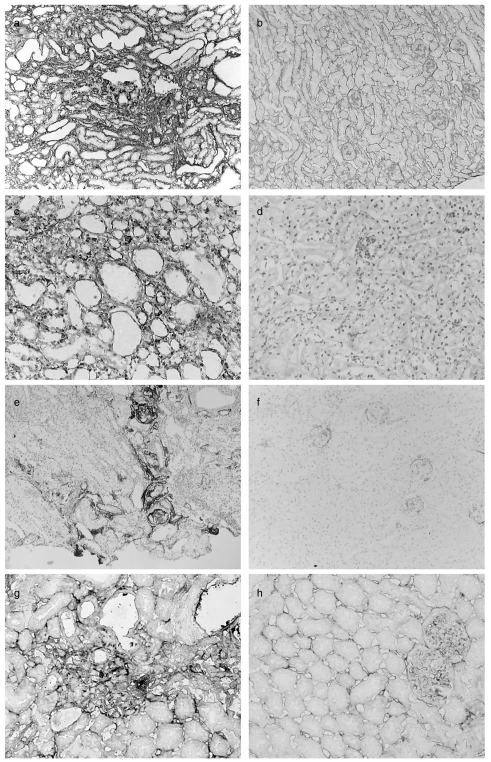
In areas of tubulointerstitial damage in the adriamycin-treated mice there was increased deposition of Col IV (Fig. 4a,b) with the strongest staining seen in areas of most severe damage. Immunochemical staining for HSP47 followed a similar pattern with a generalized increase in the intensity of staining in the adriamycin-treated group, but most evident at sites of tubulointerstitial damage (Fig. 4c,d). C3 (Fig. 4e,f) and C9 (Fig. 4g,h) deposition was also observed in the areas of tubulointerstitial damage in the adriamycin-treated mice, most evident at the site of severe tubular damage, similar to that for Col IV and HSP47.
Fig. 4.
Immunohistochemical analysis of kidneys adriamycin-treated mice (a, c, e, g) and control mice (b, d, f, h). There is increased staining for Col IV diffusely throughout the cortex in a peritubular distribution, but particularly in areas of more severe damage (a). Staining of tissue from saline-treated mice is shown as control (b). Staining for HSP47 is increased throughout the cortex of adriamycin-treated mice (c) compared to saline-treated mice (d). C3 (e) and C9 (g) deposition was seen in areas of most severe damage in adriamycin-treated mice. In control mice, very weak staining for C3 was observed only in the glomeruli. Magnification (a, b × 150; c-f × 200; g, h × 250).
In situ hybridization, to localize the site of gene transcription in the diseased kidney, showed that HSP47 mRNA was present predominantly in tubular epithelial cells. The intensity of staining was greatly increased in the adriamycin-treated compared to saline-treated mice (Fig. 5). mRNA for HSP47 could be seen in damaged areas and corresponded with immunochemical localization of the respective protein.
Fig. 5.
In situ hybridization for HSP47 mRNA in renal cortex. There is a generalized increase in the staining for HSP47 in tissue derived from the adriamycin-treated (a) compared to the saline-treated mice (b). Staining for HSP47 mRNA was seen in tubular epithelial cells (arrows). Magnification (a × 250; b × 200).
Discussion
Progressive damage to the tubulointerstitial compartment, which typifies many renal diseases, is likely to be mediated by several mechanisms. Haemodynamic changes, tubular protein overload and specific tubular toxins are all possible candidates and are not mutually exclusive. Blood pressure control, specifically with inhibitors of the renin-angiotensin axis, represents the mainstay of treatment to prevent progressive injury. The development of further interventions is limited by an incomplete understanding of the mechanisms that finally lead to injury.
Fibrosis within the tubulointerstitial compartment is a prominent feature of progressive disease. After the induction of proteinuria by adriamycin injection we demonstrated increased renal Col IV gene expression and accumulation of Col IV within the tubulointerstitial compartment. Col IV was most abundant in areas of severe tubular damage, but also present in areas that were otherwise morphologically normal. This would suggest that increased deposition of Col IV is an early event in the generation of tubulointerstitial fibrosis. Proteinuric, adriamycin-treated mice also had both increased levels of HSP47 mRNA and protein within their kidneys. This reflects increased pro-fibrotic activity within the tubulointerstitial compartment and consequent fibrosis. HSP47 and Col IV staining followed a similar distribution. It is likely therefore that HSP47 is involved in the deposition of excess collagen.
The increased Col IV seen in the tubulointerstitium of adriamycin-treated mice has 2 possible sources, epithelial and interstitial cells. In situ hybridization demonstrated HSP47 mRNA predominantly within tubular epithelial cells. Assuming that HSP47 is required for collagen synthesis this would suggest that the epithelium is a major source of new collagen synthesis.
We have shown that, in mice with adriamycin-induced proteinuria, complement C3 is deposited in areas of tubular damage. Although this does not confirm that complement activation directly contributes to the renal injury in this model it supports previous studies that demonstrate deposition of C3, the main component of the complement activation cascade, at the sites where injury is occurring.
Could the deposition of complement on the epithelial cells cause the increased levels of HSP47 and deposition of collagen? Previous studies have shown that the formation of C5b-9 (membrane attack complex) on the surface of PTECs can induce the production of proinflammatory cytokines (interleukin-6 and tumour necrosis factor-α) and matrix protein, fibronectin [25,26]. In this study we have demonstrated that C5b-9 can also increase the mRNA levels of HSP47 and Col IV, suggesting that complement activation may directly stimulate collagen production from epithelial cells. These studies do not exclude the production of biologically active intermediates, such as growth factors, by complement stimulated epithelial cells acting in an auto/paracrine manner. Whatever the mechanism the overall biological effect of complement activation is to induce pro-fibrotic phenotype in PTECs. We do predict that the use of human complement as a source of C5b-9 would affect mouse cells in any way other than the formation of membrane pores. However, C5b-9 and its precursor C5b-8 can bind the complement inhibitor CD59, a GPI linked protein that has the capacity to signal into cells. CD59 is extensively distributed in throughout the renal interstitium and is present on cultured PTEC (data not shown). We cannot exclude that CD59-mediated signalling is in part responsible for the effect observed.
Though much remains to be determined this study provides an insight into one potential mechanism of tubulointerstitial fibrosis. Complement does reach the tubular epithelium, presumably in the glomerular filtrate, but is also secreted by the epithelial cells. Once in the tubular lumen complement can be activated. In the adriamycin model of proteinuria complement activation is occurring at a time when mRNA and protein levels of both HSP47 and Col IV are increasing. Although we have not shown that these 2 observations are causally linked in vivo, in vitro complement stimulation of PTECs can increase mRNA levels for both HSP47 and Col IV.
Tubulointerstitial fibrosis certainly has a complex pathogenesis. This study describes one possible pathway. In reality complement is likely to activate several pathways and HSP47 expression be influenced by many factors. Only with a clear understanding of the relative role of each component and the hierarchy of events that lead to fibrosis can targeted interventions be planned. We suggest that both complement and HSP47 represent possible targets.
References
- 1.Ruggenenti P, Perna A, Mosconi L, et al. Urinary protein excretion rate is the best independent predictor of ESRF in non-diabetic proteinuric chronic nephropathies. Kidney Int. 1998;53:1209–16. doi: 10.1046/j.1523-1755.1998.00874.x. [DOI] [PubMed] [Google Scholar]
- 2.Cameron JS, Turner DR, Ogg CS, et al. The long term prognosis of patients with focal segmental glomerulosclerosis. Clin Nephrol. 1978;10:213–8. [PubMed] [Google Scholar]
- 3.D’Amico G, Minetti L, Ponticelli C, et al. Prognostic indicators in idiopathic IgA mesangial nephropathy. Q J Med. 1986;228:363–78. [PubMed] [Google Scholar]
- 4.Ponticelli C, Zucchelli P, Passerini P, et al. A 10-year follow-up of a randomised study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney Int. 1996;48:1600–4. doi: 10.1038/ki.1995.453. [DOI] [PubMed] [Google Scholar]
- 5.The GISEN Group. Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet. 1997;349:1857–63. [PubMed] [Google Scholar]
- 6.Anderson S, Rennke HG, Brenner BM. Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in the rat. J Clin Invest. 1986;77:1993–2000. doi: 10.1172/JCI112528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eddy AA, Giachelli CM. Renal expression of genes that promote interstitial inflammation and fibrosis in rats with protein-overload proteinuria. Kidney Int. 1995;47:1546–57. doi: 10.1038/ki.1995.218. [DOI] [PubMed] [Google Scholar]
- 8.Okada H, Danoff TM, Kalluri R, Neilson EG. Early role of Fsp1 in epithelial-mesenchymal transformation. Am J Physiol. 1997;42:F563–F574. doi: 10.1152/ajprenal.1997.273.4.F563. [DOI] [PubMed] [Google Scholar]
- 9.Cybulsky AV, Rennke HG, Feintzeig ID, Salant DJ. Complement-induced glomerular epithelial cell injury. J Clin Invest. 1986;77:1096–107. doi: 10.1172/JCI112408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheerin NS, Springall T, Carroll MC, et al. Protection against anti-glomerular basement membrane (GBM)-mediated nephriitis in C3- and C4-deficient mice. Clin Exp Immunol. 1997;110:403–9. doi: 10.1046/j.1365-2249.1997.4261438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogradowski JL, Herbert LA, Sedmak K, et al. Measurement of C5b-9 in urine in patients with the nephrotic syndrome. Kidney Int. 1991;40:1141–7. doi: 10.1038/ki.1991.326. [DOI] [PubMed] [Google Scholar]
- 12.Camussi G, Tetta C, Mazzucco G, Vercellone A. The brush border of proximal tubules of normal human kidney activates the alternative pathway of the complement system in vitro. Ann NY Acad Sci. 1983;420:321–4. doi: 10.1111/j.1749-6632.1983.tb22219.x. [DOI] [PubMed] [Google Scholar]
- 13.Mosolits S, Magyarlaki T, Nagy J. Membrane attack complex and membrane cofactor protein are related to tubulointerstitial inflammation in various human glomerulopathies. Nephron. 1997;75:179–87. doi: 10.1159/000189529. [DOI] [PubMed] [Google Scholar]
- 14.Hori Y, Yamada K, Hanafusa N, et al. Crry, a complement regulatory protein, modulates renal interstitial disease induced by proteinuria. Kidney Int. 1999;56:2096–106. doi: 10.1046/j.1523-1755.1999.00765.x. [DOI] [PubMed] [Google Scholar]
- 15.Morita Y, Nomura A, Yuzawa Y, et al. The role of complement in the pathogenesis of tubulointerstitial lesions in rat mesangial proliferative glomerulonephritis. J Am Soc Nephrol. 1997;8:1363–72. doi: 10.1681/ASN.V891363. [DOI] [PubMed] [Google Scholar]
- 16.Nomura A, Morita Y, Maruyama S, et al. Role of complement in acute tubulointerstitial injury of rats with aminonucleoside nephrosis. Am J Pathol. 1997;151:539–47. [PMC free article] [PubMed] [Google Scholar]
- 17.Nangaku M, Pippin J, Couser WG. C6 mediates chronic progression of tubulointerstitial damage in rats with remnant kidneys. J Am Soc Nephrol. 2002;13:928–36. doi: 10.1681/ASN.V134928. [DOI] [PubMed] [Google Scholar]
- 18.Kuncio GS, Alvarez R, Li S, et al. Transforming growth factor-beta modulation of the alpha 1 (IV) collagen gene in murine proximal tubular cells. Am J Physiol. 1996;271:F120–5. doi: 10.1152/ajprenal.1996.271.1.F120. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez RJ, Sun MJ, Haverty TP, et al. Biosynthetic and proliferative characteristics of tubulointerstitial fibroblasts probed with paracrine cytokines. Kidney Int. 1992;41:14–23. doi: 10.1038/ki.1992.3. [DOI] [PubMed] [Google Scholar]
- 20.Tasab M, Batten MR, Bulleid NJ. Hsp47: a molecular chaperone that interacts with and stabilizes correctly-folded procollagen. EMBO J. 2000;19:2204–11. doi: 10.1093/emboj/19.10.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng M, Razzaque MS, Nazneen A, Taguchi T. Expression of the heat shock protein 47 in gentamicin-treated rat kidneys. Int J Exp Path. 1998;79:125–32. doi: 10.1046/j.1365-2613.1998.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moriyama T, Kawada N, Ando A, et al. Up-regulation of HSP47 in the mouse kidneys with unilateral ureteral obstruction. Kidney Int. 1998;54:110–9. doi: 10.1046/j.1523-1755.1998.00964.x. [DOI] [PubMed] [Google Scholar]
- 23.Springall T, Sheerin NS, Abe K, et al. Epithelial secretion of C3 promotes colonizatiobn of the upper urinary tract by Escherichia coli. Nature Med. 2001;7:801–6. doi: 10.1038/89923. [DOI] [PubMed] [Google Scholar]
- 24.Pratt JR, Abe K, Miyazaki M, et al. In situ localization of C3 synthesis in experimental acute renal allograft rejection. Am J Pathol. 2000;157:825–31. doi: 10.1016/S0002-9440(10)64596-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montinaro V, Lopez A, Bulla R, et al. Sub-lytic terminal complement complex (TCC) stimulates renal proximal tubular epithelial cells (PTEC) to express adhesion molecules and to excrete pro-inflammatory and fibrogenic mediators. Mol Immunol. 1998;35:385. [Google Scholar]
- 26.Bürger A, Wanger C, Hug F, Hänsch GM. Up-regulation of intracellular calcium, cyclic adenosine monophosphate and fibroection synthesis in tubular epithelial cells by complement. Eur J Immunol. 1999;29:1188–93. doi: 10.1002/(SICI)1521-4141(199904)29:04<1188::AID-IMMU1188>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]