Abstract
IgA deposition in glomerular mesangium and the interaction with mesangial cells may well be the final common pathway to IgA nephropathy (IgAN). Altered hinge-region O-glycosylation of IgA1 from patients with IgAN may predispose to mesangial deposition and activation of the mesangial cell (MC) by IgA1, via a novel IgA1 receptor, and may be a key event in the pathogensis of IgAN. The aim of this study was to investigate the binding capacity and biological effects of IgA1, from both patients with IgAN and healthy controls, on human mesangial cells (HMC). Serum IgA1 was isolated with jacalin affinity chromatography, heated to aggregated form (aIgA1) and labelled with 125I. Binding capacity of aIgA1 in vitro to cultured primary HMC was evaluated by a radioligand binding assay and the specificity of binding was determined by a competitive inhibition assay. Intracellular calcium release was studied by confocal analysis and phosphorylation of extracellular signal-regulated kinase (ERK) was determined by Western blot analysis. Change of cell cycles was demonstrated by flow cytometry and HMC proliferation was evaluated by direct cell count. Expression of TGF-β mRNA and production of supernatant fibronectin were tested by RT-PCR and indirect competitive ELISA, respectively. aIgA1 from both the patients with IgAN and normal controls bound to HMC in a dose-dependent, saturable manner, and was saturated at approximately 500 pmoles per 0·5 ml of aIgA1. aIgA1 from patients with IgAN, however, bound to HMC at a higher speed and Scatchard analysis revealed a Kd of (8·89 ± 2·1) × 10−8mversus (4·3 ± 1·2) × 10−7m for aIgA1 from healthy controls (P = 0·026).The binding was specific because it was only inhibited by unlabelled Mono-IgA1 (mIgA1) and not by serum albumin or IgG. aIgA1 from patients with IgAN could induce release of intracellular calcium, phosphorylation of ERK, DNA synthesis, proliferation of HMC, expression of TGF-βmRNA and secretion of fibronectin in HMC in a similar time-dependent manner as aIgA1 from healthy controls, but the effects were much stronger and the durations were much longer (P < 0·05, respectively). We conclude that aIgA1 from patients with IgAN has a higher binding capacity to HMC and stronger biological effects than aIgA1 from healthy controls. This suggests that direct interaction between IgA1 and HMC and subsequential pathophysiological responses may play an important role in the pathogenesis for IgAN.
Keywords: IgA1, mesangial cell, IgA nephropathy, pathogenesis
Introduction
IgA nephropathy (IgAN) is the most common primary glomerulonephritis in the world [1,2]. Immunohistologically, the disease is characterized by the deposition in the glomerular mesangium in proximity to mesangial cells (MC) of IgA, which is accepted in the form of poly IgA1 (pIgA1) [3,4]. The histopathology of IgAN is predominantly abundance of mesangial matrix and proliferation of MC [5]. Clinical observations of patients who have undergone renal transplantation have provided strong support for the notion that IgA nephropathy is a systemic disease. Histological evidence of recurrent IgA nephropathy is observed in over 35% of patients who receive renal allografts as treatment for end-stage renal disease due to IgA nephropathy. When a kidney obtained from a donor with asymptomatic IgA nephropathy is transplanted into a recipient with endstage renal disease due to a disease other than IgA nephropathy, the deposits in the donor kidney rapidly disappear [6]. Static studies in IgAN patients of mucosa and marrow indicate reduced production of IgA1 and J chain in the mucosa and in contrast an increased pIgA1 production in the marrow [7]. Immunization studies is consistent with the static studies: There is a reduced mucosal IgA response to mucosal immunization [8] and systemic IgA response is exaggerated in IgAN to chronic mucosal infection with Helicobacter pylori [9], implying that in IgAN there is a failure of oral tolerance. Serum pIgA1 is increased in patients with IgAN [10–12] and has an abnormal pattern of O-glycans in hinge-region with a significant increase in N-acetylgalactosamine (GalNAc) exposure without galactose (Gal) [13,14]. It has been shown in vitro that IgA1 with reduced galactosylation decreases the ability of liver to eliminate the abundant circulating IgA1, resulting in accumulation of IgA1 in blood and self-aggregation, favouring the deposition of macromolecular IgA1 in glomerular mesangium [15,16]. pIgA1 has been demonstrated in protein eluates of biopsy specimens from IgAN patients [17,18] and a study of three kidneys indicates that mesangial pIgA1 is enriched for the Gal-deficient O-glycosylation pattern seen in serum IgA1 [19], strongly suggesting that the O-glycan abnormality is indeed directly implicated in mesangial IgA deposition.
Recently, increasing evidences have demonstrated that IgA1, isolated from healthy individuals, binds to MC in a dose dependent and saturable manner, and the binding is specific for IgA1 because only IgA1 Fc fragments could inhibit the binding whereas albumin, IgG, IgM, and IgA1 F(ab) fragments could not [20–22]. It was found that IgA1 bound to MC with 1·2 × 106 binding-sites/cell, an affinity constant (Ka) of 2·3 × 106M−1 and a dissociation constant (Kd) of 4·4 × 10−7M. Addition of various cytokines had no significant influence on Ka, but increased the number of binding sites/cell compared with unstimulated cells [23]. Moreover, binding of IgA1 to MC could induce intracellular signal transduction [22, 24, 25] and up-regulation of the secretion of pro-inflammatory cytokines, such as IL-6, TNF-α, etc. [24,26–28]. Therefore, it has been suggested that IgA1 binding to MC is via a specific Fcα receptor on MC and the interaction between IgA1 and the MC is one important aspect in the pathogenesis of IgAN.
Leung et al. [29] examined the binding characteristics of IgA1 to human mesangial cells (HMC) by flow cytometry and found that IgA1 from patients with IgAN had a higher affinity than that from normal persons. Whether IgA1 from patients might induce a more intensive pathophysiological effects on HMC have not been systemically studied. The purpose of the current study was to examine and compare the binding capacity and biological effects of serum IgA1, from both patients with IgA nephropathy and healthy controls, on HMC.
Materials and Methods
Patients and sera
Ten patients with renal biopsy-proven IgAN were enrolled in this study. All the patients clinically presented with active phase clinical presentations, such as onset of haematuria and/or proteinuria, or deteriorated haematuria and/or proteinuria, higher serum IgA concentration and without treatment with corticosteroids or immunosuppressive agents. Four patients had upper respiratory tract infection including tonsillitis or bronchitis. Renal biopsy was performed after the mucosal infections were fully controlled and the blood was drawn one day before renal biopsy. The renal function in the patient group was within normal range with a mean serum creatinine at 77 ± 24 (41–113) µmol/l. The renal pathology included diffuse mesangial proliferation glomerulonephritis (six cases), endocapillary proliferative glomerulonephritis (two cases), focal proliferative sclerotic glomerulonephritis (one case) and crescentic glomerulonephritis (one case). After obtaining informed consent, the serum samples were collected. Sera from 10 healthy individuals, without mucosal infection in recent two months, were used as healthy controls.
Isolation, aggregation and 125I labelling of human serum IgA1
IgA1 was isolated from pooled sera of 10 patients or 10 healthy controls separately by jacalin affinity chromatography [22]. Briefly, the pooled sera were diluted 1 : 1 with phosphate buffered saline (PBS), filtered through a 0·2 µm Corning syringe filter (Corning Glass Works, Corning, NY, USA) and applied to a jacalin column prepared using commercially available Jacalin immobilized on cross-linked 4% beaded agarose (Vector Laboratories, CA. USA) with an IgA1 binding capacity of 2–4 mg/ml of gel. The column was then washed with 175 mm Tris-HCl (pH 7·4) until the optical density (OD 280 nm) was less than 0·10. IgA1 was eluted with 0·1 m melibiose (Sigma, St. Louis, MO, USA) in 175 mm Tris-HCl in 3·0 ml fractions until the optical density returned to 0·1. The fractions were pooled and concentrated by ultrafiltration using 30 000 molecular weight exclusion membranes (Centriprep 30; Amicon, Beverly, MA, USA). The concentrated sample was dialysed against PBS for 24 h to remove melibiose. The sample was proven to be IgA by double immunodiffusion and immunoelectrophoresis using goat anti-human IgA, goat anti-human IgG, goat anti-human IgM (Beckman). The various molecular size forms of IgA1 were separated by molecular sieve chromatography using a 2·6 × 60 cm Sephacryl S-200 HR column mounted on a Pharmacia Smart System (AKTA-FPLC) equipped with a micropeak detector (Pharmacia Biotech, Uppsala, Sweden) and two distinct peaks in the OD280 absorption profile were noted. The molecular size of each IgA fraction was determined by SDS-PAGE and Western blot analysis using mouse anti-human IgA1 antibody, mouse anti-human IgA2 antibody, mouse anti-human IgA SC segment antibody, mouse anti-human IgA J chain antibody, alkaline phosphatase (ALP)-conjugated goat anti-mouse IgG antibody (Sigma), respectively. The protein in the second peak migrated consistent with monomeric IgA1 (mIgA1) on SDS-PAGE and could only be blotted by monoclonal mouse anti-human IgA1 antibody in Western blot analysis. To prepare aggregated IgA1 (aIgA1), the samples in PBS were passed through a 0·2 m filter and then heated at 63°C for 150 min. The samples were immediately placed on ice and microcentrifuged to remove insoluble precipitant. The soluble aIgA1 was characterized on Sephacryl S-200 HR column and demonstrated a molecular weight same as polymeric IgA1 with a molecular weight of approximate 600 kD. AIgA1 concentration in the samples was tested with Laser Velocity Dispersion Assay and the total protein concentration of the same sample was tested with Bradford method, the purity of aIgA1 was calculated as the ratio of aIgA1 concentration over total protein concentration at a level of 99·7%. AIgA1 was radiolabled with Na125I (10–20 mCi/mg iodine; Amersham corp, Arlington Heights, IL, USA) by the Idogen method [30] to a sp. act. of 0·3 mCi/mg. The radiolabelled aIgA1 had the same immunology activity on double immunodiffusion, and immunoelectrophoresis with the same molecular weight on SDS-PAGE as nonlabelled aIgA1. AIgA1 from pooled sera of all patients was prepared in the same way and the sample shown the same immunology activity and molecular weight as that from the healthy with the purity of 99·8%.
Isolation and culture of human mesangial cells and U937 cells
Normal portion of human kidney cortex was obtained from each nephrectomy specimen of six patients with kidney tumours. Primary cultures of HMC were grown from glomerular explants using a standard sequential sieving technique [31]. Briefly, cortical tissue, bathed in Hank's balanced salt solution (HBSS) at 4°C, was minced and sequentially passed through 80, 140, and 220 mesh sieves. The retained glomeruli were washed with copious amounts of HBSS to remove tubular fragments, incubated for three minutes at 37°C with 1·0 mg/ml collagenase type I (type Ia, Sigma) and centrifuged at 1000rpm for five minutes at 4°C to pellet the glomeruli. The pelleted glomeruli were resuspended in RPMI 1640 medium buffered with 25 mm HEPES at pH 7·4 and supplemented with 20% FCS (Gibco BRL, UK), 30 mg/ml penicillin, 68 mg/ml streptomycin, and 150 mg/ml glutamine, plated in 75-cm2 tissue culture flasks (Costar, Cambridge, MA, USA) and cultured in humidified 5% CO2 atmosphere at 37°C. Cells were passaged when confluent using 0·025% trypsin in 0·5 mm EDTA. The cells of first passage from each nephrectomy specimen were frozen in liquid nitrogen. Before each experiment, equal number of these cells were thawed, mixed and cultured to third or forth passage with 10%FCS in RPMI 1640 medium. All the experiments were performed on the mixed third or fourth passage. Purity and identification of mesangial cells were by cell morphology, negative staining by indirect immunofluorescence with antisera to cytokeratin and factor VIII antibody (Sigma), and positive staining of actin arrays with rhodamine phalloidin (Molecular Probes, Inc., Eugene, OR, USA). U937 cells obtained from ATCC (Rockville, MD, USA) were grown in RPMI 1640 media with 10% FCS.
Binding of aIgA1 to mesangial cells
HMC (passages 2 or 3) suspended in medium was aliquoted into 6-well tissue culture dishes. The cells were grown to confluency (about 24to 48 h) in RPMI 1640 media with 10% FCS. Cells were washed three times with PBS and preincubated at 4°C for 60 min with 0·5 ml 0·5%BSA-PBS alone or in the presence of 1000 pmol of aIgA1. After preincubation, cells were washed three times with 0·5%BSA-PBS and incubated at 4°C for 60 min with nonradioactive aIgA1 of 500 pmol alone or in the presence of increasing amounts of 125 I-aIgA1 (from 0 to 1·0 pmol) in 0·5 ml 0·5%BSA-PBS. After incubation, The cells were washed three times with PBS and solublized in 1·0 ml 0·5 N NaOH for 10 minutes. The lysates were transferred to gamma tubes and the radioactivity was quantified in a Packard 5002 gamma counter (Downer's Grove, IL, USA). The cell number from a random well in each flask was counted with an average at 5 × 105/well. In inhibition study, the cells was preincubated at 4°C for 60 min with BSA, human albumin, IgG, mIgA1 or aIgA1 of the same amount of 1000 pmol in 0·5 ml 0·5% BSA-PBS. After preincubation, cells were incubated at 4°C for 60 min with 125I-aIgA1 (1·0 pmol) in the presence of nonradioactive aIgA1 of 500 pmol in 0·5 ml 0·5%BSA-PBS. Taking the inhibition rate of BSA as zero and that of aIgA1 as 100%, other proteins inhibitive capability was calculated. The experiment with triplicate wells was repeated three times (as well as other experiments in the whole study).
Determination of intracellular Ca2+
Intracellular calcium release was studied by confocal analysis with fluorescent Ca2+ indicator Fluo-3 [32]. Briefly, 80% confluent HMC monolayers were washed with phenol red free HBSS buffer and loaded with Flou-3 acetoxymethyl ester (8 µm) and F-127 (0·07%) for 45 min at 37°C and 30 min at room temperature. The cells, rinsed and kept wet in phenol red free HBSS buffer, were placed under Confocal microscope (Leica, Manham, German) and objectives were chosen. The release of Ca2 + were monitored by recording changes in the fluorescence ratio at 525 nm (with excitation at 488 nm) at 5-second interval 30 s before and 300 s after the addition of aIgA1 at concentration of 62·5 µg/ml, 125·0 µg/ml or 250·0 µg/ml at room temperature. The release of intracellular Ca2 + was expressed as the increase of fluorescence intensity before and after the addition of aIgA1.
Western blot analysis of ERK
HMC no. 3 or 4 in 6-well tissue culture dishes, grown to confluency and in quiescent state, were incubated with 0·5%FCS in RPMI alone or in the presence of aIgA1 of 100 µg/ml from patients with IgAN or from healthy controls for additional 5 min, 15 min, 30 min and 60 min. The media was removed and the cells were lysed in 0·15 ml lysing buffer (consisting in PBS of 0·5% Triton-X100, 1·0 mm EGTA, 50 mmβ-glycerophosphate, 2·0 mm MgCl2, 100 µm Na2VO4, 1·0 mm DTT, 20 µm pepstatin, 20 µm leupeptin, 1000 µ/ml aprotinin, 1 mm PMSF) on ice for 10 min. Insoluble material was removed by centrifugation at 11 600 g for 10 min and protein concentration in supernatants was measured by the Bradford method [32] using BSA as the standard. Samples of 15 mg (for total ERK) or 30 mg (for phosphorylated ERK) were electrophoresed and transferred to polyvinylidene difluoride membranes. Detection of total ERK and phosphorylated ERK proteins was accomplished by a first incubation with 1 : 2000 dilution of rabbit anti-human ERK and mouse anti-human phosphoralated ERK (Santa Cruz Biotechnology), respectively, and followed by a 1 : 5000 dilution of horseradish peroxidase-conjugated secondary antibodies (Amersham, Buckinghamshire, UK). Membranes were washed and exposed to Kodak X-Omat S films using an ECL chemiluminescence kit (Amersham, Buckinghamshire, UK). Densitometric analysis was performed by scanning the blot on gel scan analysis system and then analysed using Imagequant (Kodak, Rochester, NY, USA).
Flow cytometry of DNA synthesis
HMC no. 3 or 4 in 6-well tissue culture dishes, grown to confluency and in quiescent state, were incubated with 0·5%FCS in RPMI alone or in the presence of aIgA1 of 100 µg/ml from patients with IgAN or from healthy controls for additional 18 h, 24 h, 36 h and 48 h before harvested using 0·025% trypsin in 0·5 mm EDTA. Cells of 2·0 × 106 were fixed with 70% ethanol at 4°C for overnight and stained with 0·8 ml staining buffer (containing propidium iodide 50 µg and RNAase 1·67–3·33 × 104U) at room temperature for 30 min. The stained cells were analysed on a Becton-Dickenson Model FACScan (San Jose, CA, USA). A minimum of 10·000 fixed cells for each sample was analysed. Fluorescence intensity was evaluated by comparing the mean fluorescence channels. The result was expressed as mean fluorescence intensity.
Total RNA extraction and reverse transcription-PCR
HMC no. 3 or 4 in 6-well tissue culture dishes, grown to confluency and in quiescent state, were incubated with 0·5%FCS in RPMI alone or in the presence of aIgA1 of 100 µg/ml from patients with IgAN or from healthy controls for 12, 24, 36, 48 and 72 h. Total RNA was extracted from HMC using Trizol Reagent kit (Life Technologies Calsbad, CA, USA). The mRNA from 5·0 µg total RNA was reverse-transcribed with Superscript II using random hexamers (Life Technologies BRL, Gaithersburg, MD, USA) at 42°C for 50 min 1·0 µl cDNA mode was amplified in a DNA thermal cycler (Perkin Elmer Cetus) using 92°C melting, 66°C annealing, 72°C extension temperature and 35 cycles according to manufacturer's instructions, using the following 5′- and 3′-primers based on the sequence data for TGF-β; 5′-TGGAACTTCTACCAGTGC GAC-3′ (sense) and 5′-AGCTTCTTAGTGAGCTTTT CTCTC-3′ (antisense). Twenty microlitres of the PCR products was analysed by agarose gel electrophoresis and staining with ethidium bromide. Integrity of the RT products from RNA was ensured by examining the gene expression of a housekeeping gene, glyceraldehydes-3- phosphate-dehydrogenase (GAPDH) with the primers 5′-CGTCTTCACCA CCATGGAGA-3′ (sense) and 5′-CGGCCATCACGCCACAGTTT-3′ (antisense). The up-regulation of TGF-β expression was expressed as the ratio of band intensity of TGF-β mRNA over GAPDH mRNA.
Fibronectin (Fn) secretion by HMC in vitro
HMC no. 3 or 4 in 6-well tissue culture dishes, grown to confluency and in quiescent state, were incubated with 0·5%FCS in RPMI alone or in the presence of aIgA1 of 100 µg/ml from patients with IgAN or from healthy controls for 12, 24, 36 and 48 h. Supernatants were collected and Fn was assessed by indirect ELISA, using the human serum Fn as a standard, rabbit anti-human Fn IgG as primary antibody and horseradish peroxidase-conjugated goat anti-rabbit IgG as the second antibody. Freshly prepared substrate solution containing OPD 0·4 mg/ml, 1·0 µl/ml 30% H2O2 in 0·1 m citrate-phosphate buffer (PH 5·0) was added and yellow colour was allowed to develop for 5–10 min before the reaction was stopped by addition of 2·0 N sulphuric acid, the absorbance at 490 nm was read in an ELISA reader (Metertech S960).
Results
Binding of aIgA1 to HMC
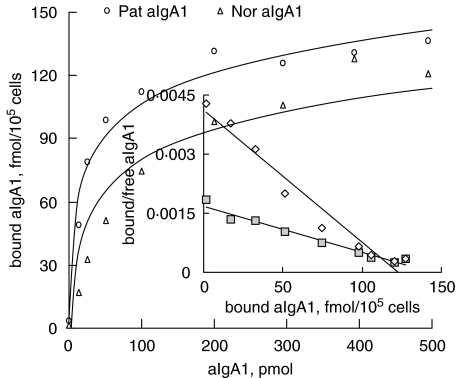
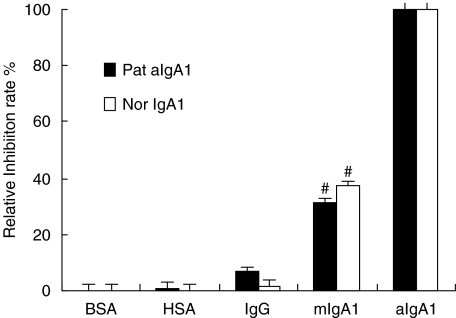
The binding of aIgA1 from both patients and healthy controls to HMC was dose dependent and saturation was achieved at approximately 500 pmol (Fig. 1). There was no significant difference in the maximal binding capacity between patients and healthy controls (136·9 ± 31·24 fmol/105cells versus 123·5 ± 21·05 fmol/105cells, P > 0·05). Quantification of aIgA1 binding to HMC was performed according to the method of Scatchard analysis [33]. The first-order linear regression fit for the Scatchard plot suggested that there was a single population of IgA receptors and the dissociation constant, Kd, for aIgA1 from patients was (8·89 ± 2·1) × 10−8mversus (4·3 ± 1·2) × 10−7m for that from healthy controls (P = 0·026). The specificity of IgA1 binding was investigated by determining the pattern of inhibition obtained with different unlabelled proteins. As shown in Fig. 2, only mIgA1 was able to inhibit the binding of aIgA1 to HMC by (30·99 ± 6·84)% for patients and (37. 3 ± 7·75)% for healthy controls. In contrast, neither human albumin nor IgG blocked the binding. All these results suggested that the receptor for IgA was class specific and aIgA1 had a higher affinity to HMC than mIgA1.
Fig. 1.
aIgA1 binding to HMC. Equilibrium binding of 125 I-aIgA1 to cultured HMC was assessed at 4°C as described in the Methods section. The experiment with triplicate wells was repeated three times in the study. The inset is a Scatchard plot. The cells were a mixture of six lines and the aIgA1 sample was from pooled sera of 10 healthy controls or 10 patients. P = 0·026, Kd value of 8·89 ± 2·1 ×10−8m for Pat. aIgA1 (patient's aIgA1) versus (4·3 ± 1·2 × 10−7m for Nor. aIgA1 (normal aIgA1).
Fig. 2.
Specificity of lgA1 binding to HMC in culture. HMC were preincubated for 60 min at 4°C with different unlabelled proteins before the addition of radio-active aIgA1: bull serum albumin (BSA), human IgG (IgG), human mIgA1 (mIgA1), human aggregated IgA1 (aIgA1). Results shown were means ± 2SE of three different experiments with triplicate wells. Pat.aIgA1 (patient's aIgA1). Nor. aIgA1 (normal aIgA1). The cells were a mixture of six lines and the aIgA1 sample was from pooled sera of 10 healthy controls or 10 patients. #P < 0·05 mIgA1 versus IgG or aIgA1.
Ca2+ mobilization in HMC stimulated with algA
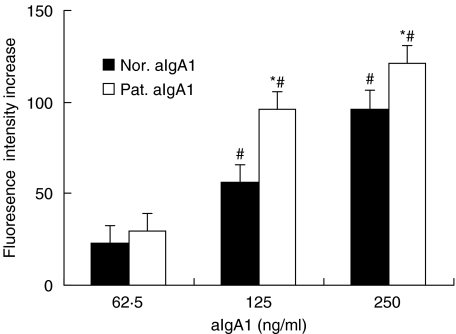
The aIgA from both patients and healthy controls could evoke a similar, dose-dependent increase in Ca2+ release lasting 2–4 min with the peak time at 20–30seconds. The increases of maximal fluorescence intensity, however, induced by aIgA1 from patients and healthy controls were 95·83 ± 11·43 and 55·88 ± 12·72 (aIgA1 at 125 µg/ml) or 120·86 ± 21·71 and 96·40 ± 15·91 (aIgA1 at 250 µg/ml), respectively (P < 0·05). The aIgA1 from patient could stimulate intracellular Ca2+ release in HMC more significantly than that from healthy controls (Fig. 3).
Fig. 3.
Intracellular Ca2+ increase of in HMC. The change of maximal fluorescence intensity at 525 nm recorded by Confocal microscopic on HMC before the stimulation of aIgA1 (base fluorescence) and 60 s after the addition (maximal fluorescence) of different concentration. The release of intracellular Ca2+ was expressed as the increase of maximal fluorescence intensity. Results shown were mean ± 2SE of three repeated experiments with triplicate wells. Pat.aIgA1 (patient's aIgA1). Nor. aIgA1 (normal aIgA1). The cells were a mixture of six lines and the aIgA1 sample was from pooled sera of 10 healthy controls or 10 patients. *P ≤ 0·05, Pat.aIgA1 versus Nor.aIgA1; #P ≤ 0·05, between different aIgA1 concentration.
Phosphorylation of ERK in cultured HMC
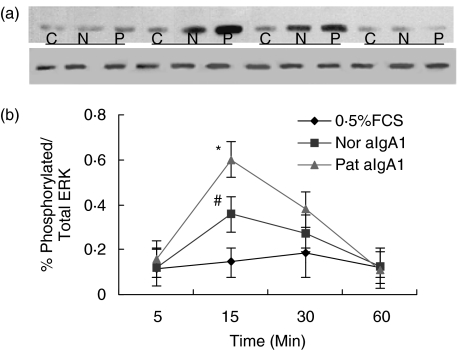
A low level (10%-12%) phosphorylation of ERK was detected in unstimulated HMC. aIgA treatment could induce a time-dependent increase in phosphorylation of ERK with maximal levels after 15–30min of incubation (Fig. 4a). The semiquantitative assessement of phosphorylation ERK was expressed as percentage in respect of density ratio of the bands of phosphorylated ERK over total ERK. At 15 min of incubation, the phosphorylation of ERK in HMC was (49·5 ± 10·1)% for aIgA1 from patients versus (30·7 ± 4·4)% for that from healthy controls, the difference was significant (P = 0·042) (Fig. 4b).
Fig. 4.
Phosphorylation of ERK in HMC. HMC were incubated in 0·5%FCS culture alone (C, 05%FCS) or with 100 µg/ml of normal aIgA1 (N, Nor.aIgA1) or patients’ aIgA1 (P, Pat. aIgA1) for different time. (a) χ film exposed using an ECL chemiluminescence kit. Upper bands were phosphorylated ERK, lower bands were total ERK (b) Densitometric analysis performed by scanning the blot of the film on scan analysis system and analysed using Imagequant. Phosphrylation of ERK was expressed as the density ratio of phosphrylated ERK over total ERK Results shown were means ± 2SE of three different experiments with triplicate wells. Pat.aIgA1 (patient's aIgA1). Nor. aIgA1 (normal aIgA1). The cells were a mixture of six lines and the aIgA1 sample was from pooled sera of 10 healthy controls or 10 patients. #P ≤ 0·05 Nor.aIgA1 versus PBS; *P ≤ 0·05 Pat.aIgA1 versus Nor.aIgA1 or P ≤ 0·01 Pat.IgA1 versus PBS.
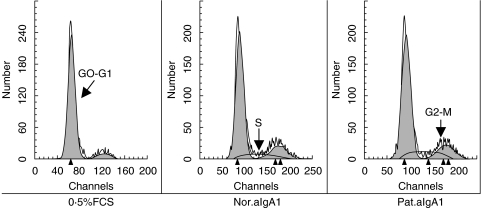
DNA synthesis and proliferation of HMC stimulated by aIgA1
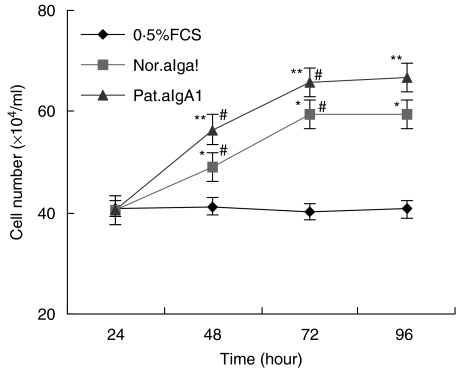
Incubation of HMC with aIgA1 induced a time-dependent DNA synthesis and mitosis in HMC, but the stimulation of aIgA1 from patients was stronger and lasted longer than that from healthy controls (Fig. 5). After 18 h of incubation, the percentage of ‘S’ phase cell in 0·5%FCS group, normal aIgA1 group and patients’ aIgA1 group were (3·48 ± 0·54)% (6·64 ± 0·96)% and (7·85 ± 0·71)%, respectively, the difference between 0·5%FCS group and aIgA1 group was significant (P < 0·05), and such trend lasted to 36 h of incubation in patients’ aIgA1 group while 24 h in normal aIgA1 group. After 24 h to 36 h of incubation, the percentage of ‘G2-M’ phase cell in 0·5%FCS group, normal aIgA1 group and patients’ aIgA1 group were (9·4 ± 1·86)% (14·35 ± 0·70)% and (17·24 ± 0·27)%, respectively, there was a significant difference between each two groups (P < 0·05). To confirm the proliferation of HMC after incubation with aIgA1, cell number was directly counted. As similar as the result of DNA synthesis, aIgA1 treatment resulted in an increase of the number of cultured HMC. After 48 h of incubation, and cell number in patients’ aIgA1 group was significantly higher than that in normal aIgA1 group (Fig. 6).
Fig. 5.
Cell cycle change of HMC£¨24 h£©. HMC, incubated for different hours in 0·5%FCS culture medium alone (0·5%FCS) or with 100 µg/ml of normal aIgA1 (Nor.aIgA1) or patients’ aIgA1 (Pat.aIgA1), were fixed and stained with Propidium iodide and RNAase at room temperature for 30 min. The stained cells were analysed on FACScan. The result was expressed as mean fluorescence intensity of HMC DNA at the incubation time of 24 h. The cells were a mixture of six lines and the aIgA1 sample was from pooled sera of 10 healthy controls or 10 patients.
Fig. 6.
Cell count of MC. HMC was incubated in 0·5%FCS culture medium alone (0·5%FCS) or with 100 µg/ml of normal aIgA1 (Nor.aIgA1) or patients’ aIgA1 (Pat.aIgA1) for different hours before count. The cell was a mixture of six lines and the aIgA1 sample was from pooled sera of 10 healthy controls or 10 patients. Results shown were means of three different experiments with triplicate wells. *P ≤ 0·05, Nor.aIgA1 versus 0·5%FCS; **P ≤ 0·05, Pat.aIgA1 versus 0·5%FCS or Nor.aIgA1; #P ≤ 0·05, 48 h versus 24 h or 72 h versus 48 h in the same group.
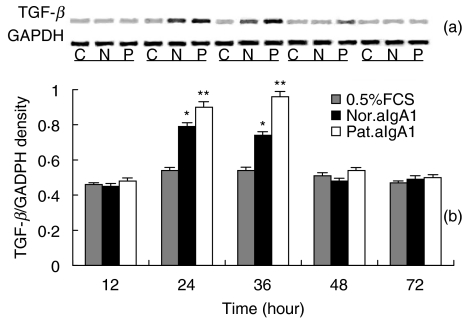
Up-regulation of TGF-β mRNA expression in HMC
A low level TGF-β mRNA expression was detected in unstimulated HMC. aIgA1 treatment induced a time-dependent up-regulation in expression of TGF-β mRNA with maximal levels after 24–36 h of incubation (Fig. 7). At 24 and 36 h of incubation, the expression of TGF-β mRNA expressed as density ratio of bands of TGF-β over GAPDH was 0·79 ± 0·02 and 0·98 ± 0·03 for aIgA1 from patients and 0·70 ± 0·01 and 0·90 ± 0·04 for that from healthy controls (both P < 0·05).
Fig. 7.
TGF-β mRNA expression in HMC. (a) Representative SDS-PAGE of RT-PCR products of mRNA isolated form serum-starved HMC exposed to 0·5%FCS culture medium alone (C, 0·5%FCS) or with aIgA1 of 100 µg/ml from healthy controls (N, Nor. aIgA1) or from patients with IgAN (P, Pat.aIgA1) for different hours. (b) Densitometric analysis of TGF-β mRNA levels, from (a), as a ratio to GAPDH. Results shown were means ± 2SE of three different experiments with triplicate wells. The cells were a mixture of six lines and the aIgA1 sample was from pooled sera of 10 healthy controls or 10 patients. *P ≤ 0·05 Nor.aIgA1 versus 0·5%FCS; **P ≤ 0·05 Pat.aIgA1 versus 0·5%FCS or Nor.aIgA1.
Production of fibronectin(Fn) in HMC
After 36 h of incubation with aIgA1 from both patients and healthy controls, Fn secretion of cultured HMC increased significantly, and HMC treated with patients’ aIgA1 secreted more Fn in the supernatant (Table 1). At 36 and 48 h of incubation, the concentration of Fn in 0·5%FCS group, normal aIgA1 group and patients’ aIgA1 group were (3·23 ± 0·22) ng/ml and (4·83 ± 0·23) ng/ml, (5·51 ± 0·15) ng/ml and (3·01 ± 0·27) ng/ml, (5·54 ± 0·43) ng/ml and (6·45 ± 0·18) ng/ml, respectively, the differences between the 0·5%FCS group, the normal aIgA1 group and the patients’ aIgA1 group were all significant (P < 0·05).
Table 1.
Secretion of fibronectin in HMC (ng/ml)
| 12 h | 24 h | 36 h | 48 h | |
|---|---|---|---|---|
| 0·5%FCS | 3·00 ± 0·05 | 3·14 ± 0·24 | 3·23 ± 0·22 | 3·01 ± 0·27 |
| Nor.aIgA1 | 3·33 ± 0·21 | 3·21 ± 0·1 | 4·83 ± 0·23*† | 5·54 ± 0·43*† |
| Pat.aIgA1 | 3·22 ± 0·12 | 3·42 ± 0·06 | 5·51 ± 0·15**† | 6·45 ± 0·18**† |
Supernant fibronectin concentration of HMC exposed to 0·5%FCS culture medium alone (0·5%FCS) or with aIgA1 of 100 µg/ml from healthy controls(Nor. aIgA1) or from patients with IgAN (Pat.aIgA1) for different hours. Results shown were means ± 2SE of three different experiments with triplicate wells. The cells were a mixture of six lines and the aIgA1 sample was from pooled sera of 10 healthy controls or 10 patients.
P ≤ 0·05 Nor.aIgA1 versus 0·5%FCS
P ≤ 0·05, Pat.aIgA1 versus 0·5%FCS or Nor.aIgA1
P ≤ 0·05 between different time with in the same group.
Discussion
IgAN is a relatively newly recognized disease, first described by Berger and Hinglais in 1968 [34]. It is now generally known to be the most common form of primary glomerulonephritis through out the world [1, 35, 36]. Although primary IgA nephropathy was considered a benign condition for many years, it is now clear that a large number of cases eventually progress to renal failure [37–40]. Indeed, IgA nephropathy is the main cause of end-stage renal disease in patients with primary glomerular disease who require renal-replacement therapy [39, 41, 42].
The pathogenensis of IgAN is still unclear. The view was widely held that mucosal immune system hyperactivity played a central role in the pathogenesis, but this view cannot now be sustained without doubt as the evidence increasing that in IgAN patients the production of IgA1 and J chain in the mucosa and mucosal IgA response to mucosal immunization is reduced while the production pIgA1 with Abnormal O-Glycosylation in the marrow and systemic pIgA response to chronic mucosal infection with Helicobacter pylori is enhanced [7–9]. It was also accepted that IgAN was an immune-complex-mediated glomerulonephritis, However, no antigen has been consistently detected in circulating immune complexes containing IgA or in biopsy specimens from the kidneys of patients with IgA nephropathy [43].
Recently, the direct interaction between deposite IgA1 and mesangial cells is being paid much attention in the pathogensis of IgAN. It has been demonstrated in mouse models of IgA immune complex nephritis [44,45] that the binding of IgA to the mesangial cells in vivo induces proliferation and deposition of excess matrix and injection of mouse IgA anti-Thy 1·1 antibody, which binds to mesangial cells, into mice results in haematuria without producing an inflammatory response [46]. Therefore, MC might be expected to express functional IgA receptors, the direct interaction between deposite IgA1 and mesangial cells may explain some of the the pathological changes seen in IgAN [47–49].
In the current study, it was confirmed that human aggregated IgA1 binding to HMC was specific, in a dose-dependent and saturable manner. Scatchard analysis revealed a population of 106 binding sites/cell with a Ka of approximately 107m−1. The interaction between aIgA1 and HMC could induce mobilization of intracellular calcium, DNA synthesis and cell proliferation, up-regulation of TGF-β mRNA, secretion of fibronectin in cultured HMC. These results support the speculation that there might be specific IgA1 receptor(s) on HMC, and direct interaction of IgA1 with HMC could enhance HMC proliferation, cytokine release, and extracellular matrix production similar to the pathological finding seen in IgA nephropathy.
Leung et al. [50] reported that poly IgA1 from patients with IgAN bound to HMC with higher affinity than aIgA1 from healthy controls. The study was performed by flow cytometry which is not the typical method to investigate the binding dynamics between receptor and ligand because labelled florescence usually has a big molecular and that might affect the binding capability of labelled ligand. Furthermore, flow cytometry can not obtain data of accurate binding sites and Ka or Kd value. In the current study, the traditional radio-ligand binding assay was performed to evaluate the binding capacity of aIgA1 and it was found that aIgA1 from patients could bind to HMC much faster and had a higher association constant than aIgA1 from healthy controls. These results indicated in details that IgA1 from patients might has higher affinity to HMC than that from healthy controls.
IgAN is characterized by proliferation of mesangial cells and abundance of extracellular matrix. For the first time, our study systemically investigated the effects of aIgA1 from patients with IgAN on cultured HMC and revealed that interaction between aIgA1 from patients with IgAN and HMC could also induce HMC to proliferate, secret inflammatory and sclerotic cytokines, and to produce extracellular matrix, and the effects were much stronger than aIgA1 from healthy controls. These data suggested that the binding between IgA1 from patients with IgAN and HMC and sequential pathophysiological response might play an important role in the pathogenesis of IgAN.
To elucidate the signal transductions after the binding, mobilization of intracellular Ca2+ and phosphorylation of ERK were also tested in the current study. The aIgA1 from both patients and healthy controls could evoke a dose-dependent, rapid and transient increase in release of Ca2+ in HMC, which was in consistant with previous reports [25]. It was also revealed that aIgA1 could phosphorylate extracellular signal-regulated kinase (ERK), indicating that interaction of IgA1 with HMC could activate multiple signal transduction pathways. Moreover, aIgA1 from patients was more powerful to induce the release of intracellular Ca2+ and phosphorylation of ERK in HMC than aIgA1 from healthy controls.
In conclusion, the current study demonstrated that aIgA1 from patients with IgAN could bind to cultured HMC with high affinity and induce signal transduct, enhance HMC proliferation, cytokine release, and extracellular matrix production. The binding capacity and pathophysiological response for aIgA1 from patients with IgAN was much stronger than IgA1 from healthy controls.
References
- 1.D’Amico G. The commonest glomerulonephritis in the world. Iga Nephropathy Q J Med. 1987;64:709–27. [PubMed] [Google Scholar]
- 2.Schena FP. A retrospective analysis of the natural history of primary IgA nephropathy worldwide. Am J Med. 1990;89:209–215. doi: 10.1016/0002-9343(90)90300-3. [DOI] [PubMed] [Google Scholar]
- 3.Conley ME, Cooper MD, Michael AF, et al. Selective deposition of immunoglobulin A1 in immunoglobulin A nephropathy, anaphylac-toid purpura nephritis, and systemic lupus erythematosus. J Clin Invest. 1980;66:1432–6. doi: 10.1172/JCI109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valentijn RM, Radl J, Haaijman JJ, Vermeer BJ, Weening JJ, Kauffmann RH, Daha MR, Van Es LA. Circulating and mesangial secretory component-binding IgA1 in primary IgA nephropathy. Kidney Int. 1984;26:760–6. doi: 10.1038/ki.1984.213. [DOI] [PubMed] [Google Scholar]
- 5.Kim P-K, Mong F-C, Lee JS, Jeong HJ, Choi IJ. Clinicopathological correlation in forty-two children with IgA nephropathy. Child Nephrol Urol. 1988;9:21–8. [PubMed] [Google Scholar]
- 6.Koselj M, Rott T, Kandus A, Vizjak A, Malovrh M. Donor-transmitted IgA nephropathy. long-term follow-up of kidney donors and recipients. Transplant Proc. 1997;29:3406–7. doi: 10.1016/s0041-1345(97)00957-3. [DOI] [PubMed] [Google Scholar]
- 7.Harper SJ, Allen AC, Pringle JH, Feehally J. Increased dimeric IgA producing B cells in the bone marrow in IgA nephropathy determined by in situ hybridization for J chain mRNA. J Clin Patho. 1996;149:38–42. doi: 10.1136/jcp.49.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Fijter JW, Eijgenraam JW, Braam CA, Holmgren J, Daha MR, van Es LA, van den Wall Bake AW. Deficient IgA1 immune response to nasal cholera toxin subunit B in primary IgA nephropathy. Kidney Int. 1996;50:952–61. doi: 10.1038/ki.1996.396. [DOI] [PubMed] [Google Scholar]
- 9.Barratt J, Bailey EM, Buck KS, Mailley J, et al. Exaggerated systemic antibody response to mucosal Helicobacter pylori infection in IgA nephropathy. Am J Kidney Dis. 1999;33:1049–57. doi: 10.1016/S0272-6386(99)70141-1. [DOI] [PubMed] [Google Scholar]
- 10.van den Wall Bake AWL, Daha MR, van den Ark A, Hiemstrps Radl J, van Es A. Serum levels and in vitro production of IgA subclasses in patients with primary IgA nephropathy. Clin Exp Immunol. 1988;74:115–20. [PMC free article] [PubMed] [Google Scholar]
- 11.Jones C, Mermelstein N, Kincaid-Smith P, Powell H, Robterson D. Quantitation of human serum polymeric IgA, IgA1 and IgA2 immunoglobulin by enzyme immunoassay. Clin Exp Immunol. 1988;72:344–9. [PMC free article] [PubMed] [Google Scholar]
- 12.Layward L, Allen AC, Hattersley JM, Harper SJ, Feehally J. Elevation of IgA in IgA nephropathy is localized in the serum and not saliva and is restricted to the IgA1 subclass. Nephrol Dial Transplant. 1993;8:25–8. doi: 10.1093/oxfordjournals.ndt.a092266. [DOI] [PubMed] [Google Scholar]
- 13.Hiki Y, Tanaka A, Kokubo T, Iwase H, Nishikido J, Hotta K, Kobayashi Y. Direct evidence for decreased sialylation and galactosylation of human serum IgA1 Fc O-glycosylated hinge peptides in IgA nephropathy by mass spectrometry. Biochem Biophys Res Commun. 2000;271:268–74. doi: 10.1006/bbrc.2000.2613. [DOI] [PubMed] [Google Scholar]
- 14.Allen AC, Bailey EM, Barratt J, Buck KS, Feehally J. Analysis of IgA1 O-glycans in IgA nephropathy by fluorophore-assisted carbohydrate electrophoresis. J Am Soc Nephrol. 1999;10:1763–71. doi: 10.1681/ASN.V1081763. [DOI] [PubMed] [Google Scholar]
- 15.Roccatello D, Sena LM. Removal systems of immunoglobulin A and immunoglobulin A containing complexes in IgA nephropathy and cirrhosis patients. The role of asialoglycoprotein receptors. Laboratory Invest. 1993;69:714–23. [PubMed] [Google Scholar]
- 16.Kokubo T, Hiki Y, Iwase H, Tanaka A, Toma K, Hotta K, Kobayashi Y. Protective role of IgA1 glycans against IgA1 self-aggregation and adhesion to extracellular matrix proteins. J Am Soc Nephrol. 1998;9:2048–54. doi: 10.1681/ASN.V9112048. [DOI] [PubMed] [Google Scholar]
- 17.Monteiro RC, Halbwachs-Mecarelli L, Roque-Barreire MC, Noel LH, Berger J, Lesavre P. Charge and size of mesangial IgA in IgA nephropathy. Kidney Int. 1985;28:666–71. doi: 10.1038/ki.1985.181. [DOI] [PubMed] [Google Scholar]
- 18.Monteiro RC, Halbwachs-Mecarelli T, Berger J, Lesavre P. Characteristics of eluted IgA in primary IgA nephropathy. Contr Nephrol. 1984;40:107–11. doi: 10.1159/000409736. [DOI] [PubMed] [Google Scholar]
- 19.Allen AC, Bailey EM, Buck KS, Barratt J, Brenchley PEC, Feehally J. O-glycosylation of mesangial IgA1 in IgA nephropathy. J Am Soc Nephrol. 1999;10:506A. [Google Scholar]
- 20.Gómez-Guerrero C, Gonzalez E, Egido J, et al. Evidence for a specific IgA receptor in rat and human mesangial cells. J Immunol. 1993;151:7172–81. [PubMed] [Google Scholar]
- 21.Gómez-Guerrero C, Duque N, Egido J, et al. Mesangial cells possess an asialo-glycoprotein receptor with affinity for human immunoglobulin A. J Am Soc Nephrol. 1998;9:568–76. doi: 10.1681/ASN.V94568. [DOI] [PubMed] [Google Scholar]
- 22.Diven SC, Caflisch CR, Hammond DK, Weigel PH, Oka JA, Goldblum RM. IgA induced activation of human mesangial cells. independent of Fc alphaR1 (CD 89) Kidney Int. 1998;54:837–47. doi: 10.1046/j.1523-1755.1998.00054.x. [DOI] [PubMed] [Google Scholar]
- 23.Bagheri N, Chintalacharuvu SR, Emancipator SN, et al. Proinflam-matory cytokines regulate Fc alpha R expression by humanmesangial cells in vitro. Clin Exp Immunol. 1997;107:404–9. doi: 10.1111/j.1365-2249.1997.264-ce1160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duque N, Gomez-Guerrero C, Egido J, et al. Interaction of IgA with Fc alpha receptors of human mesangial cells activates transcriptionfactor nuclear factor-kappa B and induces expression and synthesis of monocyte chemoattractant protein-1, IL-8, and IFN-inducible protein 10. J Immunol. 1997;159:3474–82. [PubMed] [Google Scholar]
- 25.Gomez-Guerrero C, Duque N, Egido J, et al. Stimulation of Fc (alpha) receptors induces tyrosine phosphorylation of phospholipase C-gamma (1), phosphatidylinositol phosphate hydrolysis, and Ca2+mobilization in rat and human mesangial cells. J Immunol. 1996;156:4369–76. [PubMed] [Google Scholar]
- 26.van den Dobbelsteen ME, van der Woude FJ, Schroeijers WE, van den Wall Bake AW, van Es LA, Daha MR. Binding of dimeric and polymeric IgA to rat renal mesangial cells enhances the release of interleukin 6. Kidney Int. 1994;46:512–9. doi: 10.1038/ki.1994.302. [DOI] [PubMed] [Google Scholar]
- 27.Gomez-Guerrero C, Lopez-Armada MJ, Gonzalez E, Egido J, et al. Soluble IgA and IgG aggregates are catabolized by cultured rat mesangial cells and induce production of TNF-alpha and IL-6, and proliferation. J Immunol. 1994;153:5247–55. [PubMed] [Google Scholar]
- 28.Lopez-Armada MJ, Gomez-Guerrero C, Egido J, et al. Receptors for immune complexes activate gene expression and synthesis of matrix proteins in cultured rat and human mesangial cells: role of TGF-beta. Clin Exp Immunol. 1997;110:226–32. [PubMed] [Google Scholar]
- 29.Leung JC, Tang SC, Lam MF, Chan TM, Lai KN. Charge-dependent binding of polymeric IgA1 to human mesangial cells in IgA nephropathy. Kidney Int. 2001;59:277–85. doi: 10.1046/j.1523-1755.2001.00489.x. [DOI] [PubMed] [Google Scholar]
- 30.Greenwood FC, Hunter WM, Glover JS. The preparation of (125I) labeled human growth hormone of high specific radioactivity. Biochem J. 1963;89:114. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Troyer DA, Kreisberg JI. Isolation and study of glomerular cells. Meth Enzymol. 1990;191:141–52. doi: 10.1016/0076-6879(90)91012-u. [DOI] [PubMed] [Google Scholar]
- 32.Trafford AW, Sibbring GC, Diaz ME. The effects of low concentrations of caffeine on spontaneous Ca release in isolated rat ventricular myocytes. Cell Calcium. 2000;28:269–76. doi: 10.1054/ceca.2000.0156. [DOI] [PubMed] [Google Scholar]
- 33.Catchard G. The attractions of proteins for small molecules and ions. Ann NY Acad Sci. 1991;51:660–72. [Google Scholar]
- 34.Berger J, Hinglais N. Les depots intercapillaires d’IgA-IgG. J Urol Nephrol. 1968;74:694–5. [PubMed] [Google Scholar]
- 35.Julian BA, Waldo FB, Rifai A, Mestecky J. IgA nephropathy, the most common glomerulonephritis worldwide: a neglected disease in the United States? Am J Med. 1988;84:129–32. doi: 10.1016/0002-9343(88)90019-8. [DOI] [PubMed] [Google Scholar]
- 36.Levy M, Berger J. Worldwide perspective of IgA nephropathy. Am J Kidney Dis. 1988;12:340–7. doi: 10.1016/s0272-6386(88)80021-0. [DOI] [PubMed] [Google Scholar]
- 37.D’Amico G, Colasanti G, Barbiano di Belgioioso G, et al. Long-term follow-up of IgA mesangial nephropathy: clinico-histological study in 374 patients. Semin Nephrol. 1987;7:355–8. [PubMed] [Google Scholar]
- 38.Radford MG, Jr, Donadio JV, Jr, Bergstralh EJ, Grande JP. Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol. 1997;8:199–207. doi: 10.1681/ASN.V82199. [DOI] [PubMed] [Google Scholar]
- 39.Ibels LS, Gyory AZ. IgA nephropathy: analysis of the natural history, important factors in the progression of renal disease, and a review of the literature. Medicine. 1994;73:79–102. [PubMed] [Google Scholar]
- 40.Hogg RJ, Silva FG, Wyatt RJ, Reisch JS, Argyle JC. Savino DA. Prognostic indicators in children with IgA nephropathy – report of the Southwest Pediatric Nephrology Study Group. Pediatr Nephrol. 1994;8:15–20. doi: 10.1007/BF00868251. [DOI] [PubMed] [Google Scholar]
- 41.Briganti EM, Dowling J, Finlay M, et al. The incidence of biopsyproven glomerulonephritis in Australia. Nephrol Dial Transplant. 2001;16:1364–7. doi: 10.1093/ndt/16.7.1364. [DOI] [PubMed] [Google Scholar]
- 42.Maisonneuve P, Agodoa L, Gellert R, et al. Distribution of primary renal diseases leading to end-stage renal failure in the United States, Europe, and Australia/New Zealand: results from an international comparative study. Am J Kidney Dis. 2000;35:157–65. doi: 10.1016/S0272-6386(00)70316-7. [DOI] [PubMed] [Google Scholar]
- 43.Russell MW, Mestecky J, Julian BA, Galla JH. IgA-associated renal diseases. antibodies to environmental antigens in sera and deposition of immunoglobulins and antigens in glomeruli. J Clin Immunol. 1986;6:74–86. doi: 10.1007/BF00915367. [DOI] [PubMed] [Google Scholar]
- 44.Issacs K, Miller F, Lane B, et al. Experimental model for IgA nephropathy. Clin Immunol Immunopathol. 1981;20:419–26. doi: 10.1016/0090-1229(81)90152-5. [DOI] [PubMed] [Google Scholar]
- 45.Rifai A. Experimental models for IgA-associated nephritis. Kidney Int. 1987;31:1–7. doi: 10.1038/ki.1987.1. [DOI] [PubMed] [Google Scholar]
- 46.van Dixhoorn MGA, Gorter A, Sato T, van der Wal AM, van Eendenburg JDH, Rozing J, Daha MR, de Heer E. Induction of microhematuria by an IgA isotype switch variant of a monoclonal anti-Thy-1.1 antibody in the rat. Kidney Int. 1996;50:1612–23. doi: 10.1038/ki.1996.477. [DOI] [PubMed] [Google Scholar]
- 47.Flogeg J, Feehally J. IgA Nephropathy: Recent Developments. J Am Soc Nephrol. 2000;11:2395–403. doi: 10.1681/ASN.V11122395. [DOI] [PubMed] [Google Scholar]
- 48.D’Amico G. Pathogenesis of immunoglobulin A nephropathy. Curr Opin Nephrol Hypertens. 1998;7:247–50. doi: 10.1097/00041552-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 49.Westerhuis R, Kitahara M, Flogeg J, et al. IgA-Nephropathy: how does IgA activate the mesangial cell? Clin Exp Nephrol. 2001;5:1–7. [Google Scholar]