Abstract
The ability of calcium phosphate (CaP) and calcium pyrophosphate (CaPPi) to mediate matrix metalloproteinase-2 and -9 (MMP-2 and MMP-9) binding to fibrin was evaluated. Substrate gel electrophoresis (gelatin zymography) revealed that CaP bound MMP-2 and MMP-9, forming a high molecular weight aggregate with lowered electrophoretic mobility. Formation of the CaP : MMP aggregate was necessary for fibrin binding. In contrast, CaPPi did not aggregate MMPs and did not promote uptake of MMPs into fibrin. Scatchard analysis (Ca/P ratio) revealed that CaPPi (1·96) was chemically similar to calcium pyrophosphate dihydrate (2·00) compared to amorphous CaP (1·50) or crystalline CaP, hydroxyapatite (1·66). MMP : CaP interaction appeared to be electrostatic in nature as high salt concentration (NaCl > 150 mm) reduced binding. In contrast, two non-ionic detergents (Brij-35 and Tween-20) did not prevent MMP : CaP binding. MMP : CaP interaction did not involve the C-terminal MMP region because the specific tissue inhibitor of metalloproteinases (TIMPs) also did not block MMP : CaP interaction and fibrin binding. Although MMP : CaP binding could be decreased with albumin, this effect appeared non-specific due to the high albumin concentration required. High albumin concentration could also partially dissociate preformed MMP : CaP complexes. Interestingly, type I and type IV collagen substantially increased MMP : fibrin-binding activity, whereas denatured collagen, gelatin, did not. Inflammatory joint fluid from five patients also demonstrated similar MMP fibrin-binding activity consistent with CaP mediation. The relevance of these findings to CaP and CaPPi in the pathogenesis of crystal arthropathies such as basic calcium phosphate (BCP) and calcium pyrophosphate dihydrate crystal disease (CPPD) is discussed.
Keywords: calcium phosphate, inflammation, matrix metalloproteinases, synovial fluid
Introduction
Degradation of extracellular matrix proteins by matrix metalloproteinases (MMPs) is significant in diseases states characterized by tissue destruction and inflammation (arthritis, defective wound healing) or aberrant cell proliferation and migration (tumour invasion and metastasis) [1–3]. MMPs are calcium- and zinc-dependent proteinases that degrade fibrillar and basement membrane collagens as well as other extracellular matrix proteins through selective and overlapping substrate specificities [2,3]. MMPs are synthesized as pro-forms that are activated by N-terminal proteolytic cleavage [2–4]. Although autocatalytic activation can occur with MMPs, plasmin is believed to be responsible for activation in vivo and is controlled by the plasminogen activator/inhibitor (PA/PAI) system [4,5]. Specific tissue inhibitors of metalloproteinases (TIMPs) also play a role in MMP regulation [6]. Although MMP : substrate interaction can protect MMPs from TIMP inhibition, interactions with other matrices or membranes may also modify activity [5,7–10]. As such, regulation of MMP function is multi-faceted and reflects mechanisms of activation (PA/PAI system) and inhibition (MMP/TIMP ratio) as well as other less well-defined ancillary processes that may mediate MMP interaction with matrices and membranes or other extracellular components.
Our studies have demonstrated that neutrophil MMP-9 (gelatinase B), a type IV collagenase comprised of 92-, 130- and 225-kDa forms, selectively and tightly binds fibrin [5,9]. Latent MMP forms becoming activated in a time and plasmin-dependent fashion concomitant with fibrinolysis [5]. MMP-9 binding to fibrin is mediated by calcium phosphate (CaP) in a dose- and time-dependent manner with formation of an MMP : CaP intermediate being the rate-limiting step [9]. MMP binding to fibrin may protect activated MMP from endogenous inhibitors and localizes proteolytic activity directly to sites of tissue injury or extracellular matrix turnover. Thus, the fibrin binding of latent MMP may co-ordinate MMP activation with fibrinolysis and thereby influence control of extracellular matrix degradation during wound healing or tissue remodelling. Pyrophosphate (PPi), an inhibitor of CaP formation, was shown to be an effective inhibitor of MMP : fibrin binding at physiological PPi concentrations [10]. PPi is an endogenous product of numerous metabolic pathways [11] and is considered significant for physiological bone metabolism [12] and is also known to complex calcium in pathophysiological development of crystal arthropathies [13]. In this study we sought to characterize the role of CaP and CaPPi complexes with MMP-9 and MMP-2 (fibroblast-derived gelatinase A) and the ability of both calcium-based compounds to mediate fibrin binding. Our results show that CaP can complex MMP-9 and MMP-2 and mediate fibrin binding, whereas CaPPi complexes were inactive as mediators of MMP : fibrin binding. These findings are compared to MMPs from inflammatory joint fluid that exhibit fibrin binding-activity.
Materials and Methods
Materials
Thrombin was from Parke-Davis, Inc. (Morris Plains, NJ, USA). Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) molecular weight calibration proteins and Ficoll-Hypaque were from Pharmacia-LKB, Inc. (Piscataway, NJ, USA). Gelatinase molecular weight calibration standards were prepared as described previously [14]. Sodium pyrophosphate, methanol and glacial acetic acid were obtained from J. T. Baker, Inc. (Phillipsburg, NJ, USA). Sodium citrate and acid–citrate–dextrose Vacutainer tubes were from Becton-Dickinson, Inc. (East Rutherford, NJ, USA). Dialysis membranes (12–14 kDa Mw cut-off) were from Spectrum Medical Laboratories, Inc. (Los Angeles, CA, USA). Fibrinogen sources were sodium citrate anti-coagulated human plasma collected by venipuncture or purified bovine fibrinogen obtained from Organon Teknika, Corp. (Durham, NC, USA). The tissue inhibitor of matrix metalloproteinase-1 and N-terminal TIMP were the generous gifts of Dr Kirby Bodden (University of Alabama, Birmingham, AL, USA). Acid soluble type I collagen (rat tail) and type IV collagen (human placenta), gelatin–agarose affinity chromatography media and all other reagents were from Sigma Chemical Company (St Louis, MO, USA).
Matrix metalloproteinase (MMP-9) preparation
MMP-9 (gelatinase B, type IV collagenase), and was prepared as its composite molecular weight forms (92-, 130- and 225-kDa) from human polymorphonuclear leucocytes (PMN) [5] collected from healthy volunteers. Briefly, whole blood was collected by venipuncture in acid–citrate–dextrose and PMN were isolated by dextran T-500 sedimentation, Ficoll-Hypaque density gradient centrifugation and hypotonic lysis [15]. PMNs were suspended in 1× Hanks's buffered salt solution and enumerated by an automated haematology instrument (Cell-Dyne, Model 3500, Abbott Laboratories, Inc., Abbott Park, IL, USA). Manual counting methods verified purity (averaged 95–98%) and viability by trypan blue exclusion (>95%). PMNs were aliquoted, mixed with an equal volume of 2 m NaCl at a final concentration of ∼18 000 cells/ml and stored at −30°C. The MMP-9 extract was prepared by freeze–thaw and suspension in two volumes of 50 mm Tris-HCl, pH 7·6. To remove cell debris, MMP-9 samples were centrifuged (15 000 g, 10 min). The MMP-9 extract was prepared just prior to use and stored on ice at 4–6°C. MMP preparations were characterized by gelatin zymography (see below).
Matrix metalloproteinase (MMP-2) preparation
Human plasma anticoagulated with sodium citrate served as the source of macrophage/fibroblast-derived MMP-2 (gelatinase A, 72-kDa type IV collagenase) [16,17]. This preparation has been characterized previously as having minimal contamination from neutrophil-derived MMP-9 [5]. Briefly, plasma (2·5 ml) was diluted 1 : 20 with 50 mm Tris-Cl, pH 7·6 containing 1 mm CaCl2 and loaded onto a gelatin-agarose column (5 cm × 1·0 cm) equilibrated in the same buffer. The flow-through fraction was collected and the column was washed extensively with the same buffer until absorbance measurements (415 nm) indicated negligible background levels (<0·030 absorbance units). MMP-2 was then batch-collected by application of high salt buffer (50 mm Tris-Cl, pH 7·6, 1 mm CaCl2 and 500 mm NaCl) containing 5% dimethylsulphoxide (DMSO) [17]. MMP-2 elution profile was confirmed by gelatin zymography. The column fractions containing only MMP-2 were pooled (∼4 ml, fractions 12–14) and dialysed overnight against two changes (1 l each) of 50 mm Tris-Cl, pH 7·6 containing 1 mm CaCl2 at 4–6°C. Gelatin–agarose column fractions and MMP-2 purity were assessed by SDS-PAGE and gelatin zymography.
Calcium phosphate (CaP) and calcium pyrophosphate (CaPPi) formation
CaP and CaPPi aggregation was performed in 10 mm CaCl2, containing either 0–500 µm sodium/potassium phosphate (Na2HPO4/KH2PO4, 5/1 molar ratio) or 0–500 µm sodium pyrophosphate in 50 mm Tris-HCl, pH 7·6 (950 µl final volume, 37°C, 2 h). All incubations were performed at 37°C in a thermostatically controlled water bath. CaPPi aggregate formation was monitored by turbidity at 405 nm [9,18] using a microtitre well plate reader (ThermoMAX, Molecular Devices, Inc., Sunnyvale, CA, USA). Formation kinetics of CaP with non-crystalline amorphous composition (precursor to crystalline hydroxyapatite) have been described previously [9]. For determination of non-complexed free ‘soluble’ calcium, samples were precipitated by centrifugation (15 000 g, 10 min). Calcium remaining in the supernatant was determined by arsenoazo II dye binding [19] on an automated analytical instrument (Model CX-7, Beckman Instruments, Inc., Brea, CA, USA).
MMP aggregation and fibrin-binding assay
For binding studies, 25 µl MMP-9 extract or purified MMP-2 (∼0·025 µg MMPs) was added to the CaP or CaPPi incubation mix (see above) for 15 min at 37°C. An aliquot was removed and MMP binding with CaP or CaPPi was monitored by gelatin zymography (see below). Fibrin binding assays with MMP-9 were performed using plasma as the source of fibrinogen. Because MMP-2 is a normal plasma constituent [16], fibrin-binding assays with MMP-2 were performed using purified bovine fibrinogen prepared as a stock concentration of 2 mg/ml in 50 mm Tris-Cl, pH 7·6, 135 mm NaCl. Plasma was collected in sodium citrate anticoagulant and stored at −30°C prior to use. The concentration of plasma fibrinogen was determined by activity assay [20] on an automated clinical instrument (Model ACL3000, Instrument Laboratory/Coulter, Inc., Lexington, MA, USA) and averaged 2 mg/ml (normal range 1·5–4 mg/ml). Dilute solutions of plasma (1 : 20) were used to minimize artifactual trapping of serum proteins and facilitate clot preparation [21]. Briefly, 50 µl plasma or purified bovine fibrinogen (see above) were added to the above reaction mix and fibrin polymerization catalysed by the addition of 5 µl thrombin (100 U/ml). Following incubation (15 min, 37°C) in a thermostatically controlled water bath (Model 25, Precision Scientific Instruments, Inc., Chicago, IL, USA), the tubes were vortex mixed (5 s) and the fibrin matrix spooled and condensed of a glass Pasteur pipet. The fibrin matrix was vortex washed in 1 ml 50 mm Tris-Cl, pH 7·6, transferred to 100 µl non-reducing 2× Laemmli SDS sample buffer [22] and subjected to mild heating (15 min, 37°C) with vortex mixing (3–5 times). Following equilibration in SDS, fibrin matrices were removed from the sample buffer and loaded directly in wells. Electrophoresis and gelatin zymography were performed as described below. For competition studies, fibrin matrices were generated with varying salt (25–225 mm NaCl), non-ionic detergents (0·01–0·10% Brij-35, Tween-20), non-specific protein (0–1000 µg/ml bovine serum albumin) and MMP-specific proteins including denatured collagen, i.e. gelatin (75–750 µg/ml) and native type I (fibrillar) and type IV (basement membrane) collagens (5–50 µg/ml). The specific tissue inhibitors of matrix metalloproteinases (1–100 nm TIMP-1, N-terminal TIMP) were used for blockage of the MMP C-terminal region.
Gelatin zymography
Substrate gel electrophoresis (gelatin zymography) was performed as described previously [14]. Briefly, SDS-PAGE [22,23] was performed on 7·5% polyacrylamide slab gels (11 cm resolving gel height, 0·75 mm thickness) containing co-polymerized gelatin (1·5 mg/ml). Samples containing the MMP : CaP or MMP : CaPPi aggregate were mixed with an equal volume of nonreducing 2× Laemmli sample buffer [22] and loaded into sample wells (20 µl/well). Fibrin matrices were prepared as described above and one matrix was loaded per lane. Following electrophoresis (22 mA/gel, constant current), gels were washed twice in 200 ml 2·5% (v/v) Triton X-100 (30 min each) on a platform mixer at ambient temperature. Gels were incubated in 100 ml 50 mm Tris-HCl, pH 7·6 and 5 mm CaCl2 (18–20 h, 37°C) in a closed thermostatically controlled water bath. Gelatin zymograms were stained with 0·2% (w/v) Coomassie brilliant blue R-250 in 50% methanol and 10% acetic acid and destained appropriately in 40% methanol and 10% acetic acid. Gelatinase activity appeared as cleared (unstained) regions against a dark background. Gelatinases resolved by zymography were compared to previously characterized gelatinase molecular weight standards [14]. Gelatin zymograms were photographed wet with background lighting. Gels were dried between porous cellophane sheets under vacuum with heating for storage.
SDS-PAGE
SDS-PAGE was performed as described previously [22,23]. Fibrin matrices (see above) were denatured by addition of 100 µl 2× Laemmli sample buffer containing 10% (v/v) β-mercaptoethanol. The tubes were periodically vortex mixed (3–5 times) during heating in a boiling water bath (∼10 min) to facilitate solubilization. Denatured fibrin samples (15 µl/lane) were electrophoresed on 8·5% polyacrylamide slab gels (0·75 mm thickness) at constant current (22 mA). Following electrophoresis, gels were stained with Coomassie blue, processed, and stored as described above (see gelatin zymography). Reduced fibrin subunits (γ-γ dimers, β-monomers and high molecular weight α-polymers) were identified by comparison to literature values [24]. SDS-PAGE protein molecular weight standards were: phosphorylase b (94 kDa), bovine serum albumin (67 kDa), ovalbumin (43 kDa), carbonic anhydrase (30 kDa), soybean trypsin inhibitor (20·1 kDa) and lactalbumin (14·4 kDa).
Fibrin binding MMPs in synovial fluid samples
Synovial fluids were obtained by aseptic aspiration from the knees of five patients with recurrent crystal-negative, culture-negative, inflammatory effusions (WBCs 7,000–40 000; PMNs > 75%). Patients included two males (ages 26 and 35 years) with psoriatic arthritis and females with systemic lupus erythematosus (age 42), rheumatoid arthritis (age 77) and suspected early rheumatoid arthritis (age 68 years). Synovial fluids were subjected to manual cell counting and cells were subsequently removed by centrifugation (3500 r.p.m., 15 min) in a benchtop centrifuge (Model C412, Jouan Inc, Winchester, VA, USA). The cell-free synovial fluid was then fractionated into three MMP compartments: ‘total’; ‘soluble’; and ‘pellet’. The ‘total’ was obtained by diluting the cell-free synovial fluid 10-fold in 50 mm Tris-Cl, pH 7·6. A ‘soluble’ MMP fraction was obtained following centrifugation of the ‘total’ fraction in an Eppendorf benchtop centrifuge (5 min). The ‘pellet’ was obtained by resuspension of the pelleted material in 50 µl of 50 mm Tris-Cl, pH 7·6. The ‘total’, ‘soluble’ or ‘pellet’ fractions were examined for protein content (SDS-PAGE), MMP composition (gelatin zymography) and MMP fibrin-binding activity (as described in the figure legends). To examine the dose dependence of MMP fibrin-binding activity, MMP (0–10 µl) was added to the fibrin-binding reaction mix (1 ml final volume) containing plasma (as fibrinogen source) and fibrin polymerization catalysed by the addition of 5 µl thrombin (100 U/ml), as described above. An exogenous source of fibrinogen was required in these studies as no fibrin formation was observed following the addition of thrombin directly to the joint fluid sample (data not shown). Fibrin matrices were extracted as described above and the fibrin matrix and SDS-extractable fraction analysed by gelatin zymography. An aliquot of the reaction mix prior to and following thrombin addition were also analysed by gelatin zymography.
Results
Previously, we demonstrated that MMP-9 binding to fibrin was mediated by amorphous calcium phosphate (CaP) via a transient CaP : MMP-9 complex [9] that could be disrupted by physiological pyrophosphate (PPi) concentrations [10]. These studies also demonstrated that calcium could also aggregate PPi, but identity of the Ca : PPi complex and ability to mediate MMP fibrin binding were not evaluated. Because disrupted PPi metabolism is a common finding in inflammatory joint disease, i.e. calcium pyrophosphate dihydrate deposition disease (CPPD) [25,26], we sought to determine if calcium pyrophosphate (CaPPi) could also mediate cartilage destruction via MMP binding to fibrin.
Calcium (10 mm), incubated with increasing phosphate (PO4) or pyrophosphate (PPi) (0–500 µm), resulted in the formation of an insoluble aggregate that could be monitored turbidometrically at 405 nm (Fig. 1). The sigmoidal pattern of CaP formation and dependence on ≥ 250 µm PO4 was consistent with previous studies that identified the insoluble aggregate as amorphous CaP (Table 1) [9,12]. A calcium aggregate was also obtained in the presence of Ppi; however, its formation was linear with respect to PPi concentration. Scatchard analysis of the CaPPi complex revealed a chemical composition, i.e. a Ca/P ratio (1·96), consistent with calcium pyrophosphate dihydrate (Fig. 2, Table 1).
Fig. 1.
Formation of calcium phosphate (CaP) and calcium pyrophosphate (CaPPi). Calcium (10 mm) was incubated with 0–500 µm phosphate (PO4) or pyrophosphate (PPi) (2 h, 37°C), i.e. conditions shown previously to optimize for amorphous CaP formation [9,12]. Formation of insoluble CaP (a) and CaPPi (b) was monitored turbidometrically at 405 nm.
Table 1.
Chemical composition of calcium phosphate and calcium pyrophosphate
| Calcium phosphate compound | Chemical Composition | Ca/P | Ref. |
|---|---|---|---|
| Amorphous calcium phosphate | Ca9(PO4)6 | 1·50 | [9,27] |
| Hydroxyapatite | Ca10(PO4)6(OH)2 | 1·66 | [27] |
| Calcium pyrophosphate dihydrate | Ca2P2O7. 2H2O | 2·001 | [28] |
| Ca:PPi complex2 | – | 1·96 |
Ca/PPi ratio shown
generated in this study.
Fig. 2.
Calcium pyrophosphate (CaPPi) formation and chemical composition. Calcium (10 mm) was incubated with 0–6 mm pyrophosphate (PPi) (2 h, 37°C). CaPPi formation, indicated by increased turbidity, was monitored spectrophotometrically at 405 nm (a). Scatchard analysis of CaPPi formation (b). Following incubation, reaction mixtures were centrifuged to remove CaPPi. Calcium remaining soluble in reaction supernatants was analysed by dye-binding assay, as described in Materials and methods. The PPi concentration required to completely deplete soluble calcium was determined by extrapolation to the x intercept and reflects the stoichiometry of CaPPi interaction. Ca/P ratio of 1·96 is consistent with calcium pyrophosphate dihydrate (Ca/P, 2·00) (see Table 1) [28].
Exposure of neutrophil MMP-9 (92-, 130- and 225-kDa) to CaP resulted in the formation of a high molecular weight (Mw) CaP : MMP complex with lowered electrophoretic mobility on gelatin zymograms (Fig. 3a). The 72-kDa fibroblast derived MMP-2, purified from human plasma by affinity chromatography on gelatin–agarose (see Fig. 4), also complexed CaP resulting in a shift to higher apparent Mw (Fig. 3c). No high Mw MMP complexes were noted at phosphate concentrations (<250 µm) insufficient for CaP formation. As can be seen, MMP interaction with CaP coincided with fibrin-binding activity for both MMP-2 and MMP-9 (Fig. 3b,d). In contrast, CaPPi did not complex either MMP. No fibrin-binding activity was observed for either MMP-2 or MMP-9 in the presence of CaPPi even at the highest PPi concentration tested (500 µm).
Fig. 3.
Matrix metalloproteinase (MMP) interaction with calcium phosphate (CaP) and calcium pyrophosphate (CaPPi) and effect on fibrin binding. Calcium (10 mm) was incubated with 0–500 µm phosphate (PO4) or pyrophosphate (PPi) (2 h, 37°C). Neutrophil lysate MMP-9 (92-, 130-, 225-kDa) or purified MMP-2 (72-kDa) (see Fig. 4) were then added. Following incubation (5 min, 37°C), an aliquot was removed and analysed by gelatin zymography to monitor MMP-9 (a) and MMP-2 (c) interaction with CaP and CaPPi, i.e. shift in MMP electrophoretic mobility to higher apparent molecular weight. To evaluate fibrin-binding activity, plasma (source of fibrinogen) was added to reaction mixes containing MMP-9 and purified bovine fibrinogen was added to reaction mixes containing MMP-2 to achieve a final fibrinogen concentration of 100 µg/ml. Fibrin polymerization was catalysed with 5 µl of 100 U/ml thrombin (15 min, 37°C). Fibrin matrices were syneresed, washed once and transferred to 100 µl of non-denaturing 2× Laemmli sample buffer (15 min). Following equilibration, fibrin matrices were loaded directly (one matrix/lane) on gelatin zymograms for MMP-9 (b) or MMP-2 (d). As can be seen, MMP fibrin-binding activity was consistent with CaP formation. No fibrin binding was noted for MMPs with CaPPi. Lanes are: 0, 0 µm; 1, 125 µm; 2, 250 µm; 3, 375 µm; and 4, 500 µm phosphate (PO4) or pyrophosphate (PPi). Position of MMP Mw standards (in kDa) shown on left.
Fig. 4.
MMP-2 purification from human plasma by gelatin agarose chromatography. Gelatin–agarose fractions were analysed by (a) gelatin zymography and (b) SDS-PAGE. Elution buffer (containing 5% DMSO) was changed starting with fraction 10. Fractions containing only MMP-2 (72-kDa) were pooled (fractions 12–14), dialysed and used in fibrin-binding experiments (see Fig. 3). Fraction number 11 was excluded from the pooled MMP-2 preparation due to the presence of contaminating MMP-9 (92-kDa). Starting material (S) and fraction number (1–14) shown above each lane. Arrowhead designates position of albumin. As can be seen, virtually all albumin was eliminated in the purified MMP-2 preparation as compared to the starting material (lane S).
CaP-mediated MMP uptake into fibrin matrices appeared to be highly specific as little artefactual trapping was noted in control fibrin-binding experiments or in studies performed in the presence of increasing CaP or CaPPi (Fig. 5). The small increase in ‘trapped’ albumin noted for fibrin formed in the presence of CaPPi was due probably to increased non-specific occlusion because these matrices were substantially larger (∼2 mg) than those formed with CaP (∼1 mg) (data not shown). Despite the increased size of the fibrin matrix formed in the presence of CaPPi, there was no evidence of MMP-2 or MMP-9 uptake (see Fig. 3).
Fig. 5.
Evaluation of artifactual trapping in fibrin matrices. In (a) and (b), the effect of fibrinogen source on matrices was evaluated. Fibrin was generated from plasma collected from three human volunteers, washed once and subjected to SDS-PAGE under non-reducing (a) or reducing conditions (b). Post indicates fibrin-binding mix following removal of fibrin matrix to demonstrate relative concentration of albumin and lack thereof in matrix. Fibrinogen source number indicated at top (1–3). In (c), the effect of washing on fibrin matrices was evaluated. Fibrin was generated from a single plasma source and subjected to SDS-PAGE under reducing conditions. Control lane (c) shows albumin diluted to mimic complete trapping in the fibrin matrix. Number of washes indicated top of each lane (1–4). In (d), the effect of CaP and CaPPi on trapping within fibrin matrices. Calcium was incubated in the presence of increasing phosphate (PO4) or pyrophosphate (PPi) to induce CaP or CaPPi formation, respectively. Plasma (source of fibrinogen) was added and fibrin polymerization catalysed with 5 µl of 100 U/ml thrombin (15 min, 37°C). Fibrin matrices were analysed by SDS-PAGE under reducing conditions. Lanes are: 0, 0 µm; 1, 125 µm; 2, 250 µm; 3, 375 µm; and 4, 500 µm phosphate (PO4) or pyrophosphate (PPi). SDS-PAGE calibration proteins (in kDa), albumin (arrow), fibrin γ-γ dimers and β-monomers are shown; 8·5% SDS-PAGE gels were stained with Coomassie blue.
To examine the nature of MMP interaction with CaP and subsequent effect on fibrin binding, a series of experiments were conducted in the presence of various competitors. High NaCl concentration (>150 mm) eliminated fibrin-binding activity for MMP-9 and substantially decreased fibrin binding for MMP-2 in the presence of CaP (Fig. 6). However, it should be noted that fibrin matrices formed at >150 mm NaCl from either fibrinogen source (plasma or bovine fibrinogen) were difficult to synerese for subsequent gelatin zymography. Nonionic detergents such as Brij-35 and Tween-20 used from 0·01 to 0·10% were ineffective in blocking MMP : CaP fibrin-binding activity (data not shown). MMP : CaP interaction and subsequent fibrin binding could also be blocked in the presence of high concentrations of albumin (Fig. 7). High albumin concentration also dissociated preformed MMP : CaP complexes (Figs 7d,h). This observation was probably non-specific because the albumin concentration (100–1000 µg/ml) required for this effect was substantially higher than the MMP concentration (∼0·025 µg/ml) used in these studies (Figs 7c,g). The tissue specific inhibitors of metalloproteinases (TIMPs) were also ineffective in blocking interaction with CaP, indicating that binding did not involve the C-terminal region of MMPs (data not shown). Gelatinase MMPs exposed to fibrin matrices generated in the presence of native type I fibrillar and type IV basement membrane collagens demonstrated increased gelatinase binding (Fig. 8). At the highest collagen type I concentrations, uptake of the 72-kDa MMP-2 was also noted. Gelatin, despite being a preferred substrate for gelatinases, did not block or augment MMP : fibrin interaction at any of the concentrations tested. No latent gelatinase activity was noted for the type I and type IV collagens used in this study (data not shown).
Fig. 6.
Effect of salt on MMP : fibrin binding. CaP was formed at 10 mm calcium and 250 µm phosphate (2 h, 37°C). MMP-2 and MMP-9 (∼0·025 µg) and competing NaCl (100–225 mm, final concentration) were then added (15 min, 37°C). To evaluate fibrin-binding activity, plasma (source of fibrinogen) was added to reaction mixes containing MMP-9 and purified bovine fibrinogen was added to reaction mixes containing MMP-2 to achieve a final fibrinogen concentration of 100 µg/ml. Fibrin polymerization was catalysed with 5 µl of 100 U/ml thrombin (15 min, 37°C). Fibrin matrices were syneresed, washed once and transferred to 100 µl of 2× non-denaturing Laemmli sample buffer (15 min). Following equilibration, fibrin matrices were loaded directly (one matrix/lane) on gelatin zymograms. Zymograms are: (a), MMP-2 and (b), MMP-9. Lanes are: 1, 100 mm; 2, 125 mm; 3, 150 mm; 4, 175 mm; 5, 200 mm; and 6, 225 mm NaCl. Position of MMP Mw markers (in kDa) are shown left.
Fig. 7.
Effect of albumin on MMP : CaP formation and MMP : fibrin binding. CaP was formed at 10 mm calcium and 250 µm phosphate (2 h, 37°C). MMP-2 and MMP-9 (∼0·025 µg) and competing albumin (0–1000 µg/ml, final concentration) were then added (15 min, 37°C). An aliquot was removed and evaluated by gelatin zymography (a and e) and SDS-PAGE (c and g). To evaluate fibrin-binding activity, plasma (source of fibrinogen) was added to reaction mixes containing MMP-9 and purified bovine fibrinogen was added to reaction mixes containing MMP-2 to achieve a final fibrinogen concentration of 100 µg/ml. Fibrin polymerization was catalysed with 5 µl of 100 U/ml thrombin (15 min, 37°C). Fibrin matrices were syneresed, washed once and transferred to 100 µl of non-denaturing 2× Laemmli sample buffer (15 min). Following equilibration, fibrin matrices were loaded directly (one matrix/lane) on gelatin zymograms for MMP-2 (b) or MMP-9 (f). The ability of albumin to dissociate preformed MMP : CaP complexes was also evaluated. CaP was allowed to form (as described above) and MMP-2 and MMP-9 were added (15 min, 37°C). MMP : CaP complexes were then exposed to increasing albumin concentration and dissociation evaluated after 15 min (d and h). Positions of 72-kDa MMP-2 (used in a–d) and 92-, 130- and 225-kDa MMP-9 (used in e–h) are shown. Lanes correspond to albumin concentrations of: 1, 1000 µg/ml; 2, 500 µg/ml; 3, 100 µg/ml; 4, 10 µg/ml; 5, 1 µg/ml; and 6, 0 µg/ml albumin. Triangular shape above lanes denotes albumin abundance.
Fig. 8.
Effect of collagen type on MMP : fibrin binding. Neutrophil lysate MMP-9 (∼0·025 µg) was added to plasma (source of fibrinogen and MMP-2) in the presence of denatured collagen (gelatin), type I fibrillar and type IV basement membrane collagen (15 min, 37°C). Fibrin polymerization was catalysed with 5 µl of 100 U/ml thrombin (15 min, 37°C). Fibrin matrices were syneresed, washed once and transferred to 100 µl of 2× non-denaturing Laemmli sample buffer (15 min). Following equilibration, fibrin matrices were loaded directly (one matrix/lane) on gelatin zymograms. Fibrin was formed without collagen (lane C, ‘control’) or in the presence of increasing gelatin (75, 150, 375 and 750 µg/ml, lanes 1–4, respectively), and type I and type IV collagen (5, 10, 25 and 50 µg/ml, lanes 1–4, respectively). MMP Mw standards (in kDa) are shown right.
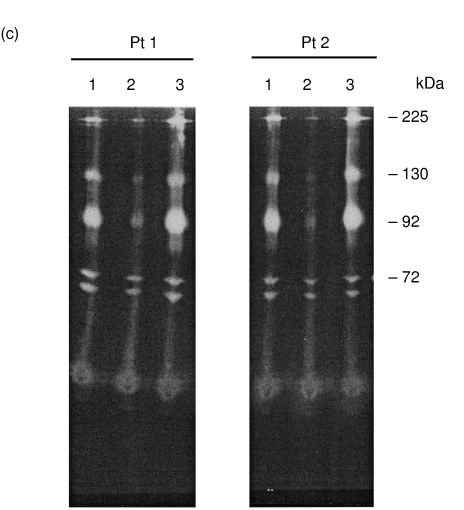
Fibrin binding activity of MMPs obtained from inflammatory joint fluids was also examined. Synovial fluid MMPs were separated into three fractions: before centrifugation (‘total’); after centrifugation (‘soluble’); and the resuspended pellet (‘pellet’). The three fractions were analysed for protein content, MMP composition and MMP fibrin-binding activity (Fig. 9). As can be seen, inflammatory joint fluid contained both MMP-2 (72-kDa) and neutrophil MMP-9 (92-, 130- and 225-kDa). Following centrifugation, MMP-9 activity was enriched in the pelleted material despite the absence of electrophoretically detectable protein. Similar results were obtained from synovial fluids collected from four other patients with recurrent, crystal-negative, culture-negative effusions (data not shown). Synovial fluids from two of these patients (patient 1 and patient 2) were examined for MMP fibrin-binding activity. As can be seen, MMP ‘total’ and ‘pellet’ fractions from both patients demonstrated substantial fibrin-binding activity. In contrast, the ‘soluble’ fraction was essentially devoid of MMP fibrin binding. To evaluate the dose dependence of fibrin-binding activity, fibrin matrices were generated in the presence of increasing synovial fluid fractions (Fig. 10). Again, MMP fibrin-binding activity was present in the ‘total’ fraction whereas the ‘soluble’ synovial fluid fraction was devoid of fibrin-binding activity. MMP fibrin-binding activity was dose-dependent on the amount of synovial fluid fraction added to the fibrin-binding assay and that a proportion MMP activity was extractable into the SDS equilibration buffer (Fig. 10d). Densitometric analysis of the reaction mix prior to fibrin formation (‘pre-’, Fig. 10a) and following removal of the fibrin matrix (‘post-’, Fig. 10b) revealed that approximately 50% of MMP-9 in the synovial fluid fraction actively bound fibrin whereas <10% of MMP-2 demonstrated fibrin-binding activity (data not shown). The ability to sediment MMPs with fibrin-binding activity is consistent with the presence of a CaP : MMP complex and has been reported previously by our laboratory [10]. Although synovial fluid 72-kDa MMP-2 demonstrated substantially less fibrin-binding activity, the binding of MMP-2 to fibrin resulted in the formation of a ‘doublet’ of gelatinase activity at 72-kDa and ∼68-kDa similar to that observed using purified MMP-2 (see above). MMP-9 : fibrin binding appeared to be very tight because most MMP remained associated the fibrin matrix (Fig. 10c), although some could be eluted by equilibration of the fibrin matrix with SDS (Fig. 10d). Similar MMP fibrin binding results were obtained with synovial fluids collected from four other patients (one male and three females) with a variety of inflammatory joint effusions, including rheumatoid arthritis, psoriatic arthritis and systemic lupus erythematosus.
Fig. 9.
Fibrin binding activity of inflammatory joint fluid MMPs. Synovial fluid MMPs were separated into three fractions: before centrifugation (‘total’); after centrifugation (‘soluble’); and the synovial fluid pellet resuspended in 100 µl 50 mm Tris-Cl, pH 7·6 (‘pellet’). The three fractions were analysed for protein (a) and MMP composition (b) and MMP fibrin-binding activity (c). For MMP fibrin-binding activity, synovial fluid was obtained from two patients with recurrent inflammatory effusions (patient 1 and patient 2). Briefly, 2 µl of each fraction (total, soluble, and resuspended pellet) was diluted with 50 mm Tris-Cl, pH 7·6 (945 µl final volume). Plasma (50 µl) was added and fibrin polymerization catalysed with 5 µl of 100 U/ml thrombin (15 min, 37°C). Fibrin matrices were syneresed, washed once and transferred to 100 µl of non-denaturing 2× Laemmli sample buffer (15 min). Following equilibration, fibrin matrices were loaded directly (one matrix/lane) on gelatin zymograms. Lanes correspond to: 1, ‘total’; 2, ‘soluble’; 3, ‘pellet’ synovial fluid fractions. Arrow indicates position of albumin. MMP Mw standards (in kDa) are shown right.
Fig. 10.
Dose dependence of fibrin binding MMPs from inflammatory synovial fluid. An aliquot (see volumes below) of synovial fluid before (‘total’) or after centrifugation (‘soluble’) was diluted with 50 mm Tris-Cl, pH 7·6 (945 µl final volume). Plasma (50 µl) was added and an aliquot was removed to evaluate ‘total’ and ‘soluble’ MMP content (prior to addition of thrombin and subsequent fibrin formation) by gelatin zymography. Fibrin polymerization was catalysed with 5 µl of 100 U/ml thrombin (15 min, 37°C). Fibrin matrices were syneresed, washed once and transferred to 100 µl of non-denaturing 2× Laemmli sample buffer (15 min). Following equilibration, fibrin matrices were loaded directly (one matrix/lane) on gelatin zymograms. An aliquot of the non-denaturing Laemmli sample buffer (30 µl/lane) was also analysed by gelatin zymography to examine SDS extactable, i.e. less tightly bound MMPs. Gelatin zymograms correspond to: (a) reaction mix before thrombin addition (‘pre-’); (b) reaction mix after thrombin addition and removal of fibrin matrix (‘post-’); (c) fibrin matrices (one matrix/lane, ‘fibrin’); and (d) MMPs extracted from fibrin matrix by the 2× non-denaturing Laemmli sample buffer (‘SDS extract’). Lanes correspond to: 1, 0 µl; 2, 2·5 µl; 3, 5 µl; 4, 7·5 µl; and 5, 10 µl of ‘total’ or ‘soluble’ synovial fluid fraction added to the standard 1 ml reaction mix. Position of MMP Mw standards are shown right.
Discussion
Although the presence of CaP and CaPPi in synovial fluid is associated strongly with degenerative joint disease, the exact mechanism by which these calcium-containing crystals influence pathological change remains to be elucidated [13, 25, 26]. Recent evidence has indicated that CaP may act to directly amplify the expression of MMPs, including MMP-9 in synovial chondrocytes and fibroblasts in basic calcium phosphate (BCP) crystal deposition disease [27,28]. In calcium pyrophosphate dihydrate (CPPD) crystal arthopathy, phagocytosis of CaPPi crystals by polymorphonuclear leucocytes and subsequent release of chemotactic factors is believed to trigger acute exacerbations [28]. Crystal composition including the binding of ancillary proteins has also been suggested as a means to influence CaP and CaPPi in the pathophysiology of erosive joint disease [13].
Our results demonstrate that fibrin binding by fibroblast-derived MMP-2 and neutrophil-derived MMP-9 is tight, dose-dependent and mediated by amorphous calcium phosphate (CaP) [9], the endogenous chemical precursor to crystalline hydroxyapatite [11,12]. Like MMP-9 [9], fibroblast 72-kDa MMP-2 binding to fibrin is associated with the formation of a high molecular weight CaP : MMP complex that resists dissociation. In contrast, calcium pyrophosphate (CaPPi) was completely inactive in complexing MMP-2 or MMP-9 or in mediating MMP fibrin binding. Although the mechanism of MMP interaction with CaP is not known, it does appear electrostatic in nature as high salt concentration could decrease or abolish this effect. In fibrin-binding studies with synovial fluid, we also found that the anionic detergent, sodium dodecyl sulphate (SDS), was partially effective in dissociating MMPs bound to fibrin matrices. In contrast, non-ionic detergents were ineffective in blocking MMP : CaP interaction. Other agents, such as high albumin, were also found to prevent CaP : MMP binding and subsequent fibrin uptake. This finding, however, was probably non-specific as the albumin concentration (100–1000 µg/ml) required for this effect was significantly greater than MMP concentration (∼0·025 µg/ml). Albumin was also found to dissociate preformed MMP : CaP complexes, but again this effect was noted only at the highest albumin concentrations tested. Interestingly, fibrin formation in the presence of collagen type I and type IV substantially increased the fibrin-binding activity of MMPs whereas gelatin, i.e. the preferred substrate for MMP-2 (gelatinase A) and MMP-9 (gelatinase B), was ineffective in mediating fibrin-binding activity.
Synovial fluid aspirated from five patients with crystal-negative, culture-negative, inflammatory effusions contained MMP-2 and MMP-9 that were also found to bind fibrin. Although the exact mechanism of synovial fluid MMP fibrin binding in these patients was not elucidated, we found that centrifugation resulted in almost total elimination in fibrin-binding activity. This finding is consistent with the previous observation that the high molecular weight CaP : MMP complex is sedimentable [10]. Although inflammatory synovial fluids were characterized as crystal-negative, small amounts of amorphous CaP would probably not be observed during routine microscopic examination. Neutrophil MMP-9 appeared to demonstrate higher fibrin-binding activity when compared to fibroblast MMP-2, despite the substantial presence of both in synovial fluid. This finding was not unexpected, as previous studies showed that impure MMP-2 did not bind fibrin [5]. The reason for lower MMP-2 fibrin-binding activity is unclear, but may be related to poorer avidity of MMP-2 for CaP (versus MMP-9) combined with non-specific competition with other, more highly abundant proteins such as albumin. Despite the sequence homology between MMP-2 and MMP-9, only MMP-9 contains a collagen type region [2,4]. Whether or not this unique region is responsible for increased MMP-9 fibrin binding, akin to that observed in the presence of type I and type IV collagen, remains to be determined. CaP-mediated MMP-9 binding to fibrin may be of considerable importance for BCP positive osteoarthritic disease because it has been shown that local MMP-9 expression is increased substantially in response to crystal exposure and interleukins [27,28]. Because amorphous CaP is precursor to more crystalline forms of BCP such as hydroxyapatite [12], CaP-mediated MMP fibrin binding may also be of relevance in early crystal-negative joint disease states [29]. Interestingly, it has been reported that prolonged hydroxyapatite exposure (> 4 h) induces inactivation of MMP-1 (collagenase) and MMP-3 (stomelysin) [30]. Despite the precursor/product relationship to hydroxyapatite [12], we did not observe any loss of MMP-2 or MMP-9 activity following CaP binding. It should be noted, however, that our studies were much shorter in terms of time (∼0·5 h) than those reported above.
Although synovial fluid PPi is elevated in patients with CPPD disease [25,26], the mechanism of crystal-induced joint erosion remains unknown. Because PPi is an effective physiological inhibitor of CaP formation [11,12], a role for PPi in regulating fibrin : CaP : MMP interaction in CPPD disease may also be envisioned. For example, fibrin deposition, neutrophil chemotaxis and local synovial tissue cell activation would occur in response to stress microfractures in cartilage similar to that observed in bone [31]. Expression of MMP-9 from activated chrondrocytes [27], fibroblasts [28] or recruited neutrophils [5] would also increase, but MMP-9 would fail to bind fibrin due to inhibition of CaP formation by elevated PPi. Subsequent plasmin-mediated fibrinolysis would be uncoupled from activation of latent MMPs. The lack of accompanying collagenolytic activity would thus impair wound healing and remodelling of the cartilagenous matrix. Damaged areas of the extracellular matrix would remain, ultimately becoming necrotic with increased susceptibility for dystrophic calcification by CPPD crystals.
Fibrin binding of MMP-2 and -9, similar to fibrin binding of tissue plasminogen activator [32], probably serves to protect enzyme from inhibitors such as tissue inhibitors of metalloproteinases (TIMPs) [6] and concentrate proteolytic activity at specific sites. Fibrin deposition occurs in response to injury when vascular breachment activates the intrinsic clotting pathway or in the interstitium when alterations in endothelial permeability result in fibrinogen transudation and tissue factor activation of the extrinsic clotting pathway [33,34]. Fibrin is ubiquitously present at sites of acute or chronic tissue injury [33,34] and fibrin binding of MMPs is probably of regulatory significance for diverse physiological and pathological processes which require extracellular matrix degradation such as bone remodelling [35], tumour invasion and metastasis [33, 36, 37]. Despite the reported presence of fibrin(ogen) in synovial fluid [33], we were unable to generate endogenous fibrin matrices from fluid aspirated from inflammatory joints. In one comprehensive study, fibrils representing collagen (or fibrin) were identified in the majority of osteoarthritic synovial fluids [29]. It should be noted that collagen fibrils also demonstrate high fibrin-binding activity and that their assimilation into the fibrin matrix may lead to reduced fibrinolysis [38]. In this study we demonstrated that native type I collagen (fibrillar) and type IV collagen (basement membrane) binding to fibrin can also substantially increase MMP uptake. In contrast, denatured collagen, i.e. gelatin, was ineffective. Deposition of fibrin, plasminogen activators and MMPs with subsequent plasmin-dependent activation of latent MMP [5] probably help to co-ordinate fibrinolytic and collagenolytic systems for physiological processes that involve extracellular matrix turnover such as normal wound healing, growth and development. Dysregulation of this process would result in pathological extracellular matrix turnover in diseases characterized by defective wound healing, chronic inflammation (arthritis) and abnormal proliferation (cancer) or regeneration. Given this pleiotropic role, fibrin probably serves a linking function to coordinate regulation of fibrinolytic and collagenolytic systems.
Complexes of fibrin : CaP : MMP provide a pathway for directed degradation of extracellular matrix during normal wound healing or other processes that involve degradation or remodelling of matrix components. Fibrin : CaP : MMP provides a unique mechanism to temporally co-ordinate fibrinolytic and collagenolytic systems. The ability of physiological concentrations of PPi to mediate CaP formation [5, 11, 12] and thereby influence MMP : fibrin interaction is likely to be of pathophysiological significance not only in crystal arthropathies (BCP and CPPD), but also in bone diseases (osteoporosis, Paget’s, ossificans petrosis).
References
- 1.Stetler-Stevenson WG, Aznavoorian S, Liotta LS. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Ann Rev Cell Biol. 1993;9:541–73. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- 2.Woessner JF., Jr The family of matrix metalloproteinases. Ann NY Acad Sci. 1994;731:11–21. doi: 10.1111/j.1749-6632.1994.tb24720.x. [DOI] [PubMed] [Google Scholar]
- 3.Nagase H. Matrix metalloproteinases. In: Hooper NM, editor. Zinc metalloproteinases in health and disease. London, UK: Taylor & Francis; 1996. pp. 153–204. [Google Scholar]
- 4.Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997;378:151–60. [PubMed] [Google Scholar]
- 5.Makowski GS, Ramsby ML. Binding of latent matrix metalloproteinase 9 to fibrin: activation via a plasmin-dependent pathway. Inflammation. 1998;22:287–305. doi: 10.1023/a:1022300216202. [DOI] [PubMed] [Google Scholar]
- 6.Willenbock F, Crabbe T, Slocombe PM, et al. The activity of the tissue inhibitors of metalloproteinases is regulated by C-terminal domain interactions: a kinetic analysis of the inhibition of gelatinase A. Biochemistry. 1993;32:4330–7. doi: 10.1021/bi00067a023. [DOI] [PubMed] [Google Scholar]
- 7.Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with the integrin αvβ3. Cell. 1996;85:683–93. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- 8.Sato H, Takino T, Okada Y, et al. A matrix metalloproteinase expressed on the surface of invasive tumor cells. Nature. 1994;370:61–5. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 9.Makowski GS, Ramsby ML. Binding of latent matrix metalloproteinase 9 to fibrin is mediated by amorphous calcium-phosphate. Inflammation. 1998;22:599–617. doi: 10.1023/a:1022314530777. [DOI] [PubMed] [Google Scholar]
- 10.Makowski GS, Ramsby ML. Amorphous calcium phosphate-mediated binding of matrix metalloproteinase 9 to fibrin is inhibited by pyrophosphate and bisphosphonate. Inflammation. 1998;23:333–60. doi: 10.1023/a:1020209616428. [DOI] [PubMed] [Google Scholar]
- 11.Russell RGG. Metabolism of inorganic pyrophosphate (PPi) Arthritis Rheum. 1976;19:465–78. doi: 10.1002/1529-0131(197605/06)19:3+<465::aid-art1780190722>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Fleisch H, Neuman WF. Mechanisms of calcification. role of collagen, polyphosphates, and phosphatase. Am J Physiol. 1961;200:1296–300. doi: 10.1152/ajplegacy.1961.200.6.1296. [DOI] [PubMed] [Google Scholar]
- 13.Halverson PB. Calcium crystal-associated diseases. Curr Opin Rheumatol. 1996;8:259–61. doi: 10.1097/00002281-199605000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Makowski GS, Ramsby ML. Calibrating gelatin zymograms with human gelatinase standards. Anal Biochem. 1996;236:353–6. doi: 10.1006/abio.1996.0179. [DOI] [PubMed] [Google Scholar]
- 15.Markert M, Andrews PC, Babior BM. Measurement of O2– production by human neutrophils. The preparation and assay of NADPH oxidase-containing particles from human neutrophils. Meth Enzymol. 1984;105:358–65. doi: 10.1016/s0076-6879(84)05048-5. [DOI] [PubMed] [Google Scholar]
- 16.Vartio T, Baumann M. Human gelatinase/type IV procollagenase is a regular plasma component. FEBS Lett. 1989;255:285–9. doi: 10.1016/0014-5793(89)81107-x. [DOI] [PubMed] [Google Scholar]
- 17.Zucker S, Lysik RM, Gurfinkel M, et al. Immunoassay of type IV collagenase/gelatinase (MMP-2) in human plasma. J Immunol Meth. 1992;148:189–98. doi: 10.1016/0022-1759(92)90172-p. [DOI] [PubMed] [Google Scholar]
- 18.Fujisawa R, Wada Y, Nodaska Y, Kuboki Y. Acidic amino acid-rich sequences as binding sites of osteonectin to hydroxyapatite crystals. Biochim Biophys Acta. 1996;1292:53–60. doi: 10.1016/0167-4838(95)00190-5. [DOI] [PubMed] [Google Scholar]
- 19.Bauer BJ. Affinity and stoichiometry of calcium binding by asenazo III. Anal Biochem. 1981;110:61–72. doi: 10.1016/0003-2697(81)90112-3. [DOI] [PubMed] [Google Scholar]
- 20.Clauss A. Rapid physiological coagulation method for the determination of fibrinogen. Acta Haematol. 1957;17:237–46. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- 21.Regoeczi E. Occlusion of plasma proteins by human fibrin: studies using trace-labelled proteins. Br J Haematol. 1968;14:279–90. doi: 10.1111/j.1365-2141.1968.tb01498.x. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Makowski GS, Ramsby ML. pH modification to enhance the molecular seiving properties of sodium dodecyl sulfate-10% polyacrylamide gels. Anal Biochem. 1993;212:283–5. doi: 10.1006/abio.1993.1324. [DOI] [PubMed] [Google Scholar]
- 24.Francis CW, Marder VJ, Barlow GH. Plasmic degradation of crosslinked fibrin. Characterization of new macromolecular soluble complexes and a model of their structure. J Clin Invest. 1980;66:1033–43. doi: 10.1172/JCI109931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan LM, McCarty DJ. Understanding inorganic pyrophosphate metabolism: toward prevention of calcium pyrophosphate dihydrate crystal deposition. Ann Rheum Dis. 1995;54:939–41. doi: 10.1136/ard.54.12.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rull M. Calcium crystal-associated diseases and miscellaneous crystals. Curr Opin Rheumatol. 1997;9:274–9. doi: 10.1097/00002281-199705000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Freemont AJ, Hampson V, Tilman R, Goupille P, Taiwo Y, Hoyland JA. Gene expression of matrix metalloproteinases 1,3, and 9 by chondrocytes in osteoarthritic human knee articular cartilage is zone and grade specific. Ann Rheum Dis. 1997;56:542–9. doi: 10.1136/ard.56.9.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCarthy GM, Marcius AM, Christopherson PA, Ryan LM, Pourmotabbed T. Basic calcium phosphate crystals induce synthesis and secretion of 92 kDa gelatinase (gelatinase B/matrix metalloproteinase 9) in human fibroblasts. Ann Rheum Dis. 1998;57:56–60. doi: 10.1136/ard.57.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nalbant S, Martinez JAM, Kitumnuaypong T, Clayburne G, Sieck M, Scumacher HR. Synovial fluid features and their relations to osteroarthritis severity: new findings from sequential studies. Osteoarth Cartil. 2003;11:50–4. doi: 10.1053/joca.2002.0861. [DOI] [PubMed] [Google Scholar]
- 30.Kremer EA, Chen Y, Sozuki K, Nagase H, Gorski JP. Hydroxyapatite induces autolytic degradation and inactivation of matrix metalloproteinase-1 and -3. J Bone Min Res. 1998;13:1890–902. doi: 10.1359/jbmr.1998.13.12.1890. [DOI] [PubMed] [Google Scholar]
- 31.Mori S, Harruf R, Ambrosius W, Burr DB. Trabecular bone volume and microdamage in the femoral heads of women with and without femoral neck fractures. Bone. 1997;21:521–6. doi: 10.1016/s8756-3282(97)00200-7. [DOI] [PubMed] [Google Scholar]
- 32.Rakoczi I, Wiman B, Collen D. On the biological signficance of the specific interaction between fibrin, plasminogen, and antiplasmin. Biochim Biophys Acta. 1978;540:295–300. doi: 10.1016/0304-4165(78)90142-3. [DOI] [PubMed] [Google Scholar]
- 33.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 34.Weinberg JB, Pippen AMM, Greenberg CS. Extravascular fibrin formation and dissolution in synovial tissue of patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 1991;34:996–1005. doi: 10.1002/art.1780340809. [DOI] [PubMed] [Google Scholar]
- 35.Reddi AH, Anderson WA. Collagenous bone matrix-induced endochondral ossification and hemopoiesis. J Cell Biol. 1976;69:557–72. doi: 10.1083/jcb.69.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown LF, Dvorak AM, Dvorak HF. Leaky vessels, fibrin deposition, and fibrosis: a sequence of events common to solid tumors and many other types of disease. Am Rev Respir Dis. 1989;140:1104–7. doi: 10.1164/ajrccm/140.4.1104. [DOI] [PubMed] [Google Scholar]
- 37.Cliffton DE, Agostino D. The effects of fibrin formation and alterations in the clotting mechanism on the development of metastases. Vasc Dis. 1965;2:43–52. [PubMed] [Google Scholar]
- 38.Mirashi M, Soria J, Lu H, Soria C, Samama M, Caen J-P. Defective thrombolysis due to collagen incorporation in fibrin clots. Thromb Res. 1988;S8:73–80. doi: 10.1016/0049-3848(88)90156-9. [DOI] [PubMed] [Google Scholar]