Abstract
We observed 42 initially non-diabetic siblings of affected children to characterize the humoral immune response to the 65 kDa isoform of glutamic acid decarboxylase (GAD65) in preclinical type I diabetes. During the observation period with a mean duration of 9·6 years 21 siblings progressed to type I diabetes. The humoral immune response to GAD65 was observed initially as a simultaneous response to the middle (M) and carboxy (C)-terminal regions of the GAD65 molecule in most cases, and if the response was restricted initially to the middle region, it spread rapidly to the C-terminal domain and in a few cases later to the amino (N)-terminal domain. There was some heterogeneity in the GAD65 isotype response, but it was composed mainly of antibodies of immunoglobulin (Ig) G1 subclass. Responses of IgG2-, IgG4-, IgM- and IgA-GAD65Ab were observed frequently, whereas IgE- and IgG3-GAD65Ab responses were seen more rarely. Initially, the non-progressors tended more often to have IgG2- and IgG4-GAD65Ab than the progressors. As a sign of a dynamic process a significant isotype spreading was seen for IgG2-GAD65Ab (P < 0·05) and close to significant for IgM (P = 0·06) among progressors and for IgM-GAD65Ab (P < 0·05) among non-progressors during the observation period. This study failed to identify any GAD65 epitope- or isotype-specific antibody reactivity that could be used as a marker for progression to disease, as such progression was not associated with any specific changes in reactivity over time. Our findings indicate that epitope- and isotype-specific GAD65 antibodies are hardly capable of separating progressors from non-progressors among GAD65Ab-positive first-degree relatives of children with type I diabetes.
Keywords: autoantibodies, epitope spreading, glutamic acid decarboxylase, isotype switching, preclinical type I diabetes
Introduction
Type I diabetes is perceived as a chronic autoimmune disease, which is thought to result from T-cell mediated destruction of the insulin-producing pancreatic β-cells [1]. During an asymptomatic destructive process of variable duration various autoantibodies to islet cell autoantigens can be detected, and these are useful for the identification of individuals with an increased risk of clinical disease [2]. Several antigens have been observed to be targets of humoral autoimmunity, the three major ones being insulin, the protein tyrosine phosphatase-related IA-2 protein and the 65 kDa isoform of glutamic acid decarboxylase (GAD65). Antibodies against glutamic acid decarboxylase, reported originally as a 64 kDa islet protein by Baekkesskov et al. [3,4], have turned out to be one of the major antibodies related to type I diabetes [5–7]. There are two isoforms of GAD, one with a molecular weight of approximately 65 kDa and the other 67 kDa, the former being the principal autoantigen in type I diabetes [8]. GAD65 antibodies are present in the majority of individuals with preclinical and recent-onset type I diabetes [6–8], and especially in older age groups GAD antibodies have shown a high diagnostic sensitivity [9].
Epitope analyses of GAD65 autoantibodies in type I diabetes indicate that antibodies to GAD65 recognize at least three major conformational epitopes located in the middle and C-terminal domains of the molecule, while a fourth linear epitope is located in the N-terminus [10–13]. There are also data suggesting that the epitope recognition of GAD65Ab changes during the preclinical phase of type I diabetes [13]. It has been reported that the middle region is an early target of the immune response to GAD65, and that the epitope profile is dynamic in prediabetic individuals during progression to clinical disease with spreading from the immunodominant middle region to the C-terminal region [14,15].
The isotype profile of antigen-specific autoantibodies might reflect the T helper 1/T helper 2 (Th1/Th2) balance of β-cell autoimmunity [16], which may change during the prediabetic phase. It has been speculated that islet cell autoimmunity may start out as a non-pathogenic Th2 response to the β-cells that turns subsequently into a pathogenic Th1 response associated with the maturation of the humoral immune response to different antigens, and most probably leads to type I diabetes in some unfortunate individuals [17–20]. Th1 immunity is associated with the generation of IgG2a and IgG3 in the mouse, while a Th2 response mainly results in the generation of IgG1 [16,21], but the equivalent antibody responses have not been clearly defined in man.
The natural history of preclinical diabetes is partly characterized, but still we have very limited information on the dynamics of the immune response to β-cell autoantigens during the course of preclinical disease. The ultimate aim of this work is to characterize the humoral immune response to GAD65 in preclinical type I diabetes by studying the occurrence of various isotypes (IgG subclasses, IgA, IgE, IgM) of GAD65 antibodies and to observe possible signs of epitope spreading within the antigen after the initial appearance of such antibodies. This could provide more sensitive and specific markers for the discrimination between early and later stages of preclinical type I diabetes.
Subjects and Methods
Subjects
The subjects were derived from the Childhood Diabetes In Finland (DiMe) Study, which is a prospective population-based family survey designed to investigate the role of genetic, immunological and environmental factors in the development of type I diabetes [22]. From September 1986 to April 1989 all newly diagnosed children with type I diabetes were invited to participate, together with their families. Blood samples were drawn from siblings of the children with recently diagnosed type I diabetes. A blood sample was available from 755 unaffected siblings of 977 (77·3%) younger than 20 years of age. A total of 58 (7·7%) siblings were observed to test positive for GAD65 autoantibody at the beginning of the study. Subsequent samples were taken at an interval of 3 months for the first 2 years and thereafter with an interval of 6–12 months, according to the study protocol. Thirteen siblings (2·0%) seroconverted to GAD65Ab positivity during prospective observation. Accordingly, the initial study population comprised 71 siblings testing positive for GAD65Ab at least once.
A subject was included in the current series if he or she had at least two serum samples available and antibodies to GAD65 had been detected at a minimum level of 15 relative units (RU) in at least one sample during the follow-up period. Altogether 42 siblings (24 females) fulfilled the inclusion criteria. All 42 subjects had at least three GAD65Ab-positive samples available for the analysis of GAD67Abs, GAD65 epitope clusters and isotype-specific antibodies. The mean age at the first GAD65Ab-positive sample analysed was 9·5 years (range 3·2–16·3 years). The number of samples per subject varied from three to 15 (median 8). The average interval between the first and last sample analysed was 3·6 years (range 0·4–8·6 years). All siblings were observed for progression to clinical type I diabetes to the end of December 1997. During that observation period, 21 (50%) siblings progressed to clinical type I diabetes. The observation time ranged from 8·7 to 11·2 years (mean 9·6 years) among the non-progressors.
Antibodies to GAD67 were observed in 17 subjects, and although the detected levels were generally low these antibodies might contribute to the binding to the chimeric constructs, and therefore we excluded all of them from the analysis of epitope-specific GAD65 antibodies. As a consequence of matching progressors to non-progressors, seven GAD67Ab-negative children were also excluded, resulting in a cohort of 18 siblings in the analysis of epitope-specific GAD65 antibodies. The 17 GAD67Ab-positive and the seven GAD67Ab-negative children excluded from the analysis of antibodies to GAD65 epitopes are shown in Table 1.
Table 1.
Epitope- and isotype-specific responses to GAD65 in initially unaffected GAD65Ab-positive siblings of children with type I diabetes
| Appearance of responses* | ||||||||
|---|---|---|---|---|---|---|---|---|
| Appearance order after initial GAD65Ab response | ||||||||
| Case no | Sex | HLA DR alleles | Age (years) at first sample analysed | Age (yearsP at last sample analysed | At he same time as initial GAD65Ab | 1 | 2 | 3 |
| Progressors | ||||||||
| 1505* | F | DR3/DR4 | 11·3 | 15·2 | ICA,IA-2 A | 1,2,4,IgM | IgA | |
| 2703 | M | DR4/non-DR3 | 15·1 | 16·6 | ICA,IA-2 A | C,M,1 | IgA | 2 |
| 8407** | F | DR4/non-DR3 | 4·8 | 9·3 | IAA,IA-2 A | 1 | 2 | |
| 12805** | M | DR3/DR4 | 5·5 | 8·6 | ICA,IA-2 A | 1,4,IgM | 2 | |
| 13105* | F | Not available | 8·0 | 11·2 | ICA,IA-2 A | 1,2,4,IgM | IgA | |
| 14512** | F | DR3/DR4 | 9·7 | 12·7 | ICA,IA-2 A | 1,IgA | 2,IgM | |
| 20004 | M | DR3/DR4 | 12·6 | 16·7 | C,M,1,IgA | 2,IgM | 4 | |
| 25603* | M | DR4/non-DR3 | 13·5 | 14·0 | ICA,IA-2 A | 1,2,3,4,IgA,IgM | ||
| 30303* | F | DR4/non-DR3 | 10·4 | 16·1 | 1,2,IgE,IgM | 4,IgA | 3 | |
| 32403 | F | DR3/DR4 | 11·7 | 14·6 | ICA,IA-2 A | C,M,1,2,3,4,IgA | IgM | |
| 42303 | F | DR3/DR4 | 15·3 | 19·4 | IA-2 A | C,M,N,1,2,4,IgA,IgM | ||
| 45303 | F | DR3/non-DR4 | 4·4 | 6·0 | ICA,IA-2 A | C,M,1,IgM | ||
| 58803* | M | DR4/non-DR3 | 9·3 | 13·2 | 1,IgA | IgM | IgE | |
| 65103 | F | DR4/non-DR3 | 7·5 | 13·4 | ICA,IA-2 A | C,M,1,2,4,IgM | N,IgA | |
| 87303 | F | DR4/non-DR3 | 16·3 | 20·9 | IA-2 A | C,M,1,2,4,IgA,IgM | ||
| 75204* | M | DR3/DR4 | 4·9 | 9·8 | ICA,IAA | 1,2,4,IgA,IgM | ||
| 68404 | F | DR4/non-DR3 | 7·8 | 9·9 | ICA | C,M,1,2,3,IgM | ||
| 28305 | M | Not available | 4·1 | 6·4 | M,N,1,IgE,IgM | C,2,4,IgA | ||
| 51605** | F | DR4/non-DR3 | 3·2 | 6·0 | ICA,IAA | 1 | 2,4,IgM | |
| 57204** | F | DR3/DR4 | 7·7 | 9·8 | ICA,IA-2 A | 1,2,4,IgA,IgE,IgM | ||
| 41904* | F | DR3/non-DR4 | 2·6 | 3·3 | ICA,IAA | 1,2,4,IgM | 3,IgE | IgA |
| Non-progressors | ||||||||
| 68705** | F | DR4/non-DR3 | 8·4 | 15·2 | ICA,IAA | 1,2,4,IgA,IgM | ||
| 14513 | M | DR3/DR4 | 8·1 | 16·6 | ICA,IAA,IA-2 A | 1,2,IgM | IgA | |
| 48003* | F | DR4/non-DR3 | 6·8 | 10·7 | ICA,IA-2 A | 1,2,4,IgA,IgM | ||
| 38605* | M | DR3/non-DR4 | 4·9 | 8·9 | ICA | 1,2,3,4,IgA,IgM | ||
| 33705* | F | DR4/non-DR3 | 8·5 | 11·5 | 1,4,IgA, | 2,IgM | ||
| 39103* | F | DR3/DR4 | 5·2 | 12·9 | 1,2,4,IgA,IgM | |||
| 29104 | M | DR4/non-DR3 | 13·6 | 17·5 | C,M,1,IgM | IgA | ||
| 44803* | M | DR4/non-DR3 | 14·7 | 15·1 | 1,2,4,IgA,IgM | |||
| 14206* | F | DR4/non-DR3 | 11·0 | 17·1 | ICA,IA-2 A | 1,2,4,IgM | ||
| 70905 | F | Not available | 17·3 | 17·4 | ICA,IAA,IA-2A | C,M,N,1,2,4,IgM | IgA | |
| 37205 | F | non-DR3/non-DR4 | 17·13 | 17·15 | ICA,IA-2 A | C,M,1,IgA | 2,IgM | 4 |
| 45205 | M | DR4/non-DR3 | 4·8 | 6·4 | ICA,IAA,IA-2A | C,1,2,IgM | M | 3,4 |
| 78503* | M | DR4/non-DR3 | 9·5 | 13·6 | 1,IgE | IgM | 2 4 | |
| 81403 | F | non-DR3/non-DR4 | 11·1 | 15·9 | ICA | C,M,1,2,4,IgM | ||
| 1904 | F | DR4/non-DR3 | 13·2 | 19·7 | C,M,N,1,2,3,4,IgA,IgM | |||
| 64704* | M | DR3/DR4 | 11·9 | 17·8 | ICA | 1,2,4,IgA,IgM | ||
| 85604 | F | DR4/non-DR3 | 13·6 | 15·4 | ICA | C,M,1,2,4,IgA | IgM | |
| 4003 | M | DR4/non-DR3 | 13·4 | 15·4 | ICA,IA-2 A | M,1,2,4 | ||
| 82804* | M | DR4/non-DR3 | 6·2 | 7·7 | ICA,IAA | 1,2,3,4,IgA,IgM | ||
| 14204* | M | DR4/non-DR3 | 13·0 | 15·4 | ICA,IA-2 A | 1,2,4,IgM | IgA | |
| 83005** | M | Not available | 6·6 | 7·5 | 1,2,3,4 | IgA,IgE,IgM | ||
N: N-terminal Abs; M: middle region Abs; C: C-terminal Abs; IgA, IgE and IgM- isotype specific Abs; the numbers indicate IgG subclass-specific Abs. Non-bold italic type represents the occurrence of the response in a single sample or fluctuating response.
GAD67Ab-positive siblings and
GAD67Ab-negative siblings excluded from the analysis of epitope-specific GAD65 antibodies to eliminate the possible contribution of GAD67 antibodies.
Assays for GAD67/GAD65 antibodies
All samples were analysed for GAD65 antibodies with a radiobinding assay, as described previously [23]. Briefly, 2 µl of serum was incubated at + 4°C overnight in 96-well plates with 20 000 cpm of 35S-labelled full-length GAD65 diluted in 50 µl of TBST buffer (50 mm Tris, 150 mm NaCl, 0·1% Tween-20, pH 7·4). After the protein A Sepharose precipitation, unbound radioactivity was washed with excess buffer. Remaining activity was counted by a liquid scintillation counter (1450 MicroBeta Trilux, PerkinElmer Wallac, Turku, Finland). The results were expressed in relative units (RU) based on a standard curve constructed from a dilution of a pool of highly positive samples with a negative sample. The cut-off limit for antibody positivity (5·35 RU) was set at the 99th percentile of 373 non-diabetic Finnish children and adolescents. The disease sensitivity of the GAD65Ab assay was 69% and the specificity 100%, based on 140 samples included in the 1995 Multiple Autoantibody Workshop [24]. Three different GAD65/GAD67 chimeras were used to analyse epitope-specific antibodies; one (GAD651−95/GAD67102−593) to measure aminoterminal-specific GAD65 antibodies (GAD65-N-Ab), another (GAD671−101/GAD6596−444/GAD67453−593) to determine antibodies specific for the middle part of GAD65 (GAD65-M-Ab) and the third (GAD671−453/GAD65445−585) to quantify carboxyterminal-specific GAD65 antibodies (GAD65-C-Ab), as described previously [15,23]. Antibodies against the in vitro transcribed and translated 35S-labelled products were measured with a radiobinding assay identical to that used to detect conventional GAD65Ab. A serum pool with high levels of epitope-specific GAD65Ab was used to construct a standard curve. A standard curve was run on each plate and the antibody levels were expressed in RU. The cut-off limit for positivity was set at the 99th percentile in 104 non-diabetic young Finnish subjects (mean age 10·9 years, range 0·5–18·2 years) in the assays for GAD67Ab (0·91 RU), GAD65-N-Ab (0·55 RU), GAD65-M-Ab (1·51 RU) and GAD65-C-Ab (1·59 RU). All samples from the same subject were analysed in the same assay run as far as possible.
Isotype-specific GAD65Ab were analysed in an assay based on the same principles as that used for total and epitope-specific GAD65Ab, except that the protein A Sepharose precipitation was replaced by monoclonal subclass-specific antibodies linked to streptavidin agarose (Pierce and Warriner, Chester, UK). The biotinylated monoclonal mouse antibodies to human IgG1 (clone G17-1), IgG2 (G18-21), IgG3 (HP6050), IgG4 (JDC-14), IgA (G20-359), IgM (G20-127) and IgE (G7-26) and to rat IgM (G53-238) were obtained from PharMingen (San Diego, CA, USA), except for IgG3 which was purchased from SouthernBiotech (Birmingham, AL, USA). The streptavidin agarose beads were washed thoroughly with phosphate-buffered saline (PBS) (50 mm phosphate buffer, 150 mm NaCl, pH 7·4) before use, and the biotinylated antibodies were linked to them by incubating 5 µg of antibody per 15 µl of beads in 50 µl of PBS with vigorous shaking at + 4°C for 1 h. Thereafter the beads were washed once with PBS and twice with TBST buffer. Finally, 15 µl of the beads were suspended in TBST buffer (total volume of suspension 50 µl) and used for the precipitation, which was performed at + 4°C for 2 h with vigorous shaking. The results were expressed as s.d. scores (s.d.s.) calculated from the following equation: s.d.s. = [delta cpm (= IgG subclass or isotype-specific cpm – unspecific antirat IgM cpm) − mean delta cpm of control subjects]/s.d. delta cpm of control samples, as described previously [25]. Forty non-diabetic young Finnish subjects [mean age 9·9 ± 4·0 (s.d.), range 0·6–16·6 years] were used as controls. The threshold for positivity was set at 3 s.d.s. This cut-off limit was exceeded by one control subject in the IgG1-GADA (5·0 s.d.s.), IgG2-GADA (3·0 s.d.s.) and IgG4-GADA (5·9 s.d.s.) assays. We participated in the serum exchange workshop carried out between the laboratories known to measure isotype responses to GAD65 (results presented at the 5th International Congress of the Immunology Diabetes Society, 2001, Chennai, India). Our results were highly concordant with the results of those laboratories, which used a method similar to that applied in our laboratory. All samples from the same subject were analysed in the same assay run as far as possible.
Assays for other diabetes-associated autoantibodies
Islet cell antibodies (ICA) were quantified by a standard indirect immunofluorescence method on sections of frozen human pancreas from a blood group O donor [26]. The end-point dilution titres of ICA-positive samples were recorded and the results expressed in Juvenile Diabetes Foundation (JDF) units. The detection limit was 2·5 JDF units. All samples initially positive for ICA were retested to confirm antibody positivity. The sensitivity of the ICA assay in our laboratory was 100% and the specificity 98% in the most relevant international standardization workshop [24].
Insulin autoantibodies (IAA) were analysed with a radiobinding assay modified from that described by Palmer et al. [27]. Endogenous insulin was removed with acid charcoal prior to the assay, and free and bound insulin were separated after incubation with mono-125I(Tyr A 14)-human insulin (Novo Research Institute (NRI), Bagsvaerd, Denmark) for 20 h in the absence or presence of an excess of unlabelled insulin. The IAA levels were expressed in nU/ml, where 1 nU/ml corresponds to a specific binding of 0·01% of the total counts. The interassay coefficient of variation was less than 8%. A subject was considered to be positive for IAA when the specific binding was 55 nU/ml (99th percentile in a reference population comprising 105 non-diabetic children and adolescents) or more. The disease sensitivity of the IAA assay was 26%, and the disease specificity 97% based on 140 samples included in the 1995 Multiple Autoantibody Workshop [24].
Antibodies to the protein tyrosine phosphatase-related protein (IA-2 A) were quantified with a radiobinding assay as described previously [28]. Antibody levels were expressed in RU based on a standard curve, as for GAD65Ab. The limit for IA-2 A positivity was set at 0·43 RU, which represents the 99th percentile in 374 non-diabetic Finnish children and adolescents. The disease sensitivity of this assay was 62% and the specificity 97%, based on 140 samples derived from the 1995 Multiple Autoantibody Workshop [24].
HLA typing
HLA typing was performed using conventional HLA serology as described previously [29]. All HLA A, B, C and DR specificities recognized by the Nomenclature Committee of the World Health Organization (WHO) in 1984 were included in the test panel [30]. HLA typing data were available for 38 subjects.
Data handling and statistical analysis
In order to compare the antibody levels between progressors and non-progressors, each progressor was matched as well as possible with one non-progressor for sex (concordance 81%), age (± 2 years; concordance 76%) and GAD65Ab-positive observation time (3·20 versus 3·19 years, respectively). The follow-up time was calculated from the first GAD65Ab-positive sample to the time of diagnosis in the progressors and to the temporally corresponding samples in the non-progressors. Integrated antibody levels over the antibody-positive observation period were calculated for each antibody by area-under-the-curve (AUC) analysis [31]. The dominant epitope and isotype-specific responses were defined based on the relative antibody values in the initial sample and on the individual AUC values during the follow-up. Student's t-test was used to compare mean ages and age differences. The distribution of GAD65Ab epitopes and isotypes between the progressors and non-progressors was evaluated by cross-tabulation and χ2 statistics. The Mann–Whitney U-test was used to compare the AUCs between the two groups studied. Correlation analyses were performed with the non-parametric Spearman's rank test (rs). A two-tailed P-value of 0·05 or less was considered to be statistically significant.
Results
Positivity for autoantibodies
As defined by the inclusion criteria, all siblings tested positive for GAD65Ab. ICA were observed initially in 14 progressors (67%) and 14 non-progressors (67%), IAA in four progressors (19%) and five non-progressors (24%) and IA-2 A in 13 progressors (62%) and in eight non-progressors (38%) (Table 1). Positivity for three or more antibodies was seen initially in 14 progressors (67%) and 10 non-progressors (48%).
Initial response to GAD65
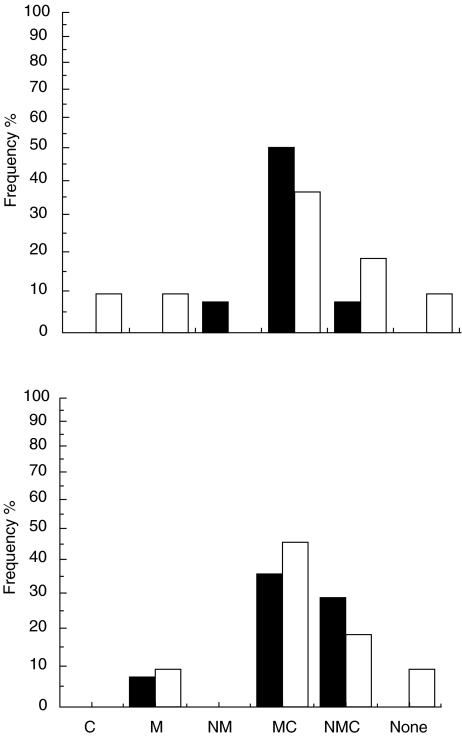
Antibodies to the middle domain of GAD65 were the most abundant in the initial response to GAD65, as 17 of the 18 siblings (94%) had GAD65-M-Abs in the first positive sample. In addition, the initial response comprised GAD65-C-Abs in 15 subjects (83%), in 14 siblings together with GAD65-M-Abs and in one case alone. GAD65-N-Abs were detected in four subjects (22%), once together with GAD65-M-Abs and in three cases in combination with both GAD65-M-Abs and GAD65-C-Abs. One subject positive for GAD65Ab were observed to have no initial response to any of the epitope clusters studied. There were no statistically significant differences between the progressors or non-progressors in the frequency of various epitopes seen in the first sample (Fig. 1). The levels of total GAD65Abs were higher among non-progressors than among the progressors, but not significantly so (P = 0·31), and a similar trend was observed for the different GAD65Ab epitopes (Table 2).
Fig. 1.
Frequency of various epitope-specific GAD65Ab profiles in the first positive sample (upper panel) and during follow-up (lower panel) in nine siblings who progressed to clinical type I diabetes during prospective observation (▪) and in nine siblings who have remained unaffected (□).
Table 2.
Levels (median; range) of epitope- and isotype-specific GAD65Ab in the first positive sample in the siblings who progressed to clinical type I diabetes (n = 9 for epitope-specific Abs and n = 21 for isotype specific Abs) and in those who have remained unaffected (n = 9 for epitope-specific Abs and n = 21 for isotype specific Abs)
| Progressors | Non-progressors | P-value | |
|---|---|---|---|
| GAD65Ab | 91·8; 7·4–396·5 | 109·5; 8·2–294·3 | 0·30 |
| GAD65-M-Ab | 4·0; 1·8–72·6 | 12·6; 0·3–65·8 | 0·90 |
| GAD65-C-Ab | 4·1; 0·6–84·5 | 6·2; 1·1–39·6 | 0·97 |
| GAD65-N-Ab | 0·0; 0·0–1·0 | 0·0; 0·0–1·8 | 0·40 |
| IgG1-GAD65 | 201·9; 3·9–769·2 | 447·0; 10·0–724·7 | 0·23 |
| IgG2-GAD65 | 5·3; 0–48·9 | 7·5; 0·3–171·3 | 0·17 |
| IgG3-GAD65 | 0·0; 0·0–8·5 | 0·3; 0·0–10·7 | 0·40 |
| IgG4-GAD65 | 4·1; 0·0–22·5 | 6·5; 0·2–33·8 | 0·20 |
| IgA-GAD65 | 2·6; 0·0–33·0 | 3·7; 0·0–12·3 | 0·74 |
| IgE-GAD65 | 0·4; 0·0–9·3 | 0·0; 0·0–3·6 | 0·51 |
| IgM-GAD65 | 5·2; 0·6–46·7 | 9·1; 0·1–44·4 | 0·22 |
Epitope responses during follow-up
Antibodies to the middle region were observed in all siblings except one non-progressor with relatively high levels of total GAD65 antibodies (Table 1, case no. 14513). He had no detectable epitope-specific antibodies. The individual spreading of the humoral immune response to GAD65 epitopes is shown in Table 1. There were no statistically significant differences between the progressors or non-progressors in the number of epitopes seen when comparing the maximal response. The median levels fell into the same order GAD65-M-Ab > GAD65-C-Ab > GAD65-N-Ab, but the median levels of GAD65-M-Ab and GAD65-N-Ab tended to be higher in the progressors than in the non-progressors in contrast to the first analysed sample. Among the progressors the GAD65-M-Ab titres (rs = 0·75; P < 0·05) correlated with the GAD65Ab levels, but no other correlations were seen.
Seroconversion to GAD65-M-Ab negativity was not seen at all, but three (20%) progressors became negative for GAD65-C-Ab before the manifestation of clinical type I diabetes. The responses to the N-terminal region remained weak during the follow-up, and negative seroconversions were frequent. Neither the number of epitopes nor the median levels of epitope-specific antibodies differed significantly between progressors and non-progressors at the time of diagnosis of overt diabetes in the progressors.
Distribution of GAD65 isotypes in the initial sample
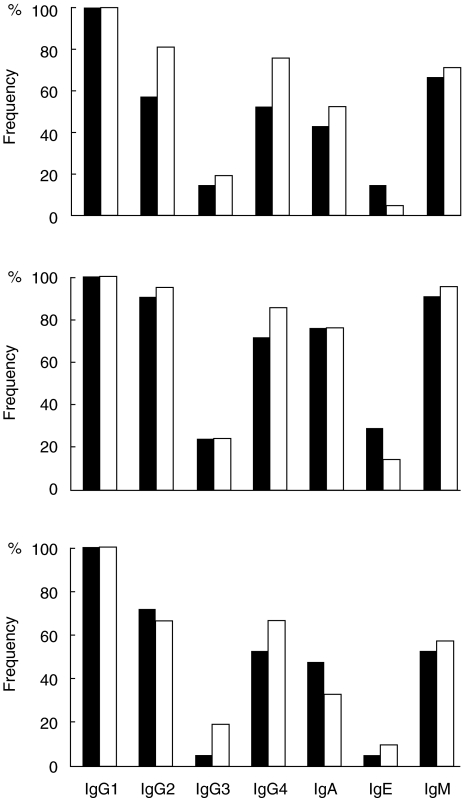
The humoral immune response to GAD65 was composed mainly of IgG1 antibodies. The initial subclass-specific response comprised IgG1-GAD65Abs in all subjects, IgG2-GAD65Abs in 29/42 (69%), IgG3-GAD65Abs in 7/42 (17%), IgG4-GAD65Abs in 27/42 (64%), IgA-GAD65Abs in 20/42 (48%), IgE-GAD65Abs in 4/42 (10%) and IgM-GAD65Abs in 29/42 (69%). There were no significant differences in the distribution of various isotype-specific antibodies between the progressors and the non-progressors, although the non-progressors tended to have IgG2- (81%versus 57%; P = 0·10) and IgG4-GAD65Abs (76%versus 52%; P = 0·11) more often than the progressors (Fig. 2). The number of isotypes was equal in the initial sample collected (median 4 in both groups; range 1–6 among progressors and 2–6 among non-progressors).
Fig. 2.
Frequency of various isotype-specific GAD65 antibodies in the first positive sample (upper panel), the maximum response seen during follow-up (middle panel) and in the last sample analysed (lower panel) in 21 siblings who progressed to clinical type I diabetes during prospective observation (▪) and in 21 siblings who have remained unaffected (□).
Immunoglobulin G subclass and isotype changes during follow-up
All the siblings experienced seroconversion to positivity for additional IgG subclasses and isotypes, as shown individually in Table 1, but no differences were observed in the distribution of the various isotypes between the progressors and non-progressors during the observation period (Table 1, Fig. 2). When comparing the proportion of positive children between the initial sample and the sample with the maximal number of detectable isotypes, a significant isotype spreading was seen for IgG2-GAD65Abs (P < 0·05) and close to significant for IgM-GAD65Abs (P = 0·06) in the progressors. Inverse seroconversions occurred in eight (38%) siblings, all of whom had initially tested positive for IgM-GAD65Abs, six for IgA-GAD65Abs, five for IgG2-GAD65Abs and four for IgG2-, IgG3- and IgG4-GAD65Abs. IgG1-GAD65Abs represented the most consistent isotype. Positive seroconversions were frequently seen among the non-progressors. Isotype spreading was also common among the non-progressors, and was found to be significant for IgM-GAD65-Ab (P < 0·05). Inverse seroconversions occurred among the non-progressors as follows: nine of them had initially tested positive for IgA-GAD65Abs, eight for IgM-GAD65Abs, six for IgG2-GAD65Abs, four for IgG4-GAD65Abs and one for IgG3- and IgE-GAD65Abs. IgG1-GAD65Ab was again the most consistent isotype. The progressors and the non-progressors had equal numbers of isotypes during the follow-up (median 5; range 2–7) and at the time of diagnosis of type I diabetes in the progressors, i.e. in the last sample (median 3; range 1–6). Neither were there significant differences in the isotype profiles between the two groups in the last sample (Fig. 2).
Dominant isotype responses
The IgG1-GAD65Ab response was initially dominant in 20 of the 21 progressors. One had a dominant IgA-GAD65Ab response. The corresponding distribution among the non-progressors was that 19 had a dominant IgG1-GAD65Ab response, one a dominant IgG2-GAD65Ab and one a dominant IgG4-GAD65Ab. The pattern was similar when relating the isotype levels to total GAD65, the IgG1-GAD65Ab dominance being obvious, with only one progressor having IgA-GAD65Ab dominance. Among the non-progressors, a 13-year-old boy with IgG2-GAD65Ab dominance and a 7-year-old boy with IgG4-GAD65Ab dominance represented the exceptions, when relating the isotype levels to total GAD65 on an individual basis. When calculating the AUC, we observed IgG1-GAD65Ab dominance in all siblings but one non-progressor, a boy with IgG2-GAD65-Ab dominance. The pattern was similar when relating the integrated isotype levels to total GAD65. Using median values, we noticed the rank order of the isotypes to be IgG1 > IgG2 > IgM > IgG4 > IgA > IgE > IgG3 at the beginning and to change to IgG1 > IgG4 > IgG2 > IgM > IgA > IgE > IgG3 during observation in the progressors, while the non-progressors had the isotypes in the order of IgG1 > IgG2 > IgM > IgG4 > IgA > IgG3 > IgE at the beginning, and it changed to IgG1 > IgM > IgG2 > IgG4 > IgA > IgE > IgG3 during observation. There were no significant differences in the integrated levels of various epitope- and isotype-specific GAD65Ab (Table 3) between the progressors and non-progressors.
Table 3.
Absolute and relative (total GAD65-corrected) integrated levels of GAD65 and GAD65 epitopes and isotypes in the siblings who progressed to clinical type I diabetes (progressors; n = 9 for epitope-specific Abs and n = 21 for isotype specific Abs) and in those who have remained unaffected (non-progressors; n = 9 for epitope-specific Abs and n = 21 for isotype specific Abs). The data are medians; interquartile range
| Progressors | Non-progressors | P-value | |
|---|---|---|---|
| Absolute AUC values | |||
| GAD65Ab | 174·9; 32·5–115·7 | 350·0; 12·5–964·1 | 0·75 |
| GAD65-M-Ab | 84·3; 20·7–236·9 | 50·3; 6·5–71·8 | 0·27 |
| GAD65-C-Ab | 5·7; 4·3–180·1 | 22·0; 2·5–33·9 | 0·97 |
| GAD65-N-Ab | 0·3; 0–0·9 | 0; 0–2·7 | 0·08 |
| IgG1-GAD65 | 561·4; 54·6–3629·3 | 974·7; 24·6–2542·4 | 0·73 |
| IgG2-GAD65 | 16·2; 1·2–67·0 | 20·5; 2·9–308·2 | 0·47 |
| IgG3-GAD65 | 1·5; 0–14·5 | 1·2; 0–20·4 | 0·85 |
| IgG4-GAD65 | 14·3; 0·7–67·6 | 10·8; 0·7–271·6 | 0·97 |
| IgA-GAD65 | 9·2; 0·7–107·1 | 6·4; 0·6–29·4 | 0·52 |
| IgE-GAD65 | 1·8; 0–7·4 | 1·4; 0–7·2 | 0·25 |
| IgM-GAD65 | 15·4; 0·8–185·9 | 17·7; 1·4–105·1 | 0·85 |
| Relative AUC values | |||
| GAD65-M-Ab/GAD65 | 0·3; 0·1–0·6 | 0·2; 0·1–0·3 | 0·27 |
| GAD65-C-Ab/GAD65 | 0·1; 0·0–0·4 | 0·1; 0·0–0·2 | 0·83 |
| GAD65-N-Ab/GAD65 | 0·0; 0·0–0·0 | 0·0; 0·0–0·0 | 0·81 |
| IgG1-GAD65/GAD65 | 2·7; 1·4–5·7 | 2·8; 1·1–7·0 | 0·55 |
| IgG2-GAD65/GAD65 | 0·1; 0·0–0·2 | 0·1; 0·0–7·1 | 0·49 |
| IgG3-GAD65/GAD65 | 0·0; 0·0–0·0 | 0·0; 0·0–0·21 | 0·98 |
| IgG4-GAD65/GAD65 | 0·1; 0·0–0·2 | 0·0; 0·0–0·9 | 0·80 |
| IgA-GAD65/GAD65 | 0·0; 0·0–0·9 | 0; 0·0–0·2 | 0·74 |
| IgE-GAD65/GAD65 | 0·0; 0·0–0·2 | 0·0; 0·0–0·1 | 0·52 |
| IgM-GAD65/GAD65 | 0·1; 0·0–0·5 | 0·0; 0·0–0·6 | 0·72 |
Discussion
As previous reports in GAD65Ab-positive subjects with type I diabetes have established [12–14], we found that the first GAD65Ab response in initially non-diabetic siblings was directed mainly towards both the middle and C-terminal domains of GAD65. More than 90% of the siblings had GAD65-M-Abs in the first GAD65Ab-positive sample, and in addition most of them (87%) also had GAD65-C-Abs. One sibling (Table 1; case no. 45205) initially had GAD65-C-Abs alone, but seroconverted soon to GAD65-M-Ab positivity. Primary reactivity to GAD65-N-Abs was clearly less common, as it was detected weakly in four subjects (22%), usually in combination with both GAD65-M-Ab and GAD65-C-Ab. These results are well in concordance with the observation in young offspring of parents with type I diabetes [15], implying that both the middle and carboxyterminal regions of GAD65 are early, perhaps even simultaneous targets of humoral GAD immunity, while the N-terminal region is a secondary epitope. The clear immunodominance of the middle domain at the beginning and spreading of the response from the middle region to the C-terminal domain speculated on in previous papers [12–14] was observed here only in a limited number of cases. Especially among the non-progressors, the median levels of GAD65-M-Ab were higher, however, at the beginning than those of GAD65-C-Ab, which could indirectly support the idea that the response to the middle region is dominant over the response to the C-terminal region. This simultaneously observed immune response instead of the middle dominance was probably seen because a majority of the siblings tested positive for GAD65Ab already in the first sample taken and only four of the 42 siblings seroconverted to GAD65Ab positivity during prospective follow-up, and we do not know for how a long time period the initially positive siblings had been positive for GAD65Ab. From the study on young genetically susceptible children recruited from the Finnish DIPP (Diabetes Prediction and Prevention) Study we know that the humoral immune response to GAD65 is very dynamic over the first 2 years after initial seroconversion and usually starts with a response to the middle domain of the GAD65 molecule. After that period the immune response to GAD65 became more stable (Ronkainen et al. submitted for publication).
None of the GAD65Ab reactivities nor any changes in reactivity over time were associated with progression to type I diabetes or diabetes presentation. Both the number of epitopes and the median levels of epitope-specific antibodies were of the same magnitude in both the progressors and the non-progressors, and also the integrated levels of various epitope-specific GAD65Ab were similar. High levels of GAD65-C-Ab were not observed to distinguish progressors from non-progressors, although such a phenomenon has been reported to predict rapid progression to insulin deficiency in adults with latent autoimmune diabetes (LADA) [32]. In fact, three progressors (33%) seroconverted to negativity for GAD65-C-Ab before the manifestation of clinical type I diabetes and the median levels of GAD65-C-Ab decreased towards diagnosis. The responses to the N-terminal region remained weak during the follow-up and negative seroconversions were frequent, even when the responses to the middle and C-terminal regions remained strong.
It has been discussed whether a Th1/Th2 imbalance would lead to type I diabetes, and further whether the distribution of different isotypes to antigens such as GAD65 might reflect the ongoing T-cell response [17–21]. In the present study we observed that the humoral immune response to GAD65 in siblings of children with type I diabetes was composed mainly of IgG1 antibodies. Significant differences in the initial distribution of various isotype-specific antibodies was not seen between the progressors and the non-progressors, although the non-progressors tended to have IgG2- and IgG4-GAD65Abs more often than the progressors. We failed to observe a dominance of immature Th2-like isotypes (IgE- and IgM-GAD65Abs) in the first samples analysed, but the rate of detectable IgM-GAD65Ab was surprisingly high; 67% of the progressors and 71% of the non-progressors initially had IgM-GAD65Ab, and in addition there was a relatively strong correlation between IgM-GAD65Ab and total GAD65. Among progressors the initial frequency of detectable IgM-GAD65Ab was higher than the frequency of any other isotype except IgG1-GAD65Ab. This observation is inconsistent with a previous report by Petersen et al., indicating a more immature isotype distribution in non-progressors who had higher levels of IgE- and IgM-GAD65 antibodies than the progressors [17]. The discrepancy between these two studies is intriguing, as the study cohorts were partly overlapping. In the former report the number of siblings was limited, however, and in addition the human monoclonal antiantibodies used were derived from a different source, which may contribute to the discrepancy.
During the prediabetic observation period there were no significant differences in isotype responses between the two groups. As a sign of a dynamic process isotype spreading was seen for IgG2-GAD65Abs and was close to significant for IgM-GAD65Abs among the progressors, while the non-progressors experienced a significant isotype spreading for the latter (P < 0·05). By using median values, we noticed the rank order of the isotypes to change during observation so that the level of IgM-GAD65Ab decreased in relation to the other isotype levels in the progressors despite simultaneous increase in the frequency of the IgM-GAD65Abs. This decreased rank order was mainly a consequence of an increase in IgG4-GAD65Ab values. Among the non-progressors the median level of IgM-GAD65Ab increased over the IgG2-GAD65Ab in the rank order list during the follow-up period and the increase in the frequency of IgG4-GAD65Ab was not so obvious.
We propose that the high levels of IgG1-GAD65Ab may reflect the dominance of the IgG1 isotype within the humoral immune response in general, rather than a bias of the response into Th1 immunity, whereas the prevalence of IgM antibodies during the early response may represent immature Th2 immunity, but here the limitations of the study have to be considered. First, a majority of the siblings had established humoral autoimmunity to GAD65 already in the first sample collected, and we do not know for how long such siblings had been positive for GAD65Ab; secondly, it is possible that IgM-GAD65Ab actually reflects the immune response in general. Previous papers on isotype-specific GAD65Abs are consistent in relation to the dominance of IgG1 antibodies and the infrequent occurrence of other IgG subclasses in type I diabetes-associated autoimmunity [17, 25, 33, 34, 35]. There is one paper, based on an enzyme-linked immunoassay (ELISA), indicating a Th2-like immune response including IgG2 and/or IgG4-GADAb dominance to be characteristic of non-diabetic antibody-positive first-degree relatives [33]. Contrasting results have also been reported, according to which isotype switching was not associated with progression to type I diabetes [25,34]. As-yet unpublished data (Ronkainen et al.) from the DIPP study show that among young genetically susceptible children monitored from birth the occurrence IgG4 antibodies to GAD65 were characteristic of those children who remained non-diabetic over the first few years of humoral GAD65 autoimmunity.
To conclude, these results show that the humoral immune response to GAD65 in siblings of subjects with type I diabetes is dynamic. It seems to start out as a simultaneous response to the middle and C-terminal regions of the GAD65 molecule, but if the response was directed initially to the middle epitope cluster, it spread rapidly to the C-terminal domain and later, in a few cases, to the N-terminal domain. The response to GAD65 is composed mainly of antibodies of IgG1 subclass, but responses of IgG2, IgG4, IgM and IgA are observed frequently, whereas IgE and IgG3 responses are seen more rarely. Our findings indicate that the analysis of epitope- and isotype-specific GAD65 antibodies do not seem to be capable of separating progressors from non-progressors among GAD65Ab-positive first-degree relatives of children with type I diabetes.
Acknowledgments
We thank Susanna Heikkilä for her skilful technical assistance. The members of the Childhood Diabetes in Finland (DiMe) Study Group are as follows: principal investigators: H.K. Åkerblom and J. Tuomilehto; coordinators: R. Lounamaa and L. Toivanen; data management: E. Virtala and J. Pitkäniemi; local investigators: A. Fagerlund, M. Flittner, B. Gustafsson, C. Häggquist, A. Hakulinen, L. Herva, P. Hiltunen, T. Huhtamäki, N.-P. Huttunen, T. Huupponen, M. Hyttinen, T. Joki, R. Jokisalo, M.-L. Käär, S. Kallio, E.A. Kaprio, U. Kaski, M. Knip, L. Laine, J. Lappalainen, J. Mäenpää, A.-L. Mäkelä, K. Niemi, A. Niiranen, A. Nuuja, P. Ojajärvi, T. Otonkoski, K. Pihlajamäki, S. Pöntynen, J. Rajantie, J. Sankala, J. Schumacher, M. Sillanpää, M.-R. Ståhlberg, C.-H. Stråhlman, T. Uotila, M. Väre, P. Varimo and G. Wetterstrand; and special investigators: A. Aro, H. Hurme, M. Hiltunen, H. Hyöty, J. Ilonen, J. Karjalainen, M. Knip, P. Leinikki, A. Miettinen, T. Petäys, L. Räsänen, H. Reijonen, A. Reunanen, T. Saukkonen, E. Savilahti, E. Tuomilehto-Wolf, P. Vähäsalo and S.M. Virtanen. This research was supported by the Medical Research Fund, Tampere University Hospital, the Foundation for Diabetes Research in Finland, Finska Läkaresällskapet, the Juvenile Diabetes Research Foundation International (grants 188517 and 197032), the Sigrid Jusélius Foundation and the Research Council for Health, Academy of Finland. The Childhood Diabetes in Finland project has been supported by grants from the National Institutes of Health (Grant DK-37957), the Sigrid Jusélius Foundation, the Association of Finnish Life Insurance Companies, the University of Helsinki and Novo Nordisk A/S, Bagsvaerd, Denmark.
References
- 1.Castano LE, Eisenbarth GS. Type I diabetes: a chronic autoimmune disease of man, mouse and rat. Ann Rev Immunol. 1990;8:647–79. doi: 10.1146/annurev.iy.08.040190.003243. [DOI] [PubMed] [Google Scholar]
- 2.Palmer JP. Predicting type I diabetes. Use of humoral immune markers. Diabetes Rev. 1993;1:104–15. [Google Scholar]
- 3.Baekkeskov S, Nielsen JH, Marner B, Bilde T, Ludvigsson J, Lernmark Å. Autoantibodies in newly diagnosed diabetic children immunoprecipitate human pancreatic islet cell proteins. Nature. 1982;298:167–9. doi: 10.1038/298167a0. [DOI] [PubMed] [Google Scholar]
- 4.Baekkeskov S, Aanstoot HJ, Christgau S, et al. Identification of the 64 K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347:151–6. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- 5.Atkinson MA, Maclaren NK, Scharp DW, Lacy PE, Riley WJ. 64 000 Mr autoantibodies as predictors of insulin-dependent diabetes. Lancet. 1990;335:1357–60. doi: 10.1016/0140-6736(90)91241-2. [DOI] [PubMed] [Google Scholar]
- 6.Sabbah E, Kulmala P, Veijola R, et al. GAD65 antibodies in relation to other antibodies and genetic risk markers in children with newly diagnosed IDDM. J Clin Endocrinol Metab. 1996;81:2455–9. doi: 10.1210/jcem.81.7.8675560. [DOI] [PubMed] [Google Scholar]
- 7.Kulmala P, Savola K, Petersen JS, et al. Prediction of insulin-dependent diabetes mellitus in siblings of children with diabetes. J Clin Invest. 1998;101:327–36. doi: 10.1172/JCI119879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagopian WA, Michelsen B, Karlsen AE, et al. Autoantibodies in IDDM primary recognize the 65 000 Mr rather than 67 000 Mr isoform of glutamic acid decarboxylase. Diabetes. 1993;42:631–6. doi: 10.2337/diab.42.4.631. [DOI] [PubMed] [Google Scholar]
- 9.Vandewalle CL, Falorni A, Svanholm S, Lernmark A, Pipeleers DG, Gorus FK. High diagnostic sensitivity of glutamate decarboxylase autoantibodies in insulin-dependent diabetes mellitus with clinical onset between age 20 and 40 years. The Belgian Diabetes Registry. J Clin Endocrinol Metab. 1995;80:846–51. doi: 10.1210/jcem.80.3.7883841. [DOI] [PubMed] [Google Scholar]
- 10.Daw K, Powers AC. Two distinct glutamic acid decarboxylase autoantibody specificities in IDDM target different epitopes. Diabetes. 1995;44:216–20. doi: 10.2337/diab.44.2.216. [DOI] [PubMed] [Google Scholar]
- 11.Ujihara N, Daw K, Gianani R, Boel EYuL, Powers AC. Identification of glutamic acid decarboxylase autoantibody heterogenity and epitope regions in type I diabetes. Diabetes. 1994;43:968–75. doi: 10.2337/diab.43.8.968. [DOI] [PubMed] [Google Scholar]
- 12.Richter W, Shi Y, Baekkeskov S. Autoreactive epitopes defined by diabetes-associated human monoclonal antibodies are localized in the middle and C-terminal domains of the smaller form of glutamate decarboxylase. Proc Natl Acad Sci USA. 1993;90:2832–6. doi: 10.1073/pnas.90.7.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falorni A, Ackefors M, Carlberg C, et al. Diagnostic sensitivity of immunodominant epitopes of glutamic acid decarboxylase (GAD65) autoantibodies in childhood IDDM. Diabetologia. 1996;39:1091–8. doi: 10.1007/BF00400659. [DOI] [PubMed] [Google Scholar]
- 14.Sohnlein P, Muller M, Syren K, et al. Epitope profiles of anti-glutamate decarboxylase reactive sera are titer-related, but not disease specific and identify EP-1 as a dominant target of autoantibody response. Diabetologia. 2000;43:210–7. doi: 10.1007/s001250050031. [DOI] [PubMed] [Google Scholar]
- 15.Bonifacio E, Lampasona V, Bernasconi L, Ziegler AG. Maturation of the humoral autoimmune response to epitopes of GAD in preclinical childhood type I diabetes. Diabetes. 2000;49:202–8. doi: 10.2337/diabetes.49.2.202. [DOI] [PubMed] [Google Scholar]
- 16.Toellner KM, Luther SA, Sze DM, et al. T helper 1 (Th1) and Th2 characteristics start to develop during T cell priming and are associated with an immediate ability to induce immunoglobulin class switching. J Exp Med. 1998;187:1193–204. doi: 10.1084/jem.187.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersen JS, Kulmala P, Clausen JT, Knip M, Dyrberg T. and the Childhood Diabetes in Finland Study GroupProgression to type I diabetes is associated with a change in the immunoglobulin isotype profile of autoantibodies to glutamic acid decarboxylase. Clin Immunol. 1999;2:276–81. doi: 10.1006/clim.1998.4641. [DOI] [PubMed] [Google Scholar]
- 18.Katz JD, Benoist C, Mathis D. T helper cell subsets in insulin-dependent diabetes. Science. 1995;26:1185–8. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 19.Pilström B, Björk L, Böhme J. Demonstration of Th1 cytokine profile in the late phase of NOD insulitis. Cytokine. 1995;8:806–14. doi: 10.1006/cyto.1995.0097. [DOI] [PubMed] [Google Scholar]
- 20.Nicholson LB, Kuchroo VK. Manipulation of the Th1/Th2 balance in autoimmune disease. Curr Opin Immunol. 1996;8:837–42. doi: 10.1016/s0952-7915(96)80013-6. [DOI] [PubMed] [Google Scholar]
- 21.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 22.Tuomilehto J, Lounamaa R, Tuomilehto-Wolf E, et al. Epidemiology of childhood diabetes mellitus in Finland − background of a nationwide study of Type I (insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:70–6. doi: 10.1007/BF00400854. [DOI] [PubMed] [Google Scholar]
- 23.Savola K, Sabbah E, Kulmala P, Vähäsalo P, Ilonen J, Knip M. Autoantibodies associated with type I diabetes mellitus persist after diagnosis in children. Diabetologia. 1998;41:1293–7. doi: 10.1007/s001250051067. [DOI] [PubMed] [Google Scholar]
- 24.Verge CF, Stenger D, Bonifacio E, et al. Combined use of autoantibodies (IA-2 autoantibody, GAD autoantibody, insulin autoantibody, cytoplasmic islet cell antibodies) in type I diabetes. Combinatorial islet autoantibody workshop. Diabetes. 1998;47:1857–66. doi: 10.2337/diabetes.47.12.1857. [DOI] [PubMed] [Google Scholar]
- 25.Bonifacio E, Scirpoli M, Kredel K, Füchtenbusch M, Ziegler AG. Early autoantibody responses in prediabetes are IgG1 dominated and suggest antigen-specific regulation. J Immunol. 1999;163:525–32. [PubMed] [Google Scholar]
- 26.Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974;2:1279–82. doi: 10.1016/s0140-6736(74)90140-8. [DOI] [PubMed] [Google Scholar]
- 27.Palmer JP, Clemons P, Lyen K, Tatpati O, Rahgu PK, Paquette TL. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983;222:1337–9. doi: 10.1126/science.6362005. [DOI] [PubMed] [Google Scholar]
- 28.Savola K, Bonifacio E, Sabbah E, et al. IA-2 antibodies − a sensitive marker of IDDM with clinical onset in childhood and adolescence. Diabetologia. 1998;41:424–9. doi: 10.1007/s001250050925. [DOI] [PubMed] [Google Scholar]
- 29.Tuomilehto-Wolf E, Tuomilehto J, Cepaitis Z, Lounamaa R. Childhood Diabetes in Finland Study Group. New susceptibility haplotype for type I diabetes. Lancet. 1989;2:299–302. doi: 10.1016/s0140-6736(89)90487-x. [DOI] [PubMed] [Google Scholar]
- 30.Albert ED, Mayr WR, editors. Histocompatibility testing. Berlin: Springer-Verlag; 1984. Nomenclature for factors of the HLA system; pp. 4–8. [Google Scholar]
- 31.Matthews JNS, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. Br Med J. 1990;300:230–5. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falorni A, Gambelunghe G, Forini F, et al. Autoantibody recognition of COOH-terminal epitopes of GAD65 marks the risk for insulin requirement in adult-onset diabetes mellitus. J Clin Endocrinol Metab. 2000;85:309–16. doi: 10.1210/jcem.85.1.6301. [DOI] [PubMed] [Google Scholar]
- 33.Couper JJ, Harrison LC, Aldis JJE, Colman P, Honeyman MC, Ferrante A. IgG subclass antibodies to glutamic acid decarboxylase and risk for progression to clinical insulin-dependent diabetes. Hum Immunol. 1998;59:493–9. doi: 10.1016/s0198-8859(98)00040-8. [DOI] [PubMed] [Google Scholar]
- 34.Hawa MI, Fava D, Medici F, et al. Antibodies to IA-2 and GAD65 in type I and type 2 diabetes: isotype restriction and polyclonality. Diabetes Care. 2000;23:228–33. doi: 10.2337/diacare.23.2.228. [DOI] [PubMed] [Google Scholar]
- 35.Lohmann T, Hawa M, Leslie RD, Lane R, Picard J, Londei M. Immune reactivity to glutamic acid decarboxylase 65 in stiffman syndrome and Type I diabetes mellitus. Lancet. 2000;356:31–5. doi: 10.1016/S0140-6736(00)02431-4. [DOI] [PubMed] [Google Scholar]