Abstract
We have investigated the effects of itraconazole (0·1–10 µm), an antimycotic which is often used prophylactically in primary and secondary immunodeficiency disorders, including chronic granulomatous disease, on mobilization of Ca2+ and restoration of Ca2+ homeostasis following activation of neutrophils with FMLP or PAF. Transmembrane fluxes of Ca2+, as well as cytosolic concentrations of the cation were measured using a combination of spectrofluorimetric and radiometric procedures. The abruptly occurring increases in cytosolic Ca2+ following activation of the cells with either FMLP (1 µm) or PAF (200 nm) were unaffected by itraconazole. However, the subsequent store-operated influx of the cation was attenuated by itraconazole at concentrations of 0·25 µm and higher. The itraconazole-mediated inhibition of uptake of Ca2+ was not associated with detectable alterations in the intracellular concentrations of cyclic AMP, ATP or inositol triphosphate, and appeared to be compatible with antagonism of store-operated Ca2+ channels. Although a secondary property, this anti-inflammatory activity of itraconazole, if operative in vivo, may be beneficial in conditions associated with dysregulation of neutrophil Ca2+ handling such as CGD.
Keywords: calcium, chemoattractants, itraconazole, neutrophils
INTRODUCTION
Itraconazole prophylaxis for fungal infections is recommended in patients with chronic granulomatous disease (CGD), and is also common practice in those with severe neutropenia, as well as in patients with advanced human immunodeficiency virus infection, and lung transplant recipients [1, 2, 3, 4, 5, 6, 7, 8]. Although itraconazole belongs to a class of compounds, the imidazole antimycotics, which have been reported to antagonize Ca2+ metabolism in a variety of mammalian cell types [9–11], relatively little is known about its effects on Ca2+ handling by leucocytes. In the case of neutrophils, receptor-mediated mobilization of Ca2+ from intracellular stores is a prerequisite for activation of several pro-inflammatory activities of these cells, while the subsequent influx of the cation is necessary not only for store refilling and reactivation of the cells, but also to sustain adhesion to vascular endothelium [12–15].
In the current study, we have investigated the effects of itraconazole on the mobilization of Ca2+ from intracellular stores following exposure of neutrophils to the chemoattractants FMLP and PAF, as well as on the subsequent store-operated influx of the cation.
MATERIALS AND METHODS
Itraconazole
Itraconazole was kindly provided by Janssen Pharmaceutica, Geel, Belgium and dissolved in dimethylacetamide (DMA) to give a stock concentration of 10 mm. Subsequent dilutions were made in the same solvent and the final concentration of DMA ± itraconazole in the various assay systems described below was 0·1%. Unless indicated, all other chemicals and reagents were purchased from the Sigma Chemical Co (St Louis, MO, USA).
Neutrophils
Purified neutrophils were prepared from heparinized blood (5 U of preservative-free heparin/ml) of healthy adult human volunteers as previously described [13,16], and resuspended to 1 × 107/ml in phosphate-buffered saline (PBS, 0·15 m, pH 7·4).
Spectrofluorimetric measurements of Ca2+ fluxes
Fura-2/AM was used as the fluorescent Ca2+-sensitive indicator for these experiments [16]. Neutrophils (1 × 107/ml) were preloaded with fura-2 (2 µm) for 30 min at 37°C in PBS, washed twice and resuspended in indicator-free HBSS, pH 7·4, containing 1·25 mm CaCl2. The fura-2 loaded cells (2 × 106/ml) were then preincubated for 10 min at 37°C with itraconazole (0·1–10 µm) or an equivalent amount of the DMA solvent (control systems) after which they were transferred to disposable reaction cuvettes which were maintained at 37°C in a Hitachi 650 10S fluorescence spectrophotometer with excitation and emission wavelengths set at 340 nm and 500 nm, respectively. After a stable wavelength was obtained (1 min), the neutrophils were activated by addition of either N-formyl-L-methionyl-L-leucyl-L-phenylalanine (FMLP; 1 µm final) or platelet-activating factor (PAF; 200 nm, final) and the subsequent increase in fluorescence intensity was monitored over a 5-min period. The final volume in each cuvette was 3 ml containing a total of 6 × 106 neutrophils.
In an additional series of experiments designed to accentuate the influx of Ca2+ into FMLP-activated neutrophils, the cells were pretreated with diphenylene iodonium chloride (DPI, 5 µm final), a selective inhibitor of NADPH oxidase [17], or adenosine deaminase (ADA; 1 unit/ml final; Roche Molecular Biochemicals, Indianapolis, USA). These agents were added to the cells 1 min after itraconazole/DMA. DPI accelerates the influx of Ca2+ into FMLP-activated neutrophils by attenuation of the restraining actions of NADPH oxidase-mediated membrane depolarization [17], while ADA antagonizes adenosine-mediated synthesis of cyclic AMP, inhibiting Ca2+ sequestration/resequestration by the endomembrane Ca2+-ATPase [18].
A modification of the fura-2 fluorescence procedure was used to investigate the effects of itraconazole on the store-operated influx of Ca2+, uncomplicated by receptor-mediated activation of phospholipase C [19,20]. Cells which had been resuspended in nominally Ca2+-free HBSS immediately following loading with fura-2 were preincubated for 3 min at 37°C followed by addition of thapsigargin (1 µm, final), a highly selective inhibitor of the endomembrane Ca2+-ATPase, which depletes intracellular Ca2+ stores [19–21]. This was followed 4 min later by addition of itraconazole (5 µm) or DMA and a further preincubation for 3 min (10 min preincubation at 37°C in total) after which the cells were transferred to a cuvette in the fluorescence spectrophotometer. Store-operated influx of Ca2+ was initiated by the addition of CaCl2 (250 µm, final) and the increase in fluorescence intensity monitored for 5 min.
Radiometric assessment of Ca2+ fluxes
45Ca2+ (calcium-45 chloride, 370 GBq; Perkin Elmer Life Sciences, Boston, MA, USA) was used as tracer to label the intracellular Ca2+ pool and to monitor Ca2+ fluxes in unstimulated and FMLP (1 µm)/PAF (200 nm)-activated neutrophils. The standardization of the procedures used to load the cells with 45Ca2+ for experiments designed to measure net efflux of the cation, as well as their application in the measurement of net influx of Ca2+ following activation of the cells with FMLP or PAF have previously been described in detail [13, 16, 22]. For these experiments itraconazole was used at a fixed, final concentration of 5 µm.
Intracellular cAMP, inositol triphosphate (IP3) and ATP
Cyclic AMP and IP3 were measured in the deproteinized extracts of unstimulated neutrophils (5 × 106/ml) and cells activated with FMLP (1 µm) or PAF (200 nm; IP3 only) using the Biotrak cAMP[125I] scintillation proximity assay system (Amersham International plc, Amersham, UK) and the IP3[3H] radioreceptor procedure (Perkin Elmer Life Sciences) both of which are competitive binding assays. Itraconazole was used at a fixed, final concentration of 5 µm added to the cells 10 min prior to the chemoattractants. Based on previous experiments, cAMP was determined 30 s after the addition of FMLP, and IP3 at 5 and 10 s after the addition of the chemoattractants when these responses are maximal [22]. The results are expressed as picomoles/107 cells.
ATP was measured in the lysates of neutrophils, which had been exposed to itraconazole (5 µm) or DMA for 10 min at 37°C, using a luciferin/luciferase chemiluminescence method [23].
Superoxide production, assembly of NADPH oxidase and oxygen consumption
The effects of itraconazole (5 µm) on superoxide generation by unstimulated neutrophils and cells activated with FMLP (1 µm) or phorbol 12-myristate 13-acetate (PMA, 25 ng/ml) were measured using lucigenin (bis-N-methylacridinium nitrate)-enhanced chemiluminescence (LECL). Neutrophils (106) were preincubated with itraconazole for 10 min at 37°C in HBSS containing 0·2 mm lucigenin after which they were activated with FMLP or PMA and the LECL responses measured with an LKB Wallac 1251 chemiluminometer (Turku, Finland). LECL readings were integrated for 5 s intervals and recorded as mV/s. This LECL procedure was also used to investigate the superoxide-scavenging potential of itraconazole (5 µm) in a cell-free xanthine oxidase (32·2 milliunits/ml, final)/xanthine (1 mm, final) superoxide-generating system.
To investigate the effects of itraconazole (5 µm) on the assembly of NADPH oxidase, neutrophils (107) were preincubated with the antimycotic for 10 min at 37°C, followed by addition of PMA (25 ng/ml) in a final volume of 10 ml HBSS. After 10 min of incubation at 37°C the reactions were terminated by transferring the tubes to an ice-bath. The cells were then pelleted by centrifugation at 4°C and the pellets resuspended in 0·34 m sucrose supplemented with 0·5 mm phenylmethylsulphonyl fluoride and disrupted by sonication. Cellular debris was removed by centrifugation and the membrane fractions in the supernatants harvested following centrifugation at 70 000 × g for 30 min. The resultant membrane pellets were dispersed in sucrose and assayed for NADPH oxidase activity using LECL. Reaction mixtures (1 ml) contained lucigenin, membrane factions (200 µl) and NADPH which was added last to initiate superoxide generation.
Oxygen consumption by PMA (25 ng/ml)-activated neutrophils was measured using a three-channel oxygen electrode (model CW1, Hansatech Ltd, King's Lynn, UK). The cells (106) were preincubated for 10 min at 37°C in HBSS in the presence or absence of itraconazole (5 µm) followed by the addition of PMA and PO2 monitored for a further 15 min
Expression and statistical analysis of results
The results of each series of experiments are expressed as the mean values ± s.e.m., with the exception of the fura-2 experiments for which the traces are shown. Statistical analysis was performed using the paired Student's t-test when comparing two groups or by analysis of variance with subsequent Tukey-Kramer multiple comparisons test for multiple comparisons.
RESULTS
Spectrofluorimetric measurement of Ca2+ fluxes
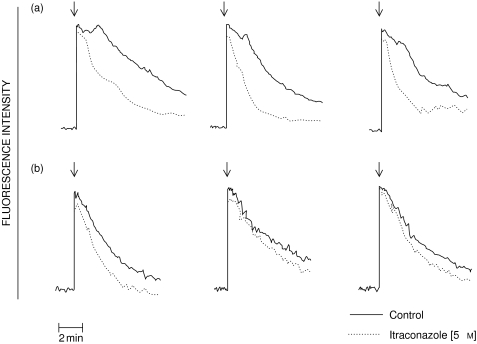
The results shown in Fig. 1 are typical traces of the FMLP- and PAF-activated fura-2 fluorescence responses of neutrophils in the absence or presence of 5 µm itraconazole. Addition of either FMLP or PAF to neutrophils was accompanied by the characteristic abrupt increase in fura-2 fluorescence due to a transient elevation in the cytosolic concentration of Ca2+. In the case of FMLP, there was a rapid decline in peak fluorescence intensity, while the peak response was sustained for about 1–2 min in PAF-activated cells, probably as a consequence of early influx of Ca2+ and delayed clearance of cytosolic Ca2+ as a result of failure of PAF to activate NADPH oxidase and adenylate cyclase, respectively [22]. Itraconazole caused a dose-related acceleration in the rate of decline in fura-2 fluorescence in FMLP-activated, but particularly PAF-activated neutrophils, which was evident at 0·25 µm and maximal at 5 µm of the antimycotic. In the case of PAF-activated cells, treatment with 5 µm itraconazole reduced the duration of the sustained peak fura-2 fluorescence response from 68 ± 4 to 22 ± 2 s (P < 0·001, data from 10 different experiments). The corresponding values for the 3 experiments shown in Fig. 1 are 70 ± 4 and 18 ± 3 s, underscoring the representative nature of these experiments.
Fig. 1.
Traces from 3 experiments using neutrophils from 3 different donors showing the effects of itraconazole (5 µm) on the fura-2 fluorescence responses of cells activated with PAF (200 nm; series A) or FMLP (1 µm; series B) added as denoted by the arrow (↓). These are representative traces of 6 (FMLP) or 10 (PAF) different experiments. —– control; ------ itraconazole (5 µm).
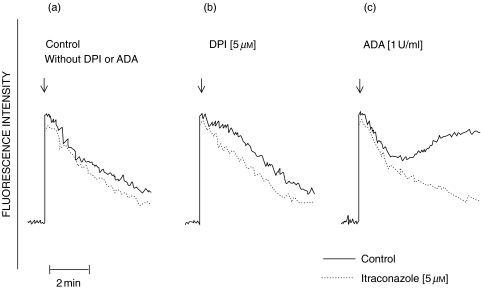
The effects of DPI and ADA on the fura-2 fluorescence responses of FMLP-activated neutrophils in the absence and presence of itraconazole are shown in Fig. 2. DPI extended the abruptly occurring peak fura-2 fluorescence responses of FMLP-activated neutrophils by 1–2 min (Fig. 2b). ADA, as previously reported [18], caused a prolonged increase in cytosolic Ca2+ which was most evident at around 2 min, and was sustained for several minutes thereafter (Fig. 2c). The prolonged increases in cytosolic Ca2+ in FMLP-activated neutrophils treated with either DPI or ADA were attenuated by itraconazole (Figs 2b,c).
Fig. 2.
Traces showing the effects of (a) the drug-free solvent control or itraconazole (5 µm) alone; (b) DPI (5 µm) alone and in combination with itraconazole (5 µm) and (c) adenosine deaminase (ADA, 1 unit/ml) alone and in combination with itraconazole (5 µm) on the FMLP (1 µm)-activated fura-2 fluorescence responses of neutrophils. The results shown are those of a single representative experiment using cells from the same donor with a total of 2 (DPI) and 3 (ADA) different experiments in each series; ↓ denotes the addition of FMLP. —– control; ------ itraconazole (5 µm).
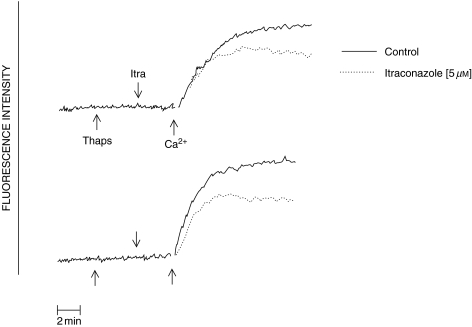
The effects of itraconazole on the store-operated influx of Ca2+ following the introduction of Ca2+ to the cell-suspending medium containing thapsigargin-treated neutrophils are shown in Fig. 3. Itraconazole reduced the influx of Ca2+ into thapsigargin-treated cells.
Fig. 3.
Traces showing the effects of itraconazole (5 µm) on the influx of Ca2+ (measured by fura-2 fluorescence) into thapsigargin (1 µm)-treated neutrophils. Thapsigargin, itraconazole and Ca2+ (250 µm CaCl2) were added sequentially as indicated by the arrows. The results shown are those of 2 different, representative experiments. —— control; ------ itraconazole (5 µm).
Efflux of 45Ca2+ from neutrophils
These results are shown in Table 1. As reported previously, addition of either FMLP or PAF to neutrophils was accompanied by an abrupt efflux of Ca2+ which was complete at 30–60 s after the addition of the chemoattractants [13,22]. Treatment of the cells with itraconazole (5 µm) significantly reduced the magnitude of efflux of Ca2+, particularly in the case of PAF-activated cells.
Table 1.
Effects of itraconazole on the net efflux of 45Ca2+ from neutrophils activated with FMLP or PAF
| System | Net efflux of 45Ca2+ (pmol/107 cells/60 s) |
|---|---|
| FMLP (1 µm) | 185·9 ± 24·0 |
| Itraconazole (5 µm) + FMLP (1 µm) | 152·6 ± 17·8** |
| PAF (200 nm) | 158·5 ± 7·1 |
| Itraconazole (5 µm) + PAF (200 nm) | 133·3 ± 8·0* |
The results are expressed as the mean values ±s.e.m. of 4 different experiments with 5 replicates in each.
P < 0·001;
P < 0·04 for comparison with the corresponding itraconazole-free system.
Influx of 45Ca2+
These results are shown in Table 2. Activation of neutrophils with FMLP or PAF was accompanied by store-operated influx of Ca2+, which was complete at 5 min after addition of the chemoattractants to the cells [13,22]. Treatment of the cells with itraconazole (5 µm) attenuated chemoattractant-activated influx of Ca2+, particularly in the case of PAF.
Table 2.
Effects of itraconazole on the net influx of 45Ca2+ in neutrophils activated with FMLP or PAF
| System | Net influx of 45Ca2+ (pmol/107 cells/5 min) |
|---|---|
| Control | 55·3 ± 5·8 |
| FMLP (1 µm) | 127·6 ± 7·3 |
| Itraconazole (5 µm) + FMLP (1 µm) | 116·1 ± 7·3 |
| PAF (200 nm) | 210·2 ± 11·8 |
| Itraconazole (5 µm) + PAF (200 nm) | 153·2 ± 9·5* |
The results are expressed as the mean values ±s.e.m. of 4 different experiments with 5 replicates in each.
P < 0·001 for comparison with the corresponding itraconazole-free system.
IP3, cAMP and ATP
The effects of itraconazole on the abruptly occurring peak IP3 responses in FMLP- and PAF-activated neutrophils are shown in Table 3. Both chemoattractants increased the levels of IP3 in neutrophils, with PAF being more potent than FMLP, but these responses were not affected by itraconazole.
Table 3.
Effects of itraconazole on inositol triphosphate (IP3) in neutrophils activated with FMLP or PAF
| IP3 concentration (pmol/107 cells) | ||
|---|---|---|
| System | 5 s | 10 s |
| Control | 62·4 ± 4·2 | ND |
| Itraconazole (5 µm) | 65·3 ± 0·8 | ND |
| FMLP (1 µm) | 87·8 ± 2·6* | 85·8 ± 2·0* |
| Itraconazole (5 µm) + FMLP (1 µm) | 101·1 ± 6·2 | 78·7 ± 6·2 |
| PAF (200 nm) | 112·2 ± fn1·7* | 70·3 ± 8·4 |
| Itraconazole (5 µm) + PAF (200 nm) | 99·7 ± 2·0 | 87·6 ± 5·0 |
The results are expressed as the mean values±s.e.m. of 4 determinations.
P ≤ 0·003 for comparison with the corresponding itraconazole-free system. ND, not done.
Likewise, itraconazole had no detectable effects on intracellular cAMP or ATP. The peak concentrations of cAMP measured 30 s after the addition of FMLP to neutrophils, were 121 ± 1 and 115 ± 7 pmols/107 cells in the absence and presence of itraconazole, respectively. The corresponding values for ATP measured 10 min after the addition of itraconazole (in the absence of the chemoattractants) were 48 ± 2 and 46 ± 2 pmols/107 cells, compatible with lack of cytotoxicity of itraconazole over the short time course of exposure of the cells to this agent.
Superoxide production, NADPH oxidase assembly and oxygen consumption
Treatment of neutrophils with itraconazole (5 µm) inhibited superoxide generation by both FMLP- and PMA-activated neutrophils. The peak LECL responses of unstimulated neutrophils and cells activated with either FMLP of PMA in the absence of itraconazole (5 µm) were 299 ± 14, 766 ± 45 and 4446 ± 528 mV/s, respectively, while the corresponding values in the presence of the antimycotic were 374 ± 40, 469 ± 24 (P < 0·001), and 2300 ± 390 mV/s (P < 0·001). However, treatment of neutrophils with itraconazole had no detectable effects on the assembly of NADPH oxidase. The peak LECL responses of isolated membranes from neutrophils activated with PMA in the absence and presence of the antimycotic being 5118 ± 110, and 5374 ± 201 mV/ s, respectively (the corresponding values for unstimulated cells were 151 ± 11, and 229 ± 24 mV/s). Likewise, oxygen consumption by PMA-activated neutrophils was not affected by itraconazole.
Inclusion of itraconazole (5 µm) in the cell-free, xanthine/xanthine oxidase superoxide-generating system resulted in a significant reduction in LECL, the values measured 5 min after initiation of the reaction being 253 ± 5, and 199 ± 5 mV/s (P < 0·001) for control and itraconazole-treated systems, respectively. Taken together, these results suggest that the itraconazole-mediated reduction in the LECL responses of activated neutrophils is achieved by a superoxide-scavenging mechanism, as opposed to itraconazole-mediated inhibition of NADPH oxidase.
DISCUSSION
Imidazole antimycotics such as miconazole and ketoconazole are well-recognized inhibitors of the store-operated influx of Ca2+ into several different mammalian cell types [24,25]. However, relatively little is known about the interactions of itraconazole with human neutrophils, specifically the effects of this agent on Ca2+ handling by these cells. Given the critical involvement of Ca2+ in the receptor-mediated activation of the pro-inflammatory activities of neutrophils and the increasing use of itraconazole as a prophylactic agent in patients with severe neutropenia or CGD, this is a potentially important topic which needs to be addressed. In the current study, we have investigated the effects of itraconazole on the Ca2+ fluxes which accompany activation of neutrophils with the chemoattractants FMLP and PAF. These chemoattractants utilize different transductional mechanisms to mobilize intracellular Ca2+ and restore Ca2+ homeostasis [22, 26, 27].
Exposure of neutrophils to either FMLP or PAF was accompanied by the characteristic abrupt increase in cytosolic Ca2+, due primarily to receptor-mediated activation of phospholipase C and IP3-mediated mobilization of Ca2+ from intracellular stores. These peak cytosolic Ca2+ responses were minimally affected by itraconazole, which is compatible with the absence of effects of this agent on peak IP3 concentrations. Whereas the peak cytosolic Ca2+ responses declined rapidly in FMLP-activated neutrophils, they were sustained for 1–2 min in PAF-activated cells. This differential response to these two chemoattractants has been attributed to the failure of PAF to activate NADPH oxidase and adenylate cyclase, resulting in prolonged peak cytosolic Ca2+ transients [22, 26, 27]. Inability to activate NADPH oxidase results in accelerated influx of extracellular Ca2+ because of consequent trivial membrane depolarization [16, 17, 22, 28, 29], while absence of activation of adenylate cyclase reduces the efficiency of clearance of Ca2+ from the cytosol [18,22].
Inclusion of itraconazole, however, accelerated the rate of decline in peak fluorescence intensity in FMLP-activated neutrophils, and particularly in PAF-activated cells. We reasoned that the less impressive responses of FMLP-activated cells were related to the efficiency of the Ca2+ exclusion and clearance mechanisms operative in these cells, as a result of activation of NADPH oxidase and adenylate cyclase, respectively. These possibilities were investigated by treating the cells with DPI which inhibits NADPH oxidase and membrane depolarization, resulting in accelerated Ca2+ influx [17], or with adenosine deaminase which inhibits the synthesis of cAMP by eliminating the interaction of neutrophil-derived adenosine with adenylate cyclase-coupled subtype A2A adenosine receptors [18]. In both cases, the elevations in cytosolic Ca2+ in FMLP-activated neutrophils were prolonged, and were impressively attenuated by itraconazole.
Treatment of neutrophils with itraconazole decreased the magnitude of efflux of Ca2+ from neutrophils activated with FMLP of PAF, without affecting the peak cAMP response in FMLP-activated neutrophils. These observations appeared to exclude enhancement of either efflux or cAMP-mediated resequestration of Ca2+ as being the mechanisms of itraconazole-mediated reduction in cytosolic Ca2+ in chemoattractant-activated neutrophils. Interestingly, however, itraconazole pretreatment of FMLP- or PAF-activated neutrophils was accompanied by decreased store-operated influx of the cation. This is compatible with the observed decrease in efflux of the cation from itraconazole-treated cells activated with FMLP or PAF, since decreased influx of Ca2+ would result in increased utilization of Ca2+ mobilized from stores for store refilling, with a consequent reduction in efflux [30].
The contention that store-operated Ca2+ influx mechanisms are the target of itraconazole is supported by the observation that the antimycotic also decreased the influx of Ca2+ into thapsigargin-treated neutrophils, a system which is less complex than those involving receptor-mediated mobilization of intracellular and extracellular Ca2+. Using this system, the magnitude of Ca2+ uptake of thapsigargin-treated neutrophils was clearly decreased by itraconazole, while the rate of influx over the initial 2 min time course of the experiment was relatively unimpeded. A possible interpretation of these findings is that store-operated influx of Ca2+ in neutrophils involves more that one type of channel [31] with differential sensitivity to itraconazole. Clearly, the exact mechanism by which itraconazole interferes with the store-operated influx of Ca2+ into neutrophils remains to be established, and we cannot exclude nonspecific effects due to interference with membrane integrity, as have previously been reported for miconazole [32].
We also observed that itraconazole, at a concentration which interfered with Ca2+ influx, did not appear to affect neutrophil NADPH oxidase activity. Although the FMLP- and PMA-activated LECL responses were reduced in the presence of itraconazole, this appeared to be due to scavenging of superoxide by the antimycotic since oxygen consumption and assembly of NADPH oxidase were unaffected, and represents an additional potential anti-inflammatory activity of this agent. The observation that itraconazole was more effective in suppressing LECL responses of neutrophils relative to those of the xanthine/xanthine oxidase system, may simply reflect the lipophilic properties of this agent. Interference with store-operated influx of Ca2+ into neutrophils therefore does not appear to either involve or affect NADPH oxidase activity. This is hardly surprising in the case of PMA, which is a Ca2+-independent activator of NADPH oxidase [33], while in FMLP-activated cells net influx of the cation is evident only after peak NADPH oxidase activity has subsided.
Although the clinical significance, if any, of the current study remains to be established, the observed effects of itraconazole on neutrophil pro-inflammatory activity, if operative in vivo, are potentially useful. In the case of patients with CGD, accelerated influx of Ca2+ into activated neutrophils as a consequence of failure of NADPH oxidase-mediated membrane depolarization [16, 17, 28], mimicked in the current and previous studies by DPI treatment of normal neutrophils [17], may explain the over-exuberant inflammatory responses which characterize this condition [34,35]. Itraconazole prophylaxis may have beneficial, albeit secondary, properties in CGD patients by attenuating the poorly regulated influx of Ca2+ into activated phagocytes. Although not included in the present study, we have been unable to detect any meaningful effects of cotrimoxazole, also used in the antimicrobial prophylaxis of CGD patients, on Ca2+ handling by chemoattractant-activated neutrophils. This antimicrobial agent may, however, possess additional properties, distinct from those of itraconazole, of benefit to CGD patients [36].
In conclusion, itraconazole, at therapeutically relevant [37], noncytotoxic concentrations, attenuates the store-operated influx of Ca2+ into activated neutrophils, a secondary property which is conceivably beneficial in patients with CGD, as well as those with allergic bronchopulmonary aspergillosis [38].
REFERENCES
- 1.Cale CM, Jones AM, Goldblatt D. Follow up of patients with chronic granulomatous disease diagnosed since 1990. Clin Exp Immunol. 2000;120:351–5. doi: 10.1046/j.1365-2249.2000.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallin JI, Alling DW, Malech HL, et al. Itraconazole to prevent fungal infections in chronic granulomatous disease. N Engl J Med. 2003;348:2416–22. doi: 10.1056/NEJMoa021931. [DOI] [PubMed] [Google Scholar]
- 3.Bohme A, Justnubling G, Bermann L, Shah PM, Stille W, Hoelzer D. Itraconazole for prophylaxis of systemic mycoses in neutropenic patients with haematological malignancies. J Antimicrob Chemother. 1996;38:953–61. doi: 10.1093/jac/38.6.953. [DOI] [PubMed] [Google Scholar]
- 4.Lamy T, Bernard M, Courois A, et al. Prophylactic use of itraconazole for the prevention of invasive pulmonary aspergillosis in high risk neutropenic patients. Leuk Lymphoma. 1998;30:163–74. doi: 10.3109/10428199809050939. [DOI] [PubMed] [Google Scholar]
- 5.Myoken Y, Sugata T, Kyo T, Fujihara M, Mikami Y. Itraconazole prophylaxis for invasive gingival aspergillosis in neutropenic patients with acute leukemia. J Peirodontol. 2002;73:33–8. doi: 10.1902/jop.2002.73.1.33. [DOI] [PubMed] [Google Scholar]
- 6.Goldman M, Cloud GA, Smedema M, et al. Does long-term itraconazole prophylaxis result in in vitro resistance in mucosal Candida albicans isolates from persons with advanced human immunodeficiency virus infection? Antimicrob Agents Chemother. 2000;44:1585–7. doi: 10.1128/aac.44.6.1585-1587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKinsey DS, Wheat LJ, Cloud GA, et al. Itraconazole prophylaxis for fungal infections in patients with advanced human immunodeficiency virus infection: Randomized placebo-controlled, double-blind study. Clin Infect Dis. 1999;28:1049–56. doi: 10.1086/514744. [DOI] [PubMed] [Google Scholar]
- 8.Kramer MR, Menin G, Rudis E, et al. Dose adjustment and cost of itraconazole prophylaxis in lung transplant recipients receiving cyclosporine and tacrolimus (FK 506) Transplant Proc. 1997;29:2657–9. doi: 10.1016/s0041-1345(97)00546-0. [DOI] [PubMed] [Google Scholar]
- 9.Favre CJ, Nüsse O, Lew DP, Krause K-H. Store-operated Ca2+ influx: what is the message from the stores to the membrane? J Laboratory Clin Med. 1996;128:19–26. doi: 10.1016/s0022-2143(96)90110-9. [DOI] [PubMed] [Google Scholar]
- 10.Hornstein EH, Vassilopoulos D, Thomas DE, Friedman FK, Tsokos GC. Modulation of human T-lymphocyte plasma membrane Ca2+ permeability by imidazole antimycotics. Immunopharmacol Immunotoxicol. 1996;18:237–45. doi: 10.3109/08923979609052734. [DOI] [PubMed] [Google Scholar]
- 11.Tran Q-M, Ohashi K, Watanabe H. Calcium signalling in endothelial cells. Cardiovasc Res. 2000;48:13–22. doi: 10.1016/s0008-6363(00)00172-3. [DOI] [PubMed] [Google Scholar]
- 12.Montero M, Alvarez J, Garcia-Sancho J. Agonist-induced Ca2+ influx in human neutrophils is secondary to emptying of intracellular Ca2+ stores. Biochem J. 1991;277:73–9. doi: 10.1042/bj2770073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson R, Goolam Mahomed A. Calcium efflux and influx in f-met-leu-phe (fMLP) -activated human neutrophils are chronologically distinct events. Clin Exp Immunol. 1997;110:132–8. doi: 10.1046/j.1365-2249.1997.5051403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dewitt S, Laffafian I, Hallett MB. Phagosomal oxidative activity during beta 2 integrin (CR3)-mediated phagocytosis by neutrophils is triggered by a non-restricted Ca2+ signal: Ca2+ controls time not space. J Cell Sci. 2003;116:2857–65. doi: 10.1242/jcs.00499. [DOI] [PubMed] [Google Scholar]
- 15.Liu LX, Hakansson L, Ridefelt P, Garcia RC, Venge P. Priming of eosinophil migration across lung epithelial cell monolayers and upregulation of CD11b/CD18 are elicited by extracellular Ca2+ Am J Respir Cell Mol Biol. 2003;28:713–21. doi: 10.1165/rcmb.4771. [DOI] [PubMed] [Google Scholar]
- 16.Tintinger GR, Theron AJ, Steel HC, Anderson R. Accelerated calcium influx and hyperactivation of neutrophils in chronic granulomatous disease. Clin Exp Immunol. 2001;123:254–63. doi: 10.1046/j.1365-2249.2001.01447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rada BK, Geiszt M, Van Bruggen R, Német K, Roos D, Ligeti E. Calcium signaling is altered in myeloid cells with a deficiency in NADPH oxidase activity. Clin Exp Immunol. 2003;132:53–60. doi: 10.1046/j.1365-2249.2003.02138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theron AJ, Steel HC, Tintinger GR, Anderson R. Endogenous adenosine regulates neutrophil pro-inflammatory activities by cyclic AMP-dependent accelerated clearance of cytosolic calcium. Inflamm Res. 2002;51:594–602. doi: 10.1007/pl00012434. [DOI] [PubMed] [Google Scholar]
- 19.Broad LM, Braun F-J, Lievremont J-P, Bird GSJ, Kurosaki T, Putney JW. Role of the phospholipase C-inositol 1,4,5-triphosphate pathway in calcium release-activated calcium current and capacitative calcium entry. J Biol Chem. 2001;276:15945–52. doi: 10.1074/jbc.M011571200. [DOI] [PubMed] [Google Scholar]
- 20.Ma H-T, Venkatachalam K, Parys JB, Gill DL. Modification of store-operated channel coupling and inositol triphosphate receptor function by 2-aminoethoxydiphenyl borate in DT40 lymphocytes. J Biol Chem. 2002;277:6915–22. doi: 10.1074/jbc.M107755200. [DOI] [PubMed] [Google Scholar]
- 21.Lytton J, Westlin M, Hanley MR. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991;266:17067–71. [PubMed] [Google Scholar]
- 22.Steel HC, Anderson R. Dissociation of the PAF-receptor from NADPH oxidase and adenylate cyclase in human neutrophils results in accelerated influx and delayed clearance of cytosolic calcium. Br J Pharmacol. 2002;136:81–9. doi: 10.1038/sj.bjp.0704685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmsen HE, Storm E, Day HJ. Determination of ATP and ADP in blood platelets: a modification of the firefly luciferase assay for plasma. Anal Biochem. 1972;46:481–501. doi: 10.1016/0003-2697(72)90323-5. [DOI] [PubMed] [Google Scholar]
- 24.Alonso-Torres SR, Garcia-Sancho J. Arachidonic acid inhibits capacitative calcium entry in rat thymocytes and human neutrophils. Biochem Biophys Acta. 1997;1328:207–13. doi: 10.1016/s0005-2736(97)00094-1. [DOI] [PubMed] [Google Scholar]
- 25.Wang JS, Wen X, Backman JT, Taavitsainen P, Neuvonen PJ, Kivist KT. Midazolem alpha-hydroxylation by human liver microsomes in vitro: inhibition by calcium blockers, itraconazole and ketoconazole. Pharmacol Toxicol. 1999;85:157–61. doi: 10.1111/j.1600-0773.1999.tb00085.x. [DOI] [PubMed] [Google Scholar]
- 26.Nick JA, Avdi NJ, Young SK, et al. Common and distinct intracellular signalling pathways in human neutrophils utilized by platelet activating factor and FMLP. J Clin Invest. 1997;99:975–86. doi: 10.1172/JCI119263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ali H, Sozzani S, Fisher I, et al. Differential regulation of formyl peptide and platelet activating factor receptors: Role of phospholipase Cβ3 phosphorylation by protein kinase A. J Biol Chem. 1998;273:11012–6. doi: 10.1074/jbc.273.18.11012. [DOI] [PubMed] [Google Scholar]
- 28.Geiszt M, Kapus A, Nemet K, et al. Regulation of capacitative Ca2+ influx in human neutrophil granulocytes: alterations in chronic granulomatous disease. J Biol Chem. 1997;272:26471–8. doi: 10.1074/jbc.272.42.26471. [DOI] [PubMed] [Google Scholar]
- 29.Hallett MB. Holding back neutrophil aggression; the oxidase has potential. Clin Exp Immunol. 2003;132:181–4. doi: 10.1046/j.1365-2249.2003.02158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson R, Goolam Mahomed A, Theron AJ, Ramafi G, Feldman C. Effects of rolipram and dibutyryl cyclic AMP on resequestration of cytosolic calcium in FMLP-activated human neutrophils. Br J Pharmacol. 1998;124:547–55. doi: 10.1038/sj.bjp.0701849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Itagaki K, Kannan KB, Livingston DH, Deitch EA, Fekete Z, Hauser CJ. Store-operated calcium entry in human neutrophils reflects multiple contributions from independently regulated pathways. J Immunol. 2002;168:4063–9. doi: 10.4049/jimmunol.168.8.4063. [DOI] [PubMed] [Google Scholar]
- 32.Yasui K, Masuda M, Matsuoka T, et al. Miconazole and amphotericin B alter polymorphonuclear leukocyte functions and membrane fluidity in similar fashions. Antimicrob Agents Chemother. 1988;32:1864–8. doi: 10.1128/aac.32.12.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goolam Mahomed A, Anderson R. Activation of human neutrophils with chemotactic peptide, opsonized zymosan and the calcium ionophore A23187, but not with a phorbol ester, is accompanied by efflux and store-operated influx of calcium. Inflammation. 2000;24:559–69. doi: 10.1023/a:1007029524141. [DOI] [PubMed] [Google Scholar]
- 34.Segal BH, Leto TL, Gallin JI, Malech HL, Holland SM. Genetic, biochemical and clinical features of chronic granulomatous disease. Medicine. 2000;79:170–200. doi: 10.1097/00005792-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Warris A, Netea MG, Wang JE, et al. Cytokine release in healthy donors and patients with chronic granulomatous disease upon stimulation with Aspergillus fumigatus. Scand J Infect Dis. 2003;35:482–7. doi: 10.1080/00365540310013009. [DOI] [PubMed] [Google Scholar]
- 36.Tsuji S, Taniuchi S, Hasui M, Yamamoto A, Kobayashi Y. Increased nitric oxide production by neutrophils from patients with chronic granulomatous disease on trimethoprim-sulfamethoxazole. Nitric Oxide. 2002;7:283–8. doi: 10.1016/s1089-8603(02)00110-6. [DOI] [PubMed] [Google Scholar]
- 37.Vogeser M, Spohrer U, Schiel X. Determination of itraconazole and hydroxyitra-conazole in plasma by use of liquid chromatography-tandem mass spectrometry with on-line solid-phase extraction. Clin Chem Laboratory Med. 2003;41:915–20. doi: 10.1515/CCLM.2003.139. [DOI] [PubMed] [Google Scholar]
- 38.Wark PA, Hensley MJ, Saltos N, et al. Anti-inflammatory effect of itraconazole in stable allergic bronchopulmonary aspergillosis: a randomized controlled trial. J Allergy Clin Immunol. 2003;111:952–7. doi: 10.1067/mai.2003.1388. [DOI] [PubMed] [Google Scholar]