Abstract
Severe acute respiratory syndrome (SARS) is a recently emerged infectious disease caused by a novel coronavirus, but its immunopathological mechanisms have not yet been fully elucidated. We investigated changes in plasma T helper (Th) cell cytokines, inflammatory cytokines and chemokines in 20 patients diagnosed with SARS. Cytokine profile of SARS patients showed marked elevation of Th1 cytokine interferon (IFN)-γ, inflammatory cytokines interleukin (IL)-1, IL-6 and IL-12 for at least 2 weeks after disease onset, but there was no significant elevation of inflammatory cytokine tumour necrosis factor (TNF)-α, anti-inflammatory cytokine IL-10, Th1 cytokine IL-2 and Th2 cytokine IL-4. The chemokine profile demonstrated significant elevation of neutrophil chemokine IL-8, monocyte chemoattractant protein-1 (MCP-1), and Th1 chemokine IFN-γ-inducible protein-10 (IP-10). Corticosteroid reduced significantly IL-8, MCP-1 and IP-10 concentrations from 5 to 8 days after treatment (all P < 0·001). Together, the elevation of Th1 cytokine IFN-γ, inflammatory cytokines IL-1, IL-6 and IL-12 and chemokines IL-8, MCP-1 and IP-10 confirmed the activation of Th1 cell-mediated immunity and hyperinnate inflammatory response in SARS through the accumulation of monocytes/macrophages and neutrophils.
Keywords: chemokines, cytokines, inflammation, severe acute respiratory syndrome (SARS)
Introduction
Severe acute respiratory syndrome (SARS) is a recently emerged infectious disease characterized by persistent fever, respiratory symptoms with lung consolidation, lymphopenia and respiratory failure in life-threatening cases [1–4]. Watery diarrhoea has also been manifested in some cases [5]. We found that lymphopenia and depletion of CD4 and CD8 T lymphocytes could be associated with disease activity and adverse outcomes in SARS [4]. The SARS-related deaths have resulted mainly from pulmonary complications, including progressive respiratory failure due to alveolar damage and acute respiratory distress syndrome (ARDS). The causative agent of SARS has been identified as a new coronavirus (SARS-CoV) [6–8] with a genome sequence that is only moderately related to other known coronaviruses [9,10]. Rapid diagnostic tests using molecular techniques for the detection of SARS-CoV RNA have been developed recently [11,12]. Administration of the antiviral drug ribarivin together with systemic corticosteroid resulted in alleviation of inflammation, reduction of organ dysfunction and improvement on survival in the majority of SARS patients [1, 5, 13], although controlled clinical trials are required to confirm this regimen's genuine efficacy.
It has been shown that the over-production of specific inflammatory cytokines [tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-10] and polymorphonuclear neutrophil (PMN) CC chemokine IL-8 is the hallmark of viral infection, probably through the activation of transcription factor nuclear factor (NF)-κB, activator protein (AP)-1 and activating factor-2 (ATF-2) [14]. For example, the H5N1 ‘avian flu’ influenza viruses are potent inducers of proinflammatory cytokines such as TNF-α in macrophages [15]. In swine pneumonia, the production of TNF-α and IL-6 was found to correlate positively with disease severity [16]. Therefore, SARS sequalae such as transendothelial migration of PMN into lung tissue, multiple organ dysfunction and ARDS have been postulated to associate with cytokine and chemokine dysregulation [17,18]. In an attempt to study possible SARS-CoV-induced cytokine and chemokine dysregulation, to identify markers for disease severity, and to explore the feasibility of designing effective treatment strategy such as anti-cytokine therapy, we have prospectively investigated a panel of circulating T helper (Th) cell cytokines, inflammatory cytokines and chemokines in patients with SARS.
Materials And Methods
Patients
Our study included 20 consecutive adult patients who were diagnosed to have SARS and admitted to the Prince of Wales Hospital, Hong Kong for treatment from 20 March to 11 April 2003. In accordance with the World Health Organization (WHO), our case definition was a fever (temperature >38°C), a chest radiograph or computed tomographic image of the thorax showing evidence of new consolidation with or without respiratory symptoms (e.g. cough and shortness of breath) and a history of close contact with a person in whom SARS had been diagnosed [19]. The diagnosis of SARS was confirmed by an indirect immunofluorescence assay with fetal rhesus kidney cells that were infected with coronavirus and fixed in acetone to detect a serological response to the SARS-CoV [7], or by a positive viral culture. Our protocol was approved by the Clinical Research Ethics Committee of the Chinese University of Hong Kong and informed consent was obtained from all participants.
Measurement of plasma cytokines and chemokines
EDTA blood samples were collected from SARS patients daily for a maximum of 19 consecutive days. They were immersed in ice and transported immediately to the laboratory for processing. Plasma were separated by centrifugation (2000 g for 10 min) at 4°C and stored in 300 µl aliquots at −70°C until analysis.
Because the more commonly used procedure of enzyme-linked immunosorbent assay (ELISA) may generate infectious droplets and aerosols, T helper (Th)1/Th2 cytokines, inflammatory cytokines and chemokines in plasma were measured serially by cytometric bead array (CBA) using a four-colour FACSCalibur flow cytometer (Becton Dickinson, CA, USA) located in a Biosafety Level II laboratory [20,21]. In CBA, five or six bead populations with distinct fluorescence intensities had been coated with capturing antibodies specific for different cytokines or chemokines. These bead populations could be resolved in the fluorescence channels of the flow cytometer. After the beads had been incubated with 50 µl of plasma, different cytokines or chemokines in the sample were captured by their corresponding beads. The cytokine/chemokine captured beads were then mixed with phycoerythrin-conjugated detection antibodies to form sandwich complexes. Following incubation, washing and acquisition of fluorescence data, the results were generated in graphical format using the BD CBA software. The concentrations of Th1/Th2 cytokines IL-2, IL-4 and IFN-γ; inflammatory cytokines IL-1β, IL-6, IL-10, TNF-α and IL-12p70; and chemokines IL-8, regulated upon activation normal T cell-expressed and secreted (RANTES), monocyte chemoattractant protein-1 (MCP-1), IFN-γ-inducible protein-10 (IP-10) and monokine induced by IFN-γ (MIG) were measured using the human Th1/Th2 cytokine, inflammatory cytokine and chemokine CBA kits (BD Pharmingen, CA, USA), respectively. The assay sensitivities of these eight cytokines and five chemokines were 2·6, 2·6, 7·1, 7·2, 2·5, 3·3, 3·7, 1·9, 0·2, 1·0, 2·7, 2·8 and 2·5 ng/l, respectively. The coefficients of variation for all cytokine and chemokine assays were less than 10%. Their respective normal ranges have been derived from measurement of ≥100 healthy subjects.
Statistical analysis
Because plasma cytokine and chemokine concentrations were not in a Gaussian distribution, the Mann–Whitney rank sum test was used for assessing their differences. Results were expressed as median (interquartile range). All analyses were performed using the Statistical Package for Social Sciences (SPSS) software for Windows, Version 9·0 (SPSS Inc., IL, USA). A probability (P) < 0·05 was considered as significantly different.
Results
SARS patients
The characteristics of the 20 SARS patients are summarized in Table 1. Coincidentally, all except one were female patients, although the female-to-male ratio of the 1755 SARS patients in this outbreak in Hong Kong, as well as that of the 290 patients treated in our Prince of Wales Hospital, were both 1·0–0·8. None of the 20 SARS patients recruited in this study died or required admission to the intensive care unit (ICU). Initial treatment included antibiotics, cefotaxime and clarithromycin (or levofloxacin) to cover common pathogens causing community-acquired pneumonia. Oseltamivir (Tamiflu) was also given initially to treat possible influenza infection. After fever had persisted for more than 48 h and leukopenia, thrombocytopenia, or both, had developed, oral ribavirin (40–60 mg/kg/day) and oral prednisolone (1 mg/kg/day) were started as a combined regimen from 2 to 9 days after disease (fever) onset. Patients who did not respond clinically to standard empirical treatment including persistent fever, increasing shortness of breath, worsening lung opacities involving >50% of lung field in chest radiograph and deteriorating oxygen saturation to <90% by pulse oximetry, were given intravenous ribavirin (400 mg every 8 h) and an additional one to three pulses of 0·5 g of methylprednisolone sodium succinate (methylprednisolone) daily.
Table 1.
Characteristics of SARS patients
| SARS patients | |
|---|---|
| Number | 20 |
| Sex (female/male) | 19/1 |
| Age, mean ± s.d. (range) years | 32·7 ± 11·7 (21–58) |
| ICU admission, % | 0 |
| Mortality, % | 0 |
| Treatment with oral ribavirin | |
| Patient number (%) | 20 (100) |
| Daily dose, mg/kg | 50 ± 10·2 |
| Treatment with oral prednisolone | |
| Patient number (%) | 20 (100) |
| Daily dose, mg/kg | 1 |
| Treatment with intravenous ribavirin | |
| Patient number (%) | 5 (25) |
| Daily dose, g | 1·2 |
| Treatment with intravenous methylprednisolone | |
| Patient number (%) | 13 (65) |
| Daily dose, g | 1·3 ± 0·3 |
Plasma cytokines of SARS patients
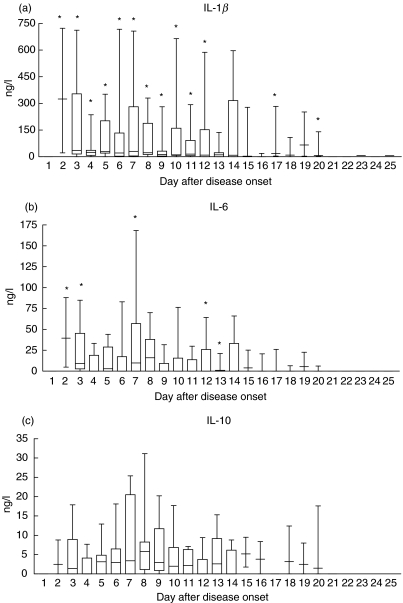
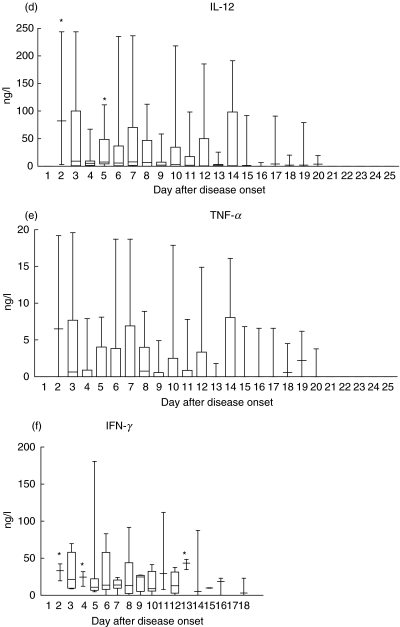
The longitudinal trends of plasma Th1/Th2 cytokines and inflammatory cytokines of the 20 SARS patients are illustrated in Fig. 1. Th1 cytokine IFN-γ and inflammatory cytokines IL-1β, IL-6 and IL-12 concentrations were significantly elevated above their normal range, respectively, within the first 12, 7 and 5 days after disease onset (all P < 0·05). In contrast, there was no significant increase in proinflammatory cytokine TNF-α in all SARS patients. Anti-inflammatory cytokine IL-10 was found to increase in some but not all patients (no overall significance). The plasma concentrations of Th1 cytokine IL-2 and Th2 cytokine IL-4 of all patients were low and within their respective normal range at all time points (data not shown).
Fig. 1.
Box & whiskers plots of changes in plasma cytokine concentrations following day of disease onset in the 20 SARS patients. Normal ranges: (a) IL-1β < 3·9 ng/l; (b) IL-6 < 3·1 ng/l; (c) IL-10 < 7·8 ng/l; (d) IL-12 < 7·8 ng/l; (e) TNF-α < 10·0 ng/l; and (f) IFN-γ < 15·6 ng/l. *Significantly elevated compared to normal values (all P < 0·05).
Plasma chemokines of SARS patients
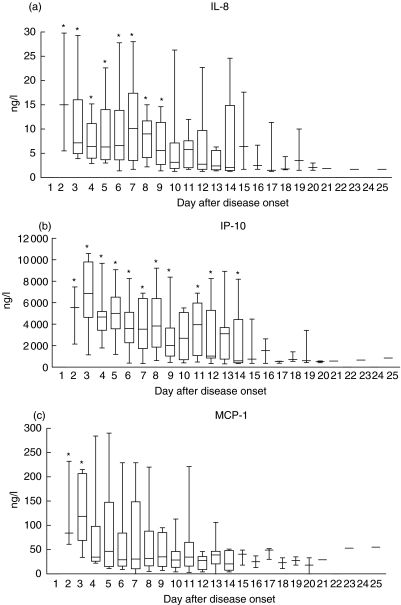
As shown in Fig. 2, plasma PMN CC chemokine IL-8, monocyte CC chemokine MCP-1 and Th1 CXC chemokine IP-10 concentrations were elevated, respectively, within the first 9, 3 and 14 days after disease onset (all P < 0·05). The overall plasma concentrations of T cell chemoattractants, namely CXC chemokine MIG and CC chemokine RANTES, were not significantly elevated.
Fig. 2.
Box & whiskers plots of changes in plasma chemokine concentrations following day of disease onset in the 20 SARS patients. Normal ranges: (a) IL-8 < 5·0 ng/l; (b) IP-10 202–1480 ng/l; (c) MCP-1 < 10–57 ng/l; (d) MIG 48–482 ng/l; and (e) RANTES 4382–18783 ng/l. *Significantly elevated compared to normal values (all P < 0·05).
Plasma cytokines and chemokines in SARS patients treated and not treated with pulsed methylprednisolone
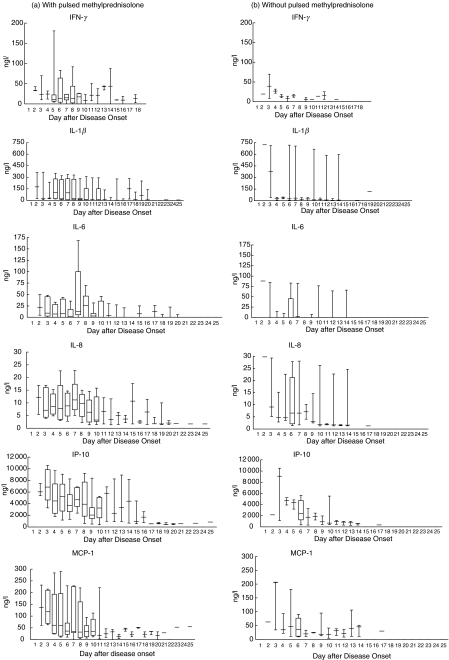
Among the 20 SARS patients in our cohort, 13 had more severe disease activity including persistent fever and worsening lung opacities; they were additionally given intravenous pulsed methylprednisolone. The other seven patients had milder disease severity and were not treated with pulsed steroid. Figure 3 shows that plasma IFN-γ, IL-1β, IL-6, IL-8, IP-10 and MCP-1 of the former group of patients had higher median concentrations than those of the latter, particularly from day 3–10 after disease onset. Following recovery, the cytokine and chemokine levels decreased progressively to normal values at about 1 week after disease onset for both groups of patients.
Fig. 3.
Box & whiskers plots of changes in plasma cytokine and chemokine concentrations following day of disease onset in SARS patients (a) treated (n = 13) and (b) not treated (n = 7) with pulsed methylprednisolone. Although not reaching statistical significance, median concentrations in (a) are higher than corresponding values in (b).
Effects of corticosteroid treatment on plasma cytokines and chemokines
As summarized in Table 2, plasma chemokine IL-8, IP-10 and MCP-1 were significantly reduced to their respective normal ranges from 5 to 8 days after corticosteroid treatment compared to the elevated pretreatment levels (all P < 0·005). These 5–8-day post-treatment concentrations were also significantly lower than those of 1–2 days after treatment (all P < 0·05). All the other measured cytokines and chemokines (IL-1β, IL-6, IL-10, IL-12p70, TNF-α, IFN-γ, RANTES and MIG) did not show any significant difference before and after corticosteroid treatment.
Table 2.
Changes in plasma chemokines of SARS patients before and after corticosteroid treatment
| Chemokine concentration (ng/l) | Immediately before corticosteroid treatment | 1–2 days after corticosteroid treatment | 5–8 days after corticosteroid treatment |
|---|---|---|---|
| IL-8 | 8·7 (4·8–14·1) | 5·9 (2·8–19·8) | 2·2 (1·7–6·1)*† |
| IP-10 | 5553·0 (4864·0–7557·0) | 4826·0 (2725·0–6098·0) | 894·5 (574·0–3117·0)*† |
| MCP-1 | 84·0 (36·5–198) | 68·5 (26·0–118·5) | 23·0 (14·0–37·0)*† |
Results as median (interquartile range).
P < 0·005 compared with concentrations before corticosteroid treatment
P < 0·05 compared with concentrations 1–2 days after corticosteroid treatment.
Discussion
This study was designed to investigate prospectively the longitudinal plasma cytokine and chemokine profile of adult patients with SARS. The progression of this potentially lethal disease may be represented by three phases: acute viral multiplication, hyperactive immune response, recovery or pulmonary destruction and death [1, 4, 5]. Accordingly, it is worthwhile to investigate cytokine and chemokine induction, which may be the cause or consequence of immune hyperactivity in SARS.
We observed that for at least 2 weeks after disease onset, all SARS patients exhibited a significant increase in the typical antiviral Th1 cytokine IFN-γ and an array of proinflammatory cytokines (IL-1β, IL-6 and IL-12), together with a moderate increase in anti-inflammatory IL-10 that was manifested in some patients. IL-1β can act as the early response cytokine to viral infection such as that caused by the human immunodeficiency virus, for mediation of inflammatory response, synthesis of acute phase proteins and release of IL-8 [14,22]. IL-6 may play a proinflammatory role in pulmonary inflammation [23]. Th2 cytokine IL-10 is an anti-inflammatory cytokine that suppresses the secretion of proinflammatory cytokines [24], therefore it was elevated in some patients 1 week after disease onset and followed the elevation of other inflammatory cytokines. IL-12, also known as natural killer (NK) cell stimulatory factor or cytotoxic lymphocyte maturation factor, is a pleiotropic proinflammatory cytokine which is produced primarily by antigen-presenting cells (monocytes, macrophages, dendritic cells and B cells) and PMN [25]. IL-12 can induce the production of IFN-γ and other Th1 cytokines with suppression of the Th2 pathway [26,27]. It has multiple effects on T lymphocytes and NK cells, including the ability to stimulate cytotoxicity, proliferation, cytokine production and the development of Th1 subsets [28,29]. Therefore, the observed early elevation of inflammatory cytokines IL-1β, IL-6, IL-12 and IFN-γ could be causative of the SARS-CoV-induced activation of Th1 cells and NK cells, release of chemokines such as IL-8, and results in pulmonary inflammation [16].
A previous study has suggested that the infiltration of proinflammatory PMN is involved in the pathogenesis of acute lung injury [30]. PMN are abundant in the airspaces of most ARDS patients with infection, and their secretory products such as myeloperoxidase and elastase in bronchoalveolar lavage (BAL) fluid can cause acute lung injury [31–33]. IL-8, the PMN α-chemokine, has been shown to be elevated in blood and alveolar spaces [34] and exhibit a positive correlation with the number of PMN in BAL fluid of patients with pneumonia and ARDS [35]. Our SARS-CoV-infected patients also showed elevation of plasma IL-8 soon after disease onset, which declined from 5 to 8 days after corticosteroid treatment. In our former clinical study, 82% of 157 SARS patients developed neutrophilia and the high neutrophil counts were associated with ICU admission and mortality from pneumonia [4]. Therefore, PMN-induced acute pneumonitis should be crucial in the pathogenesis of ARDS and pulmonary destruction in SARS patients.
Apart from the α-chemokine IL-8, we have also observed elevation of the monocyte/macrophages β-chemokine MCP-1 soon after the onset of SARS, followed by decline after corticosteroid treatment. MCP-1 concentration has been shown to increase in BAL fluid of patients with persistent ARDS [36], correlating positively with the predominance of alveolar macrophages [37] that has also been found in the postmortem examination of SARS patients [2,38]. IP-10 is a specific chemoattractant for Th1 cells for the activation of cell-mediated immune response; its expression can be up-regulated by the Th1 cytokine IFN-γ in acute lung inflammation [39]. Consistently, our results showed that both circulating IFN-γ and IP-10 were increased in SARS patients. Our observation of low or normal Th2 cytokine IL-4 in SARS patients is also similar to the finding of a previous study of viral pneumonia [40]. These suggest that SARS-CoV does not enhance the Th2 pathway and the subsequent humoral immue response. Such imbalance of Th1/Th2 cytokine profile confirms the SARS-CoV-induced Th1 predominance. In contrast to H5N1 infection [15], plasma TNF-α was not elevated in our patients. This finding, therefore, does not support the therapeutic use of TNF-α monoclonal antibody for SARS. However, it must be cautioned that the concentration of TNF-α at the pulmonary inflammatory sites has not been investigated. Previous studies have implicated that inflammatory IL-β and the acute-phase cytokine IL-6 are endogenous pyrogens or inducers of fever [41]. Therefore, elevations of IL-β and IL-6 might account for the fever onset, as both of them were found to have high plasma concentrations during the first week of disease. Moreover, the T cell chemokines MIG and RANTES also did not show any significantly elevation. Therefore, the recruitment of activated T cells in pulmonary tissue should be studied by performing chemokine assay and differential cell count in BAL fluids, although this should be difficult because of the high risk of infection.
In summary, we postulate that the elevation of the plasma chemokines IL-8, MCP-1 and IP-10, Th1-related cytokine IFN-γ and IL-12, and inflammatory cytokines IL-1β and IL-6 can induce the hyperinnate inflammatory response due to the SARS-CoV invasion of the respiratory tract. This leads to the recruitment and accumulation of alveolar macrophages and PMN [42], as well as the activation of Th1 cell-mediated immunity by the stimulation of NK and cytotoxic T lymphocytes (CTL). Treatment with immunosuppressive corticosteroid can significantly suppress the elevated chemokines IL-8, IP-10 and MCP-1, and subsequently alleviate the chemokine-associated pulmonary inflammation in SARS. Indeed, the reduction in IL-8, IP-10 and MCP-1 correlated with the improvement in general clinical condition, pulmonary function and radiological appearance in SARS patients of our present study. In addition, we also observed that higher disease severity was associated with more elevated plasma IFN-γ, IL-1β, IL-6, IL-8, IP-10 and MCP-1 concentrations (Fig. 3). It can be postulated that the addition of pulsed steroid in the severe-disease group not only controlled the rapidly deteriorating clinical condition, but basically attenuated the otherwise over-exaggerated immunological response with potentially much high median concentrations of plasma cytokines and chemokines. This further supports that the above cytokines and chemokines played important roles in the immunopathological mechanisms of SARS. We have initiated a study comparing plasma cytokines and chemokines in SARS patients with and without corticosteroid treatment. The elevated plasma cytokines and chemokines should also shed light on the possibility of using them as prognostic indicators for disease severity in SARS. For example, the presence of high anti-IL-8 : IL-8 complexes in BAL fluid has been shown to be an important prognostic indicator for ARDS [43].
Although our study has provided some understanding of the immunological mechanisms of inflammation in SARS and the therapeutic effects of corticosteroid, many further investigations are required to explore the detailed pathophysiology of this novel emerging disease with global impact. In order to correlate cytokine release with disease severity, in vitro mechanistic study of cytokine induction using SARS-CoV and immune cell culture, and eventually the measurement of cytokine and chemokine concentrations at local inflammatory sites should be contemplated. Other chemokines such as epithelial neutrophil-activating peptide (ENA)-78, a neutrophil chemoattractant [30], and macrophage inflammatory peptide-1 alpha (MIP-1α), a monocyte chemoattractant [30], should be measured. Recently, transforming growth factor (TGF)-β has been shown to be active early in acute lung injury, and potentially contribute to the development of pulmonary oedema [44]. Therefore, the role of TGF-β in SARS also requires investigation. Given the prominent roles of cytokines and chemokines in the pathogenesis of SARS, anticytokine/chemokine immunotherapy using anticytokine/chemokine antibodies (e.g. anti-IL-8, anti-MCP-1) or cytokine/chemokine antagonists may represent a novel approach for the treatment of hyperactive inflammation in SARS [45,46].
Acknowledgments
We are indebted to all medical, nursing and supportive health care workers in Hong Kong, in particular the late Dr Y. M. Tse, Dr H. Y. Cheng, Mr W. K. Lau, Ms H. M. Tang, Ms K. Y. Lau and Ms K. T. Wong for their courageous, unselfish and altruistic contributions to fighting against this deadly disease. We thank Ms Kelly Cheng of Greater China Technology Group Ltd for donation of a multifluorescence flow cytometer for SARS research.
References
- 1.Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–94. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 2.Tsang KW, Ho PL, Ooi GC, et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977–85. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 3.Poutanen SM, Low DE, Henry B, et al. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 4.Wong RSM, Wu AKL, To KF, et al. Haematological changes in patients with severe acute respiratory syndrome. Br Med J. 2003;326:1358–62. doi: 10.1136/bmj.326.7403.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peiris JS, Chu CM, Cheng VC, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–72. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peiris JS, Lai ST, Poon LL, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–25. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drosten C, Gunther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–76. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 8.Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–66. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 9.Marra MA, Jones SJ, Astell CR, et al. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 10.Rota PA, Oberste MS, Monroe SS, et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–9. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 11.Poon LL, Wong OK, Chan KH, et al. Rapid diagnosis of a coronavirus associated with severe acute respiratory syndrome (SARS) Clin Chem. 2003;49:953–5. doi: 10.1373/49.6.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng EK, Hui DS, Chan KC, et al. Quantitative analysis and prognostic implication of SARS coronavirus RNA in the plasma and serum of patients with severe acute respiratory syndrome. Clin Chem. 2003;49:1976–80. doi: 10.1373/clinchem.2003.024125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hon KL, Leung CW, Cheng WT, et al. Clinical presentations and outcome of severe acute respiratory syndrome in children. Lancet. 2003;361:1701–3. doi: 10.1016/S0140-6736(03)13364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mogensen TH, Paludan SR. Molecular pathways in virus-induced cytokine production. Microbiol Mol Biol Rev. 2001;65:131–50. doi: 10.1128/MMBR.65.1.131-150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung CY, Poon LL, Lau AS, et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 2002;360:1831–7. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- 16.Van Reeth K, Van Gucht S, Pensaert M. In vivo studies on cytokine involvement during acute viral respiratory disease of swine: troublesome but rewarding. Vet Immunol Immunopathol. 2002;87:161–8. doi: 10.1016/S0165-2427(02)00047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meduri GU. Host defense response and outcome in ARDS. Chest. 1997;112:1154–8. doi: 10.1378/chest.112.5.1154. [DOI] [PubMed] [Google Scholar]
- 18.Kunkel SL, Lukacs NW, Strieter RM, Chensue SW. The role of chemokines in the immunopathology of pulmonary disease. Forum (Genova) 1999;9:339–55. [PubMed] [Google Scholar]
- 19.World Health Organization. Severe acute respiratory syndrome (SARS) Wkly Epidemiol Rec. 2003;78:81–3. [PubMed] [Google Scholar]
- 20.Wong CK, Lam CWK. Clinical applications of cytokine assays. Adv Clin Chem. 2003;37:1–46. doi: 10.1016/s0065-2423(03)37005-2. [DOI] [PubMed] [Google Scholar]
- 21.Tarnok A, Hambsch J, Chen R, Varro R. Cytometric bead array to measure six cytokines in twenty-five microliters of serum. Clin Chem. 2003;49:1000–2. doi: 10.1373/49.6.1000. [DOI] [PubMed] [Google Scholar]
- 22.Evans SW, Whicher JT. The cytokines: physiological and pathophysiological aspects. Adv Clin Chem. 1993;30:1–88. doi: 10.1016/s0065-2423(08)60194-8. [DOI] [PubMed] [Google Scholar]
- 23.Leemans JC, Vervoordeldonk MJ, Florquin S, van Kessel KP, van der Poll T. Differential role of interleukin-6 in lung inflammation induced by lipoteichoic acid and peptidoglycan from Staphylococcus aureus. Am J Respir Crit Care Med. 2002;165:1445–50. doi: 10.1164/rccm.2106045. [DOI] [PubMed] [Google Scholar]
- 24.De Waal Malefyt R, Haanen J, Spits H, Roncarolo MG. Interleukin-10 and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen presenting capacity of monocytes via down-regulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamont AG, Adorini L. IL-12: a key cytokine in immune regulation. Immunol Today. 1996;17:214–7. doi: 10.1016/0167-5699(96)30011-x. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy MK, Picha KS, Shanebeck KD, Anderson DM, Grabstein KH. Interleukin-12 regulates the proliferation of Th1, but not Th2 or Th0, clones. Eur J Immunol. 1994;24:2271–8. doi: 10.1002/eji.1830241002. [DOI] [PubMed] [Google Scholar]
- 27.Manetti R, Gerosa F, Giudizi MG, et al. Interleukin 12 induces stable priming for interferon gamma (IFN-gamma) production during differentiation of human T helper (Th) cells and transient IFN-gamma production in established Th2 cell clones. J Exp Med. 1994;179:1273–83. doi: 10.1084/jem.179.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf SF, Sieburth D, Sypek J. Interleukin 12: a key modulator of immune function. Stem Cells. 1994;12:154–68. doi: 10.1002/stem.5530120203. [DOI] [PubMed] [Google Scholar]
- 29.Hendrzak JA, Brunda MJ. Interleukin-12. Biologic activity, therapeutic utility, and role in disease. Lab Invest. 1995;72:619–37. [PubMed] [Google Scholar]
- 30.Martin TR, Goodman RB. Chemokines in acute lung injury. In: Lenfant C, editor. Chemokines in the lung. New York: Marcel Dekker, Inc.; 2003. pp. 189–220. [Google Scholar]
- 31.Steinberg KP, Milberg JA, Martin TR, Maunder RJ, Cockrill BA, Hudson LD. Evolution of bronchoalveolar cell populations in the adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;150:113–22. doi: 10.1164/ajrccm.150.1.8025736. [DOI] [PubMed] [Google Scholar]
- 32.Weiland JE, Davis WB, Holter JF, Mohammed JR, Dorinsky PM, Gadek JE. Lung neutrophils in the adult respiratory distress syndrome. Clinical and pathophysiologic significance. Am Rev Respir Dis. 1986;133:218–25. doi: 10.1164/arrd.1986.133.2.218. [DOI] [PubMed] [Google Scholar]
- 33.Kawabata K, Hagio T, Matsuoka S. The role of neutrophil elastase in acute lung injury. Eur J Pharmacol. 2002;451:1–10. doi: 10.1016/s0014-2999(02)02182-9. [DOI] [PubMed] [Google Scholar]
- 34.Chollet-Martin S, Jourdain B, Gibert C, Elbim C, Chastre J, Gougerot-Pocidalo MA. Interactions between neutrophils and cytokines in blood and alveolar spaces during ARDS. Am J Respir Crit Care Med. 1996;154:594–601. doi: 10.1164/ajrccm.154.3.8810592. [DOI] [PubMed] [Google Scholar]
- 35.Villard J, Dayer-Pastore F, Hamacher J, Aubert JD, Schlegel-Haueter S, Nicod LP. GRO alpha and interleukin-8 in Pneumocystis carinii or bacterial pneumonia and adult respiratory distress syndrome. Am J Respir Crit Care Med. 1995;152:1549–54. doi: 10.1164/ajrccm.152.5.7582292. [DOI] [PubMed] [Google Scholar]
- 36.Goodman RB, Strieter RM, Martin DP, et al. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154:602–11. doi: 10.1164/ajrccm.154.3.8810593. [DOI] [PubMed] [Google Scholar]
- 37.Rosseau S, Hammerl P, Maus U, et al. Phenotypic characterization of alveolar monocyte recruitment in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2000;279:L25–35. doi: 10.1152/ajplung.2000.279.1.L25. [DOI] [PubMed] [Google Scholar]
- 38.Nicholls JM, Poon LL, Lee KC, et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–8. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neumann B, Emmanuilidis K, Stadler M, Holzmann B. Distinct functions of interferon-gamma for chemokine expression in models of acute lung inflammation. Immunology. 1998;95:512–21. doi: 10.1046/j.1365-2567.1998.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mo XY, Sarawar SR, Doherty PC. Induction of cytokines in mice with parainfluenza pneumonia. J Virol. 1995;69:1288–91. doi: 10.1128/jvi.69.2.1288-1291.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leon LR. Invited review. Cytokine regulation of fever: studies using gene knockout mice. J Appl Physiol. 2002;92:2648–55. doi: 10.1152/japplphysiol.01005.2001. [DOI] [PubMed] [Google Scholar]
- 42.Standiford TJ, Tsai WC. Chemokines in infectious diseases of the lung. In: Lenfant C, editor. Chemokines in the lung. New York: Marcel Dekker, Inc.; 2003. pp. 145–69. [Google Scholar]
- 43.Kurdowska A, Noble JM, Steinberg KP, Ruzinski JT, Hudson LD, Martin TR. Anti-interleukin 8 autoantibody: interleukin 8 complexes in the acute respiratory distress syndrome. Relationship between the complexes and clinical disease activity. Am J Respir Crit Care Med. 2001;163:463–8. doi: 10.1164/ajrccm.163.2.2005109. [DOI] [PubMed] [Google Scholar]
- 44.Dhainaut JF, Charpentier J, Chiche JD. Transforming growth factor-beta: a mediator of cell regulation in acute respiratory distress syndrome. Crit Care Med. 2003;31:S258–64. doi: 10.1097/01.CCM.0000057901.92381.75. [DOI] [PubMed] [Google Scholar]
- 45.Proudfoot AE, Power CA, Wells TN. The strategy of blocking the chemokine system to combat disease. Immunol Rev. 2000;177:246–56. doi: 10.1034/j.1600-065x.2000.17721.x. [DOI] [PubMed] [Google Scholar]
- 46.Luster AD. Antichemokine immunotherapy for allergic diseases. Curr Opin Allergy Clin Immunol. 2001;1:561–7. doi: 10.1097/00130832-200112000-00012. [DOI] [PubMed] [Google Scholar]