Abstract
Circadian biological clocks control many biological events, but the pathways by which these events are controlled are largely unknown. Based on a model suggesting that cytosolic-free calcium levels control the expression of the Lhcb gene in plants, we tested whether the circadian oscillation of free calcium is responsible for driving the rhythm of Lhcb expression. We found that these rhythms free-run with different periods in tobacco seedlings in constant conditions. Moreover, robust oscillations of Lhcb promoter activity continued in undifferentiated tobacco calli in the absence of Ca2+ oscillations. Therefore, these two circadian rhythms are not linked hierarchically. These data provide evidence for separate circadian pacemakers controlling molecular events in plants.
Circadian pacemakers coordinate biological events in eukaryotic and some prokaryotic organisms to optimize their adaptation to daily cycles in the environment. An important goal is to characterize the hierarchy by which the plethora of circadian events is controlled. Does a single pacemaker mechanism control all the circadian processes in organisms by intra- and intercellular interactions? Or, are there multiple pacemakers that modulate the various output rhythms, perhaps in a complicated control network? In humans, the model of multiple circadian pacemakers is strongly implicated because of experiments showing the uncoupling of two outputs (sleep/wake and body temperature) into rhythms with different periods in humans under isolation conditions (1).
In plants, desynchronization of physiological rhythms has been observed in seedlings of the bean Phaseolus vulgaris (2). In constant light (LL), the rhythm of stomatal conductance free-runs with a period close to 24 h, whereas the rhythm of leaf movement free-runs with a period close to 27 h. Therefore, in LL, these rhythms desynchronize relative to each other (2). Roenneberg and Morse (3) observed a similar phenomenon of desynchronization of output rhythms (bioluminescence and aggregation) in the dinoflagellate alga, Gonyaulax polyedra. The remarkable fact about the latter study is that Gonyaulax is a single-celled organism, and so these data indicate that there may be multiple circadian oscillators within a single cell (3). In tobacco, there is evidence for dual-oscillator control of gene expression that is elicited after light perturbations presented in a narrow developmental window (12–36 h after sowing; ref. 4). Other rhythmic phenomena in plants can be interpreted to suggest multiple circadian oscillators (5).
We previously reported a circadian oscillation of cytosolic free calcium ([Ca2+]c) in the plants Nicotiana plumbaginafolia and Arabidopsis thaliana (6). This [Ca2+]c oscillation might play a role in the central oscillator mechanism, the phototransduction path, or in controlling circadian outputs. To test whether the [Ca2+]c oscillation might control outputs, we focused on a well characterized circadian rhythm, namely, the rate of transcription of the light-harvesting complex (Lhc)b gene family (7–10), for which there is evidence of [Ca2+]c control of transcription (11, 12). Transgenic plants have been developed by using a luciferase reporter fused to the promoter of one of the Lhcb genes (Lhcb∷Luc) that enables continuous precise recording of the promoter activity (7, 8, 10). A series of experiments involving microinjection of calcium and calmodulin into tomato cells has led to a model for [Ca2+]c control of Lhc promoters (11, 12). This model and the fact that the [Ca2+]c and Lhcb∷Luc rhythms peak at about the same time led us to test the possibility that the rhythm of Lhcb transcription was controlled by the rhythm of [Ca2+]c.
Our tests of that hypothesis led to performing experiments in which we observed that the [Ca2+]c and Lhcb∷Luc rhythms free-run with different periods in tobacco seedlings in constant conditions. Moreover, robust oscillations of Lhcb∷Luc luminescence continue in undifferentiated tobacco calli in the absence of [Ca2+]c oscillations. These data mean that the [Ca2+]c oscillation does not control the rhythm of Lhcb promoter activity. Furthermore, because the rhythms of [Ca2+]c and Lhcb∷Luc free-run with different periods in LL, different circadian pacemakers control these rhythms.
MATERIALS AND METHODS
Strains and Medium.
A double reporter strain of tobacco, LAQ (for luciferase/aequorin reporter), was constructed by crossing Nicotiana tabacum var. SR-1 expressing the cauliflower mosaic virus 35S promoter fused to apoaequorin cDNA (= strain MAQ2.3, kindly provided by Kenzo Nakamura; ref. 13) with N. tabacum var. Xanthi expressing the Arabidopsis Lhcb1*1 promoter fused to the firefly luciferase gene (= Lhcb∷Luc, kindly provided by Shawn Anderson and Steve Kay; ref. 10). Both of these transgenic parental lines are kanamycin-resistant. For the double-reporter LAQ, homozygous F3 seedlings or plants were used in all experiments.
To germinate seedlings, seeds were rinsed in 70% ethanol, sterilized in 20% Clorox (5.25% sodium hypochlorite) for 15 min, and washed in sterilized water three to four times. The sterilized seeds were soaked in 10 μM gibberellic acid overnight to synchronize germination. The seeds were placed on one-half Murashige and Skoog (MS) medium (Sigma; M0153) supplemented with 0.8% (wt/vol) agar and 1× vitamin mixture (Sigma) under a 16-h light/8-h dark cycle (LD16:8) at 26°C for 7 days. If transgenic plants expressing kanamycin resistance were used, 100 mg/liter kanamycin (Sigma) also was included in the medium.
Estimation of Total Apoaequorin Activity.
Samples of 7-day-old seedlings were collected at different circadian phases (10 seedlings per time point), snap-frozen in liquid nitrogen, and stored at −80°C until all samples were collected. Aequorin in homogenates was reconstituted and discharged in vitro by injecting calcium as described previously (6, 14). The luminescence signals for the 10 s immediately after calcium injection were integrated and used to calculate aequorin activity. Data as plotted in Fig. 1E were normalized to the total protein concentrations of the extracts.
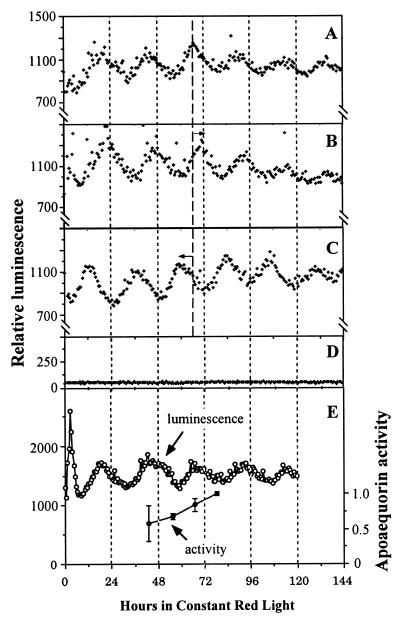
Figure 1.
Circadian oscillations of [Ca2+]c in N. tabacum monitored in the automated apparatus. (A–C) The luminescence of LAQ seedlings (six to eight seedlings per channel) in red LL (20 μE/m2⋅s) that had been incubated with coelenterazine. (A) Control (no white-light pulse). (B) One-hour white-light pulse (50 μE/m2⋅s) given at CT 13. (C) One-hour white-light pulse (50 μE/m2⋅s) given at CT 19. Before the beginning of luminescence recording, the seedlings were treated as described in Materials and Methods. Delay (B) or advance (C) phase shifts are indicated by the arrows. (D) Nontransgenic wild-type seedlings that were incubated in coelenterazine. For A–D, all treatments were done in triplicate and one representative is shown in the figure. (E) Comparison of luminescence expression of LAQ seedlings incubated in coelenterazine with total apoaequorin activity. ○, Luminescence rhythm measured as in A–D; ●, specific activity (normalized to protein concentration) of apoaequorin extracted at the indicated times from seedlings under the same conditions as the sample used for luminescence recording. Each extraction time point was done in triplicate, and error bars = SEM. The large error bar for the first data point was due to a particularly high activity in a single extract out of that triplicate.
Generation of Tobacco Calli.
Immature leaves of strain LAQ were rinsed with tap water and ethanol and then sterilized with 10% Clorox (5.25% sodium hypochlorite) for 15 min. Discs were made from the sterilized leaves and placed on solid MS medium containing 1% sucrose, 1× MS vitamin mixture (Sigma), 2 mg/liter α-naphthaleneacetic acid (NAA; Sigma), 1 mg/liter Kinetin (Sigma), 100 mg/liter kanamycin, and 0.8% agar and incubated in LD16:8 at 26°C. The calli formed from the edges of the leaf discs or strips were transferred onto fresh medium and subcultured every month. Only healthy calli were used for experiments. For monitoring the luminescence rhythms, calli were placed on medium without sucrose as described in Results.
Measurement of Luminescence Rhythms.
Seedlings were germinated and grown in LD16:8 (white light, 50 μE/m2⋅s). When the seedlings were 7 days old, they were transferred to constant darkness for 32 h. The seedlings then were transferred to an automated, multichannel photomultiplier tube apparatus (6), and recording of either Lhcb∷Luc or aequorin luminescence began under constant red light (20 μE/m2⋅s). For experiments in which white-light pulses (1-h pulses at 50 μE/m2⋅s) were administered (e.g., as in Fig. 1 B and C), those light pulses were given in the 24 h of darkness that immediately preceded the onset of constant red light. Periods and phases of the luminescence rhythms were determined by least-squares regression analyses by using the lva program (written by Takao Kondo, Nagoya Univ., Japan) and the chrono program (15).
For Lhcb∷Luc luminescence recording, two 7-day-old tobacco seedlings or about 300 mg of fresh weight of calli was placed on 2 ml of one-half MS medium (0.8% agar) supplemented with 1× vitamins and 100 mg/liter kanamycin. Thirty microliters of 2.5 μM beetle luciferin (Promega) was added directly onto the seedlings or calli at dawn of the final LD16:8 cycle and was present in the extracellular medium throughout the experiment.
For aequorin luminescence recording, apoaequorin was charged in vivo by a protocol similar to that described previously (6). Six to eight LAQ seedlings or about 500 mg fresh weight of calli was incubated with 10 μM coelenterazine (Biosynth, Basel) in darkness throughout the 8-h night of the last full LD16:8 cycle, during which time the apoaequorin was reconstituted into aequorin (6, 14). Then, the tissue was rinsed and placed on 2 ml of one-half MS medium (0.8% agar) supplemented with 1× vitamins and 100 mg/liter kanamycin. Coelenterazine was not present in the extracellular medium for the remainder of the experiment.
RESULTS
Circadian Oscillation of [Ca2+]c in N. tabacum.
The double-reporter strain, LAQ, allowed us to monitor cytosolic free calcium or the activity of the Lhcb promoter in the same genetic background (i.e., same strain) of tobacco. By selecting the appropriate incubation conditions, we could choose which parameter was measured—a preincubation with the luminophore coelenterazine for the Ca2+ reporter aequorin or a continuous incubation with beetle luciferin for the reporter of Lhcb promoter activity, firefly luciferase.
When LAQ seedlings were preincubated with coelenterazine, we observed circadian oscillations of luminescence that were equivalent to those we observed previously from Nicotiana plumbaginifolia and Arabidopsis seedlings that were transformed with the same apoaequorin construct (6). Fig. 1A shows that the luminescence emitted by reconstituted aequorin in LAQ seedlings exhibited circadian rhythms in constant red light (period 22.0–22.5 h; also see Table 1). Moreover, this rhythm could be phase-shifted by a 1-h white-light pulse, another diagnostic characteristic of circadian rhythms. White-light pulses in the early subjective night [circadian time (CT) 13] gave a phase delay of 4.5 h (Fig. 1B), whereas pulses in the late subjective night (CT 19) elicited a phase advance of 6 h (Fig. 1C). Nontransgenic N. tabacum seedlings preincubated in coelenterazine gave a low constant background signal (Fig. 1D), indicating that the luminescence signal was specific for reconstituted aequorin.
Table 1.
Free-running periods of calcium and Lhcb∷Luc rhythms
| Experiment 1
| ||||||
|---|---|---|---|---|---|---|
| Sample | Strains | Rhythms | Substrates loaded | Period (mean ± SEM) | n | Different from?* |
| a | LAQ | Calcium | coel | 22.16 ± 0.12 | 10 | b |
| b | LAQ | Lhcb∷Luc | coel + LH2 | 23.60 ± 0.18 | 10 | a |
| c | MAQ2.3 | Calcium | coel | 22.50 ± 0.19 | 3 | NS |
| d | MAQ2.3 | Calcium | coel + LH2 | 22.75 ± 0.61 | 3 | NS |
| Experiment 2 | ||||||
| e | LAQ | Calcium | coel | 22.34 ± 0.11 | 3 | f and g |
| f | LAQ | Lhcb∷Luc | LH2 | 23.89 ± 0.06 | 3 | e |
| g | LAQ | Lhcb∷Luc | coel + LH2 | 23.60 ± 0.10 | 4 | e |
| h | MAQ2.3 | Calcium | coel | 22.31 ± 0.11 | 5 | NS |
| i | MAQ2.3 | Calcium | coel + LH2 | 22.26 ± 0.08 | 4 | NS |
coel, coelenterazine; LH2, luciferin; NS, not significantly different from the paired sample (i.e., c vs. d, h vs. i).
This column shows whether the sample is different from the sample(s) indicated at the p < 0.01 level (Student’s t test).
The luminescence rhythms cannot be attributed to rhythmic levels of apoaequorin or of reconstituted aequorin. Apoaequorin content assayed from extracts of LAQ seedlings at various circadian times over 36 h displayed no circadian oscillations that could account for the rhythm of luminescence (Fig. 1E). As we found previously for N. plumbaginifolia (6), there appeared to be a progressive increase in apoaequorin levels that might result from seedling growth and continued apoaequorin synthesis. These data are consistent with the observation that the activity of the cauliflower mosaic virus 35S promoter is constitutive over the circadian cycle in LL and in constant dark in Arabidopsis (8). We conclude, therefore, that the oscillations of luminescence are a result of oscillations in [Ca2+]c.
Aequorin luminescence is specific for Ca2+ and is sensitive to free calcium in the physiological range (16). Calibration of the [Ca2+]c changes requires knowledge of the total amount of reconstituted aequorin in the plants, which necessitates a destructive assay (16). We found it difficult to achieve a complete discharge of aequorin activity in N. tabacum seedlings. However, the amplitude of the luminescence rhythms for LAQ seedlings preincubated with coelenterazine was similar to those we found previously for N. plumbaginifolia, where we estimated that [Ca2+]c ranged between approximately 100 and 150 nM at the trough phase to a peak of approximately 500–700 nM (6).
Desynchronization of [Ca2+]c and Lhcb∷Luc Rhythms in Red LL.
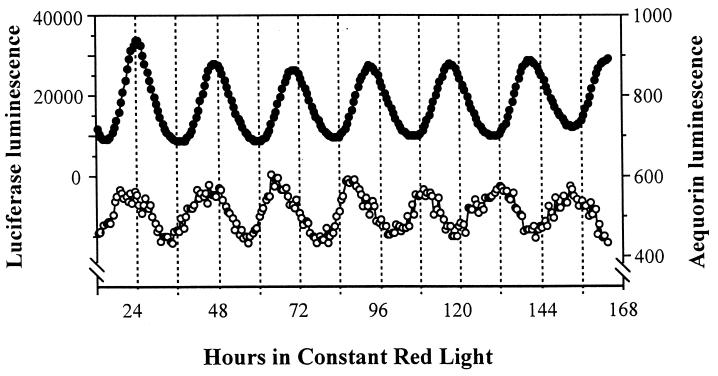
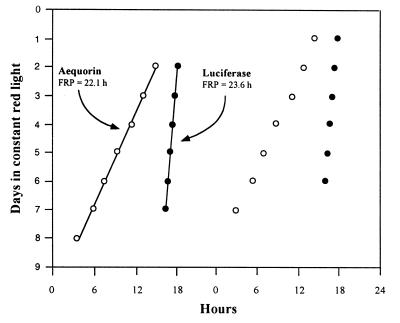
Circadian rhythms of Lhc gene expression have been well characterized at the levels of both mRNA abundance and promoter activity (4, 7–10). The rhythm of Lhcb promoter activity can be monitored noninvasively and continuously by the luminescence of plants transformed with a Lhcb∷Luc reporter construct (8, 10). To determine whether a functional relationship exists between the rhythms of [Ca2+]c and Lhcb promoter activity, we compared the luminescence rhythms of LAQ seedlings preincubated with coelenterazine (for [Ca2+]c) or incubated with luciferin (for Lhcb promoter activity). In red LL at 22°C, the period of luminescence of the LAQ seedlings preincubated with coelenterazine was 22.16 ± 0.12 h, whereas the Lhcb∷Luc luminescence rhythm had a period of 23.60 ± 0.18 h (Table 1, experiment 1). The 1.44-h difference of their periods was significant at the 1% level as tested by Student’s t test. Although the two luminescence rhythms start free-running in approximately the same phase, after 5–6 days in red LL, the two rhythms are in antiphase (Fig. 2). These period differences are consistent throughout the experiment and are not due to transients in the first few cycles (Fig. 3). Because the [Ca2+]c and Lhcb∷Luc rhythms were measured simultaneously in the same apparatus, there was no difference in the assay conditions (e.g., light intensity or temperature) that might account for these period differences.
Figure 2.
Desynchronization of [Ca2+]c and Lhcb∷Luc rhythms in the double-reporter tobacco strain LAQ in red LL at 22°C. Luminescence from the aequorin reporter is shown in the open circles and that for the Lhcb∷Luc reporter is shown in the solid circles. Two seedlings/sample were measured for the Lhcb∷Luc rhythm, and six seedlings/sample were measured for the [Ca2+]c rhythm.
Figure 3.
Consistency of differing free-running periods (FRPs) of [Ca2+]c and Lhcb∷Luc rhythms in LAQ. The timing of peaks in the [Ca2+]c rhythm is shown with open circles; peaks of the Lhcb∷Luc rhythm are shown with solid circles. Regression lines calculated with the chrono program (15) are plotted along with the estimated periods. Data are “double-plotted” (abscissa, hours in each day; ordinate, days in red LL).
Moreover, the difference in period values is not due to coelenterazine or luciferin affecting the circadian periods differentially. To test whether luciferin might affect the period, we used MAQ2.3 seedlings. MAQ2.3 is a transgenic strain of N. tabacum that expresses only the 35S promoter/apoaequorin construct and does not have the Lhcb∷Luc reporter (13). To one group of MAQ2.3 seedlings, we applied both luciferin and coelenterazine and, to another group, we applied coelenterazine alone. If luciferin directly affects the free-running period of tobacco seedlings, we would expect the coelenterazine + luciferin group to have a longer period of the aequorin luminescence rhythm than the coelenterazine group (because MAQ2.3 does not have the Lhc∷Luc construct, all the luminescence comes from aequorin). Table 1, experiments 1 and 2, show that there is no significant difference between the periods of these two groups. On the other hand, if coelenterazine shortens the period of the circadian clock, then we would expect that LAQ seedlings incubated with both coelenterazine and luciferin should have a shorter period than LAQ seedlings incubated with luciferin alone. (Because the luciferase luminescence is so much stronger than that of aequorin, we see only the luciferase signal when both substrates are presented to LAQ seedlings.) Samples f and g (Table 1, experiment 2) are not significantly different, indicating that the addition of coelenterazine does not shorten significantly the period of Lhcb∷Luc luminescence in LAQ seedlings. The data shown in Table 1 and Fig. 2 indicate that neither coelenterazine nor luciferin have a significant effect on the free-running periods and, therefore, that the period differences between LAQ seedlings incubated with coelenterazine vs. luciferin reflect different circadian pacemakers driving the [Ca2+]c vs. Lhcb∷Luc rhythms.
Circadian Rhythms in Calli Generated from LAQ.
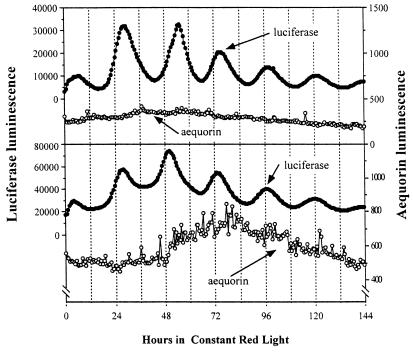
To determine whether the rhythms of [Ca2+]c and Lhcb promoter activity persist in relatively undifferentiated tissue, we developed calli from the LAQ strain. These calli grow as clumps of cells cultured in medium with 1% sucrose in light/dark cycles. They are chemomixotrophic, i.e., they derive their carbon source partially from photosynthetic fixation of CO2 and partially from sucrose in the medium. We treated calli with either coelenterazine or luciferin, transferred them to medium without sucrose, and tested their luminescence rhythms. In calli incubated with luciferin, the Lhcb∷Luc rhythm continued with a period of approximately 24 h in red LL, but no luminescence rhythm was detectable in calli preincubated with coelenterazine (Fig. 4). We confirmed that these coelenterazine-loaded calli indeed had reconstituted levels of aequorin comparable to those in the seedlings. This is another line of evidence that indicates the [Ca2+]c oscillation is not driving the rhythm of Lhcb promoter activity and further supports the dissociation of these two oscillations.
Figure 4.
Luminescence rhythms in calli of LAQ on medium without sucrose. The rhythm of Lhcb promoter activity was maintained in calli, but the [Ca2+]c rhythm disappeared in LAQ calli. Two independent experiments are depicted. Luminescence from the aequorin reporter is shown in the open circles and that for the Lhcb∷Luc reporter is shown in the solid circles.
DISCUSSION
Based on a model that hypothesized regulation of Lhcb transcription by [Ca2+]c (12), we tested whether the circadian oscillation of [Ca2+]c might be responsible for driving the rhythm of Lhcb promoter activity. Two lines of evidence indicate that there is no functional link between these rhythms: first, the Lhcb∷Luc rhythm persists, whereas the [Ca2+]c oscillation does not, in calli tissue of equivalent mass and luminescence signals to seedling tissue in which both rhythms are obvious. Second, in seedlings under red LL, the two rhythms dissociate and free-run with significantly different periods. This latter observation indicates the presence of at least two circadian pacemakers that can operate independently in tobacco. In contrast to the relative coordination observed between desynchronized rhythms in humans and Gonyaulax (1, 3), the stability of the periods of the Lhcb∷Luc and [Ca2+]c rhythms implies that the pacemakers underlying these plant rhythms do not interact. In the natural environment, the phase and period of these pacemakers will be determined by entraining light/dark and temperature signals so that these rhythms will not desynchronize. Therefore, although it may be possible that [Ca2+]c fluxes are involved in acute and/or developmental regulation of Lhcb transcription (12), our data indicate that they are not functionally linked in the circadian control pathway.
Are the [Ca2+]c and Lhcb∷Luc rhythms generated in the same cells, as in the unicellular alga Gonyaulax (3), or in different tissues? This question is difficult to answer with current technology. In Arabidopsis seedlings, imaging of the signal from the Lhcb∷Luc reporter indicates that the luminescence emanates primarily from the cotyledons, but further discrimination is not yet possible (8). The signal from the aequorin reporter is too dim at basal levels of [Ca2+]c (600 nM [Ca2+]c at the peak of the rhythm) to image effectively. In N. plumbaginifolia, the aequorin luminescence elicited by cold shock, touch, or wounding emanates from cotyledons and roots, so aequorin is distributed widely throughout the seedling (17). Because plant tissue is highly pigmented, it is probably true for both the aequorin and luciferase signals that the luminescence measured is most likely to be that emitted from the uppermost layer of cells, because photons emitted from deep within the tissue will mostly be absorbed before they can escape (17). Therefore, it is possible that the luminescence rhythms of both aequorin and luciferase come from the same cells in the cotyledons. If so, the absence of a [Ca2+]c rhythm in calli implies that the [Ca2+]c-controlling pacemaker stops or becomes uncoupled from its outputs in the relatively undifferentiated calli.
On the other hand, the [Ca2+]c rhythm may be expressed in specialized tissue. If the rhythmic changes in [Ca2+]c are localized to only specific tissues, then the 600-nM estimate of [Ca2+]c at the peak of the rhythm would be an underestimate of the true magnitude of the [Ca2+]c change in those cells. Other circadian rhythms in which the [Ca2+]c oscillation could play a role include the rhythm of stomatal conductance, because the turgor of guard cells is determined by ion fluxes, of which [Ca2+]c is a key regulator (18). By regulating changes in turgor, [Ca2+]c oscillations also could be the molecular linkage between a pacemaker and circadian movements of stems and petioles (19, 20).
If the [Ca2+]c oscillation is not the regulator of circadian changes of Lhcb transcription, what is the transduction pathway? Rhythmically expressed transcriptional factors are a likely explanation (21, 22). Rhythmically expressed CCA1 binds to the Lhcb promoter and plays a central role in its regulation (22, 23). A CCA1-null strain (cca1–1) has altered expression patterns of four clock-controlled genes, Lhcb, LHY, CAT2, and CCR2 (23). Interestingly, whereas the rhythms of Lhcb, LHY, and CAT2 expression are all altered by cca1–1 in a similar fashion (≈3-h shorter period), the expression of the CCR2 gene shows a different phenotype: an altered phase (23). Could it be that the circadian pacemakers underlying the rhythms of [Ca2+]c and CCR2 expression have a common basis and one that is different from the pacemaker controlled or mediated by CCA1 and LHY? Only time will tell.
Acknowledgments
We are grateful to Dr. Kenzo Nakamura for providing seeds of MAQ2.3, Drs. Shawn Anderson and Steve Kay for providing seeds of the Lhcb∷Luc reporter strain, Drs. Till Roenneberg and Walter Taylor for the chrono analysis program and conversion programs, and Dr. Takao Kondo for the Luminescence Vial Analysis (lva) program and extensive advice for measuring luminescence rhythms. We appreciate the advice of Drs. Marc Knight and Anthony Trewavas concerning transgenic plants expressing aequorin. This research was supported by the National Institute of Mental Health (MH43836 and MH01179 to C.H.J.).
ABBREVIATIONS
- LL
constant light
- LD
light/dark cycle
- LD16:8
16-h light/8-h dark
- [Ca2+]c
cytosolic free calcium ions
- Lhc
light-harvesting complex gene (Lhcb1*1 = CAB2)
- CT
circadian time (CT 0 = subjective dawn, CT 12 = subjective dusk)
- MS
Murashige and Skoog
- LAQ
luciferase/aequorin reporter
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Wever R. The Circadian System of Man. Berlin: Springer; 1979. [Google Scholar]
- 2.Hennessey T L, Field C B. J Biol Rhythms. 1992;7:105–113. doi: 10.1177/074873049200700202. [DOI] [PubMed] [Google Scholar]
- 3.Roenneberg T, Morse D. Nature (London) 1993;362:362–364. doi: 10.1038/362362a0. [DOI] [PubMed] [Google Scholar]
- 4.Kolar C, Fejes E, Ádám É, Schäfer E, Kay S, Nagy F. Plant J. 1998;13:563–569. doi: 10.1046/j.1365-313x.1998.00048.x. [DOI] [PubMed] [Google Scholar]
- 5.Millar A J. In: Biological Rhythms and Photoperiodism in Plants. Lumsden P J, Millar A J, editors. Oxford: Bios; 1998. pp. 51–68. [Google Scholar]
- 6.Johnson C H, Knight M R, Kondo T, Masson P, Sedbrook J, Haley A, Trewavas A. Science. 1995;269:1863–1865. doi: 10.1126/science.7569925. [DOI] [PubMed] [Google Scholar]
- 7.Millar A J, Kay S A. Plant Cell. 1991;3:541–550. doi: 10.1105/tpc.3.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Millar A J, Short S R, Chua N-H, Kay S A. Plant Cell. 1992;4:1075–1087. doi: 10.1105/tpc.4.9.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piechulla B. Plant Mol Biol. 1993;22:533–542. doi: 10.1007/BF00015982. [DOI] [PubMed] [Google Scholar]
- 10.Anderson S L, Teakle G R, Martino-Catt S J, Kay S A. Plant J. 1994;6:457–470. doi: 10.1046/j.1365-313x.1994.6040457.x. [DOI] [PubMed] [Google Scholar]
- 11.Neuhaus G, Bowler C, Kern R, Chua N-H. Cell. 1993;73:937–952. doi: 10.1016/0092-8674(93)90272-r. [DOI] [PubMed] [Google Scholar]
- 12.Bowler C, Neuhaus G, Yamagata H, Chua N-H. Cell. 1994;77:73–81. doi: 10.1016/0092-8674(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 13.Ohto M-A, Hayashi K, Isobe M, Nakamura K. Plant J. 1995;7:297–307. [Google Scholar]
- 14.Knight M R, Campbell S M, Trewavas A J. Nature (London) 1991;352:524–526. doi: 10.1038/352524a0. [DOI] [PubMed] [Google Scholar]
- 15.Roenneberg, T. & Taylor, W. (1999) Methods Enzymol.305, in press. [DOI] [PubMed]
- 16.Cobbold P M, Rink T J. Biochem J. 1987;248:313–328. doi: 10.1042/bj2480313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knight M R, Read N D, Campbell A K, Trewavas A J. J Cell Biol. 1993;121:83–90. doi: 10.1083/jcb.121.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webb A A R. In: Biological Rhythms and Photoperiodism in Plants. Lumsden P J, Millar A J, editors. Oxford: Bios; 1998. pp. 69–79. [Google Scholar]
- 19.Satter R L, Galston A W. Annu Rev Plant Physiol. 1981;32:83–110. [Google Scholar]
- 20.Kim H Y, Cote G G, Crain R C. Science. 1993;260:960–962. doi: 10.1126/science.260.5110.960. [DOI] [PubMed] [Google Scholar]
- 21.Carré I A, Kay S A. Plant Cell. 1995;7:2039–2051. doi: 10.1105/tpc.7.12.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z-Y, Tobin E M. Cell. 1998;93:1207–1217. doi: 10.1016/s0092-8674(00)81464-6. [DOI] [PubMed] [Google Scholar]
- 23.Green R M, Tobin E M. Proc Natl Acad Sci USA. 1999;96:4176–4179. doi: 10.1073/pnas.96.7.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]