Abstract
Most studies investigating the induction of oral tolerance (OT) use purified proteins such as ovalbumin (OVA), bovine serum albumin (BSA) and beta-lactoglobulin (β-LG). Little information is available regarding the induction of OT to a protein mixture, e.g. cow's milk. In this study we compared the regulatory mechanisms induced after the oral administration of a whey protein concentrate (WP) derived from cow's milk following immunization with two different adjuvants, complete Freund's adjuvant (CFA) and alum. OVA was used as a control antigen. Animals were given a single feed of these proteins at an equivalent dose of 1 mg/g body weight before they were immunized seven days later with the antigen in Freund's adjuvant or alum. Delayed type hypersensitivity (DTH) responses were suppressed by both a feed of WP and OVA after immunization with CFA. However, only OVA feeding suppressed antigen specific IgG responses. In an attempt to investigate whether WP would tolerize the more susceptible IgE responses, alum immunization replaced CFA as the adjuvant used for systemic immunizations. WP, after a single feed, significantly primed for DTH and IgE responses indicating oral sensitization to WP. In contrast, OVA suppressed DTH, IgE and IgG responses. Antigen specific proliferation of mononuclear cells was suppressed in mice fed OVA, but primed in those fed with WP. In addition cells taken from sensitized mice fed WP up-regulated levels of specific interleukin (IL) -4, -10 and -12 in vitro whereas these cytokines were suppressed in cultures from tolerant WP fed mice. Global suppression was obtained in cultures from tolerant OVA fed mice. TGF-β was not detected in draining PLN cell cultures of either tolerant or sensitized mice. These data suggest that a whey protein mixture induces divergent responses following immunization with either CFA or alum despite being fed at an identical dose. We suggest that that the choice of the adjuvant may determine the immunoregulatory outcome and this is also reflected by the systemic cytokine profile.
Keywords: Ovalbumin, whey protein, oral tolerance, sensitization, adjuvant, cytokine
Introduction
The induction of antigen specific hyporesponsiveness to soluble proteins by the mucosal route, oral tolerance (OT) is a well-documented phenomenon and normally both cellular and humoral responses are suppressed [1]. Antigens used in these studies can be both dietary in origin such as ovalbumin (OVA), beta-lactoglobulin (β-LG) and gliadin or auto-antigens as used in experimental animal models of autoimmune diseases such as experimental allergic encephalomyelitis (EAE) or collagen induced arthritis (CIA) [1,2]. A common feature amongst these antigens is that they are invariably soluble and thymus dependent so that any immunological response to them is likely to require the involvement of T cells. In contrast, thymus independent, particulate proteins such as lipopolysaccharide (LPS) may have a direct effect on B-lymphocytes and therefore are more likely to sensitize/prime via the oral route [3].
The quality and longevity of this immunological tolerance has been shown to be dependent on the time, frequency, dose of the antigen and the age of the animal [4]. OT is thought to be regulated by three mechanisms, all of which are affected by the feeding regimen. Multiple low doses of fed antigen seem to be more likely to induce active suppression [5] whereas a single high antigen dose feed (typically 1 mg/g) may result in clonal deletion and/or anergy [6,7]. Although there is an ongoing debate as to which tolerogenic mechanism is prevalent at a given dose, it is widely accepted that they are not mutually exclusive and could coexist [1, 2, 8].
Though specific antibody responses are considered less susceptible to OT induction, IgE and DTH responses seem to be equally sensitive to suppression [9].
If OT does indeed provide a mechanism to discriminate between harmless antigens and potential pathogens, its failure or breakdown might underlie some food allergies and/or inflammatory diseases. Food allergies are believed to affect 5–6% of the population at some time during their lives. The prevalence of food allergic diseases in infancy is estimated to be 2–4% with cow's milk being the most common allergen [10,11]. Since the nature of the antigen is an important factor in OT induction it is interesting to note that many experimental studies customarily feed single proteins rather than antigen mixtures such as milk. However, such mixtures are more likely to be encountered in the gut lumen [1]. As a result several hypoallergenic infant milk formulas have been proposed as therapeutic aids to alleviate symptoms in cow's milk allergic patients, based on the notion that they contain proteins/peptides of low molecular weight, which are responsible for this therapeutic effect [12].
Numerous studies, whilst using different milk proteins, such as whey and caseins, have shown suppression of IgG responses after feeding [13–15]. The doses fed, though in excess of 1 mg/g body weight, confirmed a possible difference in susceptibility to suppression of cellular and antibody responses during OT induction. Few studies have measured IgE responses to milk proteins, though other studies using OVA have shown that IgE responses are readily suppressed [1, 9, 16].
Oral tolerance (OT) induced by a single feed of ‘high dose’ soluble antigen has been attributed to clonal anergy and has been described in experimental models of autoimmune diseases [2, 6, 17–19]. This tolerant state is often characterized by the absence of antigen specific T cell proliferation, IL-2 production and increased expression of IL-2 receptors, which are reversed by exogenous administration of recombinant IL-2 [5, 6, 19]. Studies using multiple low antigen feeds to induce tolerance have suggested a role for the immunosuppressive cytokine, TGF-β[2,20–22]. This type of tolerance induction suppresses both TH1 and TH2 like immunity [23,24] as opposed to the TH1 subset which seems to be associated with clonal anergy [5,17].
In the present study we investigated the ability of a cow's milk derived whey protein preparation (WP) to induce suppressive immunoregulatory responses in vivo and in vitro in naïve animals, using the adjuvants alum and complete Freund's adjuvant to analyse specific DTH, IgG, IgE in vivo and cytokine responses in vitro. We tested the hypothesis that feeding WP antigen mixture would induce immunosuppression both in vivo and in vitro. The results presented here suggest that feeding WP at 1 mg/g body weight lead to opposing regulatory responses and was dependent on the choice of adjuvant.
Materials and Methods
Animals
Female BALB/c mice (H-2d/d) (6–8 weeks old) were purchased from Harlan Olac and maintained on an egg and milk free diet (Labsure CRM, Labsure Ltd, Maurea, UK) with water ad libitum. The study was approved by the Research Ethics Committee of ICH according to Animal Welfare Legislation.
Antigens
Ovalbumin (OVA, Fraction V N°.5503) was purchased from Sigma Chemical Co. Lacprodan 80 (WP; Danmark Protein AS, MD Foods, Skanderborgvej, Denmark); a whey protein concentrate was a gift from Nestec Ltd, Lausanne, Switzerland and was produced by ultrafiltration of acid whey. It comprised >80%β-LG with α-lactoglobulin, lactoferrin, serum albumin and immunoglobulins. Both antigens were dissolved in PBS to ensure an equivalent protein dose of 1 mg/g body weight at a single feed.
Feeding and systemic immunization
Animals were fed by gavage using a 20-gauge, 30 mm olive tipped cannula (Fine Science Tools Inc., North Vancouver, B.C., Canada). Each mouse received a single feed of antigen at 1 mg/g body weight dissolved in 0·25 ml PBS, with PBS alone for the control mice.
After 7 days the animals were parenterally immunized in the left hind footpad with 100 µg of WP or OVA emulsified in 50 µl of complete Freund's adjuvant (CFA) (Bacto H37Ra, Difco Ltd, West Moseley, Surrey, UK). This stage of the protocol was designated as Day 0 and subsequent times were described in relation to this time-point.
Preparation of alum precipitate for systemic immunization
During selected experiments, animals were immunized with antigens adsorbed to 1 mg alum precipitate. Antigens were dissolved in sterile PBS (10 µg/ml). 4·5 ml of 1 m NaHCO3 were added to every 10 ml of antigen solution and the mixtures vortexed thoroughly. The resulting solution was then centrifuged at 3000 g for 10 min. After discarding the supernatant, the remaining precipitate was washed three times in sterile PBS and stored in 0·01% w/v thiomersal/PBS at 4°C.
Measurement of systemic delayed type hypersensitivity (DTH)
Three weeks after immunization, the right hind footpad was challenged intradermally with 100 µg of heat-aggregated (65°C/30 min) OVA or native WP in 50 µl of sterile PBS. Footpad thickness was measured using a dial gauge microcaliper (Mituoyo, no. 7301, MFG Co., Tokyo, Japan) both before and 24 h after antigen challenge. The difference between these measurements was considered an index of footpad swelling and severity of DTH.
Assessment of specific anti-OVA and anti-WP IgG by direct ELISA
Serum collection by cardiac puncture was performed under nonrecovery anaesthesia.
Briefly, flat-bottomed 96 well Linbro ELISA plates (Flow ICN Biochemicals Ltd, Rickmansworth, Herts. UK) were coated with OVA or WP (100 µg/ml) and incubated at 4°C overnight. After washing, free sites were blocked with 1% NGS in PBS/Tween (200 µl/well) for 1 h at 37°C. After washing, test sera and standard affinity purified mouse anti-OVA reagents were added to the plates before incubation for 90 min at 37°C. The absorbance of a 1/1000 serum dilution was used as an arbitrary scale for anti-WP IgG determination. After washing, alkaline phosphatase-conjugated goat anti-mouse IgG antibody at a dilution of 1/3000 was added to the plates. After 90 min incubation at 37°C p-nitrophenyl phosphate (pNPP) was added and the colour reaction stopped by the addition of 25 µl of 3 N sodium hydroxide. Absorbance values were read at 405 nm with an MRX Microplate ELISA reader using Revelation™ software (Dynatech Laboratories, Billingshurst, West Sussex, UK).
Detection of total IgE by sandwich ELISA
Flat bottomed Immulon II ELISA plates (Dynatech, Virginia, USA) were coated with 100 µl of 1·0 µg/ml purified rat anti-mouse antibody overnight at 4°C. After blocking, test serum samples and purified mouse IgE isotype as a standard were incubated overnight at 4°C. Biotinylated rat anti-mouse IgE antibody (2 µg/ml) was then added for 2 h at 37°C followed by the addition of a 1/1000 dilution of streptavidin-HRPO for 1 h. Colour was developed with OPD substrate and the reaction was stopped by the addition of 25 µl 4 N H2SO4 before reading the absorbance at 490 nm.
Lymphocyte proliferation assay
2 × 105 mononuclear cells from draining lymph nodes were cultured in 96 well round bottomed tissue culture plates (Costar, Cambridge, MA, USA) with 4 × 105 irradiated (2500rads) syngeneic splenocytes from naïve BALB/c mice acting as antigen presenting cells (APC). In addition, aliquots (1 mg/ml) of specific and control antigen were also cultured with 1 µg/ml of concanavalin A (Con A) used as a positive control. The cells were then incubated at 37°C for 96 h with 1 µCi of 3H TdR added to all the wells for the final 16 h. Cells were harvested with a Skatron cell harvester onto glass fibre mats (Wallac) before being counted in a Wallac 1450 micro betacounter. Results were expressed as cpm ± 1 SD from quadruple cultures.
Specific Cytokine ELISA
Mononuclear cells (1 × 107) in 1 ml culture medium were cultured in 24 flat-bottomed well culture plates with irradiated splenocytes acting as APC (2 × 107). After centrifugation cytokines were measured in culture using a two-site sandwich ELISA after incubation for 24, 48, 72 and 168 h. Flat-bottomed Immulon IV ELISA plates (Dynatech, Virginia, USA) were coated with 100 µl of purified rat anti-mouse cytokine antibody in coating buffer overnight at 4°C. After blocking with 200 µl 10% FCS in PBS, neat supernatant samples and recombinant mouse cytokine as standards were incubated in the plates overnight at 4°C. Biotinylated rat anti-mouse cytokine antibody was added for 3 h at 37°C. This was followed by amplification with 1/1000 dilution of streptavidin-alkaline phosphatase for 1 h at 37°C. Colour was developed with substrate solution containing 1 mg/ml p-nitrophenyl phosphate (pNPP; 100 µl/well). The reaction was stopped with 25 µl 3 N NaOH and absorbance read at 405 nm.
Antigen specific TGF-β was measured with a TGF-β1 Emax™ ELISA kit (Promega) according to the manufacturer's instructions. All the results were of bioactive, rather than total, TGF-β and therefore samples were not acidified. Results were corrected for nonspecific TGF-β and culture medium containing serum were used as controls.
Antibodies and recombinant cytokine standards
Polyclonal goat anti-mouse IgG conjugated to alkaline phosphatase (Sigma) was used for specific IgG ELISA. For total IgE measurements a purified rat anti-mouse IgE (clone LO-ME-3; Serotec) was used as capture antibody with a biotinylated rat anti-mouse IgE (clone LO-ME-2; Serotec) used for detection. Purified mouse IgE, κ monoclonal isotype (Pharmingen) was used to construct the standard curve.
The following pairs of rat anti-mouse antibodies were used for cytokine ELISA and were purchased from Pharmingen (San Diego, CA, USA), unless otherwise stated, and lists capture and detection antibodies, respectively;
IFN-γ : anti-IFN-γ purified clone RA-6A2 and biotinylated clone XMG1·2;
IL-4: anti-IL-4 purified clone 11B11 and biotinylated clone BVD6–24G2;
IL-10: anti-IL-10 purified clone JES5–2A5 and biotinylated clone SXC-1;
IL-12: anti-IL-12 purified clone C17·15 (Genzyme) and hamster anti-mouse IL-12: (p35) Red T (Genzyme).
The following were used as recombinant standards (Pharmingen) and are listed with their specific activities. Murine IFN-γ (1 × 107 U/mg), murine IL-4 (1 × 107 U/mg), murine IL-10 (2 × 107 U/mg) and murine IL-12 (p70) (5 × 105 U/mg) (Genzyme).
Statistical analysis
All systemic DTH and antibody responses were expressed as means ± 1SD and all groups were compared with unpaired two-tailed Student-t-tests. Cytokine concentrations were expressed as pg/ml. All statistical comparisons were made between groups fed antigens and control groups fed PBS unless otherwise stated.
Results
Both OVA and WP feeding induces suppression of delayed hypersensitivity (DTH)
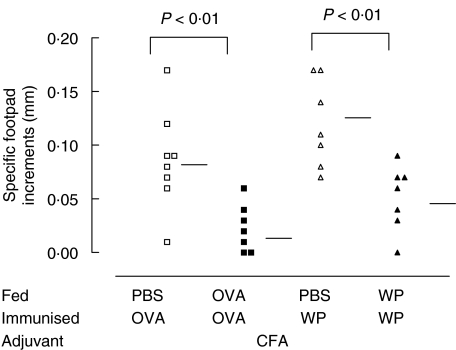
Specific DTH responses to OVA and WP were measured following systemic immunization. Mean DTH responses were significantly reduced in OVA fed mice when compared with PBS fed controls (0·09 ± 0·05 versus 0·03 ± 0·02 mm P < 0·01; Fig. 1). Similarly, feeding WP to mice also suppressed footpad responses compared with their controls (0·12 ± 0·05 versus 0·05 ± 0·03 mm P < 0·01; Fig. 1). The levels of suppression elicited with both antigens were of similar magnitude.
Fig. 1.
Delayed type hypersensitivity (DTH) responses after feeding OVA and WP. Mice (7 per group) were fed OVA (▪) or WP (▴) at an equivalent antigen dose of 1 mg/g body weight and immunized with the corresponding antigen (100 µg) in CFA. Control mice were fed PBS (□, ▵) before immunization. Twenty days later the mice were challenged with 100 µg of OVA or WP in the footpad and the swelling measured 24 h later. Horizontal bars represent group means.
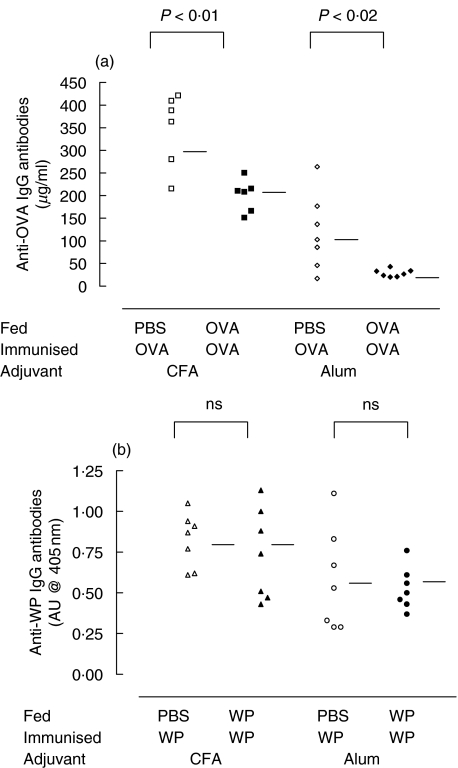
Serum from OVA fed mice contained significantly lower levels of anti-OVA IgG antibodies when compared with PBS fed controls (347·0 ± 70·9 versus 201·0 ± 40·9 µg/ml P < 0·01; Fig. 2). In contrast there was no reduction of anti-WP IgG antibody levels in WP fed mice (0·82 ± 0·13 versus 0·74 ± 0·23; Fig. 2).
Fig. 2.
Specific IgG levels were not suppressed in WP fed mice. Mice (7 per group) were immunized with OVA in CFA (▪,□), OVA in alum (r,e), WP in CFA (▴,▵) or WP in alum (•○) after prior feeding with the corresponding antigen (filled symbols) or PBS (open symbols). Twenty-four hours after challenge with OVA or WP blood was collected from the mice by terminal cardiac puncture. Specific IgG levels were measured by ELISA using alkaline phosphatase conjugated goat anti-mouse IgG antibody. (a) Anti-OVA IgG antibodies measured in µg/ml. (b) Specific WP IgG antibodies measured in absorbance units (AU) based on absorbance readings at 405 nm. Horizontal bars represent group means.
Suppression of DTH responses after a WP feed is adjuvant dependent
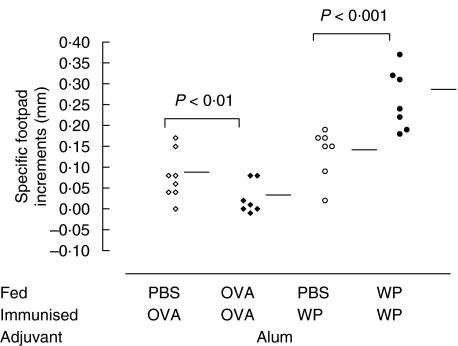
For the analysis of IgE responses, groups of animals were immunized with alum, maintaining all other experimental conditions identical to the CFA groups. Again OVA significantly reduced footpad swelling compared with PBS controls (0·08 ± 0·05 versus 0·01 ± 0·03 mm P < 0·01; Fig. 3). In contrast, WP feeding significantly increased DTH responses compared to the controls (0·13 ± 0·06 versus 0·26 ± 0·06 mm; P < 0·001; Fig. 3). This priming effect after alum adjuvant use is in contrast to the suppression observed with mice fed WP and immunized with WP in CFA (WP/WP/CFA).
Fig. 3.
WP feeding fails to suppress DTH responses in mice immunized with WP in alum. OVA and WP adsorbed to 1 mg alum were used to systemically immunize mice instead of CFA. Mice (7 per group) were fed OVA (⋄) or WP (•) at an equivalent antigen dose of 1 mg/g body weight and immunized with the corresponding antigen (100 µg) in alum. Control mice were fed PBS (◊,○) before immunization. Twenty days later the mice were challenged with 100 µg of OVA or WP in the footpad and the swelling measured 24 h later. Horizontal bars represent group means.
Specific IgG responses of OVA fed mice were significantly reduced when compared with controls (118·6 ± 92·6 versus 29·9 ± 8·5 µg/ml P < 0·02; Fig. 2) although the concentrations of anti-OVA IgG antibodies were generally lower than those seen with the CFA immunization. There was no significant difference in the absorbance units observed for the WP and PBS fed groups (0·57 ± 0·31 versus 0·53 ± 0·13, respectively; Fig. 2).
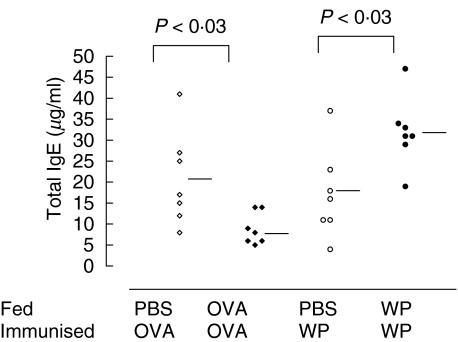
Alum immunization induced high levels of antigen specific IgE concentrations above naturally occurring low IgE levels in all of the groups treated. OVA fed mice had significantly lower total IgE concentrations than PBS fed controls (20·9 ± 13·3 versus 8·9 ± 3·5 µg/ml P < 0·03; Fig. 4). WP feeding, in contrast, increased the total IgE antibody levels compared to their respective controls (17·3 ± 12·0 versus 31·8 ± 10·9 µg/ml P < 0·03; Fig. 4).
Fig. 4.
Total IgE antibody levels were up-regulated following WP feeding. Total IgE levels were measured in sera by ELISA. Twenty-four hours after challenge with OVA (⋄,◊) or WP (•,○) blood was collected from the mice by terminal cardiac puncture. Total IgE levels in the resulting sera were measured using biotinylated rat anti-mouse IgE antibody. Absorbance was measured at 450 nm after development with streptavidin HRPO and OPD. Horizontal bars represent group means.
OVA, but not WP, feeding suppresses antigen-specific proliferation of draining lymph node mononuclear cells
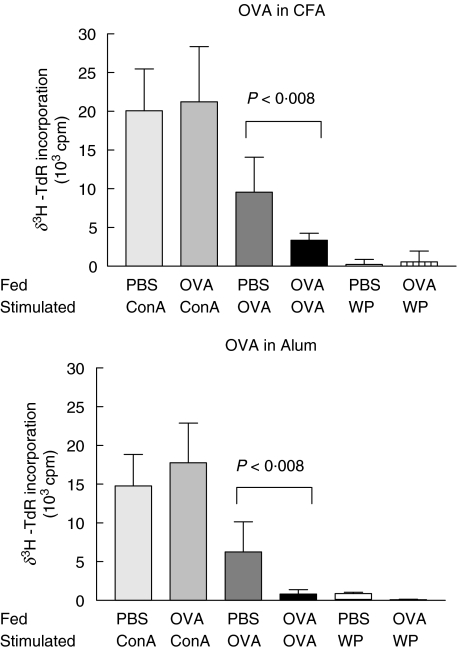
We then investigated whether the lack of suppression or priming of immune responses could also be identified at a cellular level by cell cultures in vitro. Significantly lower levels of 3H thymidine (3HTdR) incorporation were observed with PLN cells from OVA fed mice when compared with cultures from PBS fed controls (9550 ± 4540 versus 3367 ± 903 cpm and 6250 ± 3880 versus 814 ± 567 cpm for CFA and alum, respectively; P < 0·008 Fig. 5). The level of 3H TdR incorporation was approx. 50% lower than that of controls and was comparable to unfed mice immunized with OVA in CFA, or alum. This suppression was antigen specific. WP had no effect on cell cultures from either OVA or PBS fed mice or vice versa. The overall proliferative activity of these cells remained unaffected and there was no difference in their mitogenic activity compared with those from PBS fed mice after Con A stimulation.
Fig. 5.
OVA specific proliferation of PLN mononuclear cells in vitro. Mice (3–5 per group) were fed and immunized OVA. Seven days after immunization PLN were excised and cells (2 × 105 cells) were cultured with irradiated splenocytes (4 × 105cells) from syngeneic naïve mice in 96 well plates. Each well was seeded with 200 µl aliquots of cell culture and stimulated with either OVA (1 mg/ml) or WP (1 mg/ml). Con A (1 µg/ml) was added as a positive control with cells cultured alone for background controls. The cells were cultured for 96 h. The results (stimulated cpm-background cpm) are expressed as means ± 1SD from quadruple cultures and are representative of at least three different experiments.
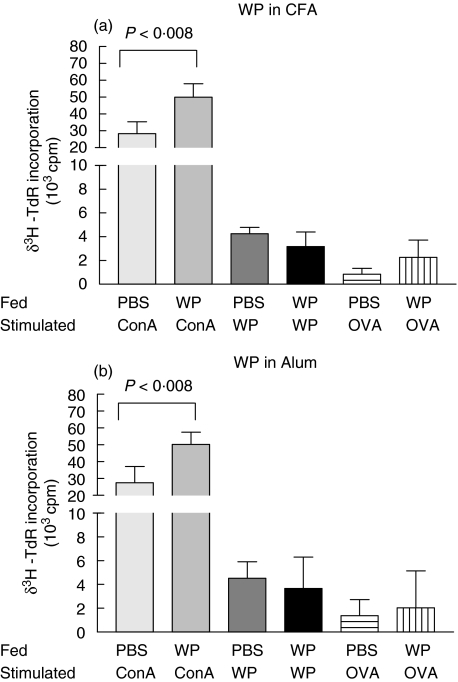
Mononuclear PLN cell cultures from WP fed mice showed no WP specific suppression when compared to PBS fed controls and this was true for both immunization schedules (4523 ± 1389 versus 3657 ± 2655 cpm and 4260 ± 527 versus 3176 ± 121 cpm for CFA and alum, respectively; P < 0·008 Fig. 6). In addition, mitogenic proliferation was markedly increased in cells from WP fed mice since the level of 3H thymidine incorporation was approximately twice that induced in cells from PBS fed mice (28270 ± 7100 versus 49900 ± 8098 cpm and 27494 ± 9567 versus 50245 ± 7180 cpm for CFA and alum, respectively; P < 0·008 Fig. 6).
Fig. 6.
WP specific proliferation of PLN mononuclear cells in vitro. Mice (3–5 per group) were fed and immunized WP. Seven days after immunization PLN were excised and cells (2 × 105 cells) were cultured with irradiated splenocytes (4 × 105cells) stimulated with WP (1 mg/ml). The cells were cultured for 96 h. The results (stimulated cpm-background cpm) are expressed as means ± 1SD from quadruple cultures and are representative of at least three different experiments.
Only WP suppresses specific immune responses to β-lactoglobulin
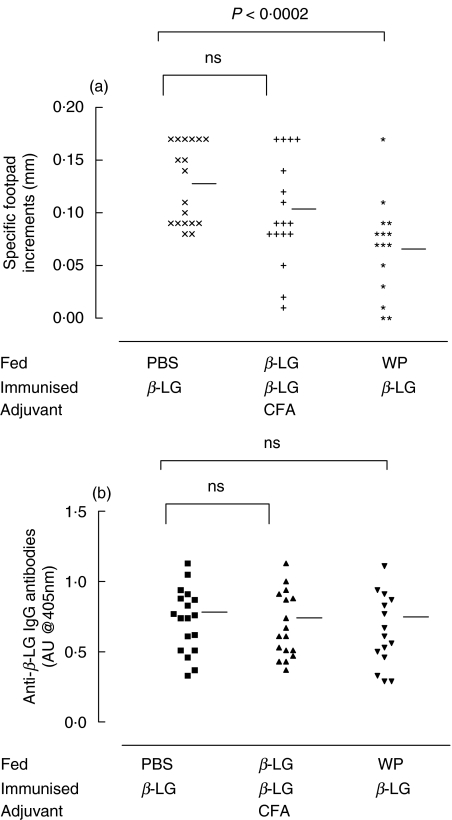
The major constituent of WP, β-LG, was fed at 1 mg/g body weight in order to compare its ability to suppress DTH and humoral responses with the effect of the whole antigen mixture. After challenge, specific antibodies and paw swelling to β-LG were measured in mice fed with both β-LG and WP. Surprisingly a single feed of β-LG was unable suppress any immune responses when compared with PBS fed controls (0·10 ± 0·05 versus 0·13 ± 0·04 mm P = ns Fig. 7). However, as with specific WP responses, a single feed of the antigen mixture was able to reduce significantly the paw swelling of mice challenged with β-LG (0·07 ± 0·04 versus 0·13 ± 0·04 mm P < 0·0002 Fig. 7). This suppression did not extend to antibody responses as there was no significant difference between this group and PBS fed controls.
Fig. 7.
Delayed type hypersensitivity (DTH) responses after feeding β-LG and WP. Mice (7 per group) were fed β-LG (+) or WP (*) at an equivalent antigen dose of 1 mg/g body weight and immunized with 100 µg β-LG in CFA. Control mice were fed PBS (×) before immunization. Twenty days later the mice were challenged with 100 µg β-LG. Blood was collected from the mice fed PBS (▪), β-LG (▴) or WP (▾) by terminal cardiac puncture. Specific anti-β-LG IgG levels were measured by ELISA using alkaline phosphatase conjugated goat anti-mouse IgG antibody and were measured in absorbance units (AU) based on absorbance readings at 405 nm. Horizontal bars represent group means.
Differences in systemic cytokine secretion patterns following whey protein feeding
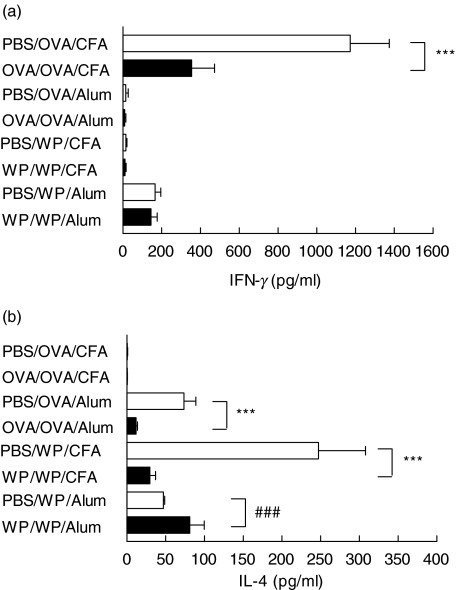
As an indicator of the systemic response to oral tolerance induction, and sensitization, cytokine patterns were analysed in mononuclear cell cultures derived from draining popliteal lymph nodes (PLN) seven days after immunization. Mononuclear cell cultures from tolerant mice fed OVA showed reduced levels of antigen specific IFN-γ, IL-4, IL-10 (Fig. 8; P < 0·001) and IL-12 (data not shown) which all peaked after 7 days in culture. Interestingly, the secretion of some cytokines was dependent on the adjuvant used. So IFN-γ was only detected after CFA immunization whereas IL-4 and IL-12 were only present with alum. Only IL-10 was detectable in cultures resulting from immunizations with both adjuvants. Cells isolated from OVA fed mice when cultured with WP or without stimulation did not release any measurable amounts of either cytokine regardless of the immunization schedule suggesting the specificity of the response.
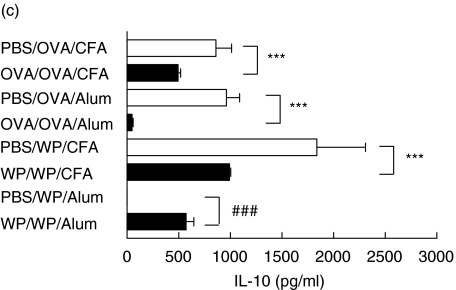
Fig. 8.
WP feeding fails to suppress specific (a) IFN-γ, (b) IL-4 and(c) IL-10 secretion by popliteal lymph node mononuclear cells in vitro after immunization with alum. Mice (n = 5/group) were fed and immunized with OVA. Seven days after immunization PLN were excised and cells (1 × 106 cells) were cultured with irradiated splenocytes (2 × 106cells) from syngeneic naïve mice in 24 well plates. Each well was seeded with 1 ml of cell culture and stimulated with either OVA (1 mg/ml) or WP (1 mg/ml) with cells cultured alone for background controls. The cells were cultured for 24–168 h and the supernatants were measured by sandwich ELISA. Suppression is indicated by ***P < 0·001 whereas priming is denoted by ###P < 0·001. The results are representative of at least three experiments.
The cytokine patterns for cultures from WP fed mice were more complex and depended on whether the mice were tolerant or sensitized and not only on the immunization schedule. WP feeding, unlike OVA, either reduced or increased specific cytokines. Interleukin-4, 10 and 12 were reduced within cultures from tolerant mice immunized with CFA while no cytokine suppression was evident in cultures from orally sensitized alum immunized mice. In contrast these cells from these sensitized mice exhibited increased levels of specific IL-4 and IL-10. Cultures from tolerant mice fed WP did not secrete any detectable levels of specific IL-12 or IFN-γ.
There were no detectable levels of bioactive TGF-β in supernatants from any cultures once the nonspecific TGF-β levels within the culture media was accounted for (data not shown).
Discussion
In this study WP, a commonly encountered milk antigen, was assessed for its ability to induce oral tolerance under different immunization schedules. We demonstrate here that the induction of oral tolerance to WP was similar to the control tolerogen, OVA, but only after immunization with CFA as the adjuvant. As expected, OVA feeding prior to systemic immunization with OVA in CFA reduced the levels of both cellular and humoral immunity compared with PBS controls [1, 4, 25–27]. A suppression of DTH responses was observed after a feed of WP but overall IgG antibody levels were indistinguishable from controls. Earlier studies feeding high OVA doses (>1 mg/g BW) to BALB/c mice suggested the induction of clonal anergy and suppression of systemic immunity, which is in general agreement with the findings presented here. Suppression of specific OVA antibodies (including IgG1 and IgG2a subclasses) and DTH responses has also been reported [1, 4, 8, 25, 27]. The inability to suppress antibody responses with WP feeding, as reported here, appears to be in contrast with other studies using similar milk proteins [13–15, 28–30]. Various mouse strains such as CH3/HeJ, BALB/c (H-2d) and C57BL/6 (H-2b) fed αs1-casein (a milk derived protein) were susceptible to tolerance induction though effects on antibody responses were variable [13]. One study described the suppression of antibody responses to αs1-casein after a high antigen dose feed. However, the mice were fed 200 mg of antigen over a 14-day period, more than 100 times greater than the dose used in our study [28].
Given WP's inability to suppress specific antibody responses, the antigen mixture was still a more effective oral tolerogen than its major constituent protein β-LG, which was unable to suppress specific DTH responses. This was in contrast to the reports of other studies using β-LG, incorporated into the diets of BALB/c mice and administered over a longer period both of which demonstrated suppression of specific antibody responses [14,30]. Again, the administered antigen dose was five to 20 times greater than the dose employed in our study. Indeed, feeding the mice over a period of 75 days at this higher dose induced detectable IgG levels in the absence of immunization, an observation not normally encountered after single or multiple feeds [14]. Pecquet and colleagues reported that feeding WP (3 mg/g body weight) and β-LG (5 mg/g body weight) to BALB/c mice suppressed both specific DTH and IgE responses while leaving IgA responses intact [30]. The same group reported suppression of specific IgE and mast cell degranulation in a rat model, again using antigen doses that exceeded 1 mg/g body weight [29]. However, we observed that in mice β-LG, given as a single feed at 1 mg/g/body weight, was unable to suppress any specific responses whereas WP suppressed specific DTH, but not antibody responses, to β-LG. This is an interesting finding since the ability of WP to induce suppression is consistent for both the antigen mixture and its single major constituent. The inability of β-LG to induce suppression under these conditions argues against the notion that OVA was a more potent ‘tolerogen’, because it was a single protein and not an antigen mixture.
In order to focus particularly on the regulation of total IgE alum was used as an adjuvant in parallel experiments. OVA feeding suppressed not only specific DTH and IgG levels but also total IgE levels within the same animal. Our study supports a previous report of specific IgE suppression with antigen feeding [9] and yields similar results as reported after intranasal administration of OVA in rats [31,32]. An unexpected observation was the substantial priming for DTH responses seen after feeding WP and immunization with alum, which was a complete ‘reversal’ of the suppression induced in mice fed WP and immunization with CFA (WP/WP/CFA). The priming of total IgE levels mirrored the enhanced DTH responses. These parameters are often considered to be equally sensitive to tolerance induction [1] and it seems from our study that they may also be equally susceptible to priming. The observation that WP, at an identical antigen dose, can elicit two contrasting responses depending on the use of the adjuvant is of great interest. Further studies would be needed to establish whether the tolerogenic dose of an antigen is also dependent on the adjuvant used.
Several studies investigating tolerance induction by feeding have compared in vivo responses and clinical scores with the in vitro proliferation of mononuclear cells taken from the spleen and draining popliteal lymph nodes [2, 17, 19, 21]. Transfer of splenocytes from orally tolerized mice [26,33] and serum retrieved one hour after a feed of OVA at 1 mg/g body weight [34–38] were able to induce suppression of DTH and humoral responses when injected into naïve recipient animals. In our study, cells isolated from tolerant OVA, but not WP, fed mice showed a significantly lower rate of proliferation following antigen specific stimulation. Stimulation of control cultures by Con A also revealed that cells from WP fed mice, both tolerant and sensitized, were more susceptible to nonspecific mitogenic stimulation than cells isolated from OVA fed mice. Based on these data, one could speculate that one of WP's constituents could be responsible for the nonspecific immunomodulatory properties. There is some evidence that lactoferrin could account for this observation. Lactoferrin has been implicated in milk's ability to boost the innate host defence mechanism in response to infections [39,40]. Furthermore the oral administration of lactoferrin may restore antibody responses following lymphocyte depletion with cyclophosphamide [41,42] enhance DTH responses [43,44] and reduce inflammation [45,46]. For these reasons it may be tempting to speculate that the nonspecific immunomodulatory actions observed with WP could be due to lactoferrin although this has not been proven. However it has to be emphasized that the demonstration of an antigen – specific cytokine pattern in our studies does suggest that the effects seen after oral WP administration are antigen specific. In addition, tolerant mice fed WP had both suppressed DTH responses and antigen-specific cytokine levels despite having enhanced mitogenic stimulation with Con A.
We compared the cytokine profile of mononuclear cells isolated from popliteal nodes of mice fed these two different antigens. A single feed of OVA (1 mg/g body weight) was able to suppress the secretion of IFN-γ, IL-4, IL-10 and IL-12 by mononuclear cells isolated from PLN. Although IFN-γ was only detected in cultures following CFA immunization and IL-4 was present in cultures where alum was the adjuvant, a consistent pattern of suppression was observed. Our findings are in agreement with other studies where high dose antigen feeding was shown to reduce the level of cytokine secretion [47,48]. This suggests that feeding OVA induces immunosuppression of in vivo and in vitro responses. However, we failed to detect TGF-β in cultures derived from popliteal lymph nodes, a cytokine secreted during tolerance mediated by active suppression and triggered by low dose antigen feeding. Immune suppression in the absence of TGF-β is considered to be due to anergy [17,19]. Although we were able to show the suppression of IL-4 and IL-10 alongside TH1 cytokines with ‘high dose’ antigen feeding, the failure to detect this immunosuppressive cytokine could be in keeping with a possible anergic mechanism.
Cultures from tolerant mice fed WP and immunized with CFA showed that both IL-4 and IL-10 were suppressed. This secretion pattern, including the absence of TGF-β closely resembled that secreted by cells isolated from tolerant OVA fed mice and substantiated the tolerogenic DTH response observed in vivo. However, a complete reversal of this immunosuppression was obtained with this antigen mixture after immunization with alum since an up-regulation of specific IL-4 and IL-10 secretion was evident confirming the priming responses in vivo. Furthermore because WP immunization increased IL-4 secretion, an archetype TH2 cytokine, we believe it supports the priming observed for IgE levels in vivo.
In summary, we have shown that immunoregulatory effects after mucosal antigen exposure are affected by the choice of adjuvant. At an identical dose, WP feeding induces suppression or priming of immune responses, depending on the adjuvant used. Tolerance induction to WP was observed with CFA and a priming response was observed with alum. This was reflected both in specific immune responses in vivo and by secretory patterns of antigen specific cytokines in vitro. This adjuvant related pattern of regulatory responses has to be considered when comparing results from different studies since the adjuvant of choice may influence the results.
Acknowledgments
The authors would like to acknowledge the financial support of the Child Health Research Appeal Trust (CHRAT) of Great Ormond Street Hospital for A.O. Afuwape and Nestec S.A. for the gift of the whey protein. Research at the Institute of Child Health and the Great Ormond Street Hospital for Children National Health Service Trust benefits from research and development funding received from the National Health Service Executive.
References
- 1.Strobel S, Mowat AM. Immune responses to dietary antigens: oral tolerance. Immunol Today. 1998;19:173–81. doi: 10.1016/s0167-5699(97)01239-5. [DOI] [PubMed] [Google Scholar]
- 2.Weiner HL, Friedman A, Miller A, et al. Oral tolerance. immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809–37. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- 3.Mowat AM, Viney JL. The anatomical basis of intestinal immunity. Immunol Rev. 1997;156:145–66. doi: 10.1111/j.1600-065x.1997.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 4.Peng HJ, Turner MW, Strobel S. The kinetics of oral hyposensitization to a protein antigen are determined by immune status and the timing, dose and frequency of antigen administration. Immunology. 1989;67:425–30. [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman A, Weiner HL. Induction of anergy or active suppression following oral tolerance is determined by antigen dosage. Proc Natl Acad Sci USA. 1994;91:6688–92. doi: 10.1073/pnas.91.14.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitacre CC, Gienapp IE, Orosz CG, Bitar DM. Oral tolerance in experimental autoimmune encephalomyelitis. III. Evidence for clonal anergy. J Immunol. 1991;147:2155–63. [PubMed] [Google Scholar]
- 7.Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995;376:177–80. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- 8.Garside P, Steel M, Liew FY, Mowat AM. CD4+ but not CD8+ T cells are required for the induction of oral tolerance. Int Immunol. 1995;7:501–4. doi: 10.1093/intimm/7.3.501. [DOI] [PubMed] [Google Scholar]
- 9.Ngan J, Kind LS. Suppressor T cells for IgE and IgG in Peyer's patches of mice made tolerant by the oral administration of ovalbumin. J Immunol. 1978;120:861–5. [PubMed] [Google Scholar]
- 10.Strobel S. Epidemiology of food sensitivity in childhood- with special reference to cow's milk allergy in infancy. In: Burr M, editor. Epidemiology of Atopic Diseases. 31. London: Karger; 1993. p. 119. [PubMed] [Google Scholar]
- 11.Host A, Halken S. Epidemiology and prevention of cow's milk allergy. Allergy. 1998;53:111–31. doi: 10.1111/j.1398-9995.1998.tb04978.x. [DOI] [PubMed] [Google Scholar]
- 12.Gortler I, Urbanek R, Forster J. Characterization of antigens and allergens in hypo-allergenic infant formulae. Eur J Pediatr. 1995;154:289–94. doi: 10.1007/BF01957364. [DOI] [PubMed] [Google Scholar]
- 13.Kim SM, Enomoto A, Hachimura S, Yamauchi K, Kaminogawa S. Serum antibody response elicited by a casein diet is directed to only limited determinants of alpha s1-casein. Int Arch Allergy Immunol. 1993;101:260–5. doi: 10.1159/000236455. [DOI] [PubMed] [Google Scholar]
- 14.Enomoto A, Konishi M, Hachimura S, Kaminogawa S. Milk whey protein fed as a constituent of the diet induced both oral tolerance and a systemic humoral response, while heat-denatured whey protein induced only oral tolerance. Clin Immunol Immunopathol. 1993;66:136–42. doi: 10.1006/clin.1993.1017. [DOI] [PubMed] [Google Scholar]
- 15.Hachimura S, Fujikawa Y, Enomoto A, Kim SM, Ametani A, Kaminogawa S. Differential inhibition of T and B cell responses to individual antigenic determinants in orally tolerized mice. International Immunol. 1994;6:1791–7. doi: 10.1093/intimm/6.11.1791. [DOI] [PubMed] [Google Scholar]
- 16.Dahlman-Hoglund A, Dahlgren U, Ahlstedt S, Hanson LA, Telemo E. Bystander suppression of the immune response to human serum albumin in rats fed ovalbumin. Immunology. 1995;86:128–33. [PMC free article] [PubMed] [Google Scholar]
- 17.Melamed D, Friedman A. In vivo tolerization of Th1 lymphocytes following a single feeding with ovalbumin: anergy in the absence of suppression. Eur J Immunol. 1994;24:1974–81. doi: 10.1002/eji.1830240906. [DOI] [PubMed] [Google Scholar]
- 18.Khoury SJ, Hancock WW, Weiner HL. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992;176:1355–64. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melamed D, Friedman A. Direct evidence for anergy in T lymphocytes tolerized by oral administration of ovalbumin. Eur J Immunol. 1993;23:935–42. doi: 10.1002/eji.1830230426. [DOI] [PubMed] [Google Scholar]
- 20.Miller A, Lider O, Weiner HL. Antigen-driven bystander suppression after oral administration of antigens. J Exp Med. 1991;174:791–8. doi: 10.1084/jem.174.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller A, Lider O, Roberts AB, Sporn MB, Weiner HL. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc Natl Acad Sci USA. 1992;89:421–5. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiner HL. Oral tolerance, an active immunologic process mediated by multiple mechanisms. J Clin Invest. 2000;106:935–7. doi: 10.1172/JCI11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Inobe J, Weiner HL. Inductive events in oral tolerance in the TCR transgenic adoptive transfer model. Cell Immunol. 1997;178:62–8. doi: 10.1006/cimm.1997.1119. [DOI] [PubMed] [Google Scholar]
- 25.Furrie E, Turner MW, Strobel S. Failure of SCID mice to generate an oral tolerogen after a feed of ovalbumin: a role for a functioning gut-associated lymphoid system. Immunology. 1994;83:562–7. [PMC free article] [PubMed] [Google Scholar]
- 26.Mowat AM, Strobel S, Drummond HE, Ferguson A. Immunological responses to fed protein antigens in mice. I. Reversal of oral tolerance to ovalbumin by cyclophosphamide. Immunology. 1982;45:105–13. [PMC free article] [PubMed] [Google Scholar]
- 27.Strobel S, Ferguson A. Modulation of intestinal and systemic immune responses to a fed protein antigen, in mice. Gut. 1986;27:829–37. doi: 10.1136/gut.27.7.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirahara K, Hisatsune T, Nishijima K, Kato H, Shiho O, Kaminogawa S. CD4+ T cells anergized by high dose feeding establish oral tolerance to antibody responses when transferred in SCID and nude mice. J Immunol. 1995;154:6238–45. [PubMed] [Google Scholar]
- 29.Fritsche R, Pahud JJ, Pecquet S, Pfeifer A. Induction of systemic immunologic tolerance to beta-lactoglobulin by oral administration of a whey protein hydrolysate. J Allergy Clin Immunol. 1997;100:266–73. doi: 10.1016/s0091-6749(97)70235-5. [DOI] [PubMed] [Google Scholar]
- 30.Pecquet S, Pfeifer A, Gauldie S, Fritsche R. Immunoglobulin E suppression and cytokine modulation in mice orally tolerized to beta-lactoglobulin. Immunology. 1999;96:278–85. doi: 10.1046/j.1365-2567.1999.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMenamin C, Holt PG. The natural immune response to inhaled soluble protein antigens involves major histocompatibility complex (MHC) class I-restricted CD8+ T cell-mediated but MHC class II-restricted CD4+ T cell-dependent immune deviation resulting in selective suppression of immunoglobulin E production. J Exp Med. 1993;178:889–99. doi: 10.1084/jem.178.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific gamma delta T cells. Science. 1994;265:1869–71. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 33.Peng HJ, Turner MW, Strobel S. Failure to induce oral tolerance to protein antigens in neonatal mice can be corrected by transfer of adult spleen cells. Pediatr Res. 1989;26:486–90. doi: 10.1203/00006450-198911000-00025. [DOI] [PubMed] [Google Scholar]
- 34.Strobel S, Mowat AM, Drummond HE, Pickering MG, Ferguson A. Immunological responses to fed protein antigens in mice. II. Oral tolerance for CMI is due to activation of cyclophosphamide-sensitive cells by gut-processed antigen. Immunology. 1983;49:451–6. [PMC free article] [PubMed] [Google Scholar]
- 35.Furrie E, Turner M, Strobel S. The absence of gut flora has no effect on the induction of oral tolerance to ovalbumin. Adv Exp Med Biol. 1995;371B:1239–41. [PubMed] [Google Scholar]
- 36.Peng HJ, Turner MW, Strobel S. The generation of a ‘tolerogen’ after the ingestion of ovalbumin is time-dependent and unrelated to serum levels of immunoreactive antigen. Clin Exp Immunol. 1990;81:510–5. doi: 10.1111/j.1365-2249.1990.tb05365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruce MG, Strobel S, Hanson DG, Ferguson A. Irradiated mice lose the capacity to ‘process’ fed antigen for systemic tolerance of delayed-type hypersensitivity. Clin Exp Immunol. 1987;70:611–8. [PMC free article] [PubMed] [Google Scholar]
- 38.Bruce MG, Ferguson A. Oral tolerance induced by gut-processed antigen. Adv Exp Med Biol. 1987;216A:721–31. doi: 10.1007/978-1-4684-5344-7_84. [DOI] [PubMed] [Google Scholar]
- 39.Ward PP, Uribe-Luna S, Conneely OM. Lactoferrin and host defense. Biochem Cell Biol. 2002;80:95–102. doi: 10.1139/o01-214. [DOI] [PubMed] [Google Scholar]
- 40.Conneely OM. Antiinflammatory activities of lactoferrin. J Am Coll Nutr. 2001;20:3895–975. doi: 10.1080/07315724.2001.10719173. [DOI] [PubMed] [Google Scholar]
- 41.Artym J, Zimecki M, Paprocka M, Kruzel ML. Orally administered lactoferrin restores humoral immune response in immunocompromised mice. Immunol Lett. 2003;89:9–15. doi: 10.1016/s0165-2478(03)00102-0. [DOI] [PubMed] [Google Scholar]
- 42.Artym J, Zimecki M, Kruzel ML. Reconstitution of the cellular immune response by lactoferrin in cyclophosphamide-treated mice is correlated with renewal of T cell compartment. Immunobiology. 2003;207:197–205. doi: 10.1078/0171-2985-00233. [DOI] [PubMed] [Google Scholar]
- 43.Zimecki M, Kruzel ML. Systemic or local co-administration of lactoferrin with sensitizing dose of antigen enhances delayed type hypersensitivity in mice. Immunol Lett. 2000;74:183–8. doi: 10.1016/s0165-2478(00)00260-1. [DOI] [PubMed] [Google Scholar]
- 44.Actor JK, Hwang SA, Olsen M, Zimecki M, Hunter RL, Jr, Kruzel ML. Lactoferrin immunomodulation of DTH response in mice. Int Immunopharmacol. 2002;2:475–86. doi: 10.1016/s1567-5769(01)00189-8. [DOI] [PubMed] [Google Scholar]
- 45.Kruzel ML, Harari Y, Chen CY, Castro GA. Lactoferrin protects gut mucosal integrity during endotoxemia induced by lipopolysaccharide in mice. Inflammation. 2000;24:33–44. doi: 10.1023/a:1006935908960. [DOI] [PubMed] [Google Scholar]
- 46.Wakabayashi H, Takakura N, Teraguchi S, Tamura Y. Lactoferrin feeding augments peritoneal macrophage activities in mice intraperitoneally injected with inactivated Candida albicans. Microbiol Immunol. 2003;47:37–43. doi: 10.1111/j.1348-0421.2003.tb02783.x. [DOI] [PubMed] [Google Scholar]
- 47.Mowat AM, Steel M, Worthey EA, Kewin PJ, Garside P. Inactivation of Th1 and Th2 cells by feeding ovalbumin. Ann N Y Acad Sci. 1996;778:122–32. doi: 10.1111/j.1749-6632.1996.tb21121.x. [DOI] [PubMed] [Google Scholar]
- 48.Garside P, Steel M, Worthey EA, Satoskar A, Alexander J, Bluethmann H, Liew FY, Mowat AM. T helper 2 cells are subject to high dose oral tolerance and are not essential for its induction. J Immunol. 1995;154:5649–55. [PubMed] [Google Scholar]