Abstract
Although complement is activated in the peritoneal cavity during chronic peritoneal dialysis (PD), little is known about its role in peritoneal defence and injury related to long-term PD. We examined the impact of glucose and commercial peritoneal dialysis solutions on complement expression in HPMCs obtained by primary culture from omental tissues of consented patients undergoing elective abdominal surgery. Constitutive expression of C3 and C4 mRNA in HPMCs was up-regulated upon exposure to 75 mm glucose in a time-dependent manner. C3 and C4 protein was secreted in both apical and basolateral directions. Glucose doses beyond 100 mm markedly down-regulated C3 and C4 expression, and stimulated LDH release dose-dependently. Such cytotoxic effects were attenuated using equivalent doses of mannitol instead of glucose. Treatment with conventional lactate-buffered dialysis solution gave rise to down-regulation of C3 and C4 expression, and heightened LDH release in HPMCs. These effects correlated with the glucose strength of the solution, persisted despite replacement with a bicarbonate-buffered solution, aggravated by glycated albumin, and were partially abrogated by supplementation with 10% fetal bovine serum in the culture system. Our findings suggest that the artificial conditions imposed by PD lead to alterations in local complement synthesis that have implications for the role of the peritoneal mesothelium in both inflammation and defence.
Keywords: complement, human peritoneal mesothelial cells, peritoneal dialysis, hypertonic glucose, mannitol, peritoneal defence
Introduction
The introduction of peritoneal dialysis (PD) over two decades ago has subjected the peritoneal cavity to a novel environment in which a whole new set of variables are likely to impact on its structure and function. The key to long-term success in PD is the maintenance of the integrity of the peritoneal membrane, which understandably is at odds with expecting it to perform a function for which it was not originally designed. There is increasing evidence that long-term PD is associated with structural changes in the peritoneal membrane that will eventually evolve into peritoneal fibrosis and loss of function [1]. To date, the exact mechanism of peritoneal membrane failure in patients on long-term PD is still unclear. One common feature that precedes the development of peritoneal fibrosis is chronic peritoneal inflammation that has been shown to be triggered by a variety of mediators including proinflammatory and profibrotic cytokines, adhesion molecules, and growth factors. Because evidence exists for complement activation in the peritoneal cavity in patients on chronic PD [2,3], the local production of complement is of potential interest as a possible effector mechanism of inflammatory injury in the causation of chronic peritoneal damage. In the present study, we first examined the impact of glucose and standard peritoneal dialysis fluid on complement expression in cultured human peritoneal mesothelial cells (HPMCs).
Another important function of the peritoneal mesothelium in the setting of chronic PD is defence against peritonitis [4]. Under normal circumstances the mesothelial lining acts as a physical barrier to microbial invasion, but this is compromised in patients on PD as a result of the artificial conditions imposed by the instillation of hypertonic glucose solutions. The peritoneal cavity itself possesses a sophisticated and complex intrinsic immune defence system against pathogenic microbes [5]. The chief components of this system are cells (leucocytes, both resident and infiltrating, peritoneal mesothelial cells, and peritoneal fibroblasts) and the inflammatory factors they secrete (cytokines, prostaglandins, leukotrienes, chemoattractants). The complement system plays a key role in innate immunity, and self defence. C3 possesses opsonic, chemotactic, anaphylotoxic, and immunoregulatory properties; while C4 is pivotal in classical and lectin pathway activation [6,7]. Thus, it is possible that locally produced complement may have a role in peritoneal defence. The second objective of our study was therefore to examine the relevance of complement as a means of local antibacterial defence in PD.
Materials and Methods
Reagents
Cell culture media, defined medium additives, trypsin-EDTA, lypopolysaccharide (LPS, serotype 0111:B4), and general chemicals were purchased from Sigma Chemicals (Poole, UK). Antibiotics, sera, agarose, and DNA size markers were obtained from Life Technologies (Rockville, MD, USA). Antibodies for cell characterization were from Sigma, for C3 and C4 ELISA from Dako (High Wycombe, UK) and the Binding Site (Birmingham, UK). ELISA kit for C5a assay was obtained from BD Biosciences Pharmingen (San Diego, CA, USA). Permeable membrane supports were supplied by Costar (Cambridge, MA, USA). Reagents for cDNA synthesis were from Pharmacia (Milton Keynes, UK), for PCR from Promega (Southampton, UK). Peritoneal dialysis solution (Gambrosol trio 40) was from Gambro Lundia AB (Lund, Sweden). The bag is made of polyvinyl chloride and consists of 3 compartments. Each litre of electrolyte solution is composed of 5·38 g sodium chloride, 4·72 sodium lactate, and minute quantities of calcium chloride, magnesium chloride, and sodium hydroxide. After reconstitution, the final glucose concentrations in 1·5%, 2·5%, and 4% solutions are 83 mm, 138 mm, and 220 mm, respectively. Bicarbonate-buffered peritoneal dialysis fluid (25 mm bicarbonate/15 mm lactate, pH 7·3) was from Baxter Healthcare (Newbury, UK).
Culture of human peritoneal mesothelial cells
Approval from the local Ethics Committee was granted for sampling of human omental tissue. HPMCs were obtained from omental tissues of consented patients undergoing elective abdominal surgery. The cells were isolated and characterized using procedures described previously [8]. Mesothelial cells showed typical cobblestone appearance at confluence. Immunohistochemical staining was performed with monoclonal antibodies (mAb) for human cytokeratin 18 and vimentin, as well as a polyclonal antibody for human factor VIII. Visualization was done with a fluorescein-conjugated secondary antibody. All cells were positive for cytokeratin and vimentin (excluding the presence of fibroblasts), but factor VIII antigen was not detected (excluding the presence of endothelial cells). The cells were maintained in medium 199 (Life Technologies, Gaithersburg, MD, USA) supplemented with penicillin (100 U/ml), streptomycin (100 µg/ml), l-glutamine (2 mm), transferrin (5 µg/ml), insulin (5 µg/ml) and 10% v/v FCS. HPMCs were incubated at 37°C in a humidified atmosphere with 5% CO2. Once monolayers of HPMCs reached confluence, the culture medium was removed and medium 199 containing 0·1% v/v FCS was added to the cells for 48 h prior to further culture experiments. Under these conditions, the HPMCs were quiesced and remained in a nonproliferative, viable condition for up to 96 h [9]. Confluent cells were split at a ratio of 1 : 3 and all experiments were performed with cells of the second passage. Experiments were performed using HPMCs derived from 10 individuals.
Growth of HPMC on inserts and permeability studies
At the second passage the cells were seeded into six-well plates on semipermeable membrane supports of 0·4 µm pore size, and grown to a confluent monolayer. The apical and basal supernatants were thus separated by the confluent layer of cells and could be sampled independently. To ensure the integrity of this cell monolayer, its permeability to 125I human albumin was measured. After a confluent cell monolayer was obtained 5 µl of 125I human albumin (0·5 µCi/µl) was added to the apical media. After 24 h, 50 µl of apical and basal supernatants were sampled and counted in a gamma counter. To distinguish between leakage of albumin between cells and metabolism with release of amino acids into the basal supernatant, basal samples were precipitated with 10% trichloroacetic acid. All experiments were performed in triplicate.
Preparation of advanced glycation end products
Bovine serum albumin (BSA) (Fraction V, Sigma Chemical Co., St. Louis, MO, USA) was passed over an affinity Gel Blue column (Bio-Rad Inc., Hercules, CA, USA), a heparin Sepharose CL6B column (Pharmacia, Arlington, IL, USA), and an endotoxin binding affinity column (Pierce, Inc.) to remove possible contaminants. BSA modified by advanced glycation (AGE-BSA) was prepared as described elsewhere [10]. Briefly, BSA was incubated with 0·5 m d-glucose in 0·2 m phosphate buffer (pH 7·4) at 37°C for 8 weeks under sterile conditions and then low molecular weight reactants and glucose were removed by dialysis against phosphate-buffered saline. BSA incubated without glucose was used as control BSA for all experiments.
RNA extraction and cDNA synthesis
Total RNA was extracted from 106 cells by a modification of the method by Chomczynski and Sacchi [11] from unstimulated and glucose-stimulated cells. Briefly, cells were lysed in 4 m guanidinium thiocyanate, 25 mm sodium citrate pH 7·0, 0·5% sarcosyl and 0·1 m 2-mercaptoethanol. This was followed by phenol/chloroform:isoamyl alcohol extraction and isopropanol precipitation. RNA was quantified by absorbance at 260 nm. Five µg total RNA was reverse transcribed to cDNA in a reaction mixture containing 160 ng oligo (dT)12−18, 500 µm of each dNTP and 200 U Moloney murine leukaemia virus reverse transcriptase in 20 µl solution for 80 min at 37°C. cDNA was stored at −20°C until further use.
PCR amplification
Primer sequences (Table 1) were based on the known sequence of human C3 and C4 cDNA [12,13]. PCR was carried out with cDNA diluted to reflect 0·2 µg RNA, 3 U Taq polymerase and 12·5 pmol each of 3′ and 5′ primers in 25 µl of a solution containing 10 mm Tris-HCl pH 9, 50 mm KCl, 2 mm MgCl2, 0·01% gelatin wt/vol, 0·1% Triton X-100, and 200 µm of each dNTP. The PCR cycle consisted of 1 min of denaturation at 94°C, 1 min of primer annealing at 65°C, and 2 min of extension/synthesis at 72°C. After 30 cycles of amplification, samples were incubated for another 10 min at 72°C. PCR reactions were carried out in a DNA thermal cycler (MJ Research, Watertown, MA, USA). The products were separated on 1·2% agarose gels and stained with ethidium bromide.
Table 1.
PCR primer sequences
| Primer* | Oligonucleotide sequence | Product size |
|---|---|---|
| C3-1 | GCT GCT CCT GCT ACT AAC CCA | 784 bp |
| C3-2 | AAA GGC AGT TCC CTC CAC TTT | |
| C4-1 | ATG GTT CCT ATG CGG CTT GGT TGT C | 256 bp |
| C4-2 | GCG ATG GTC ACA AAG GCT GTG AGT G | |
| GAPDH-1 | ACC ACA GTC CAT GCC ATC AC | 452 bp |
| GAPDH-2 | TCC ACC ACC CTG TTG CTG TA |
Primer-1 is identical to the coding strand; Primer-2 is complementary to the coding strand.
For quantification, glyceraldehyde 3-phosphate-dehydrogenase (GAPDH) [14] was amplified in every reaction as an internal control. The number of PCR cycles (28 cycles) was in the linear range of amplification for both sets of primers in accordance with preliminary experiments (data not shown). PCR products in ethidium bromide gels were photographed and the bands scanned using the Gel Doc 1000 Densitometry System and Quantity One (Bio-Rad Laboratories Ltd, Hercules, CA, USA). The product yield was expressed as a ratio of the intensity of the band of the investigated complement transcript to that of the actin band (normalized PCR product yield).
Assay of C3 and C4 in cell supernatants
To characterize the direction of C3 and C4 synthesis, the concentration of these proteins was measured in the apical and basal supernatants of HPMCs grown on cell culture inserts. To evaluate the effect of exposure to glucose, C3 and C4 secretion in both supernatants was assayed after apical incubation of HPMCs in media containing 5, 30, or 75 mmol/l glucose for 48 h. An equivalent volume of medium was added to the controls. A double ligand ELISA was used. Nunc MaxiSorp immunoplates (Nunc, Roskilde, Denmark) were coated overnight at 4°C with 100 µl of sheep anti-human C3c or C4 diluted 1/200 in PBS. After washing, they were blocked with PBS, 2% BSA for 1 h at 37°C. Appropriately diluted supernatant samples were added in duplicates. Rabbit anti-human C3c or C4 diluted 1/3000 in sample buffer (PBS, 2% BSA, 0·05% Tween) was then applied followed, after incubation at 37°C for 1 h, by horseradish peroxidase-conjugated goat anti-rabbit IgG diluted 1/5000 in sample buffer. The enzyme activity was read after incubation with o-phenylenediamine by measuring absorbance at 490 nm (MRX 1·1, Dynatech Laboratories, Inc., VG, USA). A pooled normal human serum of known C3 and C4 concentration was used to generate a standard curve and was included in every reaction. The limit of sensitivity of this assay was 0·3 ng/ml for C3, and 0·33 ng/ml for C4.
Lactate dehydrogenase assay
Cell viability was measured by LDH release. Following exposure to glucose, LDH was measured in the culture media using a commercially available method that detects the reduction of pyruvate to lactate causing a change in optical density at 340 nm (Sigma). Total cellular LDH was determined in cell lysates prepared by scraping the cells into a solution of 1% Triton X-100 in PBS followed by disruption of the cells by sonnication.
Statistical analysis
All data were expressed as means±standard deviation. Statistical analysis was performed using SPSS statistical software (Statistical Package for the Social Sciences, Inc., Chicago, IL, USA). Intergroup differences for continuous variables were assessed by one-way analysis of variance. Post hoc multiple comparisons using Tukey's HSD test was used to determine the significance of differences between groups. A P-value of less than 0·05 was considered statistically significant.
Results
Permeability of cell monolayer
125I human serum albumin was used as a marker to measure protein transport and leakage across the HPMC monolayer. In controls, 0·94 ± 0·26% of apical counts appeared in the basal media at 24 h. Albumin precipitated by trichloroacetic acid accounted for 17·4 ± 2·22% of these basolateral counts. The cell monolayer therefore remained intact with minimal protein leakage. There was no significant change in permeability after incubation with glucose, mannitol or dialysate apically (data not shown).
C3 and C4 gene expression in HPMC
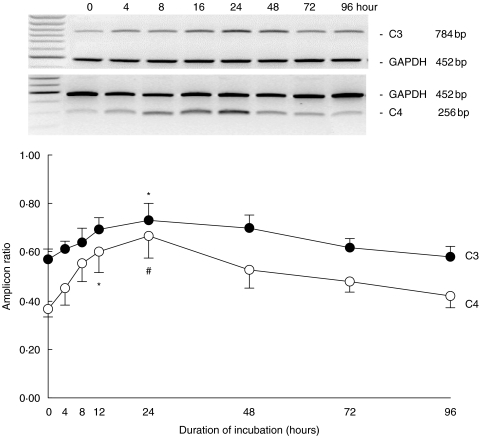
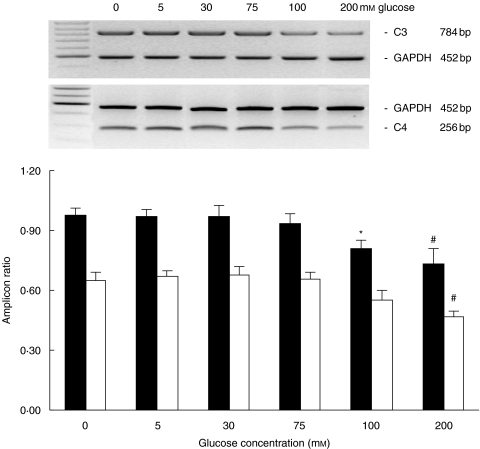
In the quiescent state, HPMCs expressed C3 and C4 mRNA constitutively. Exposure to 75 mm glucose up-regulated both C3 and C4 gene expression in a time-dependent manner for up to 24 h, thereafter the expression of both amplicons returned progressively to baseline at 96 h of incubation (Fig. 1). Exposure to doses beyond 100 mm for 24 h significantly down-regulated C3 gene expression, while C4 was down-regulated after incubation with 200 mm glucose for 24 h (Fig. 2).
Fig. 1.
Time kinetics of C3 and C4 gene expression. Growth-arrested HPMCs in M199 media were exposed to 75 mm glucose for up to 96 h. Results were obtained from triplicate experiments. A representative gel each for C3 and C4 expression in relation to GAPDH expression was shown at the top. *P = 0·02, #P = 0·003 versus time zero control.
Fig. 2.
Dose effect of glucose on C3 (▪) and C4 (□) expression. Growth-arrested HPMCs in M199 media were exposed to escalating doses of glucose for 24 h. Results were obtained from triplicate experiments. A representative gel each for C3 and C4 expression in relation to GAPDH expression was shown at the top. *P = 0·018, #P = 0·001 compared to control.
Effect of glucose on complement secretion in HPMC
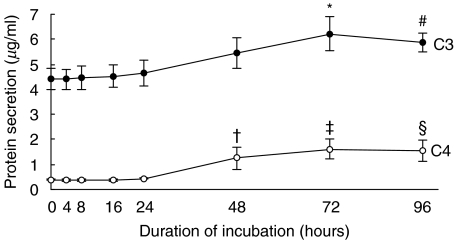
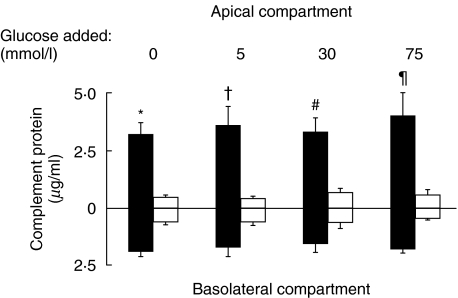
Constitutive secretion of C3 and C4 by HPMC was up-regulated after exposure to 75 mm glucose for 72 h and 48 h, respectively (Fig. 3). The polarity of C3 and C4 synthesis in HPMCs is shown in Fig. 4. HPMCs grown on permeable cell culture inserts produced C3 in both the apical and basolateral directions, with apical synthesis predominating regardless of the apical medium glucose concentration ranging from 0 to 75 mm. Apical and basolateral C3 secretion was not significantly altered after apical incubation with up to 75 mm of glucose for 24 h. C4 secretion was of a lower magnitude than C3, and C4 was secreted in both directions without polarity between the apical and basolateral media. Apical and basolateral C4 secretion was not significantly altered after apical incubation with glucose.
Fig. 3.
Time kinetics of C3 and C4 protein secretion. Growth-arrested HPMCs in M199 media were exposed to 75 mm glucose for up to 96 h. Cell supernatants were harvested for ELISA. Results were obtained from triplicate experiments. *P = 0·008, #P = 0·046, †P = 0·014, ‡P = 0·000, §P = 0·001 versus time zero control.
Fig. 4.
Polarity of C3 (▪) and C4 (□) production by HPMCs. Cells were grown to confluence on Transwell chambers and growth-arrested. Medium supplemented with different concentrations of glucose was added to the apical compartment, while medium alone was added to the basolateral compartment. After 24 h, apical and basolateral media were harvested for assay of C3 and C4 protein by ELISA. Results are means ±SD obtained from triplicate experiments. *P = 0·002, †P = 0·021, #P = 0·016, ¶P = 0·02 versus corresponding basolateral compartment.
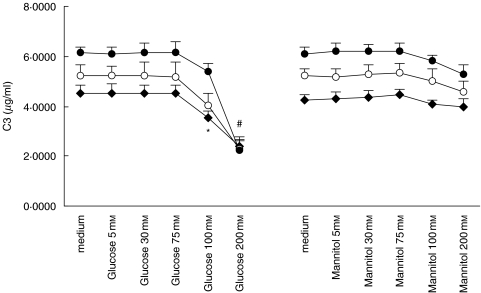
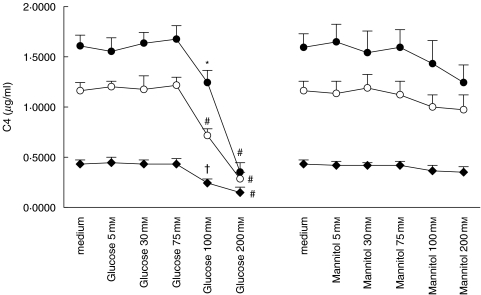
Exposure to glucose doses at 100 mm and beyond significantly down-regulated both C3 (Fig. 5) and C4 (Fig. 6) secretion in a time-dependent manner. To investigate whether these effects were due to glucose per se and to the hyperosmolar milieu it creates, we used equivalent doses of mannitol as an osmotic control. Constitutive C3 and C4 secretion was unchanged when exposed to mannitol instead of glucose, although there was a trend for reduced C3 (Fig. 5) and C4 (Fig. 6) secretion when the dose of mannitol reached 100 mm and beyond.
Fig. 5.
Effect of glucose and mannitol on C3 expression. Quiescent HPMCs were incubated with escalating doses of glucose or mannitol in M199 media for 24 h (⋄), 48 h (○), or 72 h (•), after which culture supernatants were harvested for C3 ELISA. Results were obtained from triplicate experiments. *P = 0·032, #P = 0·000 compared to medium control.
Fig. 6.
Effect of glucose and mannitol on C4 expression. Quiescent HPMCs were incubated with escalating doses of glucose or mannitol in M199 media for 24 h (⋄), 48 h (○), or 72 h (•), after which culture supernatants were harvested for C4 ELISA. Results were obtained from triplicate experiments. *P = 0·028, #P = 0·000, †P = 0·028 compared to medium control.
Apart from C3 and C4, the anaphylatoxin C5a is of potential interest because recent evidence suggests that C5a mediates an early step in the onset of sepsis by enhancing the production of various proinflammatory mediators in different cell types [15]. The level of C5a was below the detection limit of 0·625 ng/ml under all experimental conditions (data not shown).
Viability of HPMCs after exposure to glucose
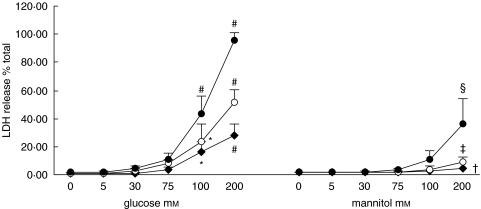
There was no appreciable change in cell number or LDH release in cell supernatant after exposure to 5, 30, or 75 mm of glucose for up to 72 h. Exposure to doses beyond 100 mm led to a significant dose- and time-dependent release of LDH. Parallel experiments using mannitol as an osmotic control showed marked attenuation in LDH release at doses above 100 mm compared to glucose as the stimulant (Fig. 7).
Fig. 7.
Effect of glucose and mannitol on LDH release. Quiescent HPMCs were incubated with escalating doses of glucose or mannitol in M199 media for 24 h (⋄), 48 h (○), or 72 h (•), after which culture supernatants were harvested for assay of LDH release. Results were obtained from triplicate experiments. *P = 0·01, #P = 0·000, †P = 0·026, ‡P = 0·001, §P = 0·002 compared to medium control.
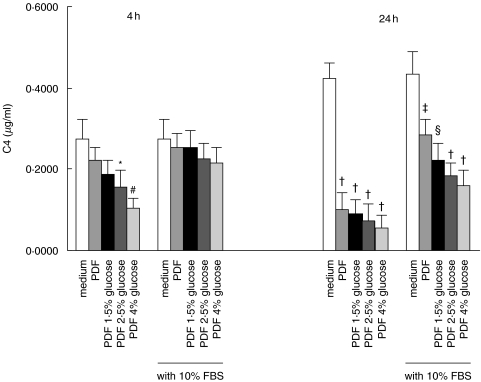
Effect of therapeutic peritoneal dialysis fluid (PDF)
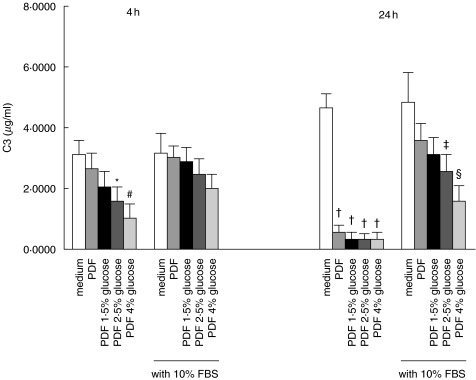
Treatment of HPMC with Gambrosol Trio dialysis fluid for 4 h attenuated C3 (Fig. 8) and C4 (Fig. 9) secretion compared to incubation in M199 culture medium. This effect was dependent on the strength of the dialysis solution used, and was markedly exaggerated upon extending the duration of incubation to 24 h. To investigate whether the inhibitory effect of PDF on HPMC C3 and C4 production was due to cytotoxicity, we supplemented the culture medium with serum. Supplementation with 10% FBS partially nullified the effect of PDF at 24 h. The effect of bicarbonate-buffered PDF (pH = 7·3) on C3 (Fig. 10) and C4 (Fig. 11) secretion in HPMC was not different from conventional lactate-buffered PDF.
Fig. 8.
Effect of peritoneal dialysis fluid (PDF) on C3 secretion. Quiescent HPMCs were incubated with serum-free M199 medium, or glucose-free PDF, or PDF of various glucose strengths, or equivalent solutions supplemented with 10% FBS for 4 h or 24 h. Culture supernatants were harvested for C3 ELISA. The glucose concentrations of 1·5%, 2·5%, and 4% solutions are 83 mm, 138 mm, and 220 mm, respectively. Results were obtained from triplicate experiments. *P = 0·018, #P = 0·002, †P = 0·000, ‡P = 0·011, §P = 0·001 compared to medium control.
Fig. 9.
Effect of peritoneal dialysis fluid on C4 secretion. Quiescent HPMCs were incubated with serum-free M199 medium, or glucose-free PDF, or PDF of various glucose strengths, or equivalent solutions supplemented with 10% FBS for 4 h or 24 h. Culture supernatants were harvested for C4 ELISA. The glucose concentrations of 1·5%, 2·5%, and 4% solutions are 83 mm, 138 mm, and 220 mm, respectively. Results were obtained from triplicate experiments. *P = 0·02, #P = 0·002, †P = 0·000, ‡P = 0·009, §P = 0·001 compared to medium control.
Fig. 10.
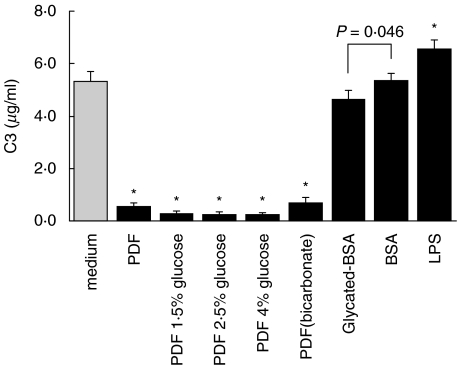
Effect of bicarbonate PDF and glycated albumin on C3 secretion. Quiescent HPMCs were treated with PDF of different strengths, glycated BSA (0·25 µg/ml), or BSA (0·25 µg/ml) in M199. Incubation with 10 ng/ml lipopolysaccharide (LPS) in M199 served as positive control. After 48 h, culture supernatants were harvested for C3 ELISA. The glucose concentrations of 1·5%, 2·5%, and 4% solutions are 83 mm, 138 mm, and 220 mm, respectively. Results were obtained from triplicate experiments. *P = 0·000 compared to medium control.
Fig. 11.
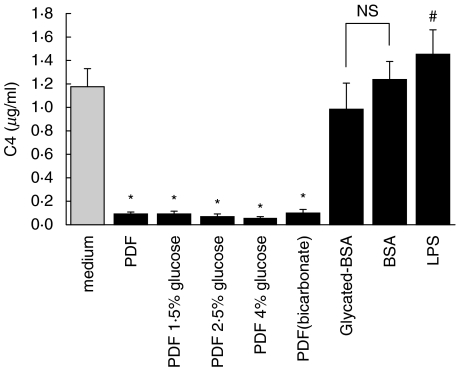
Effect of bicarbonate PDF and glycated albumin on C4 secretion. Quiescent HPMCs were treated with PDF of different strengths, glycated BSA (0·25 µg/ml), or BSA (0·25 µg/ml) in M199. Incubation with 10 ng/ml lipopolysaccharide (LPS) in M199 served as positive control. After 48 h, culture supernatants were harvested for C4 ELISA. The glucose concentrations of 1·5%, 2·5%, and 4% solutions are 83 mm, 138 mm, and 220 mm, respectively. Results were obtained from triplicate experiments. *P = 0·000, #P = 0·007 compared to medium control.
Treatment with BSA modified by advanced glycation (AGE-BSA) reduced C3 secretion as compared to BSA alone (Fig. 10). There was also a trend for down-regulated C4 secretion by AGE-BSA, although the difference did not reach statistical significance (Fig. 11). LPS (10 ng/ml) stimulated both C3 and C4 secretion by HPMC and served as a positive control. C5a remained undetectable under all experimental conditions (data not shown).
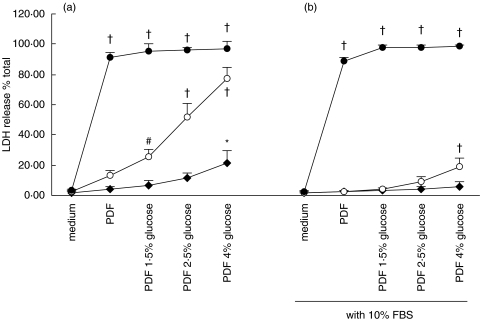
The effect of PDF on LDH release in HPMC is depicted in Fig. 12. LDH release from HPMC was a function of both the glucose concentration of the dialysis fluid and the duration of exposure to PDF. The LDH response at 1 h and 4 h, but not at 24 h, of incubation was markedly attenuated when PDF was supplemented with 10% FBS.
Fig. 12.
Effect of peritoneal dialysis fluid on LDH release. Quiescent HPMCs were incubated with serum-free M199 medium, or glucose-free PDF, or PDF of various glucose strengths (a), or equivalent solutions supplemented with 10% FBS (b) for 1 h (⋄), 4 h (○), or 24 h (•). Culture supernatants were harvested for assay of LDH release. The glucose concentrations of 1·5%, 2·5%, and 4% solutions are 83 mm, 138 mm, and 220 mm, respectively. Results were obtained from triplicate experiments. *P = 0·001, #P = 0·005, †P = 0·000 compared to medium control.
Discussion
Various studies have indicated that the morphological changes in the peritoneal mesothelium that accompany long-term PD are heralded by a state of chronic inflammation which subsequently progresses to denudation of peritoneal mesothelial cells, and duplication of submesothelial and capillary basement membranes [1, 16, 17]. The terminal events are represented by mesenchymal transdifferentiation, fibrosis and, ultimately, ultrafiltration failure. The molecular mechanisms underlying these immunopathological processes are not well understood, and are likely to be multifactorial. Despite the continuous development of new dialysis solutions over the past two decades [18], one fundamental property that has hardly changed is the use of hypertonic glucose as an osmotic agent. The complement cascade is a major mediator of inflammation and host protection, and plays an instrumental role in the development of an effective inflammatory response by generating important peptides, known collectively as complement ‘split products’, of which C3a, C4a, and C5a are the most pro-inflammatory [7]. The capacity of peritoneal mesothelial cells to synthesize complement components during PD [2, 3, 19] implies that complement may play a role in immunoregulation in the peritoneal cavity during PD. Yet, limited data is available in the literature on the role of complement in the evolution of peritoneal membrane structure and function in the context of chronic PD.
In the present study, we examined three major components of the complement system in HPMCs – C3, C4, and C5a. C3 is the most abundant complement protein in circulation and plays a pivotal role in complement activation, as the classical, the alternative and lectin pathways have to proceed through cleavage of the C3 molecule [20]. C4 functions as an intermediary component of the classical and lectin pathways. Its local production could facilitate response against pathogens or aggravate immune-mediated injury by releasing the anaphylotoxin, C4a [21], and stimulating antigen-specific T-cells during antigen presentation [20]. C5a is also an anaphylatoxin and can cause toxic oxygen radical formation [15]. Here, we showed that HPMCs expressed C3 and C4 constitutively. In contrast, C5a was not generated during these experiments. Exposure to 75 mm glucose up-regulated both C3 and C4 production. However, exposure to glucose beyond 75 mm significantly down-regulated C3 and C4 expression and stimulated LDH release in HPMCs, suggesting that these concentrations of glucose are cytotoxic. The detrimental effects of supraphysiological concentrations of glucose on HPMCs are further supported by the failure of equivalent doses of mannitol as an osmotic control to reproduce the impact of glucose alone. This concurs with the finding by Gotloib et al. [22] in a mouse model of peritoneal dialysis that the harmful effects of hyperosmolality per se on the mesothelium are only modest as opposed to those of hypertonic glucose.
Although the glucose content of even the lowest strength of neat commercial dialysis solution exceeds 75 mm, our unpublished experience obtained from performing the peritoneal equilibration test [23] in over 350 subjects receiving maintenance PD showed that the steady state glucose level of spent dialysate is in the range 71·3 ± 16·8, 46·1 ± 21·7, and 24·0 ± 13·3 mm for 4%, 2·5%, and 1·5% dialysis solutions, respectively. This implies that C3 and C4 expression in mesothelial cells, upon prolonged exposure to a glucose load of less than 75 mm during the steady-state in patients undergoing maintenance PD, may be chronically stimulated to promote a proinflammatory environment. Our finding of basal, as well as apical secretion of C3 and C4 is consistent with the speculation that complement may participate in the process of chronic inflammation of the peritoneal membrane during maintenance PD. Further studies to validate or refute such contention would be best carried out in a complement knockout animal model, such as the C3-deficient mice [24].
Another important function of the peritoneal mesothelium is defence against and clearance of peritonitis in PD. Despite substantial improvements in bag connection technology, peritonitis remains an important cause of morbidity and dialysis failure in patients undergoing PD. A large body of evidence indicates that conventional peritoneal dialysate fluids cause a functional impairment of peritoneal host defence mechanisms [25], which consist mainly of resident mononuclear phagocytes, mesothelial and dendritic cells [5]. The initial phase of the inflammatory response to infection involves activation of the innate immune system, in particular the complement system and neutrophils [7]. Bacteria are able to activate both the classical and alternative pathways, leading to opsonization of bacterial cells by C3. This in turn enhances their phagocytosis by neutrophils and macrophages. The major organisms causing PD-related peritonitis include Staphylococcus aureus, Staphylococcus epidermidis, and other Gram-negative bacteria such as Escherichia coli and Pseudomonas aeruginosa [26]. These microbes have been shown to infect the peritoneum by bacterial attachment followed by internalization [27]. Furthermore, Celik et al. [28] has demonstrated that an effective antimicrobial immune defence in a mouse model of peritonitis is complement-dependent. Thus, we undertook to investigate whether PD may affect complement synthesis in the peritoneal mesothelium.
To mimic the in vivo environment in PD, HPMCs were incubated with various strengths of commercial PD solutions with glucose content ranging from 83 mm to 220 mm. Regardless of the strength of the solution used, constitutive C3 and C4 expression was markedly suppressed, while LDH release was stimulated. These effects were partially abrogated by supplementation with 10% FBS, signifying the cytotoxic nature of PD solutions. This enhanced viability of HPMCs with FBS supplementation is consistent with our previous in vitro findings [29].
To investigate the effect of the lactate component of dialysate, we used a bicarbonate-buffered solution and found little change in the inhibitory capacity of the fluid on complement production by HPMCs as compared with conventional lactate-buffered dialysate. Such finding is in keeping with previous report on the suboptimal biocompatibility of bicarbonate solutions [30].
Apart from tonicity, pH, and high glucose contents, the formation of glucose degradation products during heat-sterilization and storage of the dialysate is another important consideration. Glucose as a highly reactive substance is easily degraded into glucose degradation products (GDP), which are toxic [31] and can give rise to early or advanced glycation end products. Exposure of HPMCs to glycated BSA marginally inhibited C3 expression compared with control, while C4 expression was also decreased though the reduction did not achieve statistical significance. Taken together, our findings suggest that hypertonic glucose, lactate, and possibly the bicarbonate components of commercial PD dialysates as well as AGEs are all toxic to HPMCs. The chief components of the peritoneal defence system are resident macrophages, neutrophils that are recruited from the systemic circulation, mesothelial cells, and fibroblasts [5, 32, 33]. In view of the link between complement fragments and the opsonic and phagocytic property of macrophages [7, 21, 34], it would be logical to postulate that the down-regulation of complement production by PD solutions leads to increased susceptibility to infection due to ineffective clearance of any invading bacteria. In conclusion, our findings suggest that the artificial conditions imposed by PD lead to alterations in local complement synthesis that have implications for the role of the peritoneal mesothelium in both inflammation (through up-regulated basolateral complement production in the steady state) and defence (through cytotoxicity and down-regulated complement production upon acute exposure to dialysis solutions).
Acknowledgments
This study is supported by the Seed Funding for Basic Research Programme of the University of Hong Kong. The authors thank Mr J Buckley, Department of Urology, Guy’s, King's College and St Thomas’ Hospitals’ Medical School, King's College London, United Kingdom, and Drs PC Tam, SM Chu, and B Wong, Division of Urology, Department of Surgery, Queen Mary Hospital, Hong Kong, and Drs M Que, HS So and Ly Ho, Division of Urology, Department of Surgery, United Christian Hospital, Hong Kong, for harvesting human omental tissue.
References
- 1.Coles GA, Topley N. Long-term peritoneal membrane changes. Adv Ren Replace Ther. 2000;7:289–301. doi: 10.1053/jarr.2000.16268. [DOI] [PubMed] [Google Scholar]
- 2.Young GA, Kendall S, Brownjohn AM. Complement activation during CAPD. Nephrol Dial Transplant. 1993;8:1372–5. [PubMed] [Google Scholar]
- 3.Reddingius RE, Schroder CH, Daha MR, Willems HL, Koster AM, Monnens LA. Complement in serum and dialysate in children on continuous ambulatory peritoneal dialysis. Perit Dial Int. 1995;15:49–53. [PubMed] [Google Scholar]
- 4.Faull RJ. Peritoneal defenses against infection: winning the battle but losing the war? Semin Dial. 2000;13:47–53. doi: 10.1046/j.1525-139x.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- 5.Broche F, Tellado JM. Defense mechanisms of the peritoneal cavity. Curr Opin Crit Care. 2001;7:105–16. doi: 10.1097/00075198-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 7.Barrington R, Zhang M, Fischer M, Carroll MC. The role of complement in inflammation and adaptive immunity. Immunol Rev. 2001;180:5–15. doi: 10.1034/j.1600-065x.2001.1800101.x. [DOI] [PubMed] [Google Scholar]
- 8.Lai KN, Leung JC, Chan LY, et al. Expression of aquaporin-3 in human peritoneal mesothelial cells and its up-regulation by glucose in vitro. Kidney Int. 2002;62:1431–9. doi: 10.1111/j.1523-1755.2002.kid564.x. [DOI] [PubMed] [Google Scholar]
- 9.Topley N, Jorres A, Luttmann W, et al. Human peritoneal mesothelial cells synthesize interleukin-6: induction by IL-1 beta and TNF alpha. Kidney Int. 1993;43:226–33. doi: 10.1038/ki.1993.36. [DOI] [PubMed] [Google Scholar]
- 10.Yang CW, Vlassara H, Peten EP, He CJ, Striker GE, Striker LJ. Advanced glycation end products up-regulate gene expression found in diabetic glomerular disease. Proc Natl Acad Sci USA. 1994;91:9436–40. doi: 10.1073/pnas.91.20.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 12.de Bruijn MH, Fey GH. Human complement component C3: cDNA coding sequence and derived primary structure. Proc Natl Acad Sci USA. 1985;82:708–12. doi: 10.1073/pnas.82.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neubauer A, Neubauer B, Liu E. Polymerase chain reaction based assay to detect allelic loss in human DNA. loss of beta-interferon gene in chronic myelogenous leukemia. Nucl Acids Res. 1990;18:993–8. doi: 10.1093/nar/18.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung JC, Tsang AW, Chan DT, Lai KN. Absence of CD89, polymeric immunoglobulin receptor, and asialoglycoprotein receptor on human mesangial cells. J Am Soc Nephrol. 2000;11:241–9. doi: 10.1681/ASN.V112241. [DOI] [PubMed] [Google Scholar]
- 15.Riedemann NC, Guo RF, Ward PA. Conceptual perspectives. A Key Role C5a/C5aR activation for the development of sepsis. J Leukoc Biol. 2003;74:966–70. doi: 10.1189/jlb.0403137. [DOI] [PubMed] [Google Scholar]
- 16.Williams JD, Craig KJ, Topley N, Williams GT. Peritoneal dialysis: changes to the structure of the peritoneal membrane and potential for biocompatible solutions. Kidney International. 2003;63(S84) Suppl:S158–S161. doi: 10.1046/j.1523-1755.63.s84.46.x. [DOI] [PubMed] [Google Scholar]
- 17.Yanez-Mo M, Lara-Pezzi E, Selgas R, et al. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Engl J Med. 2003;348:403–13. doi: 10.1056/NEJMoa020809. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Lopez E, Lindholm B, Tranaeus A. Biocompatibility of new peritoneal dialysis solutions: clinical experience. Perit Dial Int. 2000;20:S48–S56. [PubMed] [Google Scholar]
- 19.Barbano G, Cappa F, Prigione I, et al. Peritoneal mesothelial cells produce complement factors and express CD59 that inhibits C5b-9-mediated cell lysis. Adv Perit Dial. 1999;15:253–7. [PubMed] [Google Scholar]
- 20.Erdei A, Fust G, Gergely J. The role of C3 in the immune response. Immunol Today. 1991;12:332–7. doi: 10.1016/0167-5699(91)90011-H. [DOI] [PubMed] [Google Scholar]
- 21.Frank MM, Fries LF. The role of complement in inflammation and phagocytosis. Immunol Today. 1991;12:322–6. doi: 10.1016/0167-5699(91)90009-I. [DOI] [PubMed] [Google Scholar]
- 22.Gotloib L, Wajsbrot V, Shostak A, Kushnier R. Effect of hyperosmolality upon the mesothelial monolayer exposed in vivo and in situ to a mannitol-enriched dialysis solution. Nephron. 1999;81:301–9. doi: 10.1159/000045297. [DOI] [PubMed] [Google Scholar]
- 23.Twardowski ZJ. PET – a simpler approach for determining prescriptions for adequate dialysis therapy. Adv Perit Dial. 1990;6:186–91. [PubMed] [Google Scholar]
- 24.Springall T, Sheerin NS, Abe K, Holers VM, Wan H, Sacks SH. Epithelial secretion of C3 promotes colonization of the upper urinary tract by Escherichia coli. Nat Med. 2001;7:801–6. doi: 10.1038/89923. [DOI] [PubMed] [Google Scholar]
- 25.Jorres A, Gahl GM, Frei U. Peritoneal dialysis fluid biocompatibility: does it really matter? Kidney Int. 1994;48(Suppl):S79–S86. [PubMed] [Google Scholar]
- 26.Voinescu CG, Khanna R. Peritonitis in peritoneal dialysis. Int J Artif Organs. 2002;25:249–60. doi: 10.1177/039139880202500402. [DOI] [PubMed] [Google Scholar]
- 27.Visser CE, Brouwer-Steenbergen JJ, Schadee-Eestermans IL, Meijer S, Krediet RT, Beelen RH. Ingestion of Staphylococcus aureus, Staphylococcus epidermidis, and Escherichia coli by human peritoneal mesothelial cells. Infect Immun. 1996;64:3425–8. doi: 10.1128/iai.64.8.3425-3428.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Celik I, Stover C, Botto M, et al. Role of the classical pathway of complement activation in experimentally induced polymicrobial peritonitis. Infect Immun. 2001;69:7304–9. doi: 10.1128/IAI.69.12.7304-7309.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai KN, Ho SK, Leung J, Tang SC, Chan TM, Li FK. Increased survival of mesothelial cells from the peritoneum in peritoneal dialysis fluid. Cell Biol Int. 2001;25:445–50. doi: 10.1006/cbir.2000.0664. [DOI] [PubMed] [Google Scholar]
- 30.Schambye HT, Pedersen FB, Wang P. Bicarbonate is not the ultimate answer to the biocompatibility problems of CAPD solutions: a cytotoxicity test of CAPD solutions and effluents. Adv Perit Dial. 1992;8:42–6. [PubMed] [Google Scholar]
- 31.Martinson E, Wieslander A, Kjellstrand P, Boberg U. Toxicity of heat sterilized peritoneal dialysis fluids is derived from degradation of glucose. ASAIO J. 1992;38:M370–M372. doi: 10.1097/00002480-199207000-00057. [DOI] [PubMed] [Google Scholar]
- 32.Jones S, Holmes CJ, Mackenzie RK, et al. Continuous dialysis with bicarbonate/lactate-buffered peritoneal dialysis fluids results in a long-term improvement in ex vivo peritoneal macrophage function. J Am Soc Nephrol. 2002;13:S97–103. [PubMed] [Google Scholar]
- 33.Mackenzie RK, Topley N, Neubauer A, Coles GA, Williams JD. Staphylococcal exoproducts down-regulate cyclooxygenase 1 and 2 in peritoneal macrophages. J Laboratory Clin Med. 1997;129:23–34. doi: 10.1016/s0022-2143(97)90158-x. [DOI] [PubMed] [Google Scholar]
- 34.Faust D, Akoglu B, Zgouras D, Scheuermann EH, Milovic V, Stein J. Anti-inflammatory drugs modulate C1q secretion in human peritoneal macrophages in vitro. Biochem Pharmacol. 2002;64:457–62. doi: 10.1016/s0006-2952(02)01183-8. [DOI] [PubMed] [Google Scholar]