Abstract
Autoantibodies to thyroid peroxidase (TPO) recognize predominantly conformational epitopes, which are restricted to two distinct determinants, termed immunodominant domain region (IDR) A and B. These dominant determinants reside in the region with structural homology to myeloperoxidase (MPO)-like domain and may extend into the adjacent complement control protein (CCP) domain. We have explored the location of these determinants on the MPO-like domain of the structural model of TPO, by identifying exposed hydrophilic loops that are potential candidates for the autoantigenic sites, generating rabbit antipeptide antisera, and competing with well characterized murine monoclonal antibodies (mabs) specific for these two IDRs. We recently defined the location of IDR-B, and here report our findings on the location of IDR-A and its relationship to IDR-B, defined with a new panel of 15 antipeptide antisera. Moreover, in combination with single amino acid replacements by in vitro mutagenesis, we have defined the limits of the IDR-B region on the TPO model. The combination of antisera to peptides P12 (aa 549–563), P14 (aa 599–617) and P18 (aa 210–225) inhibited the binding of the mab specific for IDR-A (mab 2) by 75. The same combination inhibited the binding of autoantibodies to native TPO from 67 to 94% (mean 81·5%) at autoantibody levels of 5 IU. Fabs prepared from the antipeptide IgG and pooled in this combination were also effective in competition assays, thus defining the epitopes more precisely. IDR-A was found to lie immediately adjacent to IDR-B and thus the two immunodominant epitopes form an extended patch on the surface of TPO. Finally, by single amino acid mutagenesis, we show that IDR-B extends to residue N642, thus further localizing the boundary of this autoantigenic region on the structural model.
Keywords: thyroid peroxidase, autoimmunity, immunodominant epitopes, thyroid peroxidase autoantibodies
Introduction
Autoantibodies to thyroid specific antigens are a classical hallmark of thyroid autoimmune disease. The thyroid proteins to which the humoral response is directed include thyroid peroxidase (TPO), the enzyme responsible for the synthesis of thyroid hormones [1–3]. Autoantibodies to TPO are frequently of high titre and recognize predominantly conformational epitopes on the surface of the molecule [3]. A small proportion of the autoantibodies also recognize linear determinants on TPO, which are believed to arise at the late stage of the inflammatory disease process [3]. The role of autoantibodies to TPO in disease pathogenesis is not clear, although their direct role in the destruction of the thyroid follicular cells by cytotoxic mechanisms cannot be excluded [3,4]. In addition, the antibodies may facilitate presentation of TPO to autoreactive T cells [5], as recently shown for antithyroglobulin antibodies [6]. Thus, knowledge of the regions on TPO recognized by these autoantibodies is important, and may lead to greater understanding of why immune tolerance to these determinants is lost. It may also lead to the development of novel therapeutic intervention strategies for the prevention or treatment of these disorders.
TPO is a large multidomain enzyme whose structure is presently unknown, but its high degree of sequence homology to other peroxidases of known structure, such as myeloperoxidase (MPO), has allowed models of the structure to be built [7]. Competition experiments with a panel of murine anti-TPO monoclonal antibodies (mabs), initially defined two principal conformational, discontinous epitopes on TPO, termed immunodominant domain regions (IDR)-A and -B [8]. Later studies, with recombinant human Fabs prepared from autoimmune thyroid disease patients, used the converse terminology for these epitopes [2]. In this communication we follow the terminology used in our earlier reports [7,9], based upon the murine mab definitions [8]. Other regions on TPO recognized by conformational dependent autoantibodies, are likely to represent minor determinants [10–18]. A variety of strategies have been employed to map the key residues within the IDR-A and -B epitopes in TPO [7–12, 18–26]. One study identified lysine 713 as an essential residue in IDR-A [19], and this residue is part of an epitope recognized by anti-TPO murine mab 47 which is specific for aa713–720 [23,24]. Other studies extended the boundaries of the IDR-A and -B epitopes beyond the MPO-like region of TPO to the complement control protein (CCP) and the EGF-like domains [18, 20, 21]. More recently, a recombinant human anti-TPO autoantibody was used to screen random peptide libraries together with sequence alignment of the mimotopes on the TPO structural model to locate the important residues of IDR-A [22]. The recombinant antibody T15 was shown to bind three loops located in the MPO-like domain (including the aa713–720) and one loop in the CCP-like domain, giving new insight into the localization of IDR-A [22].
By identifying potential surface loops and raising antipeptide antisera, we previously localized the IDR-B domain on the surface of TPO to a region around residues 599–614 (peptide P14), and the IDR-A domain to be located close by [7,9]. In the present study we have continued with this approach, focusing on the MPO-like domain and have identified a mixture of antipeptide antibodies, including antipeptide 14 antibodies, that define a region encompassing both IDR-A and -B epitopes. This combination of antipeptide antibodies inhibits almost all human autoantibody binding to TPO. Furthermore, we have performed single amino acid substitutions in the vicinity of IDR-B to identify residues that define the boundary of IDR-B on TPO.
Materials and Methods
Antibodies
Mouse mabs to TPO were obtained from Dr J Ruf [8]. Serum from patients with thyroid autoimmune disease was obtained from the Warsaw outpatient endocrine clinic. Diagnosis was made by standard clinical and biochemical criteria and the samples comprised sera from patients’ with Graves’ disease (n = 19) and lymphocytic hypothyroid disease (Hashimoto's thyroiditis) (n = 10). Pooled serum from normal healthy individuals (n = 20) was used a control. Pooled sera from 20 patients with thyroid autoimmune disease, positive for TPO antibodies. were used as positive control. Autoantibodies to TPO were measured by ELISA, standardized to the WHO/MRC international standard 66/387 [7]. Rabbit antibodies in reaction with peptides and TPO were also measured by ELISA [7]. Peptides were conjugated to maleimide activated keyhole limpet haemocyanin (KLH) (1 mg peptide/1 mg KLH) and further purified by chromatography on Sephadex G-100 chromatography in PBS [7]. Two New Zealand White rabbits per peptide were injected according to the described schedule [7]. Rabbit IgG Fab preparations were prepared using immobilized papain (Perbio Science, Tattenhall, UK) followed by chromatography through protein A Sepharose to remove the undigested IgG and Fc fragments [27]. All antisera were tested for reactivity to human proteins such as bovine serum albumin, IgG and thyroglobulin and failed to show any binding.
Modelling of TPO structure; selection and synthesis of accessible peptides
The molecular model of TPO, based upon the homologous structure of MPO has been described [7]. All the synthetic peptide sequences used in this study (Table 1) correspond to sequences in the MPO-like domain of TPO. The location and solvent accessibility of some of these peptides such as P6, P14, P16 and P17 have been detailed previously [7]; the other peptides were selected by inspection of the model. All peptides were synthesized by F-moc chemistry with C-terminal amides and a cysteine residue at the N- or the C-terminus for coupling to carrier protein and checked for purity by mass spectrometry [7]. Selection of amino acids for mutagenesis was performed by examination of TPO model and selecting residues in or around P14 sequence which would be likely to contribute to interaction with antibody.
Table 1.
Anti-peptide antibody titre in reaction with peptide and human TPO assayed by direct ELISA
| Peptide number and TPO sequence | Antibody titre in reaction with peptide | Antibody titre in reaction with human TPO |
|---|---|---|
| Peptide 4 (451–469) | 1 : 6400 | 1 : 51200 |
| Peptide 5 (489–507) | 1 : 6400 | 1 : 8000 |
| Peptide 7 (515–531) | 1 : 12800 | 1 : 4000 |
| Peptide 8 (618–636) | 1 : 12800 | 1 : 32000 |
| Peptide 9 (662–680) | 1 : 3200 | 1 : 2000 |
| Peptide 10 (679–696) | 1 : 3200 | 1 : 1000 |
| Peptide 18 (210–225) | 1 : 32000 | 1 : 4000 |
| Peptide 19 (468–477) | 1 : 160000 | 1 : 32000 |
| Peptide 22 (536–546) | 1 : 256000 | 1 : 32000 |
| Peptide 24 (375–387) | 1 : 320000 | 1 : 320000 |
| Peptide 28 (222–229) | 1 : 8000 | 1 : 32000 |
| Peptide 30 (461–476) | 1 : 256000 | 1 : 64000 |
| Peptide 32 (273–286) | 1 : 256000 | 1 : 64000 |
| Peptide 35 (642–650) | 1 : 64000 | 1 : 500 |
| Peptide 43 (702–721) | 1 : 256000 | 1 : 256000 |
| Peptide 6 (503–516) | 1 : 51200 | 1 : 6400 |
| Peptide 12 (549–563) | 1 : 64000 | 1 : 256000 |
| Peptide 14 (599–617) | 1 : 256000 | 1 : 256000 |
| Peptide 16 (189–201) | 1 : 320000 | 1 : 64000 |
| Peptide 17 (179–190) | 1 : 640000 | 1 : 8000 |
Site directed mutagenesis
Mutagenesis was carried out using the Altered Sites II in vitro Mutagenesis System (Promega, Southampton, UK), as described previously [28]. Full length human TPO cDNA [29] was subcloned into pALTER-1 vector and two stop codons were generated at positions 2617–2619 bp and 2620–2622 bp. To facilitate further subcloning the NheI restriction site at the 5′-end (66–71 bp) and the XbaI restriction site at the 3′-end (2623–2628 bp) were added in the same mutagenesis reaction. The resulting truncated hTPO cDNA encoding the extracellular domain of TPO served as a template to generate following site-specific mutations: K713A (nucleotide change: AAA→gcc), E716A (GAA→Gct), E461T (GAG→aca), D553N (GAT→aAc), D624S (GAC→tcC), N642D (AAC→gAC). All mutations were verified by nucleotide sequencing of both the strands.
In vitro transcription/translation and immunoprecipitation
Wild type and all mutant TPO cDNAs encoding TPO ectodomain were subcloned into pCIneo (Promega) using NheI and XbaI restriction sites, and protein produced by in vitro transcription/translation in a single tube reaction using TNT T7 Quick Coupled Transcription/Translation System (Promega) in the presence of 35S-methionine [28]. The sizes of all translated proteins were confirmed by SDS polyacrylamide gel electrophoresis and autoradiography. All translated products were freshly prepared and used without freezing. The 35S-methionine labelled TPO (20 000 cpm) was incubated with the mouse mabs in a total volume of 20 µl in immunoprecipitation buffer (10 mm HEPES, pH 7·4, 150 mm NaCl, 20 mm methionine, 0·5% Triton X-100, 0·5% BSA) at 4C overnight. Then 10 µl of a 50% slurry of protein G-Sepharose (Sigma-Aldrich, Gillingham, UK) was added and the reaction mixture was incubated at 4C for 1 h with shaking. After transfer of the reactions on Multiscreen filtration plates (Millipore, Watford, UK), pellets were washed five times with immunoprecipitation buffer and twice with water, dried at room temperature and counted. Data presented for mouse mabs are the mean of duplicate samples. For background binding, an irrelevant IgG murine mab was used and subtracted from the binding of the anti-TPO mabs. The binding of the mabs to TPO mutants was considered to be substantially affected if the reactivity represented <50% of wild type binding [28]. The results of the binding of mabs to mutant TPO were analysed by students t-test and P-values of <0·05 were considered significant.
Purification of hTPO
Human TPO microsomes were prepared from pooled Graves’ thyroid tissue as described [7]. TPO preparations used for ELISA were further purified by affinity chromatography on protein L-Sepharose. A column containing 2 ml of protein L-Sepharose (Actigen Ltd, Cambridge, UK) was washed with 20 ml of Tris-buffered saline, pH 8·0 containing 0·05% deoxycholate (DOC) followed by solubilized TPO in the same buffer. The column was incubated for 1 h at room temperature, washed with TBS containing 0·05% DOC and the non retained fraction collected and concentrated for use in ELISA experiments. This step removes almost all Ig contamination from the TPO preparation.
Competitive inhibition ELISA
The inhibition of autoantibody binding to TPO by the rabbit antipeptide antisera or their Fab fragments was performed exactly as described previously [7]. Briefly, to microtitre plates coated with purified human TPO (1 µg/ml) in carbonate-bicarbonate buffer, 100 µl of various dilutions of rabbit antipeptide antiserum (1 : 100 and 1 : 250) were added and incubated overnight at room temperature. After washing, patients’ sera diluted to give varying amounts of anti-TPO autoantibodies (corresponding to 1–25 IU/ml of anti-TPO), were added to the wells and incubated for 1 h at room temperature. Each patient's serum was tested at 4 or 5 dilutions. After washing three times in TBST, HRP-conjugated rabbit antihuman IgG (Dako, Ely, UK) (diluted 1 : 2000) was added and incubated for 1 h followed by three washes with PBS. The plates were developed with TMB solution and optical density measured at 450 nm. Pre-immune rabbit serum was used for controls, and wells without addition of human serum were considered as blank. Inhibition of autoantibody binding was calculated in comparison to preimmune serum according to the formula described previously [7]. All experiments were repeated at least 3 times. Any inhibition over 3SD (12·3%) for preimmune serum was considered as significant. ELISA for determination of antibody binding to TPO coated wells was performed as described [7].
Results
Competition studies with antipeptide antibodies
We have previously reported a model of the three-dimensional structure of TPO and demonstrated that it is built up from three domains; an MPO-like domain (residues 142–738), a CCP-like domain (residues 739–795) and an EGF-like domain (residues 796–841) [7]. From the model, we were able to predict potential surface loops that might serve as antibody binding regions and thus locate IDR-B on the structure of TPO. In order to localize the IDR-A epitope, 15 new peptides corresponding to potential surface loops in the remainder of the MPO-like domain of TPO model were synthesized (Table 1), and rabbit antisera generated. By ELISA, all the rabbit antipeptide antisera react with the corresponding immunizing peptide and with purified human TPO, but at different titres (Table 1). The rabbit antipeptide antisera were tested for their ability to inhibit the binding of serum autoantibodies of a pool of 20 sera positive for anti-TPO autoantibody. None of the rabbit antisera to the 15 new peptides significantly inhibited the binding of the anti-TPO autoantibodies in the pooled serum; data for P12, P18 and P43 are shown in Table 2 and none of the others alone were higher than these. As a positive control, antibodies to P14, which was shown previously to define the IDR-B epitope [7] inhibited the binding of the anti-TPO autoantibodies in the serum pool by 41·2% (SD 7·8%) (Table 2). Fab fragments prepared from antipeptide 14 IgG also inhibited the binding of the anti-TPO autoantibodies in the patient serum pool, but at a lower level of 32·2% (SD 4·2%) compared with whole IgG (Table 2).
Table 2.
Inhibition of binding of anti-TPO autoantibodies in pooled patient sera by rabbit antipeptide antisera, their Fab preparations or their mixtures
| Rabbit antiserum or Fab preparations | % Inhibition (SD) (n = 20 serum pool) |
|---|---|
| Preimmune serum | 4·1 (3·8) |
| P14 serum | 41·2 (7·8) |
| P14 Fab | 32·2 (4·2) |
| P12 serum | 11·2 (4·3) |
| P18 serum | 10·0 (3·8) |
| P14 + P12 serum | 63·0 (6·2) |
| P14 + P18 serum | 58·0 (5·4) |
| P12 + P18 serum | 29·0 (6·4) |
| P14 + P12 + P18 serum | 75·3 (8·4) |
| P14 + P12 + P18 Fab mixture | 64·6 (6·5) |
| P4 + P30 + P32 serum | 11·6 (7·4) |
| P4 + P30 + P32 Fab mixture | 8·3 (5·8) |
| P43 serum | 12·0 (7·6) |
To determine possible co-operative effects of mixing different antipeptide antisera with anti-P14 antiserum, various combinations were assessed for their ability to inhibit anti-TPO autoantibody binding. Anti-P12 and anti-P18 antisera, when mixed with anti-P14 antisera, increased the inhibitory capacity to 63·0% (SD 6·2%) and 58·0% (SD 5·4%), respectively (Table 2). All three antisera together increased the inhibition of binding of the anti-TPO autoantibodies in the serum pool to 75·3% (SD 8·4%). A mixture of Fab preparations derived from purified IgG from all three antipeptide antisera also inhibited binding of the TPO antibodies in the serum pool by 64·6% (SD 6·5%) (Table 2). As a control, a similar mixture of three antisera to P4 + P30 + P32, or their Fab preparations, did not lead to any significant inhibition of anti-TPO autoantibodies in the serum pool (Table 2).
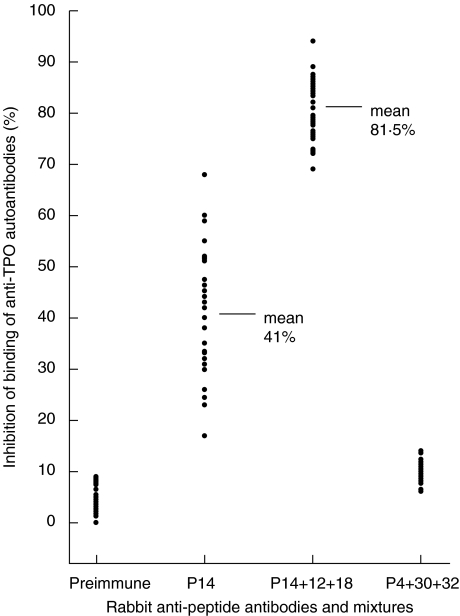
The mixture of anti-P12 + P14 + P18 antisera was then tested for its ability to inhibit the binding of anti-TPO autoantibodies in individual patient's serum. A total of 29 patients’ sera positive for anti-TPO autoantibody were tested. Different patients’ sera were inhibited to varying degrees by the mixture, ranging from 67 to 94% (mean 81·5%) at autoantibody levels of 5 IU (Fig. 1). This is significantly greater than for anti-P14 antiserum alone, ranging from 17 to 68% (mean 41%) [7]. The ability of the mixture of anti-P12 + P14 + P18 antisera to inhibit the binding of anti-TPO autoantibodies in individual patient's serum, as well as in the pool of the 20 positive sera provided compelling evidence that this mixture was defining the major immunodominant epitopes on TPO. Additional experiments were conducted to test this interpretation, using murine mabs specific for IDR-A and IDR-B regions of TPO [8]. The binding of mabs 15, 18 and 64, which are specific for IDR-B, was inhibited strongly by anti-P14 antisera, while mab 2 specific for IDR-A was not inhibited (Table 3). However, when either anti-P12 or anti-P18 was mixed with anti-P14, the inhibition of mabs 2 and 47 increased (as did the inhibition of the IDR-B specific mabs). When all three antisera were mixed (anti-P12 + P14 + P18), an even more prounced inhibition of 75% for the IDR-A specific mab 2 was achieved (Table 3). The results confirm that the mixture of anti-P12 + P14 + P18 antisera recognize both IDR-A and -B on TPO.
Fig. 1.
Inhibition of autoantibody binding to TPO by rabbit antipeptide sera by ELISA. Each point represent an individual patient. The mean inhibition values with P14 and with P14 + P12 and P18 mixture are also indicated. Human sera were used at an autoantibody level of 5 IU/ml. Preimmune serum and antisera to peptide 4, 18, 30 and 32 were diluted 1/100, while antisera to peptides 12 and 14 were used at 1/250 dilution. In all experiments a panel of 29 individual patient sera was studied in the inhibition assay.
Table 3.
Inhibition of monoclonal antibody binding to TPO by rabbit antipeptide antibodies and pooled Graves’ patients’ serum positive for anti-TPO antibodies
| IDR-B region specific mabs (% inhibition *) | IDR-A region specific mabs (% inhibition *) | ||||
|---|---|---|---|---|---|
| Antibodies | mab 15 | mab 18 | mab 64 | mab 2 | mab 47 |
| Pre-immune | 4 | 5 | 5 | 6 | 4 |
| P12 | 5 | 6 | 11 | 20 | 0 |
| P14 | 97 | 93 | 86 | 3 | 1 |
| P18 | 6 | 7 | 8 | 17 | 2 |
| P43 | 4 | 10 | 10 | 27 | 99 |
| P12 + P14 | 100 | 100 | 87 | 48 | 5 |
| P18 + P14 | 100 | 100 | 93 | 22 | 8 |
| P14 + P43 | 100 | 100 | 95 | 40 | 92 |
| P12 + P18 | 10 | 13 | 11 | 45 | 12 |
| P12 + P43 | 12 | 24 | 18 | 70 | 95 |
| P18 + P43 | 10 | 20 | 15 | 46 | 96 |
| P12 + P14 + P18 | 100 | 100 | 92 | 75 | 17 |
| P12 + P14 + P43 | 100 | 100 | 93 | 78 | 99 |
| P18 + P14 + P43 | 100 | 100 | 98 | 50 | 98 |
| P12 + P14 + P18 + P43 | 100 | 100 | 98 | 72 | 99 |
| P4 + P30 + P32 | 10 | 14 | 13 | 11 | 7 |
| Blood donor serum pool | 0 | 0 | 1 | 2 | 0 |
| Graves’ patients’ serum pool (n = 20) | 97 | 96 | 77 | 82 | 14 |
standard deviation for all results in Table 1 were between 4 and 8%.
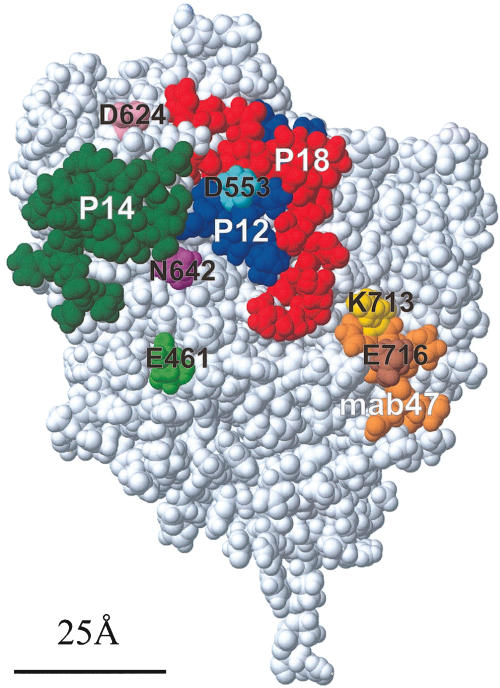
P43 (aa702–721) includes the amino acid sequence (aa713–721) recognized by mab 47, and thus as expected, complete inhibition of binding of mab 47 by anti-P43 antiserum was obtained (Table 3). Addition of anti-P43 to a mixture of anti-P14 + P18 led to a substantial increase in inhibition of mab 2 from 22% to 50%, but more strikingly, addition of anti-P43 to a mixture of anti-P12 + P14 increased the inhibition of mab 2 binding even further to 78% (Table 3). Interestingly, the inhibition of mab 2 by mixing anti-P43 with the anti-P12 + P14 antisera (78%) was comparable to the mixture with anti-P12 antisera (70%), showing that the mab 2 epitope must be adjacent to both the epitopes recognized by anti-P12 (i.e. P12) and anti-P43 (i.e. P43, the epitope for mab 47) (Table 3). The location of the peptide sequences P12, P14 and P18 on the surface of the TPO model is shown in Fig. 2. It is clear that the IDR-A and -B epitopes on the surface of TPO are immediately adjacent to each other.
Fig. 2.
Space filling representation of the modelled MPO-like domain of TPO [7]), showing the location of the single amino acids replacements and the peptides used in this study. Peptide P14 (green) principally defines IDR-B [7]. P12 (blue) and P18 (red), together with P14, define a region that encompasses both IDR-A and -B domains. The sequence comprising the epitope for mab 47 (residues 713–721), is shown in orange, including the single amino acid replacement residues within this region, K713 (yellow) and E716 (brown) highlighted. Peptide P43 (residues 702–721) includes this sequence. Also shown are the other single amino acid replacements D624 (pink), D553 (light blue), N642 (purple) and E461 (light green). The bar (25 Å long) represents the approximate diameter of an antibody combining site.
Binding of anti-TPO antibodies to single amino acid mutants of TPO
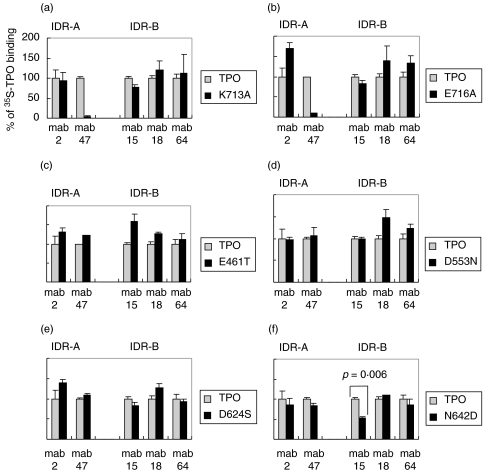
We selected amino acid residues for mutagenesis within the vicinity of the IDR-B domain on the structural model of TPO. In order to ensure that the amino acid change would have a minimal effect on the folding of the polypeptide chain, the selected amino acid was replaced by the residue present in the MPO sequence at that position. Four residues were selected for this purpose (E461, D553, D624, N642) and are depicted on the structural model in Fig. 2. We compared the binding of the IDR-A and -B specific murine mabs to the 35S-labelled mutants relative to the binding to wild type TPO. To ensure the integrity of the conformational epitopes, we used fresh translates, since freezing of other autoantigens such as the type 1 diabetes antigen, glutamic acid decarboxylase can adversely effect the conformational determinants [28]. To evaluate the immunoprecipitation system, we initially tested two mutations within the epitope of mab 47 (aa713–721). K713A and E716A mutants showed a complete loss of binding to mab 47 in comparison to wild TPO binding (Fig. 3a, b). This showed that the translation and immunoprecipitation method was effective in determining the loss of an antibody epitope by this assay. The mutations at K713A and E716A had no influence on the binding of the remaining mab specific for IDR-A (mab 2) or those specific for IDR-B (mabs 15, 18 and 64) (Fig. 3a, b). Analysis of the other single amino acid mutants E461T, D553N, D624S and N642D to the IDR-A and -B specific mabs (Fig. 3c–f) showed that only the N642D mutation significantly reduced the binding of mab 15 by <50% of the binding to wild type TPO (P = 0·006) (Fig. 3f). In sum, the results demonstrate that N642, which lies in close proximity to P14 (Fig. 2) may be part of IDR-B. Furthermore, the negative results for residues E461, D553 and D624 serve to limit the extent of IDR-B.
Fig. 3.
Immunoprecipitation analyses of murine mabs specific for IDR-A and -B with single amino acid mutants of TPO using 35S-methionine labelled translated proteins. The results are expressed as percentage values of wild type TPO ectodomain precipitated in the same experiment. The TPO mutants used were: (a) K713A and (b) E716A, selected as controls within the epitope of mab 47 (c) E461T (d) D553N (e) D624S and (f) N642D selected to probe the vicinity of IDR-B. Background binding was assessed using an irrelevant IgG mab. Generally, the background binding obtained in the assay with different mutants ranged from 361cpm to 580cpm. Specific binding to wild type TPO ectodomain with the variety of anti-TPO mabs used in the study ranged from 8997cpm to 1287cpm. The binding of mab 47 was abrogated for the K713A (a) and E716A (b) mutants respectively), whilst the binding of mab 15, specific for IDR-B, was significantly reduced (p = 0·006) in the N642D mutant (f). Data represents the results of at least two experiments.
Discussion
In our earlier study, we successfully localized the IDR-B epitope of TPO in the MPO-like domain, using a rabbit antiserum to a single peptide (P14, aa599–617) [7]. However, the size of the IgG molecule and the possibility that steric hindrance blocked binding of anti-TPO autoantibodies to a wider region, limited the precision with which this determinant was mapped. In this report, we show that Fab fragments of the anti-P14 IgG also inhibit the binding of the autoantibodies from patients with thyroid autoimmune disease, confirming that the P14 sequence is indeed a substantial component of the IDR-B epitope. The dimensions of the epitope represented by P14 on the surface of TPO (green in Fig. 2) are comparable to those of a Fab combining site, which typically has a diameter of 25 Å (shown in Fig. 2).
We have now identified an additional number of peptides (n = 15) exposed or partially exposed on the surface of the TPO model. These potential antibody binding regions were not explored in our earlier study [7] and we therefore generated high titre rabbit antisera to the corresponding synthetic peptide sequences. However, none of the rabbit antisera to these synthetic peptide sequences significantly inhibited the binding of anti-TPO autoantibodies present in a pool of sera from Graves’ patients’ positive for TPO antibodies, confirming that they do not individually contribute to the IDR-A (or IDR-B) domain. However, we observed that mixing antisera to P14 with antisera to P12 (aa 549–563), resulted in a significant augmentation of the inhibitory activity of the former, greater than the sum of the two antipeptide antisera alone (Table 2). This co-operative effect was explored further by mixing other antipeptide antisera. Addition of anti-P18 antiserum (aa 210–225) also significantly enhanced the level of inhibition achieved with anti-P14 alone (Table 2) and strikingly, the mixture of antipeptide antibodies to P12 + P14 + P18 also inhibited the binding to TPO of murine mabs directed to the IDR-A, as well as to the -B epitope (Table 3). Addition of anti-P43 to anti-P12 or anti-P12 + P14 mixture led to a substantial increase in the inhibition for mab 2 specific for IDR-A, confirming that anti-P43 also contributes to IDR-A.
The combination of antisera to P12, P14 and P18 effectively blocks the binding of both human anti-TPO autoantibodies and the murine mabs that define both IDR-A and -B domains, except for mab 47 which requires the addition of anti-P43. Since the anti-P14 antisera principally define IDR-B, this suggests that anti-P12 and anti-P18 antisera together with anti-P14 antisera delineate an area on the TPO surface that also harbours IDR-A. On the model of TPO, peptides P14 (green in Fig. 2), P12 (blue) and P18 (red) lie immediately adjacent to each other. The proposition that this contiguous surface patch encompasses both principal immunodominant regions is confirmed by the fact that these three antisera together are capable of inhibiting between 67 and 94% of the human anti-TPO autoantibody response in patients with thyroid autoimmune disease (Fig. 1).
We also examined the role of amino acids in the vicinity of peptide 14 that might contribute to IDR-B, by using cell free translation of nascent TPO, which has previously been used for mapping TPO determinants [12,14]. We used mab 47 as a positive control antibody for the mutagenesis and translation/immunoprecipitation reaction, since the peptide sequence that it recognizes is well characterized (aa713–721) [23,24]. Single amino acid replacements at K713 and E716 lead to loss of binding of mab 47, while binding of another mab specific for IDR-A (mab 2) and the panel of mabs specific for IDR-B (mabs 15, 18 and 64) was unaffected (Fig. 3a,b), thus demonstrating the utility of this approach. Of the four single amino acid mutations in the vicinity of P14 that were examined in this study, only N642D influenced the binding of any IDR-domain specific mab; the binding of mab 15 specific for IDR-B was significantly reduced (Fig. 3f). These data therefore allow us to extend the boundary of IDR-B to include N642 which lies immediately adjacent to P14 (Fig. 2), but the negative results for E461, D553 and D624 serve to define the limits of this epitope.
The epitope on TPO recognized by mab 47 (aa713–720) has been studied extensively and has been used as an ‘handle’ to define at least a part of IDR-A [23,24]. Recombinant human anti-TPO Fabs specific for the IDR-A are blocked from binding to TPO if incubated with mab 47, giving compelling evidence that the IDR-A domain resides near to the aa713–720 region of TPO [25,26]. The inhibition of recombinant Fabs specific for IDR-A domain by mab 47 is entirely consistent with our model, since the mab 47 epitope (orange in Fig. 2) lies adjacent to the region defined by P12 and P18, but distant from P14 and thus the IDR-B epitope. Furthermore, the recent finding that Lys713 is an important residue contributing to the IDR-A epitope [19] is also consistent with our localization of the two epitopes. Lys713 (at the N-terminal end of the peptide that defines the mab 47 epitope) is shown in Fig. 2 (yellow), and can be seen to lie immediately alongside P18 (red in Fig. 2). It can thus contribute to IDR-A, but not to IDR-B. This is also consistent with our finding that antisera to P43, which contains the mab 47 epitope, when added to the anti-P12 + P14 + P18 antisera mixture increases the level of inhibition of the recombinant Fabs specific for IDR-A, but not IDR-B domains (Table 3).
Recently, regions outside the MPO-like domain such as the CCP-like domain [20], and in particular Tyr772 in the latter domain [18], or both the CCP-like and EGF-like domains [16, 18, 21] have been implicated in contributing to the IDR epitopes. Since we do not know the quaternary structure and hence the packing, of the three structural domains that constitute the TPO ectodomain, we cannot comment on the contribution of epitopes outside the MPO-like domain. It may be that the entire TPO ectodomain is folded so that amino acids such as Tyr772, or indeed other peptide sequences in the CCP- or EGF-like domains, contribute to the IDR-A and -B epitopes in the MPO-like domain. However, we have in this study been able to determine the location and extent of the two major immunodominant regions, IDR-A and -B, in terms of the P12, P14 and P18 peptides and found that these two epitopes lie close together on the surface of TPO, forming a contiguous patch. A mixture of antisera to these three peptides can inhibit up to 94% of a patient's anti-TPO autoantibody response, and thus the region of TPO defined by these peptides (P12, P14, P18) together with a contribution from the mab 47 epitope (residues 713–721, contained within P43) accounts for the vast majority of the epitopes recognized by autoantibodies in patients with autoimmune thyroid disease.
Acknowledgments
This work was supported by grants from the Wellcome Trust (UK) and Medical Centre of Postgraduate Education (Poland) (number CMKP 501-06-99). We are also grateful to the Polish-British Research Partnership Programme of the British Council for support. Finally, we wish to thank Dr Jean Ruf for the continued provision of his valuable TPO specific mabs to allow this work to progress and to Dr Shioko Kimura for the TPO cDNA.
References
- 1.Banga JP, Barnett PS, McGregor AM. Immunological and molecular characteristics of the thyroid peroxidase autoantigen. Autoimmunity. 1991;8:335–43. doi: 10.3109/08916939109007642. [DOI] [PubMed] [Google Scholar]
- 2.Rapoport B, McLachlan S. Thyroid peroxidase as an autoantigen in autoimmune thyroid disease: update. Endocrine Revs. 1994;3:96–102. [Google Scholar]
- 3.Banga JP. Thyroid peroxidase: developments in our understanding of its structure and the relevance of these findings to autoimmunity. Curr Opinion Endocrinol Diabetes. 1998;5:275–81. [Google Scholar]
- 4.Stassi G, De Maria R. Autoimmune thyroid disease: new models of cell death in autoimmunity. Nature Rev Immunol. 2002;2:195–204. doi: 10.1038/nri750. [DOI] [PubMed] [Google Scholar]
- 5.Guo J, Wang B, Rapoport B, McLachlan S. Evidence for antigen presentation to sensitised T cells by thyroid peroxidase (TPO)-specific B cells in mice injected with fibroblasts co-expressing TPO and MHC class II. Clin Exp Immunol. 2000;119:38–46. doi: 10.1046/j.1365-2249.2000.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai Y, Carayanniotis KA, Eliades P, et al. Enhancing or suppressive effects of antibodies on processing of a pathogenic T cell epitope in thyroglobulin. J Immunol. 1999;162:6987–92. [PubMed] [Google Scholar]
- 7.Hobby P, Gardas A, Rodomski R, et al. Identification of an immunodominant region recognised by human autoantibodies in a three dimensional model of thyroid peroxidase. Endocrinol. 2000;141:2018–26. doi: 10.1210/endo.141.6.7506. [DOI] [PubMed] [Google Scholar]
- 8.Ruf J, Toubert ME, Czarnocka B, et al. Relationship between immunological structures and biochemical properties of human thyroid peroxidase. Endocrinol. 1989;125:1211–8. doi: 10.1210/endo-125-3-1211. [DOI] [PubMed] [Google Scholar]
- 9.Gardas A, Watson PF, Hobby P, et al. Human thyroid peroxidase: mapping of autoantibodies, conformational epitopes to the enzyme surface. Redox Report. 2000;5:237–41. doi: 10.1179/135100000101535681. [DOI] [PubMed] [Google Scholar]
- 10.Estienne V, Duthoit C, Vinet L, et al. A conformational B-cell epitope on the C-terminal end of the extracellular part of human thyroid peroxidase. J Biol Chem. 1998;273:8056–62. doi: 10.1074/jbc.273.14.8056. [DOI] [PubMed] [Google Scholar]
- 11.Estienne V, Blanchet C, Niccoli-Sire P, et al. Molecular model, calcium sensitivity and disease specificity of a conformational thyroperoxidase B cell epitope. J Biol Chem. 1999;274:35313–7. doi: 10.1074/jbc.274.50.35313. [DOI] [PubMed] [Google Scholar]
- 12.Grennan Jones F, Ziemnicka K, Sanders J, et al. Analysis of autoantibody epitopes on human thyroid peroxidase. Autoimmun. 1999;30:157–69. doi: 10.3109/08916939908993850. [DOI] [PubMed] [Google Scholar]
- 13.Guo J, Wang B, Jaume JC, Rapoport B, McLachlan S. Rarity of autoantibodies to a major autoantigen, thyroid peroxidase, that interact with denatured antigen or with epitopes outside the immunodominant region. Clin Exp Immunol. 1999;117:19–29. doi: 10.1046/j.1365-2249.1999.00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong Z, Farilla L, Guo J, McLachlan S, Rapoport B. Does the autoantibody immunodominant region include amino acids residues 742–771. Thyroid. 2001;11:227–31. doi: 10.1089/105072501750159598. [DOI] [PubMed] [Google Scholar]
- 15.Chapal N, Peraldi-Roux S, Bresson D, et al. Human anti-thyroid peroxidase single chain fragment variable of Ig isolated from a combinatorial library assembled in-cell: insight into the in vivo situation. J Immunol. 2000;114:4162–9. doi: 10.4049/jimmunol.164.8.4162. [DOI] [PubMed] [Google Scholar]
- 16.Pichurin PN, Guo J, Estienne V, et al. Evidence that the complement control protein-epidermal growth factor like domain of thyroid peroxidase lies on the fringe of the immunodominant region recognised by autoantibodies. Thyroid. 2002;12:1085–95. doi: 10.1089/105072502321085180. [DOI] [PubMed] [Google Scholar]
- 17.Chapal N, Chardes T, Bresson D, et al. Thyroid peroxidase autoantibodies obtained from random single chain Fv libraries contain the same heavy/light combinations as occur in vivo. Endocrinol. 2001;142:4740–50. doi: 10.1210/endo.142.11.8473. [DOI] [PubMed] [Google Scholar]
- 18.Estienne V, Duthoit C, Blanchin S, et al. Analysis of a conformational B cell epitope of human thyroperoxidase: identification of a tyrosine residue at a strategic location for immunodominance. Int Immunol. 2002;14:359–66. doi: 10.1093/intimm/14.4.359. [DOI] [PubMed] [Google Scholar]
- 19.Guo J, Yan XM, McLachlan S, Rapoport B. Search for the autoantibody immunodominant region on thyroid peroxidase: epitopic footprinting with a human monoclonal autoantibody locates a facet on the native antigen containing a highly conformational epitope. J Immunol. 2001;166:1327–33. doi: 10.4049/jimmunol.166.2.1327. [DOI] [PubMed] [Google Scholar]
- 20.Guo J, McLachlan SM, Rapoport B. Localisation of the thyroid peroxidase autoantibody immunodominant region to a junctional region containing portions of the domains homologous to complement control protein and myeloperoxidase. J Biol Chem. 2002;277:40189–95. doi: 10.1074/jbc.M205524200. [DOI] [PubMed] [Google Scholar]
- 21.Blanchin S, Estienne V, Guo J, et al. Human thyroid peroxidase folds in one complex B cell immunodominant region. Biochem Biophys Res Commun. 2002;295:1118–24. doi: 10.1016/s0006-291x(02)00827-6. [DOI] [PubMed] [Google Scholar]
- 22.Bresson D, Cerutti M, Devauchelle G, et al. Localisation of the discontinuous immunodominant region recognised by human thyroperoxidase autoantibodies in autoimmune thyroid disease. J Biol Chem. 2003;278:9560–9. doi: 10.1074/jbc.M211930200. [DOI] [PubMed] [Google Scholar]
- 23.Finke R, Seto P, Ruf J, Carayon P, Rapoport B. Determination at the molecular level of a B cell epitope on thyroid peroxidase likely to be associated with autoimmune thyroid disease. J Clin Endocrinol Metab. 1991;73:919–21. doi: 10.1210/jcem-73-4-919. [DOI] [PubMed] [Google Scholar]
- 24.Chazenbalk GD, Costante G, Portolano S, McLachlan S, Rapoport B. The immunodominant region on human thyroid peroxidase recognised by autoantibodies does not contain the the monoclonal antibody 47/c21 linear epitope. J Clin Endocrinol Metab. 1993;77:1715–9. doi: 10.1210/jcem.77.6.7505290. [DOI] [PubMed] [Google Scholar]
- 25.Czarnocka B, Janota-Bzowski M, McIntosh RS, et al. Immunoglobulin Gk antithyroid peroxidase antibodies in Hashimoto's thyroiditis: Epitope mapping analysis. J Clin Endocrinol Metab. 1997;82:2639–44. doi: 10.1210/jcem.82.8.4124. [DOI] [PubMed] [Google Scholar]
- 26.Guo J, McIntosh RS, Czarnocka B, et al. Relationship between autoantibody epitopic recognition and immunoglobulin gene usage. Clin Exp Immunol. 1998;111:408–14. doi: 10.1046/j.1365-2249.1998.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coulter A, Harris R. Simplified preparation of rabbit Fab fragments. J Immunol Meths. 1983;59:199–205. doi: 10.1016/0022-1759(83)90031-5. [DOI] [PubMed] [Google Scholar]
- 28.Tree TIM, Morgenthaler NG, Duhindan N, et al. Two distantly spaced amino acids in glutamic acid decarboxylase act in concert for maintainance of conformational determinants recognised by heterogeneous autoantibodies in type 1 diabetes. Diabetologia. 2000;43:881–9. doi: 10.1007/s001250051465. [DOI] [PubMed] [Google Scholar]
- 29.Kimura S, Kotani T, McBride OW, et al. Human thyroid peroxidase: complete cDNA and protein sequence, chromosome mapping, and identification of two alternately spliced mRNAs. Proc Natl Acad Sci USA. 1987;84:5555–9. doi: 10.1073/pnas.84.16.5555. [DOI] [PMC free article] [PubMed] [Google Scholar]