Abstract
Parathyroid hormone (PTH) stimulates osteoblasts to produce the proinflammatory cytokine interleukin-6 (IL-6), causing bone resorption. In patients with primary hyperparathyroidism, elevated serum levels of IL-6 normalize after resection of parathyroid tumours. Because IL-6 is also expressed in normal parathyroids and in other endocrine cells (adrenal and islet), we hypothesized that parathyroid tumours might contribute directly to the elevated serum IL-6 levels in patients with hyperparathyroidism. Immunohistochemistry identified IL-6, PTH, and chromogranin-A (an endocrine and neuroendocrine tumour marker) in normal, adenomatous and hyperplastic parathyroids. Using immunofluorescence and confocal microscopy, IL-6 co-localized with PTH and with chromogranin-A in parathyroid cells. All cultured parathyroid tumours secreted IL-6 at levels markedly higher than optimally stimulated peripheral blood mononuclear cells. Supernates from cultured parathyroids stimulated proliferation of an IL-6-dependent cell line, and anti-IL-6 MoAb abolished this stimulatory effect. IL-6 mRNA was documented in cultured parathyroid tumours, cultured normal parathyroids, fresh operative parathyroid tumours and fresh operative normal specimens. In conclusion, these data show that parathyroid tumours and normal parathyroids contain, produce and secrete IL-6. Our findings present a novel pathway by which human parathyroids may contribute markedly to IL-6 production and elevation of serum IL-6 levels in patients with hyperparathyroidism. The physiological relevance of IL-6 production by human parathyroids remains to be determined, but IL-6 secretion by parathyroid tumours may contribute to bone loss and to other multi-system complaints observed in these patients.
Keywords: cytokines, hyperparathyroidism, interleukin-6, parathyroid, parathyroid hormone
Introduction
Primary hyperparathyroidism is an increasingly common endocrine disorder [1]. Although many patients with this diagnosis have few symptoms, there is increasing evidence that significant bone loss is present in a majority of cases and that parathyroidectomy results in marked improvement in bone mass in patients with primary hyperparathyroidism [2,3]. The mechanisms by which parathyroid tumours promote increased bone turnover are not fully understood, although several recent studies have found that parathyroid hormone stimulates the production and release of cytokines from monocyte-macrophage-like cells as well as from osteoblasts, and that these cytokines affect osteoblast and osteoclast functions [4]. For example, some cytokines such as IL-1, IL-6, IL-11, transforming growth factor (TGF) and tumour necrosis factor (TNF)-α, promote bone resorption [5–9].
The direct effects of IL-6 on bone resorption have been well characterized. IL-6 stimulates osteoclastogenesis and bone resorption in vitro, and IL-6 is produced by osteoblasts in response to PTH [5, 10–14]. In a recent clinical study, 16-fold higher serum levels of IL-6 were reported in patients with primary hyperparathyroidism than in controls, and these elevated IL-6 levels normalized after parathyroidectomy [15]. Serum levels of IL-6 and IL-6 soluble receptor (IL-6sR) have been shown to correlate strongly with rates of bone turnover in patients with primary hyperparathyroidism [16]. Furthermore, circulating levels of IL-6 and IL-6sR can predict rates of bone loss in patients with hyperparathyroidism [16,17].
IL-6, a cytokine with pleiotropic activities, was originally identified as B cell stimulatory factor 2 (BSF2) in supernates of mitogen-treated leucocytes that induced terminal differentiation of immunoglobulin-producing B cells [18]. IL-6 expression has been reported in T cells, fibroblasts, adipocytes, endothelial cells, pancreatic islets and in a variety of tumours (prostate, astrocytic, colorectal, biliary and adrenal) [18–22]. Recently, IL-6 immunoreactivity has been demonstrated in normal parathyroid cells [23]. Therefore, we postulated that human parathyroid tumours might express and secrete IL-6, contributing directly to the elevated serum IL-6 levels noted in patients with primary hyperparathyroidism.
Materials and Methods
Patients and tissues
Included in this study were portions of parathyroid operative tissue specimens from 80 patients shown in Table 1 with clinical and histopathological diagnoses of solitary adenoma (n = 26, ID 1, 2, 17–19, 27–42, 67–70, 74), double adenoma (n = 4, ID 20, 21, 43 and 44), primary hyperplasia (n = 8, ID 3, 22, 47–50, 72 and 73), familial primary hyperplasia (n = 4, ID 4, 5, 46 and 71), renal failure-related secondary hyperplasia (n = 13, ID 6, 23–25 and 51–59), multiple endocrine neoplasia (MEN-I) (n = 2, ID 7 and 45) and carcinoma (n = 2, ID 8 and 26). Normal parathyroid tissue was obtained from surgeries on thyroid goitres and tumours in instances where these normal parathyroids were intimate with the capsule of the thyroid tumours (n = 21, ID 9–16, 60–66 and 75–80). After resection, these normal parathyroids were routinely dissected from the surface of the thyroid goitres and tumours, minced finely in a Petri dish, and returned to the patients as autografted parathyroid fragments. Afterwards, small numbers of residual normal parathyroid cells left in the Petri dish that would have been discarded were suspended in HBSS for study.
Table 1.
All patients listed by diagnosis and type of assay performed
| Culture supernates ng/mg protein/24 h | IL-6 mRNN | ||||||
|---|---|---|---|---|---|---|---|
| ID | Diagnosis | Single stain IHC† | Dual stain if‡ | PTH/IL-6§ | IL-6 bioassay†† | n.d. Cuatured | n.d. Frozen |
| 1 | Sol. Adenoma‡‡ | IL-6 +, PTH +, CG +, VWF + | n.d.* | n.d. | n.d. | n.d. | n.d. |
| 2 | Sol. Adenoma | IL-6 +, PTH + | |||||
| 3 | 1° Hyperplasia** | IL-6 +, PTH +,CG +, VWF + | n.d. | +/+++ | n.d. | n.d. | n.d. |
| 4 | Fam. 1° Hyperpl¶ | IL-6 +, PTH +, CG + | IL-6/PTH +, CG + | n.d. | n.d. | n.d. | n.d. |
| 5 | Fam. 1° Hyperpl | IL-6 +, PTH + | |||||
| 6 | 2° Hyperplasia§§ | IL-6 +, PTH +,CG +, VWF + | n.d. | n.d. | n.d. | n.d. | n.d. |
| 7 | MEN I | IL-6 +, PTH + | |||||
| 8 | Carcinoma | IL-6 +, PTH-,CG +, VWF- | n.d. | n.d. | n.d. | n.d. | n.d. |
| 9 | Normal | IL-6 +, PTH +, CG-, VWF- | n.d. | n.d. | n.d. | n.d. | n.d. |
| 10 | Normal | IL-6-, PTH + | n.d. | n.d. | n.d. | n.d. | n.d. |
| 11 | Normal | IL-6-, PTH +, VWF + | n.d. | n.d. | n.d. | n.d. | n.d. |
| 12 | Normal | IL-6 +, PTH + | n.d. | n.d./+++ | n.d. | n.d. | n.d. |
| 13 | Normal | IL-6 +, PTH + | n.d. | n.d. | n.d. | n.d. | n.d. |
| 14 | Normal | IL-6 + | |||||
| 15 | Normal | IL-6 + | |||||
| 16 | Normal | IL-6 + | |||||
| 17 | Sol. Adenoma | n.d. | IL-6/PTH +, CG + | n.d. | n.d. | n.d. | n.d. |
| 18 | Sol. Adenoma | n.d. | IL-6/PTH +, CG + | n.d. | n.d. | n.d. | n.d. |
| 19 | Sol. Adenoma | n.d. | IL-6/CD45 - | n.d. | n.d. | n.d. | n.d. |
| 20 | Dbl. Adenoma¶¶ | n.d. | IL-6/PTH +, CG + | n.d. | n.d. | n.d. | n.d. |
| 21 | Dbl. Adenoma | n.d. | IL-6/PTH +, CG + | n.d. | n.d. | n.d. | n.d. |
| 22 | 1° Hyperplasia | n.d. | IL-6/PTH +, CG + | n.d. | n.d. | n.d. | n.d. |
| 23 | 2° Hyperplasia | n.d. | IL-6/PTH +, CG + | n.d. | n.d. | n.d. | n.d. |
| 24 | 2° Hyperplasia | n.d. | IL-6/PTH +, CG + | n.d. | n.d. | n.d. | n.d. |
| 25 | 2° Hyperplasia | n.d. | IL-6/PTH +, CG +,IL-6/CD45 - | n.d. | n.d. | n.d. | n.d. |
| 26 | Carcinoma | n.d. | IL-6/PTH +, CG + | n.d. | n.d. | n.d. | n.d. |
| 27 | Sol. Adenoma | n.d. | n.d. | ++/+++ | n.d. | positive | undetectable |
| 28 | Sol. Adenoma | n.d. | n.d. | ±/++ | n.d. | n.d. | undetectable |
| 29 | Sol. Adenoma | n.d. | n.d. | ±/+++ | n.d. | n.d. | n.d. |
| 30 | Sol. Adenoma | n.d. | n.d. | + ++/+++ | n.d. | n.d. | n.d. |
| 31 | Sol. Adenoma | n.d. | n.d. | ±/++ | n.d. | n.d. | n.d. |
| 32 | Sol. Adenoma | n.d. | n.d. | ±/+++ | n.d. | n.d. | n.d. |
| 33 | Sol. Adenoma | n.d. | n.d. | ±/++ | n.d. | positive | n.d. |
| 34 | Sol. Adenoma | n.d. | n.d. | ±/+++ | n.d. | n.d. | n.d. |
| 35 | Sol. Adenoma | n.d. | n.d. | ±/++ | n.d. | n.d. | n.d. |
| 36 | Sol. Adenoma | n.d. | n.d. | ±/+++ | n.d. | n.d. | n.d. |
| 37 | Sol. Adenoma | n.d. | n.d. | +/++ | n.d. | n.d. | n.d. |
| 38 | Sol. Adenoma | n.d. | n.d. | ±/+++ | n.d. | n.d. | n.d. |
| 39 | Sol. Adenoma | n.d. | n.d. | ±/++ | n.d. | positive | (positive)††† |
| 40 | Sol. Adenoma | n.d. | n.d. | ±/+++ | n.d. | n.d. | n.d. |
| 41 | Sol. Adenoma | n.d. | n.d. | +/+++ | n.d.. | n.d. | n.d. |
| 42 | Sol. Adenoma | n.d. | n.d. | ±/++++ | n.d. | n.d. | n.d. |
| 43 | Dbl. Adenoma | n.d. | n.d. | ±/++ | n.d. | positive | n.d. |
| 44 | Dbl. Adenoma | n.d. | n.d. | ±/+++ | n.d. | n.d. | n.d. |
| 45 | MEN-I | n.d. | n.d. | ±/+++ | + | positive | undetectable |
| 46 | Fam. 1° Hyperpl × 2‡‡‡ | n.d. | n.d. | +/+++ | n.d. | n.d. | n.d. |
| 47 | 1° Hyperplasia | n.d. | n.d. | ±/++ | n.d. | positive | undetectable |
| 48 | 1° Hyperplasia | n.d. | n.d. | ±/+++ | n.d. | n.d. | n.d. |
| 49 | 1° Hyperplasia | n.d. | n.d. | ++/++ | n.d. | n.d. | n.d. |
| 50 | 1° Hyperplasia × 2 | n.d. | n.d. | ±/+++ | n.d. | n.d. | n.d. |
| 51 | 2° Hyperplasia × 4 | n.d. | n.d. | ±/++ | + | positive | positive |
| 52 | 2° Hyperplasia × 3 | n.d. | n.d. | ±/+++ | + | positive | positive |
| 53 | 2° hyperplasia × 4 | n.d. | n.d. | ±/+++ | n.d. | n.d. | n.d. |
| 54 | 2° Hyperplasia × 3 | n.d. | n.d. | + ++/+++ | n.d. | n.d. | n.d. |
| 55 | 2° Hyperplasia × 4 | n.d. | n.d. | + ++/++ | n.d. | n.d. | n.d. |
| 56 | 2° Hyperplasia × 3 | n.d. | n.d. | ±/++ | n.d. | positive | undetectable |
| 57 | 2° Hyperplasia × 1 | n.d. | n.d. | + ++/++ | n.d. | n.d. | n.d. |
| 58 | 2° Hyperplasia × 3 | n.d. | n.d. | + ++/++ | n.d. | positive | n.d. |
| 59 | 2° Hyperplasia × 3 | n.d. | n.d. | ±/+++ | n.d. | n.d. | n.d. |
| 60 | Normal | n.d. | n.d. | + ++/+ | n.d. | n.d. | n.d. |
| 61 | Normal | n.d. | n.d. | ++/++ | n.d. | n.d. | n.d. |
| 62 | Normal | n.d. | n.d. | ++/++ | n.d. | n.d. | n.d. |
| 63 | Normal | n.d. | n.d. | ++/+++ | n.d. | n.d. | n.d. |
| 64 | Normal | n.d. | n.d. | + ++/+++ | n.d. | n.d. | n.d. |
| 65 | Normal | n.d. | n.d. | n.d./+ | n.d. | n.d. | n.d. |
| 66 | Normal | n.d.. | n.d. | n.d./++ | n.d. | n.d. | n.d. |
| 67 | Sol. Adenoma | n.d. | n.d. | n.d. | + | n.d. | n.d. |
| 68 | Sol. Adenoma | n.d. | n.d. | n.d. | + | n.d. | n.d. |
| 69 | Sol. Adenoma | n.d. | n.d. | n.d. | + | n.d. | n.d. |
| 70 | Sol. Adenoma | n.d. | n.d. | n.d. | + | n.d. | n.d. |
| 71 | Fam. 1° Hyperpl | n.d. | n.d. | n.d. | + | n.d. | n.d. |
| 72 | 1° Hyperplasia | n.d. | n.d. | n.d. | + | n.d. | n.d. |
| 73 | 1° Hyperplasia | n.d. | n.d. | n.d. | + | n.d. | n.d. |
| 74 | Sol. Adenoma | n.d. | n.d. | n.d.. | n.d. | positive | undetectable |
| 75 | Normal | n.d. | n.d. | n.d.. | n.d. | positive | positive |
| 76 | Normal | n.d. | n.d. | n.d. | n.d. | positive | positive |
| 77 | Normal | n.d.. | n.d. | n.d. | n.d. | n.d. | undetectable |
| 78 | Normal | n.d. | n.d. | n.d. | n.d. | n.d. | undetectable |
| 79 | Normal | n.d. | n.d. | n.d. | n.d. | n.d. | positive |
| 80 | Normal | n.d. | n.d. | n.d. | n.d. | n.d. | (positive) |
IHC indicates immunohistochemistry.
IF indicates immunofluorescence.
PTH(RIA)/IL-6 (EIA): ± ≤ 1 ng/mg tissue protein/24 h, += 1–10 ng/mg tissue protein/24 h, + + = 10–100 ng/mg tissue protein/24 h, ++ + = 100–1000 ng/mg tissue protein/24 h, + + + + ≥ 1000 ng/mg tissue protein/24 h.
IL-6 bioassay, + = positive.
n.d.: The test was not done.
Sol. Adenoma = solitary adenoma
1° Hyperplasia = primary hyperplasia.
Fam. 1° Hyperpl = familial primary hyperplasia.
2° Hyperplasia = secondary hyperplasia.
Dbl. Adenoma = double adenoma.
(positive) = weakly positive. †††×2 = two glands from this patient.
Immunohistochemical staining of parathyroid tumour sections for IL-6, PTH, chromogranin-A and von Willebrand factor
Formalin-fixed, paraffin-embedded specimens of human parathyroid glands were immunostained for IL-6, PTH, chromogranin-A (an endocrine and neuroendocrine tumour marker) and von Willebrand factor (factor VIII-related antigen, an endothelial cell marker). Immunohistochemical staining was performed using an avidin–biotin–peroxidase complex technique and steam heat-induced antigen retrieval. Rabbit polyclonal antibodies to human IL-6, 1/40 dilution (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), and PTH, 1/80 dilution (Biogenex, San Ramon, CA, USA) were used, as well as monoclonal antibodies to chromogranin-A, 1/80 dilution (clone DAK-A3) (Dako, Carpinteria, CA, USA) and to von Willebrand factor, 1/100 dilution with enzyme pretreatment (clone F8/68) (Dako). For negative controls, the specific antibody was replaced with buffer. Cytoplasmic immunostaining was assessed (by C. C.) using two parameters: (a) intensity using a 0, +, + +, or + ++ scale and (b) percentage of cells staining positively, with <5% regarded as negative, >50% regarded as diffuse positive and >5% but <50% as focal positive.
Immunofluorescent labelling of parathyoid cells for IL-6, PTH, chromogranin-A and CD45
Cytospins of fresh, finely minced human parathyroid tissue were dually labelled with fluorescent antibodies for IL-6 and PTH, IL-6 and chromogranin-A or IL-6 and CD45 (a marker for haematopoietic cells) followed by confocal microscopy. Cytospins were then rehydrated in Tris-buffered saline (TBS), quenched with 0·02% hydrogen peroxide, washed, permeabilized with 0·5% Triton X-100 in TBS, washed again, and incubated in serum-free blocking solution (protein block, Dako) followed by overnight incubation in a humid chamber at 4°C with rabbit polyclonal antibodies to human IL-6 (Santa Cruz Biotechnology, Inc.) or rabbit isotype control (Dako) in ICC diluent buffer (BD PharMingen, San Diego, CA, USA). For dual labelling, cytospins were further incubated with rat antihuman PTH MoAb (clone 3B3, Dako) or rat isotype control or murine antihuman chromogranin-A MoAb (clone DAK-A3, DAKO), murine antihuman CD45 (clone HI30, BD PharMingen) or murine isotype control diluted 1 : 20 in TBS with 1% BSA. Cytospins were then washed three times in TBS with 1% BSA and incubated with fluorophore-conjugated secondary antibodies, goat antirabbit IgG (Alexa fluor 594, Molecular Probes, Eugene, OR, USA), goat antirat IgG or goat antimouse IgG (Alexa fluor 488, Molecular Probes). Cytospins were then washed and mounted in aqueous mounting medium (Vector Laboratories, Burlingame, CA, USA). Coverslips were applied. Positive controls included human lymphoid Hick-3 cells for IL-6, human parathyroid tissue for PTH and human duodenal tissue for chromogranin-A. Confocal microscopy was performed on a scanning confocal microscope system (LSM 510; Carl Zeiss Inc., Thornwood, NY, USA).
Parathyroid cell culture
Human parathyroid glands were minced, washed twice with Hanks's balanced salt solution (HBSS) and incubated in 2 mg/ml collagenase (CLS4, Type 4, Worthington Biochemical Corp., Lakewood, NJ, USA) or endotox in-free liberase (Roche Diagnostics Corp., Indianapolis, IN, USA) for 1–1·5 h in a 37°C shaking water bath (170 rpm) with vigorous hand shaking at 30-min intervals. The dissociated cells were passed through sterile nylon mesh (500 µm), washed in HBSS and resuspended in RPMI-1640 (0·45 mm/l calcium, 0·4 mm/l magnesium) plus 10% fetal bovine serum (FBS), 2 mm l-glutamine, 10 mm Hepes, 0·5 mm Na pyruvate, 100 IU/ml penicillin and 100 µg/ml streptomycin. The cells were plated at 0·5–1 × 106/ml in 12-well dishes and cultured for 1–9 days at 37°C, 5% humidified CO2.
Detection of intact PTH (iPTH) and IL-6
For detection of iPTH and IL-6, parathyroid cell culture supernates were collected after 1–9 days of culture and stored at −20°C until assay. IL-6 and iPTH values were reported per tissue protein (mg) per 24-h intervals. Upon termination of culture, cell monolayers were solubilized in 0·1% Triton X-100 in 1 N NaOH for protein determination using a BCA Protein Assay Kit (Pierce Chemical Co., Rockford, IL, USA). For iPTH analysis, the samples were diluted 1 : 10 in culture media and analysed using an RIA kit (40–2170, Nichols Institute Diagnostics, San Juan Capistrano, CA, USA) with culture medium as a negative control. This immunoradiometric assay utilizes two different goat polyclonal antibodies to human PTH. One antibody that binds only the mid-region and C-terminal PTH 38–84 is immobilized onto plastic beads, and the other antibody binds only the N-terminal PTH 1–34 and is radiolabelled for detection [24]. IL-6 was quantified using a sandwich ELISA described previously [25] using a pair of monoclonal antibodies (M-620-E for capture and biotinylated M-621-B for detection, Endogen).
Detection of IL-6 bioactivity
IL-6 bioactivity in parathyroid culture supernates was assayed using the IL-6-dependent B cell hybridoma B13·29 (B-9) in a proliferation assay as previously described [8]. B9 cells were maintained in media containing 80 U/ml recombinant human IL-6 (r-IL-6) (Genzyme). After culturing the B9 cells for 24 h in media without IL-6, triplicate cultures of 2 × 104 cells/well were exposed to serial dilutions of parathyroid culture supernates. After 48 h culture, the cells were pulsed with [3H]-thymidine ([3H]Tdr, 1 µC/well) and harvested 18 h later onto filtermats. [3H]Tdr incorporation was measured using a beta counter (Wallac 1450 Microbeta Trilux). A standard curve was derived using dilutions of a recombinant IL-6 standard. IL-6 neutralization was performed by preincubating parathyroid culture supernates with decreasing concentrations of a polyclonal antihuman antiserum (P-620, Endogen) 3 h prior to B9 stimulation.
Measurement of IL-6 mRNA
RNA was isolated from freshly excised, frozen human parathyroid tissue or from primary parathyroid cell cultures and analysed for IL-6 mRNA by reverse transcriptase-polymerase chain reaction (RT-PCR) as described previously [26]. When comparing IL-6 message in cultured parathyroid glands and in de novo tissue, all reagents used in sample preparation were endotoxin free. Total RNA was extracted using 4 m guanidium isothiocyanate and precipitated with isopropanol. For reverse transcription (RT), 1 µg of parathyroid RNA was reverse transcribed in 50 µl RT cocktail (50 mm Tris pH 8·3, 6 mm MgCl2, 40 mm KCl, 10 mm dithiothreitol, 0·01% nonidet P-40, 50 µm random hexamers, 25 µm deoxynucleotide triphosphates (dNTP), 3 U RNasin, and 30 U murine leukaemia virus reverse transcriptase (Promega, Madison, WI, USA). The reverse transcription was allowed to proceed for 10 min at room temperature followed by 1 h at 42°C and terminated by heat inactivation at 95°C for 5 min. PCR amplification for cytokine message was performed as described previously [27,28] with primers specific for human GAPDH and IL-6. A 264 base-pair fragment was amplified using forward (ATGAACTC CTTCTCCACAAGC) and reverse (GTTTTCTGCCAGTGC CTCTTTG) IL-6 primers [29]. Serial 10-fold dilutions of a plasmid containing the IL-6 sequence were included for comparison (0·1–1000 copies/reaction). Thirty cycles of denaturation (95°C), annealing (60°C) and elongation (72°C) were performed and the amplification products were fractionated by electrophoresis through 1·6% agarose gels and visualized with ethidium bromide staining. The identity of the amplified IL-6 fragment was verified by Southern blotting and hybridization to an internal digoxigenin-labelled oligoprobe (TGCTCCTGGTGTTGCCT GCTGCCTT).
Statistical analysis
All data were analysed using the GraphPad InStat program (GraphPad Software, Inc., San Diego, CA, USA) and SAS. The statistical significance of differences in quantitative variables among groups was analysed by the Kruskal–Wallis test (non-parametric anova), rather than by parametric anova, as the data did not follow a Gaussian distribution and the variance for each group was not similar. Specific comparisons between two groups were analysed by the Wilcoxon rank-sum test. A P-value = 0·05 was considered statistically significant. Statistical tests were two-sided.
Results
Identification of IL-6, PTH, chromogranin-A and von Willebrand factor in parathyroid cells
IL-6 was detected in eight of eight ex vivo parathyroid tumours stained for IL-6 (ID 1–8, Table 1) and also in six of eight ex vivo normal parathyroids (ID 9–13, Table 1), confirming a previous report by Kontogeorgos et al. describing the histological detection of IL-6 in multiple endocrine tissues [23]. The specificity of our IL-6 staining was verified by competitive inhibition using recombinant human IL-6. The intensity of staining for IL-6 (on a scale of 0 to +++) was rated as + + + for six of eight parathyroid tumours and two of eight normal glands, + + for two of eight parathyroid tumours and three of eight normal glands, and + in one of eight normal parathyroids with only two normal glands negative for IL-6 (Table 2). In addition, at least 50% of the cells were positive in eight of eight parathyroid tumours and in four of eight normal glands studied. In two normal parathyroid glands that were rated ++ for IL-6, 20% and 40% of the cells stained positively in the respective glands (Table 2). Thus, immunostaining documented relatively high levels of IL-6 in vivo in all parathyroid tumours and in four of eight normal glands studied and demonstrated lower levels of IL-6 production, but nevertheless positivity, in two of eight normal parathyroid glands.
Table 2.
IL-6 immunostaining in parathyroid tumors and normal parathyroids*
| IL-6 | ||
|---|---|---|
| Diagnosis | Intensity | % cells positive |
| Carcinoma | + + + | 80 |
| Solitary Adenoma | + + | 60 |
| Solitary Adenoma | + + + | 80 |
| Solitary Adenoma | + + + | >50 |
| Familial 1° Hyperplasia | + + + | 90 |
| Familial 1° Hyperplasia | + + + | >50 |
| 2° Hyperplasia | + + | >50 |
| MEN I | + + + | >50 |
| Normal | + + + | 90 |
| Normal | + | 70 |
| Normal | + + | 70 |
| Normal | + + + | >50 |
| Normal | + + | 20 |
| Normal | + + | 40 |
| Normal | Neg | <5 |
| Normal | Neg | <5 |
By visual inspection using a light microscope, the intensity of staining was rated on a scale of 0 to +++, with 0 meaning no staining and +++ representing maximum intensity staining. To determine percentage cells positive, the entire section was viewed and the percentage of cells with positive staining was estimated.
PTH production was demonstrated in seven of eight parathyroid tumours (ID 1–8, Table 1) and in five of five normal glands stained for PTH (ID 9–13, Table 1). The one parathyroid tumour that did not stain positive for PTH was a carcinoma (ID 8, Table 1), and this should not be unexpected because non-productive parathyroid carcinomas have been reported by others [30]. Chromogranin-A was found in five of five tumours stained for chromogranin-A (ID 1, 3, 4, 6, 8, Table 1), but not in one normal gland tested (ID 9, Table 1). Von Willebrand factor was detected in endothelial cells lining blood vessels of three of four tumours tested (ID 1, 3, 6 and 8, Table 1), and in one of two normal glands tested (ID 9 and 11, Table 1).
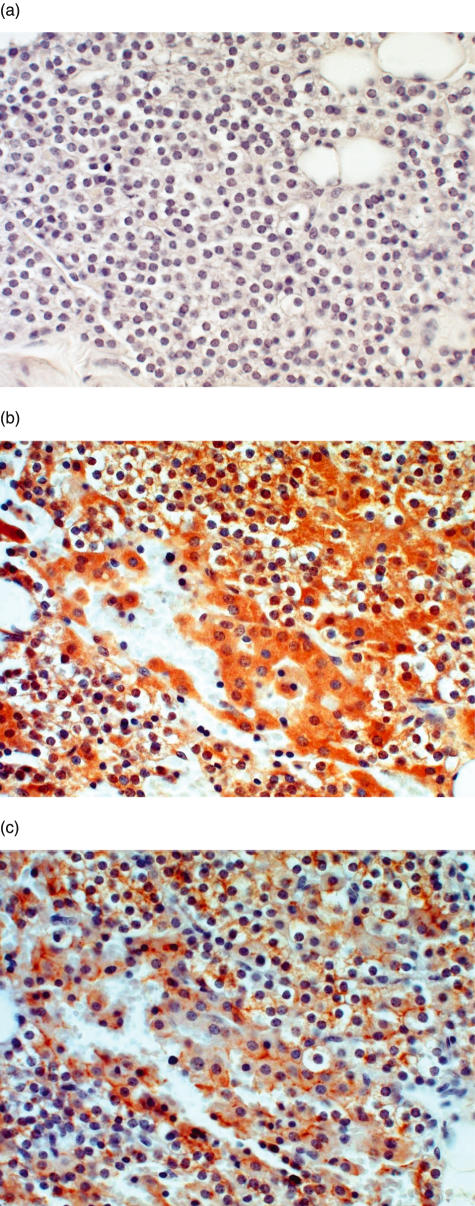
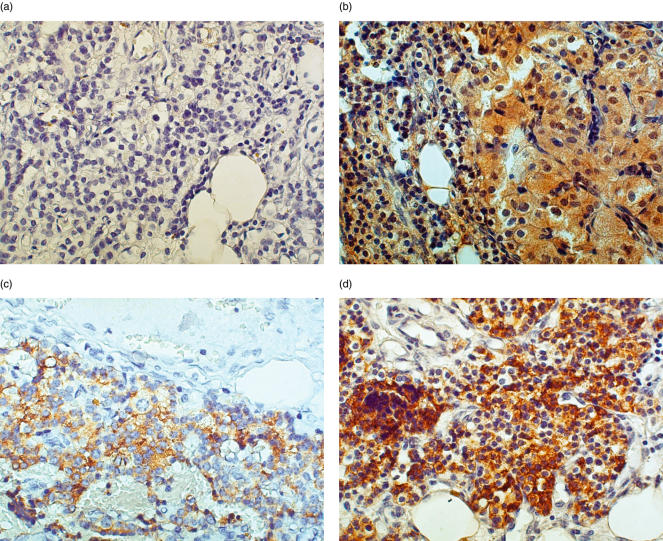
Figure 1 shows serial sections of normal parathyroid tissue, including the isotype negative control (Fig. 1a), positive staining for IL-6 (Fig. 1b) and positive staining for PTH (Fig. 1c). A majority of cells (90%) stained positively for PTH, as expected, and a majority of cells (90%) in these normal glands also were positive for IL-6. Figure 2 demonstrates a representative parathyroid tumour with isotype negative control staining (Fig. 2a), and positive staining for IL-6 (Fig. 2b), PTH (Fig. 2c) and chromogranin-A (Fig. 2d). A high frequency of cells (>50%) stained positively for IL-6, PTH or chromogranin-A throughout the tissue. In the four tumours stained for von Willebrand factor, the relatively few cells that stained positively were localized to endothelial cells of blood vessel walls and did not overlap with the IL-6-, PTH- or chromogranin-A-positive cells (not shown).
Fig. 1.
A normal parathyroid gland (ID 12, Table 1) was embedded in paraffin and stained by immunohistochemistry as described in Materials and Methods. (a) Isotype negative control, 400×; (b) IL-6 staining; 400×; (c) PTH staining; 4000×.
Fig. 2.
A representative sample of parathyroid tumour (ID 4, Table 1) stained for IL-6, PTH and chromogranin-A. Tissue from a patient with familial primary adenoma was embedded in paraffin and stained by immunohistochemistry as described in Materials and Methods. (a) Negative control, 400×; (b)IL-6 staining, 400×; (c) PTH staining, 400×; (d) Chromogranin-A staining, 400×.
Co-expression of IL-6 and PTH or IL-6 and chromogranin-A in parathyroid cells
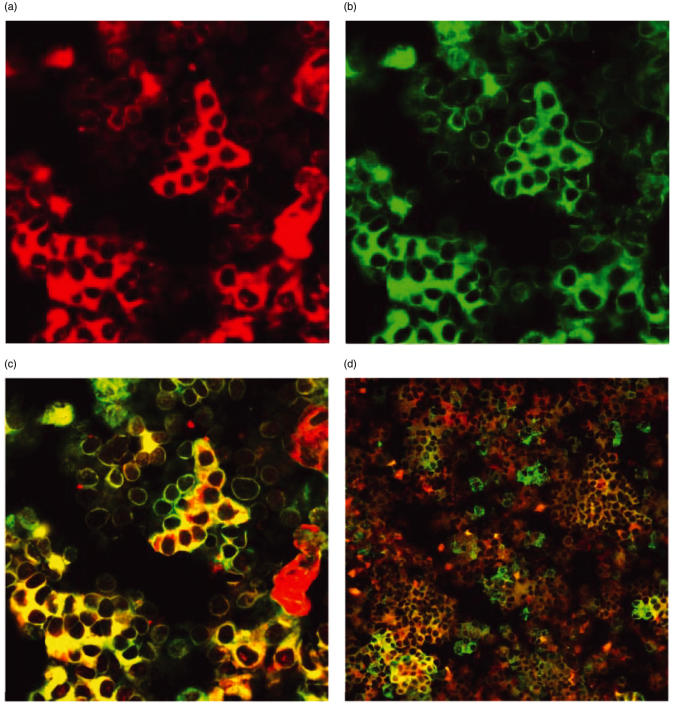
Many different types of cells are known sources of IL-6. Because histological analyses may not always be fully conclusive [23], it was necessary to determine whether the IL-6 detected by immunohistochemistry was present in parathyroid cells, haematopoietic cells or some other cell type found in parathyroid glands. Therefore, cytospins of fresh human parathyroid tissue were dually labelled using monoclonal antibodies for IL-6 and PTH, as well as IL-6 and chromogranin-A (n = 10, ID 4, 17, 18, 20–26, Table 1) or IL-6 and CD45 (n = 2, ID 19 and 25, Table 1) followed by fluorophore-conjugated secondary antibodies and confocal microscopy. As positive controls, human lymphoid cells (Hick-3 cells) were labelled using anti-IL-6 MoAb, human parathyroid tissue was labelled using anti-PTH MoAb, and human duodenal tissue was labelled using antichromogranin-A MoAb (not shown). Isotype control MoAb served as negative controls (not shown). In 10 of 10 parathyroid tumours, positive dual labelling for IL-6/PTH as well as for IL-6/chromogranin-A was observed in single parathyroid cells. Figure 3 shows representative confocal images of cultured parathyroid cells immunofluorescently labelled for IL-6 (red, Fig. 3a) and PTH (green, Fig. 3b). In this example, IL-6 and PTH co-localized to the cytoplasm of many parathyroid cells, appearing yellow by confocal microscopy (Fig. 3c). Similarly, immunofluoresent labelling for IL-6/chromogranin-A in parathyroid cells from the same patient demonstrates clearly that IL-6 is coexpressed with chromogranin in neuroendocine cells of these parathyroid glands (Fig. 3d). In addition, a few cells can be identified that were singly labelled red for IL-6 or green for PTH, suggesting either that PTH and IL-6 expression are not co-ordinated or that different subsets of parathyroid cells express PTH versus PTH/IL-6. In addition, to test for the possibility that lymphocytes (or other haematopoietic cells present in parathyroid cell cultures) might be an important source of IL-6, we dually stained cytospins of parathyroid cells for both CD45 and IL-6. In samples from two parathyroids (ID 19 and 25, Table 1) less than 2% of cells were CD45 positive, and only rare cells exhibited dual staining for both CD45 and IL-6 (data not shown). This finding indicates that IL-6-secreting haematopoietic cells were extremely sparse, compared with the high frequency of PTH/IL-6 co-expressing cells (Fig. 3).
Fig. 3.
Confocal microscopic analysis of cultured parathyroid cells labelled with MoAb against IL-6, PTH, and/or chromogranin-A followed by fluorophore-conjugated secondary antibodies. Cytospins were prepared of cultured parathyroid cells from a patient with a clinical diagnosis of renal failure-related secondary hyperplasia (ID 25, Table 1). The cells shown in Fig. 3a,b and c were stained as described in Materials and methods with both anti-IL-6 with Alexa fluor 594-conjugated secondary antibodies and anti-PTH with Alexa fluor 488-conjugated secondary antibodies. (a) Cells expressing IL-6 (red), emission wavelength 615 nm; 400×. (b) Cells expressing PTH (green), emission wavelength 515 nm, 400×. (c) Cells co-expressing IL-6 and PTH (bright yellow), emission wavelength 545 nm, 400×. The cells shown in Fig. 3d are cytospins of parathyroid cells from the same patient labelled as described for IL-6, but doubly labelled with antichromogranin-A and Alexa fluor 488-conjugated secondary antibodies. Cells coexpressing IL-6 and chromogranin-A appear bright yellow, emission wavelength 545 nm, 200×.
Secretion of PTH and IL-6 by cultured human parathyroids
PTH was detected by immunoradiometric assay in the culture supernates of all normal parathyroid glands tested (ID 12 and 60–66, Table 1) and in all parathyroid tumours (ID 3 and 27–59, Table 1). The levels of PTH secreted by parathyroid tumours varied widely (range = 0·003–1079 ng/mg tissue protein/24 h, median = 0·216 ng/mg tissue protein/24 h, n = 57). Notably, culture supernates from individual secondary hyperplasias (n = 28) contained diverse levels of PTH (range = 0·002–1079 ng/mg tissue protein/24 h) resulting in a median of 0·4 and a mean of 154·7 ± 55·8 (Table 3). In contrast, PTH secretion by normal parathyroid glands exhibited less variation (range = 58–185·5, median = 86·9 ng/mg tissue protein/24 h, n = 5) (Table 3). A comparison of PTH secretion by various types of parathyroids (adenomas, primary hyperplasia, secondary hyperplasia and normal glands) revealed that the adenomas secreted significantly less PTH than normal parathyroids per mg tissue protein (Table 3). PTH release by familial primary hyperplastic glands and one gland from a patient diagnosed with MEN-1 were also lower than normal, but statistical analysis was not possible due to the small number of samples. Thus, while three of 28 secondary hyperplastic glands secreted markedly higher PTH levels (>800 ng/mg tissue protein/24 h), in general tumorigenesis correlated with a profound decrease in PTH production when compared with cultures of normal parathyroid cells.
Table 3.
PTH and IL-6 secreted by parathyroid glands in vitro*
| PTH | IL-6 | ||||
|---|---|---|---|---|---|
| Diagnosis | n | Mean | Median | Mean | Median |
| Adenoma | 20 | 15·9 ± 13·7 | 0·1† | 235·3 ± 63·2 | 135·4§ |
| MEN-I | 1 | 0·061 | 193·2 | ||
| Familial 1° Hyp | 2 | 3·06, 0·39 | 1·7 | 206·6, 28·5 | 117·6 |
| 1° Hyperplasia | 6 | 6·9 ± 6·3 | 0·2 | 173·0 ± 54 | 0142·7 |
| 2° Hyperplasia | 28 | 154·7 ± 55·8 | 0·4 | 109·5 ± 23·6 | 64·1 |
| Normal | 5, 8‡ | 131·6 ± 77 | 86·9 | 62·5 ± 18 | 55·3 |
Shown for each diagnosis are numbers of glands (n) studied, the mean PTH ± s.e., the median value of PTH, the mean IL-6±s.e. and the median value of IL-6. Units are ng/mg tissue protein/24 h. Dissociated parathyroid glands were plated at 0·5–1 × 106/ml in 12-well dishes and cultured for 1–9 days at 37°C, 5% humidified CO2. Upon termination of culture, supernates were harvested for PTH and IL-6 analysis, and cell monolayers were solubilized for total protein quantification as described in Materials and methods.
Five normal glands were analysed for PTH, and eight were analysed for IL-6.
Using the Kruskal–Wallis non-parametric test, differences among PTH levels secreted by adenomas, 1° hyperplasia, 2° hyperplasia and normal glands were significant (P = 0·0290), with P < 0·05 between PTH in culture supernates of adenomas and normal parathyroids.
Using the Kruskal–Wallis non-parametric test, differences among IL-6 levels secreted by adenomas, 1° hyperplasia, 2° hyperplasia, and normal glands were significant (P = 0·0415), with P < 0·05 between IL-6 in culture supernates of adenomas and normal parathyroids using the Wilcoxon rank-sum test.
In contrast, all types of parathyroid tumours released high amounts of IL-6 as determined by enzyme-linked immunosorbent assay (ELISA) (mean values 109·5 ± 23·6–235·3 ± 63·2 ng/mg tissue protein/24 h, Table 3), whereas normal parathyroids produced about one-fourth to one-half as much IL-6 as parathyroid tumours. Using non-parametric anova, there was a significant difference in IL-6 secretion among adenomas, primary hyperplasias, secondary hyperplasias and normal glands (P = 0·0415) (Table 3). In addition, using the Wilcoxon rank-sum test, adenomas (but not primary or secondary hyperplasias) produced significantly more IL-6 than normal glands (P = 0·04).
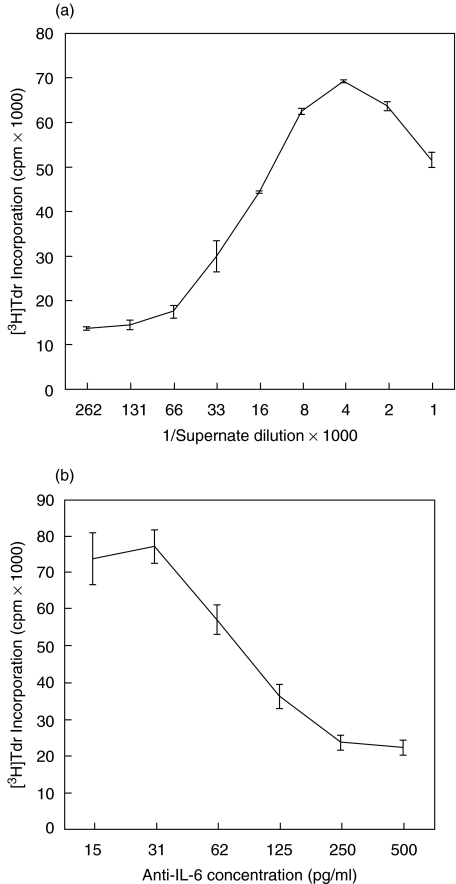
Functional activity of IL-6 produced by human parathyroids
To confirm that the IL-6 secreted by human parathyroids is biologically active, an IL-6-dependent cell line, B9, was cultured in the presence or absence of parathyroid culture supernates from 10 parathyroid tumours (ID 45, 51, 52, 67–73, Table 1), and proliferation of the B9 cells was measured by [3H]Tdr uptake. This assay was performed qualitatively, whereby single dilutions of culture supernates from 10 of 10 glands were found to markedly induce the proliferation of B9 cells (data not shown). As shown in Fig. 4a, the B9 cells proliferated in a dose-dependent fashion to increased concentrations of parathyroid culture supernates. Furthermore, pretreatment with anti-IL-6 antibodies inhibited the stimulatory activity of a representative parathyroid culture supernate in a dose-dependent manner (Fig. 4b).
Fig. 4.
Proliferation of IL-6-dependent B9 cells is stimulated by parathyroid culture supernates and inhibited by anti-IL-6 MoAb. (a) A representative experiment is shown, one of five experiments using culture supernates from a patient with primary hyperplasia (ID 72, Table 1). Means of triplicate wells ± s.d. are plotted. (b) A representative experiment is shown (one of three using optimal concentration of parathyroid culture supernate pretreated with titrations of anti-IL-6 antibodies). Parathyroid culture supernates were preincubated for 3 h with anti-IL-6 MoAb at the indicated concentrations. B9 cell proliferation was determined exactly as described in Fig. 4a. Means of triplicate wells ± s.d. are plotted.
IL-6 mRNA expression in cultured parathyroids and in frozen operative parathyroid specimens
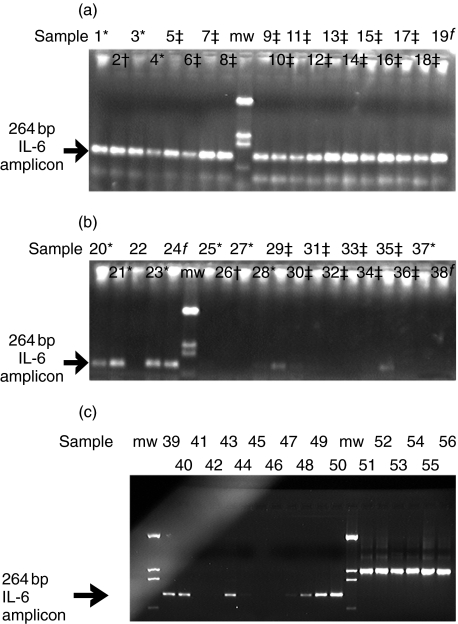
In order to confirm the in vivo IL-6 production demonstrated by immunohistochemical analysis, IL-6 mRNA expression was investigated in cultured parathyroids and in frozen operative specimens by RT-PCR, using primers specific for human IL-6. As shown in Fig. 5a and b, a 264 bp IL-6 amplicon was present in six cultured parathyroids from five individuals with a clinical diagnosis of adenoma (samples 1, 3, 4, 20, 21 and 23), one gland from a patient with MEN-I (sample 2), one gland from an individual with primary hyperplasia (sample 19) and 6 glands from four patients with secondary hyperplasia (samples 5–8, 9–11, 12–14 and 15–18). In contrast, detection of IL-6 mRNA from 14 parathyroid glands collected ex vivo from nine individuals and frozen immediately after resection (samples 25–38) showed a much lower frequency of positivity and intensity of signal (Fig. 5b), suggesting amplification and/or induction of IL-6 production upon culture initiation. As shown in Fig. 5b, the 264 bp IL-6 amplicon was detected strongly in one frozen, uncultured gland from a patient with secondary hyperplasia (sample 29) and detected weakly in the other gland from that same patient (sample 30). IL-6 mRNA expression was also clearly detected in one frozen, uncultured gland (sample 35) from a patient with secondary hyperplasia, less well in a second gland from the same patient (sample 34) and not detected in a third gland from that patient (sample 36). In addition, there was weak IL-6 mRNA signal in frozen parathyroid tissue from one patient with adenoma (sample 28). IL-6 mRNA was not detected in uncultured tissue from four other patients (samples 25–27 and samples 31–33), while cultures of the parathyroid glands from the same patients led to clearly detectable IL-6 signals (Fig. 5a, samples 1, 2, 3 and 9–11, respectively). Induction of such signals was rapid upon culture initiation (<6 h) and was not secondary to the presence of endotoxin in the sample cultures (data not shown).
Fig. 5.
(a) Assessment of IL-6 expression in collagenase-dispersed, cultured human parathyroids by RT-PCR. RT-PCR was performed on total RNA from collagenase-dispersed, cultured human parathyroids using primers that amplified 264 bp of human IL-6. Samples 1 and 2 (ID 74 and 45, respectively, Table 1) cells collected at 24 h; samples 3 and 4 (ID 27 and 39, respectively, Table 1) cells collected at 48 h; samples 5–8 (ID 51, Table 1), cells collected 2, 6, 10 and 14 days, respectively; lane 9 = molecular weight markers (pTZ19 digested with DdeI); samples 9–11 (ID 56, Table 1), cells harvested 1, 5 and 9 days, respectively; samples 12–14 (ID 52, Table 1) three separate glands, cells harvested at 48 h; samples 15–18 (ID 58, Table 1) cells harvested at 2, 6, 10 and 12 days; sample 19 (ID 47, Table 1) cells harvested at 9 days. *Adenoma; †MEN-1; ‡secondary hyperplasia; fprimary hyperplasia.(b)Assessment of IL-6 expression in cultured or frozen operative specimens of human parathyroids by RT-PCR. RT-PCR was performed on total RNA from collagenase-dispersed, cultured (samples 20–24) or frozen, operative (samples 25–38) human parathyroids using primers that amplified 264 bp of human IL-6. Samples 20 and 21 (ID 43, Table 1) two separate glands, cells collected at 24 h; sample 22, not performed; sample 23 (ID 33, Table 1) cells collected at 24 h; sample 24 (ID 47, Table 1) cells collagenase-dispersed and not cultured, RNA prepared immediately after dispersion; lane 6 = molecular weight markers; sample 25 (ID 74, Table 1); sample 26 (ID 45, Table 1); sample 27 (ID 27, Table 1); sample 28 (ID 39, Table 1); samples 29, 30 (ID 51, Table 1), two glands; samples 31–33 (ID 56, Table 1), three glands; samples 34–36 (ID 52, Table 1), three glands, sample 37 (ID 28, Table 1), sample 38 (ID 47, Table 1). *Adenoma; †MEN-1; ‡secondary hyperplasia; fprimary hyperplasia. (c) Assessment of IL-6 expression in normal human parathyroids by RT-PCR. RT-PCR was performed on total RNA from frozen, operative normal human parathyroids using primers that amplified 264 bp of human IL-6. Sample 39 (ID 75, Table 1); sample 40 (ID 76, Table 1); sample 41(ID 77, Table 1); sample 42 (ID 78, Table 1); sample 43 (ID 79, Table 1); sample 44 (ID 80, Table 1); lanes 45–49: 10-fold serial dilutions of IL-6 transcripts corresponding to 0·1, 1, 10, 100 and 1000 copies/reaction, respectively. GAPDH control amplification was performed on the same samples, 39–43 (lanes 50–55, respectively).
Samples of tissue from six normal parathyroids were also tested for the presence of IL-6 mRNA (Fig. 5C, samples 39–44), along with GAPDH as a control for the RNA. IL-6 message was detected in four of six of these normal parathyroids (samples 39, 40, 43 and low signal for 44), confirming the in vivo expression of IL-6 in normal glands already demonstrated by immunohistochemistry (Fig. 1). IL-6 mRNA was undetectable in two of six normal parathyroids even though these samples showed comparable GAPDH signals, suggesting that IL-6 transcription is regulated in vivo, and that these repression mechanisms may be relieved upon in vitro culture.
Discussion
The most significant finding of this study is that human parathyroid tumours and normal human parathyroid cells contain, produce and secrete the proinflammatory cytokine IL-6. The impetus for our study was the recent report that IL-6 concentrations in serum are elevated markedly in patients with primary hyperparathyroidism, and that successful parathyroidectomy normalized serum IL-6 levels [15]. It has been suggested that some of the symptoms of hyperparathyroidism, such as fatigue, lethargy, bone pain, arthralgia and muscle aches and pains may be due in part to elevated serum levels of proinflammatory cytokines such as IL-6 [15]. We observed routinely prompt and dramatic improvement in multi-system complaints of patients with hyperparathyroidism after resection of parathyroid tumours (data not shown).
IL-6 is a pleiotropic cytokine with a diverse range of biological activities in immune and inflammatory responses. IL-6 has been linked to the pathogenesis of a number of diseases, including multiple myeloma [31,32], Castleman's disease [33,34], postmenopausal osteoporosis [35–37], rheumatoid arthritis [38] and multiple sclerosis [39]. It has been shown clearly that osteoblasts secrete IL-6 under the stimulus of PTH, and that IL-6 is an important mediator of bone resorption, both directly [6, 10, 11] and by regulating the activities of PTH [40]. In addition, IL-6 has a role in osteoclast activation and the subsequent induction of bone resorption [35]. While PTH-stimulated IL-6 release from osteoblasts may be the predominant source of IL-6 in the serum of patients with primary hyperparathyroidism, there are other potential sources, such as T cells, endothelial cells, monocytes or fibroblasts that secrete IL-6 when activated during inflammatory responses [41]. The novelty of our study is the finding that parathyroids themselves contain, produce and secrete IL-6. As a variety of tumours have also been shown to express IL-6 [18–22], we hypothesized that parathyroid tumours may produce and secrete IL-6 and thus contribute to the markedly elevated serum levels associated with hyperparathyroidism. During the course of our studies, a report of immunohistochemical staining for IL-6 in normal human parathyroid cells was published that clearly supports our findings [23]. To our knowledge, the potential physiological impact of IL-6 secretion by human parathyroids has not been addressed previously.
Based on our findings presented herein, there are several issues that deserve further investigation. First, while evidence was provided for IL-6 production by parathyroid cells in vivo and in vitro, the production appears to be tightly regulated in vivo based on mRNA analysis. Thus, the nature and physiological bases for this regulation should be examined. Next, while the data in Table 3 suggest that IL-6 production and PTH production are controlled differentially in vitro, our data indicate that parathyroid tumours tend to produce higher levels of IL-6 and lower levels of PTH than normal glands. While not providing formal proof that the elevated serum IL-6 levels noted in patients with primary hyperparathyroidism [15] is due to parathyroid IL-6 production, the relatively lower PTH production by these cells suggests that PTH-mediated IL-6 induction in haematopoietic cells would be a minor contributor. This observation also suggests that chronic IL-6 elevation could exert an important effect on bone metabolism, even if PTH production is diminished.
Human parathyroids are composed predominantly of two different cell types, chief cells and oxyphilic cells. Chief cells, the major cell type, are responsible for PTH synthesis and secretion, and PTH is co-stored and co-secreted with chromogranin-A in this parathyroid lineage [42]. In our experiments, immunofluorescence localized IL-6 to PTH- and chromogranin-A-containing cells, most probably chief cells, in normal, adenomatous and hyperplastic parathyroids. In addition, we also detected in each gland a large number of chromogranin A-positive cells that contained IL-6, but not PTH, suggesting that neuroendocrine cells other than chief cells in parathyroid tumours may produce IL-6. Our results correlate with those of Kontogeorgos et al. [23], who found that parathyroid chief cells in normal parathyroids were mildly positive for IL-6 by immunohistochemistry, while oxyphilic cells stained more intensely for IL-6.
Cultures of dispersed parathyroid glands contain endothelial cells and a small percentage of haematopoietic cells, including lymphocytes, monocytes and macrophages. Therefore, it is possible that cells of non-parathyroid lineages may have contributed to the IL-6 we have detected in culture media. However, in serial sections the same cells that stained positive by immunohistochemistry for von Willebrand factor were negative for IL-6, and vice-versa, implying that endothelial cells in the parathyroid tumours we studied were not the source of IL-6. Because dual staining for IL-6 and CD45 was detected rarely in the glands studied by immunofluorescence, it is unlikely that haematopoietic cells within the parenchyma of the parathyroid tumours could be responsible for the large amounts of IL-6 found in culture media. In fact, culture supernates from parathyroid cells contained approximately 100-fold higher IL-6 levels than supernates from similar numbers of mitogen-stimulated haematopoietic cells [29], suggesting that parathyroid cells are far more potent IL-6 producers than haematopoietic cells in vitro.
IL-6 message appears to be markedly amplified and/or induced upon culture of parathyroid tumours, based on mRNA analysis. However, this does not mean that IL-6 production by cultured parathyroid cells is merely an artefact. First, IL-6 mRNA also was detected in five of 14 frozen operative tumour specimens and in four of six normal glands analysed ex vivo (Fig. 5b,c). IL-6 mRNA did not amplify in the remaining samples, possibly because the sensitivity of our RT-PCR procedure is too low and/or because IL-6 message is degraded rapidly. IL-6 message degradation seems to be a plausible explanation, since the amount of signal observed in the cultured cells appeared modest compared to the abundant protein released by these cells (Fig. 5a and Table 3). Secondly, the very rapid induction of IL-6 production (IL-6 protein is detected within 6 h of culture, data not shown) suggests that these cells may be ‘programmed’ for the production of this factor and that culture conditions merely release potential in vivo transcriptional repression. Thirdly, as five of 14 tumours and four of six normal samples evaluated ex vivo scored a positive signal, expression of IL-6 may reflect a cycling pattern in vivo. Fourthly, the IL-6 production by these cells in vitro is not likely to be an artefact, as the levels are about 100-fold higher than optimally stimulated monocyte/macrophage cultures [27], which are construed as the major IL-6 source during inflammatory processes. Fifthly, histological analysis of freshly excised parathyroid normal and tumour tissue show clear evidence of IL-6 production (Fig. 1) [23]. Therefore, we submit that our data provide compelling evidence that parathyroid cells most likely secrete IL-6 in vivo as well as in vitro, albeit clearly at lower levels in vivo.
Another provocative question is the potential contribution of IL-6 to the overall immune dysfunction documented in hyperparathyroidism [29]. Patients with renal failure-related hyperparathyroidism have impaired humoral immunity, including reduced B cell proliferation and antibody production [43]. Lymphocytes have receptors for PTH [44], and it has been reported that PTH inhibits production of IgG, IgA and IgM by cultured human B cells, while the inactivation of PTH abolishes this inhibitory effect [45]. The respective contributions of these various mechanisms to immune dysfunction in patients with hyperparathyroidism need to be investigated. For example, as IL-6 (originally identified as B cell stimulatory factor) is well known to activate B cells, it may mitigate the down-regulatory effect of PTH on humoral responses in vivo.
In conclusion, this study suggests strongly that parathyroid cells may be an important source of IL-6 not only in vitro but also in vivo. IL-6 production may be modulated during tumorigenesis and the increased number of parathyroid cells during hyperplasia or transformation may be sufficient to contribute clinically relevant levels of this proinflammatory cytokine. While the physiological functions of IL-6 from human parathyroids and the regulatory mechanism(s) for IL-6 secretion by parathyroids remain to be determined, IL-6 released directly by parathyroid tumours may contribute to symptoms of hyperparathyroidism and to bone loss in these patients. Because there is compelling evidence for an important role of IL-6 in bone loss and other symptoms of patients with hyperparathyroidism, we conclude that IL-6 production by parathyroid cells themselves may contribute directly to the deleterious effects of these tumours.
Acknowledgments
We acknowledge Drs Michael Kutner and Kirk Easley, Emory University, Department of Biostatistics, for careful statistical analysis of our data. We express sincere appreciation to Dr. Clay Chappell, Emory University, for excellent immunofluorescent staining and confocal microscopy and to Dr. Emil Hagström, University Hospital, Uppsala, Sweden, for valuable contributions to this study. We would also like to thank Dr Dominique Weber for her excellent contributions to the studies of IL-6 mRNA expression in cultured parathyroids. We thank Dr Judith A. Kapp for critical reading of the manuscript and for valuable suggestions. This research was made possible by a generous gift supporting the Elizabeth Brooke Gottlich Laboratory.
References
- 1.Silverberg SJ, Bilezikian J. Clinical presentation of primary hyperparathyroidism in the United States. In: Bilezikian J, Marcus R, Levine M, editors. The parathyroids: basic and clinical concepts. 2. New York: Academic Press; 2001. pp. 349–60. [Google Scholar]
- 2.Silverberg S, Shane E, Jacobs T, Siris E, Bilezikian J. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 1999;341:1249–55. doi: 10.1056/NEJM199910213411701. [DOI] [PubMed] [Google Scholar]
- 3.Kulak C, Bandeira C, Voss D, Sobieszczyk S, Silverberg S, Bandeira F. Marked improvement in bone mass after parathyroidectomy in osteitis fibrosa cystica. J Clin Endocrinol Metab. 1998;83:732–5. doi: 10.1210/jcem.83.3.4655. [DOI] [PubMed] [Google Scholar]
- 4.Silverberg S, Bilezikian J. Cytokines in primary hyperparathyroidism – factors that matter [Editorial] J Clin Endocrinol Metab. 1996;81:3448–9. doi: 10.1210/jcem.81.10.8855782. [DOI] [PubMed] [Google Scholar]
- 5.Greenfield E, Shaw S, Gornik S, Banks M. Adenyl cyclase and interleukin 6 are downstream effectors of parathyroid hormone resulting in stimulation of bone resorption. J Clin Invest. 1995;96:1238–44. doi: 10.1172/JCI118157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishimi Y, Miyaura C, Jin C, et al. IL-6 is produced by osteoblasts and induces bone resporption. J Immunol. 1990;145:3297–303. [PubMed] [Google Scholar]
- 7.Rifas L, Kenney J, Marcelli M, et al. Production of interleukin-6 in human osteoblasts and human bone marrow stromal cells: evidence that induction by interleukin-1 and tumor necrosis factor-α is not regulated by ovarian steroids. Endocrinology. 1995;136:4056–67. doi: 10.1210/endo.136.9.7649114. [DOI] [PubMed] [Google Scholar]
- 8.Horowitz M, Brown M, Insogna K, et al. Molecular and cellular biology of cytokines. New York: Wiley-Liss, Inc.; 1990. PTHrP and PTH induce the secretion of IL-6 by a clonal osteosarcoma cell line; pp. 471–6. [Google Scholar]
- 9.Nakchbandi IA, Mitnick MA, Masiukiewicz US, Sun BH, Insogna KL. IL-6 negatively regulated IL-11 production in vitro and in vivo. Endocrinology. 2001;142:3850–6. doi: 10.1210/endo.142.9.8368. [DOI] [PubMed] [Google Scholar]
- 10.Lowik C, van der Plujm G, Hoekman K, Bijvoet O, Aarden L, Papapoulos S. Parathyroid hormone (PTH) and PTH-like protein (PLP) stimulate interleukin-6 production by osteogenic cells: a possible role of interleukin-6 in osteoclastogenesis. Biochem Biophys Res Commun. 1989;162:1546–52. doi: 10.1016/0006-291x(89)90851-6. [DOI] [PubMed] [Google Scholar]
- 11.Greenfield E, Gornik S, Horowitz M, Donahue H, Shaw S. Regulation of cytokine expression in osteoblasts by parathyroid hormone: rapid stimulation of interleukin-6 and leukemia inhibitory factor mRNA. J Bone Miner Res. 1993;8:1163–71. doi: 10.1002/jbmr.5650081003. [DOI] [PubMed] [Google Scholar]
- 12.Li N, Ouchi Y, Okamoto Y, et al. Effect of parathyroid hormone on release of interleukin-1 and interleukin-6 from cultured mouse osteoblastic cells. Biochem Biophys Res Commun. 1991;179:236–42. doi: 10.1016/0006-291x(91)91360-o. [DOI] [PubMed] [Google Scholar]
- 13.Sakagami Y, Girasole G, Yu X-P, Boswell H, Manolagas S. Stimulation of interleukin-6 production by either calcitonin gene-related peptide or parathyroid hormone in two phenotypically distinct bone marrow-derived murine stromal cell lines. J Bone Miner Res. 1993;8:811–6. doi: 10.1002/jbmr.5650080706. [DOI] [PubMed] [Google Scholar]
- 14.Jilka R, Hangoc G, Girasole G, et al. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992;257:88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- 15.Jacob T, Mitnick M, Shapses S, Ellison A, Gundberg C, Insogna K. Circulating levels of interleukin-6 and tumor necrosis factor-α are elevated in primary hyperparathyroidism and correlate with markers of bone resporption − a clinical research center study. J Clin Endocrinol Metab. 1996;81:3450–4. doi: 10.1210/jcem.81.10.8855783. [DOI] [PubMed] [Google Scholar]
- 16.Insogna K, Mitnick MA, Pascarella J, Nakchbandi I, Grey A, Masiukiewicz U. Role of the interleukin-6/interleukin-6 soluble receptor cytokine system in mediating increased skeletal sensitivity to parathyroid hormone in perimenopausal women. J Bone Miner Res. 2002;17:N108–N116. [PubMed] [Google Scholar]
- 17.Nakchbandi IA, Mitnick MA, Lang R, Gundberg C, Kinder B, Insogna K. Circulating levels of interleukin-6 soluble receptor predict rates of bone loss in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 2002;87:4946–51. doi: 10.1210/jc.2001-011814. [DOI] [PubMed] [Google Scholar]
- 18.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–79. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Chow K, Wu C. Expression and up-regulation of interleukin-6 in oesophageal carcinoma cells by n-sodium butyrate. Br J Cancer. 1999;80:1617–22. doi: 10.1038/sj.bjc.6690571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori S, Murakami-Mori K, Bonavida B. Dexamethasone enhances expression of membrane and soluble interleukin-6 receptors by prostate carcinoma cell lines. Anticancer Res. 1998;18:4403–8. [PubMed] [Google Scholar]
- 21.Heese M, Dimitriades-Schmutz B, Roe-John S. Role of interleukin-6 and soluble IL-6 receptor in region-specific induction of astrocytic differentiation and neurotrophin expression. Glia. 1999;26:191–200. doi: 10.1002/(sici)1098-1136(199905)26:3<191::aid-glia1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Goydos J, Brumfield A, Frezza E, Booth A, Lotze M, Carty E. Marked elevation of serum interleukin-6 in patients with cholangiocarcinoma. Ann Surg. 1998;227:398–404. doi: 10.1097/00000658-199803000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kontogeorgos G, Messini I, Kyrodimou E, et al. Immunohistochemical localization of interleukin-6 in peripheral human endocrine glands. Endocrine. 2002;17:135–40. doi: 10.1385/ENDO:17:2:135. [DOI] [PubMed] [Google Scholar]
- 24.Nussbaum SR, Zahradnik RJ, Lavigne JR, et al. Highly sensitive two-site immunoradiometric assay of parathyrin, and its clinical utility in evaluating patients with hypercalcemia. Clin Chem. 1988;33:1364–7. [PubMed] [Google Scholar]
- 25.Tzanno-Martins C, Futata E, Jorgetti V, Duarte AJ. Immune response in hemodialysis patients: is there any difference when low and high iPTH levels are compared? Clin Nephrol. 2000;54:22–9. [PubMed] [Google Scholar]
- 26.Villinger F, Rollin PE, Brar SS, et al. Markedly elevated levels of interferon (IFN)-γ, IFN-α, interleukin (IL)-2, IL-10, and tumor necrosis factor-α asssociated with fatal Ebola virus infection. J Infect Dis. 1999;178:S188–S191. doi: 10.1086/514283. [DOI] [PubMed] [Google Scholar]
- 27.Villinger F, Brar S, Mayne A, Chikkala N, Ansari AA. Comparative analysis of cytokine genes from nonhuman primates. J Immunol. 1995;155:3945–54. [PubMed] [Google Scholar]
- 28.Hoffman SL, Crutcher JM, Puri SK, et al. Sterile protection of monkeys against malaria after administration of interleukin-12. Nat Med. 1997;3:80–3. doi: 10.1038/nm0197-80. [DOI] [PubMed] [Google Scholar]
- 29.Villinger F, Hunt D, Mayne A, et al. Qualitative and quantitative studies of cytokines synthesized and secreted by non-human primate peripheral blood mononuclear cells. Cytokine. 1993;5:469–79. doi: 10.1016/1043-4666(93)90038-7. [DOI] [PubMed] [Google Scholar]
- 30.Merlano M, Conte P, Scarsi P, et al. Nonfunctioning parathyroid carcinoma. A case report. Tumori. 1985;71:193–6. doi: 10.1177/030089168507100216. [DOI] [PubMed] [Google Scholar]
- 31.Hilbert DM, Kopf M, Mock BA, Kohler G, Rudikoff S. Interleukin 6 is essential for in vivo development of B lineage neoplasm. J Exp Med. 1995;182:243–8. doi: 10.1084/jem.182.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lattanzio G, Libert C, Aquilina M, et al. Defective development of pristane-oil-induced plasmacytomas in interleukin-6-deficient BALB/c mice. Am J Pathol. 1997;151:689–96. [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshizaki K, Mutsuda T, Nishimoto N, et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman's disease. Blood. 1989;74:1360–7. [PubMed] [Google Scholar]
- 34.Beck JT, Hsu SM, Wijdenes J, et al. Alleviation of systemic manifestation of Castleman's disease by monoclonal anti-interleukin-6 antibody. N Engl J Med. 1994;330:602–5. doi: 10.1056/NEJM199403033300904. [DOI] [PubMed] [Google Scholar]
- 35.Tamura T, Udagawa N, Takahashi N, et al. Soluble interleukin 6 receptor triggers osteoclast formation by interleukin 6. Proc Natl Acad Sci. 1993;90:11924–8. doi: 10.1073/pnas.90.24.11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poli V, Balena R, Fattori E, et al. Interleukin 6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J. 1994;13:1189–96. doi: 10.1002/j.1460-2075.1994.tb06368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheidt-Nave C, Bismar H, Leidig-Brickner G, et al. Serum interleukin 6 is a major predictor of bone loss in women specific to the first decade past monopause. J Clin Endocrinol Metab. 2001;86:2032–42. doi: 10.1210/jcem.86.5.7445. [DOI] [PubMed] [Google Scholar]
- 38.Alonzi T, Fattori E, Lazzaro D, et al. Interleukin-6 is required for the development of collagen-induced arthritis. J Exp Med. 1998;187:468. doi: 10.1084/jem.187.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eugster HP, Frei K, Kopf M, Lassmann H, Fontana A. IL-6-deficient mice resist myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. Eur J Immunol. 1998;28:2178–87. doi: 10.1002/(SICI)1521-4141(199807)28:07<2178::AID-IMMU2178>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 40.Grey A, Mitnick MA, Masiukiewicz U, et al. A role for interleukin-6 in parathyroid hormone-induced bone resorption in vivo. Endocrinology. 1999;140:4683–90. doi: 10.1210/endo.140.10.7036. [DOI] [PubMed] [Google Scholar]
- 41.Biffl WL, Moore EE, Moore FA, Peterson VM. Interleukin-6 and the injured patient. Marker of injury or mediator of inflammation? Ann Surg. 1996;224:647–64. doi: 10.1097/00000658-199611000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kronenberg HM, Bringhurst FR, Segre GV, Potts JT. Parathyroid Hormone Biosynthesis and Metabolism. In: Bilezikian JP, Marcus R, Levine MA, editors. The parathyroids: basic and clinical concepts. New York: Academic Press; 2001. pp. 17–30. [Google Scholar]
- 43.Smogorzewski M, Massry SG. Defects in B-cell function and metabolism in uremia: role of parathyroid hormone. Kidney Int. 2001;78(Suppl):S186–S189. doi: 10.1046/j.1523-1755.2001.59780186.x. [DOI] [PubMed] [Google Scholar]
- 44.Klinger M, Alexiewicz JM, Linker-Israeli M, et al. Effect of parathyroid hormone on human T cell activation. Kidney Int. 1990;37:1543–51. doi: 10.1038/ki.1990.147. [DOI] [PubMed] [Google Scholar]
- 45.Gaciong Z, Alexiewicz JM, Linker-Israeli M, Shulman IA, Pitts TO, Massry SG. Inhibition of immunoglobulin production by parathyroid hormone. Implications in chronic renal failure. Kidney Int. 1991;40:96–106. doi: 10.1038/ki.1991.186. [DOI] [PubMed] [Google Scholar]