Abstract
Down-regulation of receptors involved in the recognition or transmission of inflammatory signals and a reduced responsiveness support the concept that macrophages are ‘desensitized’ during their differentiation in the intestinal mucosa. During inflammatory bowel disease (IBD) intestinal macrophages (IMACs) change to a reactive or ‘aggressive’ type. After having established a method of isolation and purification of IMACs, message for cathepsin D was one of the mRNAs we found to be up-regulated in a subtractive hybridization of Crohn's disease (CD) macrophages versus IMACs from control mucosa. The expression of cathepsin D in intestinal mucosa was analysed by immunohistochemistry in biopsies from IBD and control patients and in a mouse model of dextran sulphate sodium (DSS)-induced acute and chronic colitis. IMACs were isolated and purified from normal and inflamed mucosa by immunomagnetic beads armed with a CD33 antibody. RT-PCR was performed for cathepsin D mRNA. Results were confirmed by Northern blot and flow cytometrical analysis. Immunohistochemistry revealed a significant increase in the cathepsin D protein expression in inflamed intestinal mucosa from IBD patients compared to non-inflamed mucosa. No cathepsin D polymerase chain reaction (PCR) product could be obtained with mRNA from CD33-positive IMACs from normal mucosa. Reverse transcription (RT)-PCR showed an induction of mRNA for cathepsin D in purified IMACs from IBD patients. Northern blot and flow cytometry analysis confirmed these results. Cathepsin D protein was also found in intestinal mucosa in acute and chronic DSS-colitis but was absent in normal mucosa. This study shows that expression of cathepsin D is induced in inflammation-associated IMACs. The presence of cathepsin D might contribute to the mucosal damage in IBD.
Keywords: cathepsin D, inflammatory bowel disease, macrophages
Introduction
Over the past 10 years, it has become evident that immunosurveillance and immune defence is a concerted performance by a vast number of mucosal cell-types in the intestine. It is clear that antigen processing and antigen presentation must be well regulated, especially at the border between self and the multitude of bacteria in the gut lumen.
Despite an intact epithelial barrier the mucosa may be exposed to a low but constant level of bacterial antigens. Down-regulation of surface receptors involved in the recognition or transmission of inflammatory signals contributes to mucosal tolerance. On intestinal macrophages (IMACs) this refers to CD14 [1,2], toll-like receptors (TLRs)2 and 4 [3], HLA-DR [4], the integrins CD11b/CD18 and CD11c/CD18, which are discussed as alternative binding proteins for Gram-negative bacteria [5,6] as well as for the T cell co-stimulatory molecules CD80 (B7-1) and CD86 (B7-2) [4] indicating a low ability of IMACs to mediate immune responses. Functional data, e.g. a diminished oxidative burst activity [7–10] support the concept that IMACs are ‘desensitized’ during differentiation in the intestinal mucosa. However, mucosal inflammation changes the situation for IMACs. The inflamed mucosa is populated by IMACs that are fully responsive to bacterial antigens [3]. Furthermore, the expression of T cell co-stimulatory molecules CD80 and CD86 indicates that IMACs in inflamed mucosa have the ability to mediate immune responses [4,11]. In addition, activation of NF-kappaB during human mucosal inflammation indicates an involvement in the inflammatory process [12].
Several cathepsins have been shown to be implicated in antigen processing in antigen presenting cells (APCs) [13–15]. Cathepsin D plays a key role during antigen presentation by contributing to lysosomal proteolysis and degradation of material into smaller fragments [16]. In cathepsin D-deficient mice, which die around postnatal day 25 from intestinal necrosis, a lack of proteolysis of proteins that regulate cell growth and tissue homeostasis was suggested [17]. Vesicles with cathepsin D-digested peptide fragments merge with vesicles containing newly synthesized major histocompatibility complex (MHC) class II molecules. MHC class II-molecules bind peptides derived from internalized degraded material and are transported subsequently to the cell surface where they present these peptides to T cells. IMACs from patients with inflammatory bowel disease show expression of both MHC class I and II antigens, thus allowing the presentation of antigens to mucosal T cells. However, the necessity of cathepsin D and other cathepsins for antigen presentation is still under discussion [18].
In addition to its role during antigen presentation cathepsin D is capable of digesting extracellular matrix proteins [19]. Similar to the intracellularly located cathepsin D, the secreted enzyme also shows a proteolytic potential in the extracellular space. Cathepsin D has the potential to initiate a proteolytic cascade, to degrade and remodel extracellular matrix resulting in the release of fibroblast growth factor (FGF) and FGF-similiar growth factors associated with the matrix proteins [20].
Because proteases can alter extracellular matrices and basement membranes [20–22] cathepsin D was also suggested to be involved in the course of severe diseases. The usefulness of cathepsin D levels as a prognostic factor during disease course, however, is controversial. It has been postulated that cathepsin D levels should be monitored as a prognostic indicator in breast cancer [19]. Abnormal cathepsin D immunostaining patterns can be used to predict a potential for lymphatic invasion in colorectal carcinoma [23]. Cathepsin D protein was also found by enzyme-linked immunosorbent assay (ELISA) in biopsy specimens from patients with normal colon and colonic disease but a lack in cathepsin D protein expression was found in inflamed specimens [24].
The role of cathepsin D in antigen presentation and degradation of extracellular matrices may be of great importance for immune reactions in the intestinal mucosa. Moreover, cathepsin D was one of the mRNAs we found to be up-regulated in a subtractive hybridization of CD macrophages versus IMACs from control mucosa [8]. This led us to investigate cathepsin D expression in normal and inflamed intestinal mucosa.
Materials and Methods
Animal model
Eighteen female Balb/c mice weighing 20–22 g (Charles River, Germany) were used for the experiments. Except for the periods of induction of colitis they had food and water ad libidum. The animal studies were approved by the local Institutional Review Board.
Induction of acute and chronic colitis
For the induction of acute colitis, six mice received 5% dextran sulphate sodium (DSS) in drinking water for 7 days. For the induction of chronic colitis, six mice received four cycles of DSS treatment, as described [25,26]. Each cycle consisted of 5% DSS in drinking water for 7 days, followed by a 10-day interval with normal drinking water. Mice used for experiments were age-matched and had received DSS treatment simultaneously.
Patients
Endoscopic biopsies or surgical specimens were taken either from healthy areas of the colonic mucosa of patients undergoing surveillance colonoscopy or surgery for colorectal carcinoma (control patients, non-inflamed (NI) patients) or from the inflamed colonic mucosa of patients with Crohn's disease (CD) or ulcerative colitis (UC). Histology was performed on additional biopsies or on the normal mucosa of surgical specimens by a pathologist. Inflammatory bowel disease (IBD) patients were treated with 5-ASA, or steroids, or both. One patient with CD was treated with azathioprin. The different therapies had no apparent influence on the results of our assays. The study was approved by the University of Regensburg Ethics Committee.
Assessment of an endoscopic inflammation score in colitis
The degree of inflammation at the site where specimens were taken by biopsy was graded endoscopically [12]: –, no inflammation: +, low degree of inflammation (increased granularity and friability of the mucosa in ulcerative colitis (UC) and single small aphthous lesions in CD); + +, moderate inflammation (mucous membranes, spontaneous bleeding and small ulcers in UC, multiple aphtous lesions and small ulcers in CD) and +++, severe inflammation (large ulcers in UC and large ulcerous lesions in CD).
Twenty-four samples from colon without detectable inflammation in terms of the described endoscopic score were included in the study as non-inflamed. Twenty-five CD and four UC samples with endoscopic scores of ++and +++ were used. Specimens from patients with UC were taken from descending colon or sigmoid colon, specimens from patients with CD were taken from colon or sigmoid colon.
Assessment of histological score in mice
For the assessment of the histological score 1 cm of the distal third of the colon was removed and scored as described [27]. Three sections obtained each at 100 µm distance were evaluated. Mice were scored individually. Each score represents the mean of three sections. The histological examination was performed blinded.
Histology was scored as follows:
epithelium (E), 0: normal morphology; 1: loss of globlet cells, 2: loss of globlet cells in large areas; 3: loss of crypts; 4: loss of crypts in large areas.
Infiltration (I), 0: no infiltrate; 1: infiltrate around crypt basis; 2: infiltrate reaching to L. muscularis mucosae; 3: extensive infiltration reaching the L. muscularis mucosae and thickening of the mucosa with abundant oedema; 4: infiltration of the submucosa.
The total histological score represents the sum of the epithelium and infiltration score (total score = E + I).
Six control mice with a histological score of 0–1 were included in the study as non-inflamed. Six acute and six chronic colitis mice were included with a histological score of 4–8.
Antibodies
For the identification of cathepsin D in human mucosa rabbit antihuman cathepsin D (A0561, IgG, DAKO, Hamburg, Germany) and biotin-conjugated goat antirabbit secondary antibody (E0432, Dako) were used (isotype control: rabbit immunoglobulin fraction, X0903, Dako).
Monoclonal mouse antihuman macrophage CD68 (M0814, clone KP1, IgG1 κ, Dako) and biotin-conjugated rabbit antimouse IgG secondary antibody (Jackson Immunoresearch, Hamburg, Germany) were applied for the identification of human IMACs (isotype control: mouse IgG1 κ, M-5284, Sigma, Taufkirchen, Germany).
For isolation of human IMACs monoclonal mouse antihuman macrophage CD33 MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) were used.
For flow cytometry analysis cell suspensions were immunostained with rabbit antihuman cathepsin D and fluoroscein isothiocyanate (FITC)-conjugated antirabbit secondary antibody (swine F(ab′)2, Dako).
The following polyclonal antibody was used for the identification of cathepsin D in mouse mucosa: rabbit antimouse cathepsin D [28] (isotype control: rabbit immunoglobulin fraction, X0903, Dako). Secondary antibody: peroxidase-conjugated antirabbit (goat, polyclonal, P0448, Dako). Peroxidase catalyses the deposition of a fluorophore-labelled tyramide amplification reagent (NEL741, TSA™ Plus, PerkinElmer, Boston, MA, USA). For chromogenic visualization a peroxidase conjugated antifluoroscein antibody (NEF720, NEN®, Boston, MA, USA) was applied.
Rat antimouse Mac-3 (IgG1κ, clone M3/84, PharMingen, Heidelberg, Germany) and biotin-conjugated rabbit antirat secondary antibody (E0467, Dako) were applied for immunohistochemical identification of mouse macropohages (isotype control: rat IgG1κ, clone R3-34, PharMingen).
Demasking of paraffin embedded sections
Paraffin embedded human or mouse sections were cut (5 µm), floated on demineralized water, placed on slides and baked for 30 minutes (min) at 60°C. Slides were dewaxed for 10 min with xylene and rehydrated in a graded ethanol series (99%, 95%, 70% ethanol and phosphate buffered saline (PBS) pH 7·4 for 5 min each). For demasking sections were incubated for 30 min with target retrieval solution (S3307, Dako, Hamburg, Germany) at 95°C in a microwave oven. Endogenous peroxidase was quenched for 30 min with 1% hydrogen peroxide in PBS buffer. Slides were washed three times in PBS.
Immunohistochemistry in human specimens
For the identification of cathepsin D-positive cells in human tissue, specimens were incubated with primary rabbit antihuman cathepsin D antiserum (final concentration 5 µg/ml). A rabbit immunoglobulin fraction (final concentration 5 µg/ml) was used as isotype control. Biotin-conjugated goat antirabbit secondary antibody (1/500 dilution) and consecutively the Vectastain ABC elite standard system (#PK-6100, Vector Laboratories, Burlingame, CA, USA) were applied. After washing the tissue was incubated with a freshly prepared solution of NovaRED® (AEC, Vector) for brown immunostaining.
To prove that mucosal macrophages express cathepsin D, the slides were incubated for 30 min with 1% hydrogen peroxide in PBS buffer to suppress remaining peroxidase. After three washing steps, the specimens were incubated with mouse antihuman CD68 (final concentration 0·5 µg/ml). Purified mouse isotype (final concentration 0·5 µg/ml) was used as control. Subsequently biotin-conjugated rabbit antimouse IgG secondary antibody (1/500 diluted) and the Vectastain ABC elite standard system were applied. Slides were preincubated for 8 min in 0·01% benzidine dihydrochloride (BDHC, Sigma) with 0·03% sodium nitroprusside (Sigma). This was followed by incubation in the reaction medium (0·01% BDHC, 0·005% hydrogen peroxide and 0·03% sodium nitroprusside) for blue immunostaining. The formation of the dark blue, granular reaction product was monitored under the microscope. Immunostaining was interrupted with water. Cells were not counterstained. For semiquantitative analysis brown cells, blue cells and double immunostained cells was determined separately by evaluating four high-power fields at a magnification of 400×. The percentage of double immunostained cells was calculated.
Immunohistochemistry in mouse specimens
For the identification of cathepsin D in mouse mucosa, specimens were incubated for 30 min with blocking buffer (TNB, 0·1 m Tris-HCl, pH 7·5, 0·15 m NaCl and 0·5% blocking reagent FP1020, PerkinElmer™, Boston, MA, USA). Specimens were then placed in TNB buffer with 1/2000 (final concentration 0·5 µg/ml) diluted primary antiserum for 30 min. A rabbit immunoglobulin fraction (final concentration 0·5 µg/ml) was used as isotype control. Slides were washed in triton buffer (TNT, 0·1 m Tris-HCl pH 7·5, 0·15 m NaCl, 0·3% Triton X-100) and incubated for 30 min with 1/500 diluted peroxidase-conjugated antirabbit secondary antibody in TNB buffer. Peroxidase catalyses the deposition of a fluorophore-labelled tyramide amplification reagent (NEL741, TSA™ Plus, PerkinElmer, Boston, MA, USA). A working solution of the amplification reagent was applied as recommended by the manufacturer.
Slides were washed in TNT wash buffer. For chromogenic visualization slides were incubated for 30 min with antifluoroscein-peroxidase antibodies 1/25 diluted in TNB buffer. After washing the tissue was incubated with NovaRED® for brown immunostaining.
To clarify whether macrophages express cathepsin D the slides were incubated with hydrogen peroxide in PBS buffer and incubated with rat antimouse Mac-3 antibody or isotype control (final concentration 0·5 µg/ml). Biotin-conjugated rabbit antirat secondary antibody (1/500 diluted) were applied. The Vectastain ABC elite standard system were used and sections were incubated with benzidine dihydrochloride (BDHC), hydrogen peroxide and sodium nitroprusside for blue immunostaining. For semiquantitative analysis brown cells, blue cells and double immunostained cells were determined separately by evaluating four high-power fields at a magnification of 400×. The percentage of double immunostained cells was calculated.
Flow cytometry
Human monocytes and IMACs were immunostained according to a modified protocol described by Mikulka et al. [29]. For flow cytometry analysis 1 × 106 cells were resuspended in PBS. For fixation cells were kept in 70% ice-cold methanol at −20°C for 60 min, washed and resuspended. To block unspecific binding sites human AB serum was added to a final concentration of 2%. For each immunostaining 100 µl of cell suspension was placed into 1·5 ml polypropylene tubes and 2 µl of each antibody solution was added. Incubation was performed in the dark for 60 min on ice to ensure specific immunostaining. Cells were washed with PBS and resuspended in 100 µl PBS for further immunostaining with secondary antibody.
Flow cytometry was performed utilizing a Coulter® EPICS® XL-MCL™ (Coulter, Immunotech, Krefeld, Germany) equipped with an argon ion laser with an excitation power of 15 mW at 488 nm. The fluorescence of 1 × 104 cells was collected on a four decades log scale through forward light scatter (FSC) and linear scale through right angle scatter (SSC). Fluorescence for FITC was collected at 530 nm (FL1). Analysis gates were set around debris and intact cells on a FSC versus SSC dot plot. The fluorescence histograms were generated availing the gated data. Data analysis was performed with the WIN-MDI software.
Isolation of human lamina propria mononuclear cells (LPMNCs)
Pieces of intestinal mucosa from surgical biopsy specimens were incubated in calcium- and magnesium-free Hanks's balanced salt solution (HBSS) with 1 mmol/l ethylene diamine tetraacetic acid (EDTA) for 30 min at 37°C under gentle shaking to remove the intestinal epithelial cells (IEC). Human LPMNCs were isolated according to a protocol described recently [30]. Specimens were incubated for 30 min in 2 ml PBS with 1 mg/ml collagenase type I (= 336 U/ml), 0·3 mg/ml deoxyribonuclease (DNase I, Boehringer, Mannheim, Germany) and 0·2 mg/ml hyaluronidase without fetal calf serum (FCS) at 37°C. Cells were dispersed by passing through a 27-gauge needle by a 1–2 ml syringe, washed in 1·5 ml PBS with 500 µl FCS and finally submitted to Ficoll density gradient centrifugation for 20 min at ≈690 g (without brake) in a Heraeus centrifuge for the isolation of mononuclear cells. The interphase was removed carefully and washed with PBS.
Isolation and purification of IMACs
LPMNCs were isolated from normal and CD mucosa specimens. IMACs were labelled with immunomagnetic MicroBeads armed with CD33 antibody and purified two times with the help of type AS separation columns (Miltenyi Biotec), as described recently [30]. Briefly, LPMNCs containing magnetically labelled IMACs were passed through an AS separation column which was placed in the permanent magnet SuperMACS®. The magnetically labelled cells were retained in the column. After removal of the column from the magnetic field, the retained fraction was eluted. Eluted cells were passed through a second AS separation column to increase purity of IMACs [30]. Final purity of >95% IMACs was confirmed by FACS analysis using PE-conjugated goat antimouse IgG antibody (Caltag, Medac, Hamburg, Germany).
Semiquantitative reverse transcription-polymerase chain reaction (RT-PCR) for cathepsin D
Poly(A)-RNA was isolated by polyT magnetic beads (Dynal, Oslo, Norway) from CD33+ cells according to the manufacturer's protocol. Briefly, IMACs were resuspended in lysis/binding buffer and a repeated passage of the solution through a pipette tip was performed to obtain complete lysis. The vial was placed in the magnetic field of a permanent magnet (MCP®-E-1, Dynal, Oslo, Norway) for 5 min at room temperature to remove immunomagnetic MicroBeads armed with CD33 antibody. The suspension was mixed with Dynabeads Oligo (dT)25 and rotated on a roller for 5 min at room temperature. The lysate was washed three times with washing buffer. Elution of mRNA from Dynabeads Oligo (dT)25 was performed at 65°C for 2 min in 20 µl elution solution.
Poly(A)-RNA was reverse transcribed with the Reverse Transcription System (Promega, Madison, WI, USA) according to the manufacturer's protocol in a 15-min reaction at 42°C. For PCR, the primers used were as follows: β-actin upstream TGA CGG GGT CAC CCA CAC; β-actin downstream CTA GAA GCA TTT GCG GTG GAA; cathepsin D upstream CAA CAG CGA CAA GTC CAG C; cathepsin D downstream CTG AAT CAG CGG CAC GGC.
To test cDNAs for representation and full length genes RT-PCR with a 5′β-actin, GAPDH, 6K Clathrin and 2K Clathrin primer set from the Gene Checker™ Kit (Invitrogen, Leek, the Netherlands) was performed. The PCR for cathepsin D comprised 25–35 cycles with denaturing at 94°C for 30 s, annealing at 60°C for 30 s and extension at 72°C for 30 s. The PCR for the genes from the Gene Checker™ Kit comprised 25 cycles with denaturing at 94°C for 30 s, annealing at 55°C for 30 s and extension at 72°C for 30 s. The reactions were performed in a TRIO-Thermoblock (Biometra, Goettingen, Germany). The PCR products were then subjected to 1% agarose gel electrophoresis together with a size standard ΦX 174 Hae III (Stratagene, Amsterdam, the Netherlands).
SMART® PCR and blotting
To verify results from semiquantitative PCR, RT-PCR products were hybridized with a 32P labelled probe. Poly(A)-RNA was reverse transcribed and amplified with the SMART® PCR cDNA synthesis kit (K1052-1, Clontech, Palo Alto, CA, USA). RT was performed at 42°C for 60 min cDNA was amplified with the modified oligo(dT)- and oligo(dG) primers as described in the manual. PCR was optimized for each poly(A)-RNA isolate. To determine the optimal number of cycles: 12, 15, 18, 21 and 24 PCR cycles were performed. Aliquots of each PCR were transferred onto a 1% agarose/EtBr gel. Optimal numbers of PCR cycles were determined as two cycles less than were needed to overcycle the cDNA. Successful reverse transcription and amplification was further tested with the Gene Checker™ Kit (Invitrogen, Leek, the Netherlands).
For blotting cDNA was size-fractionated in a 1% agarose gel, transferred onto a nylon membrane (msi, Westborough, USA) and covalently cross-linked to the nylon membrane with a Stratalinker™ 1800 (Stratagene, La Jolla, USA). Prehybridization was performed in formamide-free buffer (6×SSC, 5×Denhardt’s, 0·5% (w/v) SDS and 100 µg/µl tRNA) for 2 h at 65°C. The oligonucleotides for the production of 32P radioactively labelled probes were as described above. For hybridization radioactively labelled probe (0·5–2 106 cpm/ml final concentration) was added and incubated overnight at 65°C. To determine the relative amounts of cDNA, the intensities of individual cDNA bands were quantified using a densitometer (Molecular Dynamics, Sunnyvale, USA) and then normalized to that of β-actin cDNA.
Statistical analysis
Data are expressed as mean (± s.d.). Statistical analyses were performed using the Mann–Whitney U rank-sum test. Differences were considered significant with a P-value of < 0·05.
Results
Cathepsin D mRNA expression in human IMACs
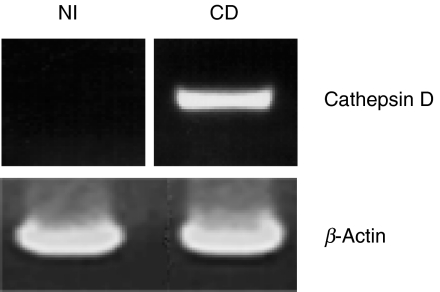
Human IMACs were isolated from mucosa specimens with immunomagnetic MicroBeads armed with CD33 antibody as described in Materials and methods. No specific cathepsin D product could be amplified with RT-PCR from IMACs from control patients with 35 cycles (Fig. 1, left lane), whereas a strong signal was obtained with mRNA from CD IMACs (Fig. 1, right lane). Integrity of the mRNA was verified by Gene Checker™ kit. RT-PCR for five different housekeeping genes was performed and proved integrity of the RNA (only actin shown). The experiment was repeated three times with IMACs from different patients.
Fig. 1.
Cathepsin D mRNA expression in CD33-positive IMACs from a patient with non-inflamed (NI) mucosa and a CD patient. RT-PCR for cathepsin D and β-actin as indicated. Only IMACs from mucosa of the CD patient express significant amounts of cathepsin D mRNA. This figure is representative for two more experiments.
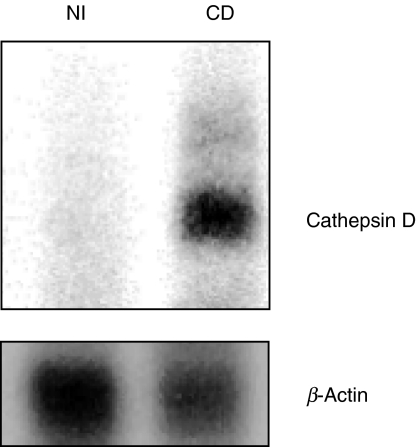
To demonstrate the reliability of the RT-PCR we twice performed blotting of linearly amplified RT-PCR products from IMACs purified from normal mucosa and inflamed CD mucosa specimen and hybridized with a 32P labelled probe. With PCR products different from those used for simple RT-PCR, no specific product was obtained from control mucosa (Fig. 2, left lane) whereas a clear signal was visible after hybridization of radioactively labelled probes with linearly amplified cDNA from CD IMACs (Fig. 2, right lane). Densitometry revealed 0 versus 312 relative density units.
Fig. 2.
Analysis of linearly amplified cDNA of IMACs from NI patients and IMACs from CD patients hybridized with a 32P labelled probe. Blot for cathepsin D and β-actin as indicated. To avoid individual differences responsible for results, IMACs from four inflamed specimens and from four normal specimens were pooled. Only IMACs from mucosa of CD patients express significant amounts of cathepsin D mRNA. This figure is representative for one more experiment.
To investigate the mRNA expression pattern of other cathepsins we performed a comparative analysis of cathepsins B, F, L and S mRNA expression. IMACs from patients with non-inflamed mucosa (n = 10) showed mRNA expression for cathepsins B and L. In four samples weak cathepsin F mRNA expression was detectable. In IMACs from mucosa of CD patients (n = 7) no expression of cathepsins B, F, L or S mRNA was detectable (data not shown).
Flow cytometrical detection of cathepsin D in human IMACs
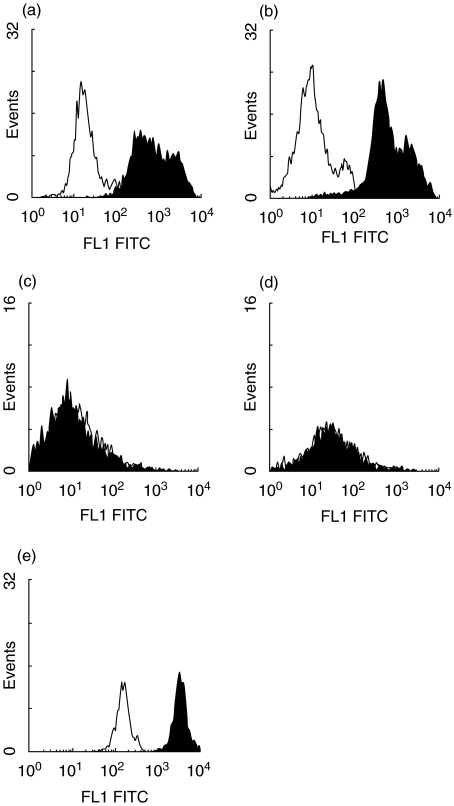
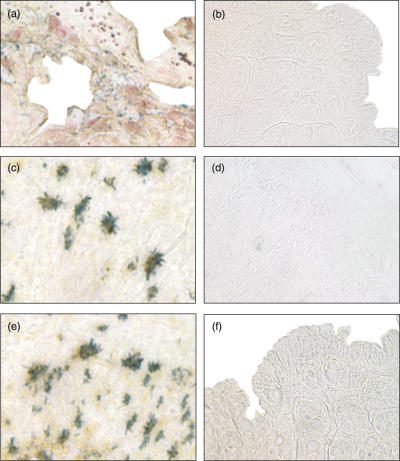
By flow cytometrical analysis the data on cathepsin D mRNA expression could be confirmed. IMACs were isolated and purified from human LPMNCs from three normal, two inflamed CD and two inflamed UC specimens as described in Material and methods. IMACs from specimens different from those used for RT-PCR and in blotting procedures were used. No cathepsin D protein was detectable in IMACs obtained from control mucosa (Figs 3c,d). However, IMACs isolated from patients with CD (Fig. 3a) and UC (Fig. 3b) clearly showed intracellular expression of cathepsin D. The fluorescence ratio of cells immunostained with anti-cathepsin D antibody versus cells immunostained with isotype control antibody was 1·02 ± 0·03 in IMACs from non-inflamed patients indicating a lack of cathepsin D expression. In patients with IBD this ratio was increased to 66·56 ± 8·52 confirming cathepsin D expression. An anti-CD68 immunostaining is shown as positive control for intracellular immunostaining (Fig. 3e).
Fig. 3.
Flow cytometry analysis of cathepsin D in human IMACs from NI patients and IMACs from CD and UC patients. Cells were immunostained with anti-cathepsin D antibody (black histogram) or control (white histogram). (a) Cathepsin D expression in IMACs from CD patients and (b) UC patients. (c and d) Cathepsin D expression in IMACs from NI patients. (e) Intracellular anti-CD68 immunostaining as positive control. Only IMACs from mucosa of IBD patients showed expression of cathepsin D. Flow cytometry analysis was performed three times for non-inflamed and two times for CD and UC patients each.
Expression of cathepsin D protein in inflamed human mucosa
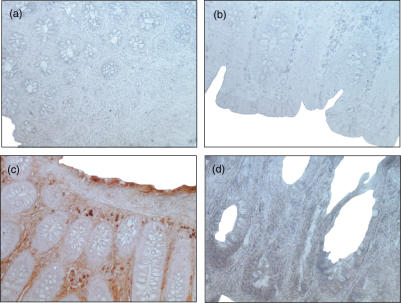
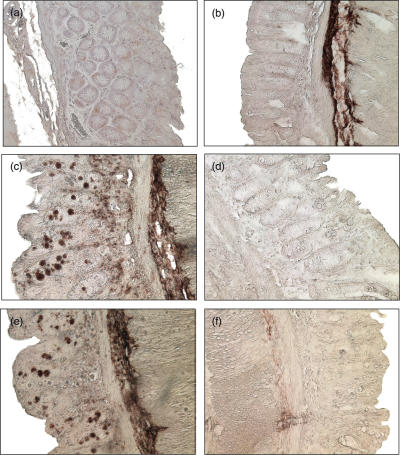
Immunohistochemistry with frozen sections from three non-inflamed patients and three patients with CD with moderate to severe inflammation (endoscopic score ++and +++) was performed to determine cathepsin D protein in the mucosa. In specimens obtained from macroscopical normal mucosa from patients without IBD no cathepsin D (Fig. 4a) could be detected. In contrast, in the lamina propria of CD patients approximately 10–20% of the cells immunostained positive for cathepsin D (Fig. 4c) with an inhomogeneous pattern of distribution. Positively immunostained cells accumulated subepithelially and were preferentially detectable close to the crypts. In the isotype controls from all patients no immunostaining could be detected (Figs 4b,d).
Fig. 4.
Immunohistochemical detection of cathepsin D in control mucosa and inflamed intestinal mucosa. Frozen human sections were cut and fixed in acetone for immunoperoxidase staining with a rabbit antihuman antibody. (a) No cells containing cathepsin D were detectable in control mucosa from NI patients. (b) Isotype control in control mucosa from NI patients. (c) Cells with cathepsin D were detectable in the lamina propria in patients with CD. (d) Isotype control in the lamina propria in patients with CD (original magnification × 400). This figure is representative for two more experiments from each group.
Identification of the cell type expressing cathepsin D in human mucosa
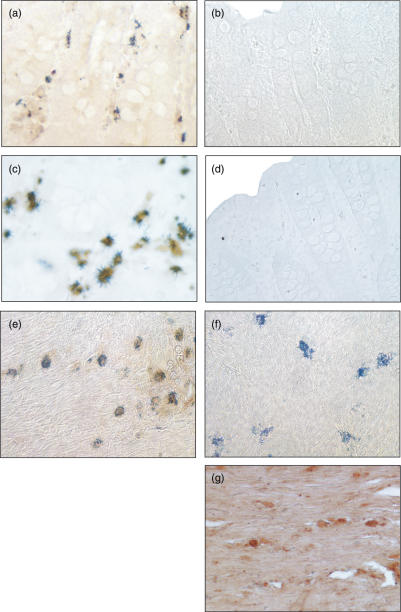
To elucidate which cell types in the human inflamed mucosa express cathepsin D, we applied double-labelling immunohistochemistry. The distribution of immunostained cells (Fig. 4) led us to assume that cathepsin D-positive cells might be IMACs. Therefore we used an antibody against a typical intracellular IMACs marker (CD68) to identify this cell population. A sequential double-immunostaining procedure using NovaRED® and BDHC was applied. The antibodies for the identification of IMACs were either used for the first or the second immunostaining step. The first immunostaining step was visualized with NovaRED® (diffuse brown reaction product). The second immunostaining step was visualized with BDHC. Immunostaining of the macrophage-specific, intracellular antigen CD68 resulted in an intracellular granular or needle-like dark blue BDHC deposit.
Immunohistochemistry with paraffin-embedded sections of four patients without inflamed mucosa, four patients with CD and two patients with UC with an endoscopic score of ++and +++ was performed. In macroscopically normal specimens obtained from non-inflamed patients no cathepsin D (Fig. 5a) could be detected in IMACs. In contrast, in the lamina propria of CD and UC patients 74–84% IMACs immunostained positive for cathepsin D (Figs 5c,e). This immunostaining pattern was consistent when the cathepsin D antibody was applied in the second immunostaining step.
Fig. 5.
Identification of the cell types containing cathepsin D in the inflamed human mucosa. Cathepsin D was immunostained in a first step. CD68 was immunostained in a second step. Positive cells were visualized with NovaRED (brown) and BDHC (dark blue) reaction product. (a) No Cathepsin D was detected in macrophages (blue) in control mucosa from NI patients. (b) Isotype control in control mucosa from NI patients. (c) Cathepsin D (brown) could be co-localized with macrophages (blue) in the lamina propria from a CD patient. (d) Isotype control in the lamina propria from a CD patient. (e) Cathepsin D (brown) could be co-localized with macrophages (blue) in the submucosa from a UC patient. (f) No immunostaining (blue) was detected in a UC patient with cathepsin D isotype control antibody in the first immunostaining step. (g) No immunostaining of cathepsin D-positive cells (brown) was detected in a UC patient with macrophage isotype control antibody in the second immunostaining step (original magnification × 400). This figure is representative for three more experiments.
Three isotype controls were performed: substitution of the anticathepsin D antibody, the antimacrophage CD68 antibody or both with the homologous isotype control abolished specific immunostaining. In addition, no immunostaining was obtained if both primary antibodies were substituted with isotype controls in mucosa from non-inflamed patients (Fig. 5b) or CD patients (Fig. 5d). Isotype controls with only one antibody left are shown as an example: only blue immunostaining of IMACs was detected in the lamina propria of a UC patient if cathepsin D isotype control antibody was applied (Fig. 5f). Only brown immunostaining of cathepsin D-positive cells was detected in the submucosa if, instead of anti-CD68, an isotype control antibody was applied (Fig. 5g).
Expression of cathepsin D protein in acute and chronic DSS colitis
Immunohistochemistry on paraffin-embedded sections from three mice suffering from DSS-induced acute colitis with a histological score of 6–8, three mice with chronic DSS-induced colitis (histological score of 4–6) and three healthy control mice (histological score of 0–1) was performed to determine cathepsin D protein expression in the mouse colon. In specimens obtained from control mice with macroscopically normal mucosa no cathepsin D protein (Fig. 6a) could be detected. In contrast, in the lamina propria of mucosa from mice with DSS-induced acute colitis (Fig. 6c) and mice with chronic DSS-induced colitis (Fig. 6e) approximately 10–20% of the cells immunostained positive for cathepsin D with an heterogeneous pattern of distribution. No cathepsin D protein was detectable in intestinal epithelial cells (IEC) in all samples under the conditions used (Fig. 6). In the isotype controls from all samples no immunostaining could be detected (Figs 6b,d,f).
Fig. 6.
After antigen unmasking immunoperoxidase staining was applied for immunohistochemical detection of cathepsin D in control and inflamed mucosa. Paraffin-embedded mouse sections were cut, dewaxed and rehydrated. (a) No cells containing cathepsin D were detectable in control mucosa from untreated mice. (b) Isotype control in control mucosa from untreated mice. (c) In acute DSS-induced colitis cells with cathepsin D were detectable in the lamina propria. (d) Isotype control in acute DSS-induced colitis. (e) In chronic DSS-induced colitis cells with cathepsin D were detectable in the lamina propria. (f) Isotype control in chronic DSS-induced colitis (original magnification × 400). This figure is representative for two more experiments from each group.
Identification of the cell type expressing cathepsin D in the murine mucosa
To elucidate which cell types in the mouse inflamed mucosa expressed cathepsin D, we used an anti-Mac-3 antibody to identify murine IMACs. Immunohistochemistry with paraffin-embedded sections from three mice with DSS-induced acute colitis with a histological score of 6–8, three mice with chronic DSS-induced colitis with a histological score of 4–6 and three control mice with a histological score of 0–1 was performed to determine cathepsin D protein in MAC-3-positive cells. In specimens obtained from control mice with macroscopically normal mucosa no cathepsin D (Fig. 7a) could be detected in MAC-3-positive cells. In contrast, in the lamina propria of mucosa from mice with DSS-induced acute colitis (Fig. 7c) and mice with chronic DSS-induced colitis (Fig. 7e) most of the MAC-3-positive cells immunostained positive for cathepsin D and vice versa.
Fig. 7.
Identification of the cell types containing Cathepsin D in the inflamed mouse-mucosa. Cathepsin D was immunostained in a first step. MAC-3 was immunostained in a second step. Positive cells were visualized with NovaRED (brown) and BDHC (dark blue) reaction product. (a) No Cathepsin D was detected in macrophages (blue) in control mucosa from untreated mice. (b) Isotype control in control mucosa from untreated mice. (c) Cathepsin D (brown) could be co-localized in macrophages (blue) in the lamina propria of mice with acute DSS-induced colitis. (d) Isotype control in acute DSS-induced colitis. (e) Cathepsin D (brown) could be co-localized in macrophages (blue) in the lamina propria of mice with chronic DSS-induced colitis. (f) Isotype control in chronic DSS-induced colitis (original magnification × 400). This figure is representative for two more experiments from each group.
Three isotype controls were performed for each sample: substitution of the antimouse cathepsin D antibody, the antimouse Mac-3 antibody, or both, with the homologous isotype controls abolished the respective specific immunostaining. No immunostaining was obtained if both primary antibodies were substituted in control mucosa from untreated mice (Fig. 7b), in the lamina propria of mice with DSS-induced acute colitis (Fig. 7d) or mice with chronic DSS-induced colitis (Fig. 7f).
Discussion
In the present study we demonstrate an induction of cathepsin D mRNA and protein expression in IMACs from inflamed mucosa compared with healthy controls, as well as in a mouse model of colitis. To elucidate possible differences of IMACs from normal and inflamed mucosa we performed a subtractive screening by subtracting cDNA from normal IMACs from those of CD macrophages with the Clontech PCR-Select cDNA subtraction kit [8]. Overall 70 differentially expressed genes were obtained.
A search of the expressed sequence tag database revealed that a subtracted product had > 99% homology to homo sapiens cathepsin D (lysosomal aspartyl protease) (CTSD) mRNA (accession number NM_001909). To show the presence of cathepsin D in inflammation we performed immunohistochemistry in human and mouse tissue. Immunohistochemistry clearly showed cathepsin D in cells of human patients with inflamed mucosa. In the lamina propria from mice with acute or chronic DSS-induced colitis inflammation, as demonstrated by macroscopic and microscopic parameters, was accompanied by cathepsin D presence. Macrophage-like distribution and frequency of the immunostained cells led us to determine further the cathepsin D expression in this specific cell population.
Inflammation-associated human IMACs showed cathepsin D mRNA and protein, as revealed by both RT-PCR and flow cytometry analysis. We were not able to find any extracellular immunostaining for cathepsin D. Immunohistochemistry showed no signal for cathepsin D in IEC. On the other hand, inflammation associated IMACs showed no cathepsins B, F, L and S mRNA expression in RT-PCR.
The aspartic proteinase cathepsin D is a member of the large family of cysteine proteases. Unlike other proteases, which are mostly secretory proteins, procathepsin D is sorted to the lysosomes. Under normal physiological conditions neither procathepsin D nor cathepsin D are secreted and are found only intracellularly. Cathepsin D is involved in antigen processing in concerted action with the cysteine proteases cathepsins B and L [31–34]. Cathepsins D, B and L have an endoproteolytical activity involved in antigen processing, finely balanced in APCs, generating certain antigenic epitopes [35]. By altering the balance between cathepsins, changes of epitopes from exogeneous antigens may occur during antigen presentation. However, lysosomal bulk proteolysis is maintained in mice deficient for cathepsin D [17]. Despite that, a lack of limited proteolysis of proteins regulating cell growth and/or tissue homeostasis was suggested [17].
Macrophages mobilize proteinases and participate in the pathophysiological remodelling of the extracellular matrix in tissue-destructive diseases such as arthritis, bone resorption, metastasis and chronic lung damage. The tissue-damaging proteinases cathepsins B and L expressed by CD68-positive human mononuclear cells were shown to play important roles in joint destruction and bone erosion in patients with rheumatoid arthritis [36]. Cathepsin K is one of the key proteinases determining where and when bone resorption will be initiated [37,38]. Levels of cathepsins B and L may also provide prognostic values in certain cancers [39–41]. The pathophysiological process resulting in emphysema was suggested to involve remodelling of the extracellular matrix by proteinases including cathepsins L [42] and S [43] synthesized in human alveolar macrophages. Furthermore, in an animal model of bleomycin-induced pulmonary fibrosis in rat changes in the activities of several proteinases were described [44]. During chronic inflammation, the activities of the cathepsins L, B, H and S in isolated alveolar macrophages were found to be increased strongly [44]. Cathepsin B activity was shown to be stimulated in peritoneal macrophages by induction of an acute inflammation in mice [45].
An induction of cathepsin D expression in LPMNCs from IBD-patients has been controversial in the past. No up-regulation of cathepsin D protein was found by Galandiuk and coworkers in 1993 [24]. Lugering and coworkers found cathepsin D both precursor and mature forms to be elevated in IBD patients compared to normal controls [46]. Cathepsin D levels in diseased osteoarthritic and rheumatoid synovium were elevated compared to controls [47], although only a limited correlation was observed between the extent of inflammation present in the synovia and the levels of enzyme. A significant correlation between cathepsin D activity and the degree of gingival inflammation was found supporting the hypothesis of participation in the destruction of periodontal tissues during gingivitis and periodontitis [48].
An induced production and secretion of cathepsin D in the mucosa of IBD patients could contribute to the destruction of extracellular matrix and basement membrane followed by a loss of epithelial cells and an impairment of the intestinal barrier. However, an influence of cathepsin D induction on epithelial damage cannot be demonstrated easily by knock-out technique as a deletion of this enzyme is followed by early death of cathepsin D-deficient mice [17].
Following secretion of procathepsin D into the extracellular space and activation, mature cathepsin D can develop proteolytic potential [19]. Cathepsin D can degrade growth inhibitors or liberate angiogenic factors or growth factors such as the matrix associated fibroblast growth factor (FGF) and FGF-similar growth factors [20].
Down-regulation of the macrophage-specific surface antigens [3,30] and diminished functional properties [8] may be part of an immunological disarmament for the benefit of peripheral non-responsiveness to abundant antigens to which the mucosa is continuously exposed. IMACs fully responsive to bacterial antigens [4,12] with up-regulated catalytic enzymes capable of tissue destructive activity [8] may initiate or perpetuate a dysregulated immune response, as demonstrated in IBD.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (SFB 585, Ro 1236/3–2) and by the BMBF Kompetenznetz – CED (G.R., H.H). We thank the endoscopists and nurses of our endoscopy division for their excellent co-operation.
References
- 1.Pavli P, Maxwell L, Van de PE, et al. Distribution of human colonic dendritic cells and macrophages. Clin Exp Immunol. 1996;104:124–32. doi: 10.1046/j.1365-2249.1996.d01-642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grimm MC, Pavli P, Van de PE, et al. Evidence for a CD14+ population of monocytes in inflammatory bowel disease mucosa − implications for pathogenesis. Clin Exp Immunol. 1995;100:291–7. doi: 10.1111/j.1365-2249.1995.tb03667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hausmann M, Kiessling S, Mestermann S, et al. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- 4.Rogler G, Hausmann M, Spottl T, et al. T cell co-stimulatory molecules are upregulated on intestinal macrophages from inflammatory bowel disease mucosa. Eur J Gastroenterol Hepatol. 1999;11:1105–11. doi: 10.1097/00042737-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Ingalls RR, Monks BG, Savedra R, Jr, et al. CD11/CD18 and CD14 share a common lipid A signaling pathway. J Immunol. 1998;161:5413–20. [PubMed] [Google Scholar]
- 6.Ingalls RR, Arnaout MA, Delude RL, et al. The CD11/CD18 integrins: characterization of three novel LPS signaling receptors. Prog Clin Biol Res. 1998;397:107–17. [PubMed] [Google Scholar]
- 7.Rogler G, Andus T, Schölmerich J, et al. Innovative concepts in inflammatory bowel disease. Dordrecht: Kluwer Academic Publishers; 1999. Intestinal macrophages; pp. 111–119. [Google Scholar]
- 8.Hausmann M, Spottl T, Andus T, et al. Subtractive screening reveals up-regulation of NADPH oxidase expression in Crohn's disease intestinal macrophages. Clin Exp Immunol. 2001;125:48–55. doi: 10.1046/j.1365-2249.2001.01567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LeFevre ME, Hammer R, Joel DD. Macrophages of the mammalian small intestine: a review. J Reticuloendothel Soc. 1979;26:553–73. [PubMed] [Google Scholar]
- 10.Rugtveit J, Haraldsen G, Hogasen AK, et al. Respiratory burst of intestinal macrophages in inflammatory bowel disease is mainly caused by CD14+L1+ monocyte derived cells. Gut. 1995;37:367–73. doi: 10.1136/gut.37.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rugtveit J, Bakka A, Brandtzaeg P. Differential distribution of B7.1 (CD80) and B7.2 (CD86) co-stimulatory molecules on mucosal macrophage subsets in human inflammatory bowel disease (IBD) Clin Exp Immunol. 1997;110:104–13. doi: 10.1046/j.1365-2249.1997.5071404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogler G, Brand K, Vogl D, et al. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–69. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh CS, deRoos P, Honey K, et al. A role for cathepsin L and cathepsin S in peptide generation for MHC class II presentation. J Immunol. 2002;168:2618–25. doi: 10.4049/jimmunol.168.6.2618. [DOI] [PubMed] [Google Scholar]
- 14.Bennett K, Levine T, Ellis JS, et al. Antigen processing for presentation by class II major histocompatibility complex requires cleavage by cathepsin E. Eur J Immunol. 1992;22:1519–24. doi: 10.1002/eji.1830220626. [DOI] [PubMed] [Google Scholar]
- 15.Hewitt EW, Treumann A, Morrice N, et al. Natural processing sites for human cathepsin E and cathepsin D in tetanus toxin: implications for T cell epitope generation. J Immunol. 1997;159:4693–9. [PubMed] [Google Scholar]
- 16.Tsukuba T, Okamoto K, Yasuda Y, et al. New functional aspects of cathepsin D and cathepsin E. Mol Cells. 2000;10:601–11. doi: 10.1007/s10059-000-0601-8. [DOI] [PubMed] [Google Scholar]
- 17.Saftig P, Hetman M, Schmahl W, et al. Mice deficient for the lysosomal proteinase cathepsin D exhibit progressive atrophy of the intestinal mucosa and profound destruction of lymphoid cells. EMBO J. 1995;14:3599–608. doi: 10.1002/j.1460-2075.1995.tb00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villadangos JA, Bryant RA, Deussing J, et al. Proteases involved in MHC class II antigen presentation. Immunol Rev. 1999;172:109–20. doi: 10.1111/j.1600-065x.1999.tb01360.x. [DOI] [PubMed] [Google Scholar]
- 19.Rochefort H. Biological and clinical significance of cathepsin D in breast cancer. Acta Oncol. 1992;31:125–30. doi: 10.3109/02841869209088891. [DOI] [PubMed] [Google Scholar]
- 20.Briozzo P, Badet J, Capony F, et al. MCF7 mammary cancer cells respond to bFGF and internalize it following its release from extracellular matrix: a permissive role of cathepsin D. Exp Cell Res. 1991;194:252–9. doi: 10.1016/0014-4827(91)90362-x. [DOI] [PubMed] [Google Scholar]
- 21.Montcourrier P, Mangeat PH, Salazar G, et al. Cathepsin D in breast cancer cells can digest extracellular matrix in large acidic vesicles. Cancer Res. 1990;50:6045–54. [PubMed] [Google Scholar]
- 22.Tryggvason K, Hoyhtya M, Salo T. Proteolytic degradation of extracellular matrix in tumor invasion. Biochim Biophys Acta. 1987;907:191–217. doi: 10.1016/0304-419x(87)90006-0. [DOI] [PubMed] [Google Scholar]
- 23.Arao J, Fukui H, Ono Y, et al. Immunohistochemical localization of cathepsin D in colorectal tumors. Dis Colon Rectum. 2000;43:396–401. doi: 10.1007/BF02258308. [DOI] [PubMed] [Google Scholar]
- 24.Galandiuk S, Miseljic S, Yang AR, et al. Expression of hormone receptors, cathepsin D, and HER-2/neu oncoprotein in normal colon and colonic disease. Arch Surg. 1993;128:637–42. doi: 10.1001/archsurg.1993.01420180035007. [DOI] [PubMed] [Google Scholar]
- 25.Obermeier F, Kojouharoff G, Hans W, et al. Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol. 1999;116:238–45. doi: 10.1046/j.1365-2249.1999.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okayasu I, Hatakeyama S, Yamada M, et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 27.Steidler L, Hans W, Schotte L, et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352–5. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- 28.Kasper D, Dittmer F, von Figura K, et al. Neither type of mannose 6-phosphate receptor is sufficient for targeting of lysosomal enzymes along intracellular routes. J Cell Biol. 1996;134:615–23. doi: 10.1083/jcb.134.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mikulka WR, Bolton WE. Methodologies for the preservation of proliferation associated antigens PCNA, p120, and p105 in tumor cell lines for use in flow cytometry. Cytometry. 1994;17:246–57. doi: 10.1002/cyto.990170308. [DOI] [PubMed] [Google Scholar]
- 30.Rogler G, Hausmann M, Vogl D, et al. Isolation and phenotypic characterization of colonic macrophages. Clin Exp Immunol. 1998;112:205–15. doi: 10.1046/j.1365-2249.1998.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez GM, Diment S. Role of cathepsin D in antigen presentation of ovalbumin. J Immunol. 1992;149:2894–8. [PubMed] [Google Scholar]
- 32.Van Noort JM, Boon J, Van der Drift AC, et al. Antigen processing by endosomal proteases determines which sites of sperm-whale myoglobin are eventually recognized by T cells. Eur J Immunol. 1991;21:1989–96. doi: 10.1002/eji.1830210904. [DOI] [PubMed] [Google Scholar]
- 33.McCoy K, Gal S, Schwartz RH, et al. An acid protease secreted by transformed cells interferes with antigen processing. J Cell Biol. 1988;106:1879–84. doi: 10.1083/jcb.106.6.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diment S. Different roles for thiol and aspartyl proteases in antigen presentation of ovalbumin. J Immunol. 1990;145:417–22. [PubMed] [Google Scholar]
- 35.Rodriguez GM, Diment S. Destructive proteolysis by cysteine proteases in antigen presentation of ovalbumin. Eur J Immunol. 1995;25:1823–7. doi: 10.1002/eji.1830250705. [DOI] [PubMed] [Google Scholar]
- 36.Kaneko M, Tomita T, Nakase T, et al. Expression of proteinases and inflammatory cytokines in subchondral bone regions in the destructive joint of rheumatoid arthritis. Rheumatology (Oxford) 2001;40:247–55. doi: 10.1093/rheumatology/40.3.247. [DOI] [PubMed] [Google Scholar]
- 37.Delaisse JM, Engsig MT, Everts V, et al. Proteinases in bone resorption: obvious and less obvious roles. Clin Chim Acta. 2000;291:223–34. doi: 10.1016/s0009-8981(99)00230-2. [DOI] [PubMed] [Google Scholar]
- 38.Saftig P, Hunziker E, Everts V, et al. Functions of cathepsin K in bone resorption. Lessons from cathepsin K deficient mice. Adv Exp Med. 2000;477:293–303. doi: 10.1007/0-306-46826-3_32. [DOI] [PubMed] [Google Scholar]
- 39.Duffy MJ. Proteases as prognostic markers in cancer. Clin Cancer Res. 1996;2:613–8. [PubMed] [Google Scholar]
- 40.Harbeck N, Alt U, Berger U, et al. Prognostic impact of proteolytic factors (urokinase-type plasminogen activator, plasminogen activator inhibitor 1, and cathepsins B, D, and L) in primary breast cancer reflects effects of adjuvant systemic therapy. Clin Cancer Res. 2001;7:2757–64. [PubMed] [Google Scholar]
- 41.Yano M, Hirai K, Naito Z, et al. Expression of cathepsin B and cystatin C in human breast cancer. Surg Today. 2001;31:385–9. doi: 10.1007/s005950170126. [DOI] [PubMed] [Google Scholar]
- 42.Shapiro SD, Campbell EJ, Welgus HG, et al. Elastin degradation by mononuclear phagocytes. Ann NY Acad Sci. 1991;624:69–80. doi: 10.1111/j.1749-6632.1991.tb17007.x. [DOI] [PubMed] [Google Scholar]
- 43.Shi GP, Munger JS, Meara JP, et al. Molecular cloning and expression of human alveolar macrophage cathepsin S, an elastinolytic cysteine protease. J Biol Chem. 1992;267:7258–62. [PubMed] [Google Scholar]
- 44.Koslowski R, Knoch KP, Wenzel KW. Proteinases and proteinase inhibitors during the development of pulmonary fibrosis in rat. Clin Chim Acta. 1998;271:45–56. doi: 10.1016/s0009-8981(97)00228-3. [DOI] [PubMed] [Google Scholar]
- 45.Lesser M, Chang JC, Orlowski M. Cathepsin B and D activity in stimulated peritoneal macrophages. Mol Cell Biochem. 1985;69:67–73. doi: 10.1007/BF00225928. [DOI] [PubMed] [Google Scholar]
- 46.Lugering N, Kucharzik T, Stein H, et al. IL-10 synergizes with IL-4 and IL-13 in inhibiting lysosomal enzyme secretion by human monocytes and lamina propria mononuclear cells from patients with inflammatory bowel disease. Dig Dis Sci. 1998;43:706–14. doi: 10.1023/a:1018845526434. [DOI] [PubMed] [Google Scholar]
- 47.Kar NC, Cracchiolo A, III, Mirra J, et al. Acid, neutral, and alkaline hydrolases in arthritic synovium. Am J Clin Pathol. 1976;65:220–8. doi: 10.1093/ajcp/65.2.220. [DOI] [PubMed] [Google Scholar]
- 48.Jotterand H, Cimasoni G. Cathepsin D in the connective tissue and epithelium of inflammed human gingiva. J Biol Buccale. 1977;5:333–42. [PubMed] [Google Scholar]