Abstract
We have developed a solid-phase enzyme-linked immunosorbent assay (ELISA) to study the vaccination responses to Vi capsular polysaccharide of Salmonella typhi (S. typhi Vi) vaccine. Purified S. typhi Vi polysaccharide was biotinylated and bound to streptavidin coated microtitre plates. Reproducibility was determined across a range of IgG antibody levels: mean interassay coefficients of variation (CVs) were <11·9% for non-vaccinated sera with low levels and <11·1% for sera with very high levels of anti-S. typhi Vi IgG. Specificity was assessed by inhibition studies using salmonella antigen. We have developed the ELISA based on normal adult serum responses to test immunization with S. typhi Vi vaccine. We also report here anti-S. typhi Vi IgG levels in a group of healthy preschool children. In non-vaccinated adult sera (n = 104), the median value of anti-S. typhi Vi IgG, expressed in S. typhi Vi arbitrary units (AU/ml), was 5·3 AU/ml and in non-vaccinated sera from children (n = 44) the median value was 1·4 AU/ml. The data from immunization of healthy volunteers (n = 23) show that geometric mean levels of anti-S. typhi Vi IgG were significantly higher (P < 0·0001) for post-vaccination subjects (39·2 AU/ml) compared to paired prevaccination (3·9 AU/ml) values. A total of 21/23 vaccine recipients had <8 AU/ml S. typhi Vi IgG in their sera prior to vaccination and of these 20/21 (95%) exhibited threefold increases and 14/21 (67%) fourfold increases in their S. typhi Vi IgG following vaccination. Based on the data in this study, we propose a threefold increase in anti-S. typhi Vi IgG post-vaccination to be considered a positive vaccination response. The ability to demonstrate clearly an antibody rise in response to immunization with S. typhi Vi capsular polysaccharide vaccine suggests that this is likely to be a useful vaccine for the assessment of B cell function in patients with suspected immune deficiency.
Keywords: immunodeficiency, polysaccharide, Salmonella typhi antigen, test immunogen
INTRODUCTION
The assessment of specific antibody levels is an important tool in the interpretation of responses to test immunization in primary antibody deficiency diseases [1]. Tetanus toxoid, which is a strong immunogen, has been used to assess the response to proteins and lack of an IgG antibody response is highly significant [2]. The first carbohydrate vaccine to be used was Haemophilus influenzae type b (Hib-PRP) and enzyme-linked immunosorbent assays (ELISAs) (using an international reference preparation) were developed to measure IgG responses [3]. Significant responses as well as protective levels based on epidemiological data are defined and available [4]. The development and universal adoption of conjugate Hib vaccines resulted in the pure carbohydrate form of the vaccine being withdrawn and this vaccine is no longer available to test polysaccharide immune responses. Pneumovax, a 23-valent mixture of capsular carbohydrates, is currently used as a test immunogen for polysaccharide responses [2]. However, the development and universal adoption of conjugate pneumococcal vaccines [5] may result in the pure carbohydrate form of the vaccine being withdrawn in the future. If this were to happen, the ability to assess B cell function in terms of ability to respond to pure polysaccharide antigens in patients with suspected antibody deficiency would be jeopardized.
In the event of such a possibility, we investigated the potential use of another candidate polysaccharide vaccine for test immunization. Typhim Vi is a carbohydrate extract given by injection which was licensed in 1988 for use in adults and children >18 months of age. A single dose followed by a booster every 3 years is recommended [6,7].
The main aim of the current work was to identify the usefulness of Typhim Vi as a test immunogen by developing a reproducible and reliable ELISA to measure serum IgG responses to S. typhi Vi polysaccharide antigen. In so doing, we have used this ELISA to determine the range of antibody levels in non-vaccinated normal adults and assessed the magnitude of IgG responses in normal adults after immunization in order to define a positive carbohydrate test immunization response.
MATERIALS AND METHODS
Ethical permission
Ethical permission to study anti-S. typhi Vi IgG serum antibody levels in adults and children was obtained from the Central Oxfordshire Research Ethics Committee (COREC). Ethical permission to vaccinate healthy adult volunteers with Vi capsular polysaccharide typhoid vaccine and to study their serum immune response to the vaccine was also obtained from COREC.
Human sera
Normal healthy donor's serum was obtained from the Birmingham Blood Transfusion Service and from laboratory staff in the clinical immunology department. Blood from the Transfusion Service was obtained from 43 females (age range 20–70 years) and 38 males (21–68 years). At least five men and five women from each decade from 30 to 70 years were included in the study. No travel immunization histories or salmonella infection histories are available for any of the healthy blood donors obtained from the blood transfusion service. Blood was obtained from 14 female (age range 20–57 years) and nine male (age range 20–54 years) members of staff and was also used to help define normal S. typhi Vi IgG levels in serum. In addition, all 23 staff volunteers were immunized intramuscularly with 25 µg of purified Vi capsular polysaccharide of S. typhi (Aventis Pasteur, Maidenhead, UK) and their serum IgG responses assessed 4–5 weeks later. Prior to vaccination, no staff volunteer recalled a recent history of salmonella infection or had been immunized previously with the Vi capsular typhoid vaccine. Normal stored sera (n = 44) from 4–5-year-old children (24 males and 20 females) participating in a meningococcal AC polysaccharide vaccine immunogenicity study in Oxford were used to define a paediatric range. Preimmunization sera from each child were used in this study. Sera was spun down and stored at −80°C until used in ELISA assay.
Biotinylation of S. typhi antigen
The method is adapted from Diaz Romero and Outschoorn, 1993 [8]. Briefly, 1·5 ml Vi capsular polysaccharide antigen (1 mg/ml; Aventis Pasteur, Lyon, France) was injected into a Slide-A-Lyser® dialysis cassette (Pierce Chemical Company, UK), labelled and placed in 0·1 m sodium acetate coupling buffer at pH 5·5 (BDH, UK) overnight. Following dialysis the dialysed antigen was removed, placed in a bijou and diluted to 1 mg/ml with dH2O. One ml of diluted antigen was mixed with 1 ml of 20 mm periodate solution (Sigma, UK) and incubated at 0°C for 5 min before being dialysed for a second time overnight in the coupling buffer. On removal from the dialysis cassette, the antigen was mixed with a 50 mm biotin–hydrazide solution (Sigma, UK) by agitation in the dark for 2 h at room temperature (RT) and this biotinylated antigen dialysed for a third time in phosphate buffered saline (PBS) (Sigma, UK) overnight. On the third morning, the biotinylated antigen was adjusted to 20 mm concentration and stored at −20°C. In preliminary experiments (data not shown), concentrations of biotinylated S. typhi Vi coating antigen varying from 0·2 to 20 mm were assessed in the ELISA using known vaccinated sera to assess anti-S. typhi Vi IgG levels. A 2 mm antigen solution gave reproducible results over a range of optical densities (OD). For use, the 20 mm solution of biotinylated antigen was diluted to a 2 mm molarity in sodium acetate buffer (pH 5·5) and this is the coating concentration used in all assays within this report.
Anti- S. typhi IgG ELISA
Anti-S. typhi Vi IgG in serum was measured using an ELISA. Briefly, streptavidin-coated ELISA plates (Roche Diagnostics, UK) were coated with 50 µl/well of 2 mm biotinylated S. typhi Vi antigen in 25 mm Tris buffer and incubated for 16 h in a sealed moist specimen bag at RT. Following four washes with Tris buffer (Tris-T, BDH Supplies, Poole, UK) containing 0·05% Tween 20 Tris (Tris-T, BDH Supplies, Poole, UK) 100 µl of the standard curve sera at QC sera and test sera at three different dilutions (1/50, 1/100, 1/200) in Tris-T were added in duplicate to the wells and incubated for 2 h at RT. The mean of the three dilutions was used to calculate IgG levels. Eight doubling dilutions of sera (beginning at 1 in 20 in TRIS buffer) from an individual who had been immunized recently with 25 µg of purified Vi capsular polysaccharide of S. typhi were used to create the standard curve (Fig. 1). Following four washes, 100 µl of goat antihuman IgG alkaline phosphatase (Sigma, Poole, Dorset, UK) at 1/2000 concentration was added to each well and the plate incubated for a further 1 h at RT. After four further washes, 100 µl of p-nitrophenylphosphate substrate in EO16 buffer (Don Whitley Scientific, UK) was added to the wells of the plate. The colour reaction was allowed to develop at RT and monitored at 405 nm on an ELISA plate reader (Thermo Life Sciences, Basingstoke, UK). After 30 min the reaction was stopped by addition of 50 µl 3M NaOH to each well and the plate read again.
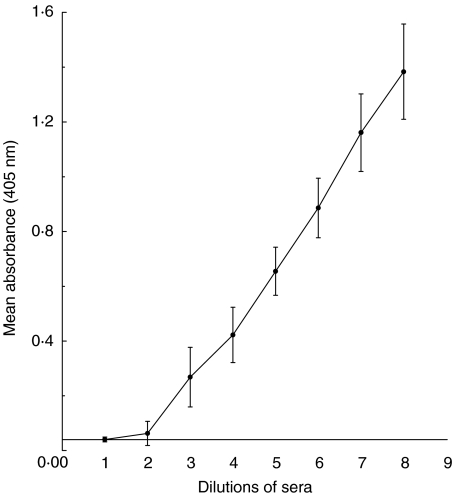
Fig. 1.
Reproducibility of the S. typhi Vi ELISA for specific IgG using sera from a recently vaccinated individual. Mean absorbance at 405 nm for the Tris buffer control, dilution 1 on the x-axis, and sera (from a recently vaccinated individual), was used to create the standard curve in the ELISA. Eight doubling dilutions of standard sera from one in 20 to one in 2560 (dilutions 2–9) were used. The mean absorbance (405 nm) ± 1 s.d. for each dilution over 18 assays is shown.
Anti-S. typhi Vi IgG levels are expressed as arbitrary units with reference to a standard curve. The top standard (dilution 1 in 20 in TRIS buffer) was assigned an arbitrary value of 2 AU/ml. OD values were read off the standard curve and converted into S. typhi Vi arbitrary units (AU/ml).
Statistical analysis
Normality tests to assess Gaussian distribution of antibody levels were performed using graphpad prism 4 software (GraphPad Software Inc., San Diego, CA, USA). As the distribution of antibody levels in adult sera was skewed, where appropriate results are expressed as median values plus interquartile ranges. Although S. typhi Vi IgG antibody levels in children's sera were normally distributed, to allow comparison with adults where appropriate, results for paediatric are presented as median values plus interquartile ranges. Unless stated otherwise, the Mann–Whitney U-tests and two-tailed P-values are quoted throughout. The Wilcoxon matched-pairs signed rank-sum test was used to assess the statistical significance between pre- and post-vaccination antibody levels in matched serum pairs. Analysis was performed using graphpad prism 4 software.
Specificity
Specificity was assessed by direct inhibition of the antigen. Test sera were preincubated overnight in a test tube at room temperature with increasing concentrations of non-biotinylated purified S. typhi Vi antigen (25–100 µg/ml, Aventis Pasteur, Lyon, France) or with control buffer and the supernatant assayed in the ELISA the following day. Results are expressed as OD at A405 nm or percentage inhibition calculated as A405 nm non-adsorbed sera –A405 nm adsorbed sera/OD non-adsorbed sera (×100).
RESULTS
Reproducibility of the ELISA
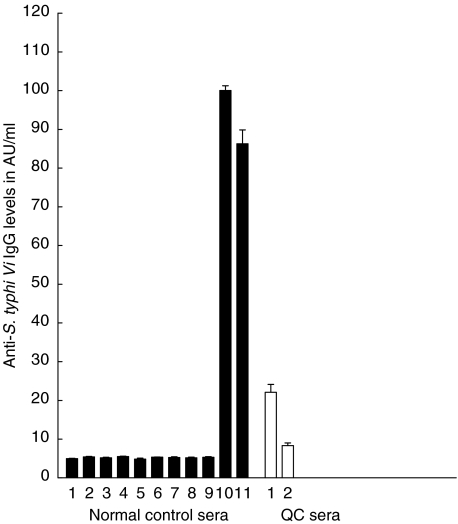
The intra-assay CVs for five different sera distributed over six different wells on the same plate ranged from 3·7 to 13·1% (data not shown). Figure 1 shows good reproducibility in the S. typhi Vi ELISA of the standard curve which is derived using sera from a recently vaccinated individual. Using this serum over 18 consecutive assays gave interassay CVs ranging between 4 and 19%. Mean background absorbance of the ELISA using Tris buffer alone in place of sera was 0·04 ± 0·009 (Fig. 1). Reproducibility of 11 normal sera, two of whom had been vaccinated recently with S. typhi Vi vaccine, was then determined. Mean interassay CVs for all 11 subjects over seven different assays ranged from 4·5 to 11·9%. Figure 2 demonstrates that interassay CVs were similar for those sera with low and high antibody levels. For sera with low levels, <10AU/ml (n = 9) the mean interassay CV was <11·9% and for two sera with >80AU/ml of anti-S. typhi IgG, the mean interassay CV was <11% (Fig. 2). The values and reproducibility of the QC sera used in the ELISA assay are indicated in Fig. 2. Data presented in this report have been drawn from the work of three different operators who developed this ELISA over a period of 2 years. During this time, one batch of antigen from Aventis was used. A standard operating procedure for the ELISA has been written-up in our laboratory and will be followed by all operators when conducting the assay in future.
Fig. 2.
Reproducibility of the S. typhi Vi ELISA for specific IgG using sera from healthy donors (n = 11). Anti-S. typhi Vi IgG levels expressed in AU/ml for 11 healthy donors (hatched bars). Anti-S. typhi Vi IgG values are expressed as the mean AU/ml ± 1 s.d. over seven different assays. Two of the 11 (nos 10 and 11) had been vaccinated recently with S. typhi Vi vaccine and their sera used in assays 4–6 weeks post-vaccination. The reproducibility of the two internal QC sera (run in each assay) is indicated by the clear bars. The QC values were 22·1 ± 5·0 and 8·3 ± 1·5 AU/ml, respectively.
Specificity by direct inhibition
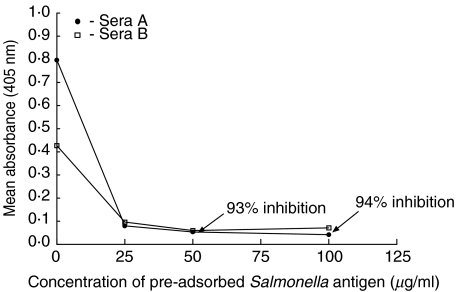
To demonstrate specificity of the anti-S. typhi Vi IgG ELISA by direct inhibition, sera (from recently vaccinated individuals) were chosen to represent medium to high levels of anti-S. typhi Vi IgG. In Fig. 3, sera A containing 45 AU/ml and sera B containing 23AU/ml of anti-S. typhi Vi IgG were used in the direct inhibition assay. Figure 3 shows that it was possible to inhibit between 70 and 79% (using sera B) and between 88 and 94% specific S. typhi Vi IgG binding in the ELISA when sera A was preincubated overnight with concentrations of S. typhi Vi at 100, 50 and 25 µg/ml prior to using in the ELISA. Figure 3 shows that inhibition was consistent at all concentrations of S. typhi Vi polysaccharide used, as evidenced by reduced absorbance at 405 nm. In this series of experiments, 100 µ/ml was the highest concentration of salmonella antigen used to inhibit activity in the ELISA. Future work will determine whether preincubation with higher concentrations of antigen (> 100 µg/ml) can abolish all activity in the ELISA.
Fig. 3.
Specificity of S. typhi Vi ELISA to detect IgG in sera from vaccinated individuals by direct inhibition.The mean absorbance (A405 nm) and inhibition (%) of IgG anti-S. typhi Vi in sera (one in 50 dilution) from two vaccinated subjects A and B was assessed following an overnight preadsorption with or without varying concentrations of S. typhi Vi antigen. Following preadsorption, sera A and B were used in the ELISA immediately. Sera A and B contained 45 AU/ml and 23 AU/ml anti-S. typhi Vi IgG, respectively. Results from a representative experiment are shown.
Levels of anti-S. typhi Vi IgG in adult and paediatric sera
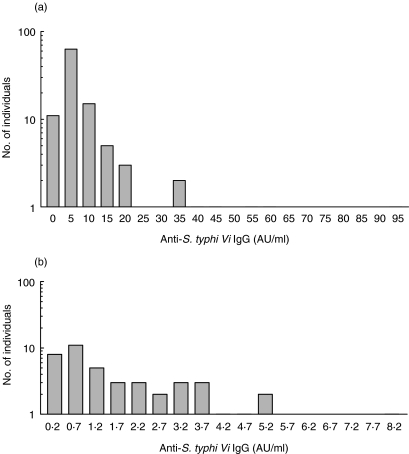
Tests for Gaussian distribution demonstrated that S. typhi Vi IgG levels from a group of 104 adult blood donors were not normally distributed (Fig. 4a). The median S. typhi Vi IgG value plus interquartile ranges for adult sera was 5·3 AU/ml (4·6–7·7 AU/ml) (Fig. 4a); In general, preschool children's sera had lower levels of anti-S. typhi Vi IgG, and the distribution was shown to be normal (Fig. 4b). The median value plus interquartile ranges of S. typhi Vi IgG in children's sera was 1·4 AU/ml (0·6–3·3 AU/ml). Concentrations of anti-S. typhi Vi IgG antibodies in the sera of healthy adults were significantly higher (P < 0·0001, Mann–Whitney U-test) than found in preschool children.
Fig. 4.
(a,b) Levels of anti-S. typhi Vi IgG levels in adult and child sera. (a) Distribution of anti-S. typhi Vi IgG in normal sera from healthy adult donors (n = 104, age range 20–70 years); (b) distribution from 4–5-year-old preschool children (n = 44). Values are shown as S. typhi Vi IgG AU/ml. Different scales are used in (a) and (b) due to significantly lower concentrations of S. typhi Vi IgG in preschool sera.
S. typhi Vi IgG levels pre- and post-vaccination in 23 adult sera
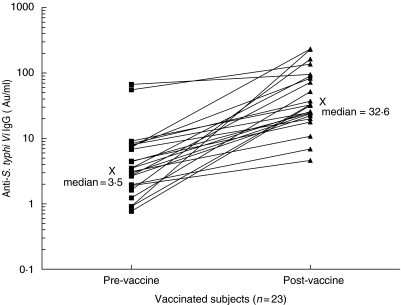
Levels of anti-S. typhi Vi IgG were significantly higher for post-vaccination subjects (n = 23) compared to paired prevaccination samples (P < 0·0001, Wilcoxon's signed rank sum test). The geometric mean value (with 95% confidence limits) prevaccination was 3·9 AU/ml (2·4, 6·5) and this increased post-vaccination to 39·2 (24·8, 61·3) (Fig. 5). The increase in anti-S. typhi Vi IgG for individual paired serum samples is shown in Fig. 5. Of the 23 serum samples, two had greater than 55 AU/ml anti-S. typhi Vi IgG prior to vaccination and on vaccination their serum anti-S. typhi Vi IgG increased by 1·4- and 2·5-fold, respectively (Fig. 5). Further questioning of these two subjects post-vaccination revealed that both may have had contact with the Salmonella organism in the previous 2 years (see Discussion).
Fig. 5.
Anti-S. typhi Vi IgG serum levels pre- and post-S. typhi Vi vaccination. Anti-S. typhi Vi IgG levels in 23 paired serum samples from normal adults taken pre- and 4–6 weeks post-S. typhi Vi vaccination. Median values pre- and post-vaccination are given and labelled with X. Two of the 23 serum samples had greater than 55 AU/ml anti-S. typhi IgG prior to vaccination.
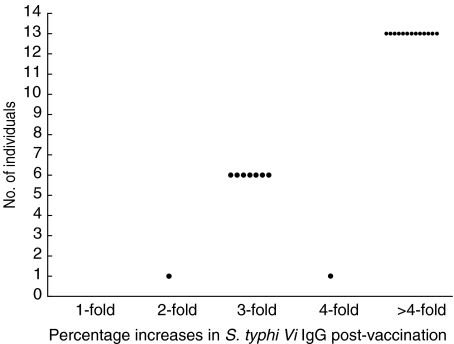
Of the remaining 21 serum samples, all responded to vaccination with two-, three-, four- and between five- and 254-fold increases in their serum S. typhi Vi IgG levels. Twenty of 21 (95%) exhibited post-vaccination responses greater than three times their prevaccination level and 13/21 (67%) had vaccination responses greater than four times their prevaccination level (Fig. 6). Ninety-five per cent of post-vaccination S. typhi Vi IgG levels were greater than the 75th percentile for normal adult values in non-vaccinated adults (Fig. 4b). Quadruplicate testing of pre- and post-sera from seven of the 23 vaccinees demonstrated the reproducibility of the data and good discrimination between pre- and post-antibody levels (data not shown).
Fig. 6.
Responses to S. typhi Vi vaccination in healthy adults. Increases in S. typhi Vi IgG post-vaccination compared to prevaccination levels are shown for 21 of 23 vaccinated individuals. Percentage increases are expressed as two-, three- and fourfold and as > fourfold.
DISCUSSION
Did we develop a reproducible ELISA?
In this study, we have shown the application of a solid-phase ELISA using biotinylated purified Vi capsular polysaccharide on avidin-coated microtitre plates to detect serum IgG antibodies to S. typhi Vi polysaccharide. At present, we do not have an international reference preparation for this ELISA and used an in-house reference preparation. We are actively investigating the possibility of obtaining one from the vaccine manufacturers.
The ELISA was reproducible across all ranges of IgG anti-S. typhi Vi antibody levels as indicated by intra- and inter-CVs of <12% irrespective of high or low serum anti-S. typhi Vi IgG levels (Figs 1 and 2). One of the most important variables affecting reproducibility in ELISA is consistent coating of the solid phase. A number of factors can affect polysaccharide-coating concentrations in ELISA [8–10]. The biotin–avidin method of coating was chosen on the basis of its performance against a number of other coating methods (data not shown; Simmonds V. A study to design an ELISA to measure Salmonella typhi antibodies in humans. MSc dissertation, 2000. University of London, Kings College Department of Immunology). These included dissolving the polysaccharide in carbonate buffer, mixing with methylated human serum albumin and dissolving the Vi capsular polysaccharide in 1%N-(3-dimethylaminopropyl)-N-ethyl-carbondiimide hydrochloride (EDC) before immobilizing on Covalink plates [10].
Using an avidin–biotin complex to attach antigen to the solid phase has several advantages over other ELISA systems. The high affinity that avidin has for biotin means that low concentrations of polysaccharide can be attached to avidin-coated plates rapidly and efficiently, background absorbance is minimal or absent and polysaccharide modification can be controlled to ensure minimal alterations to antigenic determinants [8–10].
The specificity of the assay for detecting anti-S. typhi Vi IgG antibodies in serum was demonstrated in part by the in vivo immunization responses observed (see below) and also by direct inhibition assays where purified salmonella antigen was shown to absorb between 70 and 90% of anti-S. typhi Vi IgG from hyperimmune sera following an overnight incubation prior to use in the ELISA (Fig. 3). The failure to totally inhibit recently vaccinated adult sera with purified salmonella antigen may be due to the heterogeneity of the specificities and affinities and of these antibodies. It may also reflect the high quantities of anti-S. typhi Vi IgG present in the sera from these recently vaccinated individuals.
Determining the range of pre-existing antibody levels in adults and children
In the populations of blood donors assessed in the current study the 75th percentile value was 7·7 AU/ml S. typhi Vi IgG for adults and 3·3 AU/ml for preschool children (Fig. 4a,b). Forty per cent of children's values were below 1·25AU/ml (Fig. 4b). At present this ELISA at assay may not be able to discriminate accurately AU S. typhi Vi IgG levels below this amount. However, as the slope in Fig. 1 demonstrates, it is probably fairly accurate down to 1·25 AU/ml, becoming more grey below this value and this would cover 60% of the preschool children's values. Work is ongoing to increase the sensitivity of the ELISA. We detected significantly higher concentrations of anti-S. typhi Vi IgG antibodies in the sera of healthy adults compared to children (P < 0·0001), suggesting age to be an important factor in antibody levels detected using this ELISA. This could be consistent with an age-dependent increase and increased natural exposure to S. typhi organisms or cross-reacting antigens by adults (Fig. 4b). Another possible explanation is the slow ontogeny of antipolysaccharide responses during childhood; a fully effective response to carbohydrate antigens is seen around 5 years of age in humans [11]. We did not test sera from children under the age of 2 years because of the known physiological immaturity of the immune system in handling pure polysaccharides in this age group [11–13]. Future studies will aim to examine baseline antibody levels across all age ranges.
Could we establish a good immunization response?
This ELISA assay was able to clearly distinguish significantly higher median levels of anti-S. typhi Vi IgG in 23 adults following Vi polysaccharide vaccination (Fig. 5). Two of the 23 vaccinated individuals had greater than 55 AU/ml anti-S. typhi IgG prior to vaccination. However, one of the two had suffered a severe systemic illness while holidaying in Asia less than 2 years prior to being vaccinated and it had been suggested at the time that poor drinking water was to blame. Although no organism was isolated, it is possible that S. typhi may have been the cause. The other subject frequently visited the tropics where she had been brought up and it is possible that she was more likely than the other vaccinees to have encountered the salmonella organism there.
Of the remaining 21 vaccinees, the vaccination data showed a threefold rise in IgG levels between pre- and post-vaccination sera for 95% of subjects (Fig. 6). All of these had post-vaccination anti-S. typhi Vi IgG levels greater than the 75th percentile for normal non-vaccinated adult values (Figs 4a and 5). One of the 21 vaccinees (5%) demonstrated a 2·5 rise in IgG levels in her post-vaccination sera. This is not unusual for carbohydrate responses, where a small proportion of poor responders to other carbohydrate molecules have been seen [14].
Attempts to define normal antibody responses to polysaccharides have proved difficult. A ‘modified meta analysis’ of 23 studies to define the normal antibody response to pneumococcal vaccine showed wide variation in antibody responses to pneumococcal vaccine among normal subjects [21]. Based on the data in this study, we propose a threefold increase in anti-S. typhi Vi IgG post-vaccination to be considered a positive vaccination response. We suggest that serum prevaccination levels greater than 24·8 AU/ml anti-S. typhi Vi IgG (the lower limit of the 95% confidence limits post-vaccination) may exclude the need to vaccinate. However, more data on a larger cohort of pre- and post-vaccinated subjects will confirm the accuracy of these figures.
The efficacy of the S. typhi Vi capsular polysaccharide vaccine has been demonstrated in field trials in highly endemic areas [6,22]. In these studies, antibody levels were measured using radioimmune assays. In one study in Nepal where 275 subjects were immunized with the S. typhi Vi vaccine, 75% of vaccinees responded with a fourfold rise in serum antibody levels, which compares closely to level found in this study where 67% of 21 vaccinated subjects responded with a fourfold increase in serum antibodies.
The use of pure polysaccharide vaccines in the form of Hib PRP and 23 valent pneumococcal polysaccharide (Pneumovax) to define selective anti polysaccharide antibody deficiencies is well documented (reviewed in [2,15]). While the advent of conjugate Hib vaccines led to the discontinuation of Hib PRP, Pneumovax continues to be widely used in clinical immunology practice at present. Test immunization with Pneumovax has been valuable in the assessment of primary immunodeficiency and its widespread use has highlighted patient populations who may have subtle defects in handling polysaccharide antigens [14, 16, 17, 18, 19, 20]. Our results suggest that S. typhi Vi vaccine is also likely to be an useful test immunogen in the assessment of antibody responses to polysaccharide antigens and we plan to validate the use of S. typhi Vi vaccine in patients with suspected primary and secondary antibody deficiency in order to test this hypothesis.
Acknowledgments
We would like to thank the staff in the immunology laboratory for organizing the blood transfusion samples and Dr A. Aslam for help in immunizing some of the volunteers.
REFERENCES
- 1.Conley ME, Notorangelo D, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Clin Immunol. 1999;93:190–7. doi: 10.1006/clim.1999.4799. [DOI] [PubMed] [Google Scholar]
- 2.Chapel HM, Misbah SA, Webster D. Assessment of the immune system. In: Ochs H, Puck J, Smith CIE, editors. Primary immunodeficiency diseases. 2. Oxford University Press; 2004. in press. [Google Scholar]
- 3.Booy R, Taylor SA, Dobson SRM, et al. Immunogenicity and safety of PRP-T conjugate vaccine given according to the British accelerated immunisation schedule. Arch Dis Child. 1992;67:475–8. doi: 10.1136/adc.67.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kayhty H, Peltola H, Karanko V, Makela PH. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1983;147:1100. doi: 10.1093/infdis/147.6.1100. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Pediatrics Committee on Infectious Diseases Policy statement. Recommendations for the prevention of pneumococcal infections, including the use of pneumococcal conjugate vaccine (Prevnar), pneumococcal polysaccharide vaccine and antibiotic prophylaxis. Pediatrics. 2000;106:362–6. doi: 10.1542/peds.106.2.362. [DOI] [PubMed] [Google Scholar]
- 6.Klugman KP, Gilbertson IT, Koornhof HJ, et al. Protective activity of Vi capsular polysaccharide vaccine against typhoid fever. Lancet. 1987;330:1165–9. doi: 10.1016/s0140-6736(87)91316-x. [DOI] [PubMed] [Google Scholar]
- 7.Salisbury D, Begg NT. Immunisation against infectious disease. London: HMSO; 1996. pp. 243–9. ch. 33, Typhoid. [Google Scholar]
- 8.Diaz Romero J, Outschoorn I. Selective biotinylation of Neisseria meningitides group B capsular polysaccharide and application in an improved ELISA for the detection of specific antibodies. J Immunol Meth. 1993;160:35–47. doi: 10.1016/0022-1759(93)90006-s. [DOI] [PubMed] [Google Scholar]
- 9.Sutton AF, Vann W, Karpas AB, Stein KE, Schneerson R. An avidin–biotin based ELISA for quantitation of antibody to bacterial polysaccharides. J Immunol Meth. 1985;82:215–24. doi: 10.1016/0022-1759(85)90353-9. [DOI] [PubMed] [Google Scholar]
- 10.Zielen S, Broker M, Strnad N, et al. Simple determination of polysaccharide specfic antibodies by means of chemically modified ELISA plates. J Immunol Meth. 1996;193:1–7. doi: 10.1016/0022-1759(96)00033-6. [DOI] [PubMed] [Google Scholar]
- 11.Peltola H, Kayhty H, Sivonen A, Makela PH. Haemophilus influenzae type b capsular polysaccharide vaccine in children. A double blind field study of 100,000 vaccinees 3 months to 5 years of age in Finland. Paediatrics. 1977;60:730–7. [PubMed] [Google Scholar]
- 12.Barrett DJ, Lee CG, Ammann AJ, Ayoub EM. IgG and IgM pneumococcal polysaccharide antibody responses in infants. Paediatr Res. 1984;18:1067–71. doi: 10.1203/00006450-198411000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Timens W, Boes A, Rozeboom-Uiterwijk T, Poppema S. Immaturity of the human splenic marginal zone in infancy. Possible contribution to the deficient infant immune response. J Immunol. 1989;143:3200–6. [PubMed] [Google Scholar]
- 14.Misbah SA, Griffiths H, Mitchell T, Freeland A, Haeney MR, Chapel HM. Anti-polysaccharide antibodies in 450 children with otitis media. Clin Exp Immunol. 1997;109:67–72. doi: 10.1046/j.1365-2249.1997.4291322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rijkers GT, Lieke AM, Sanders AM, Zegers BJM. Anti-capsular polysaccharide antibody deficiency states. Immunodeficiency. 1993:1–21. [PubMed] [Google Scholar]
- 16.Gennery AR, Barge D, O'Sullivan JJ, Flood TJ, Abinun MA, Cant AJ. Antibody deficiency and autoimmunity in 22q11.2 deletion syndrome. Arch Dis Child. 2002;86:422–5. doi: 10.1136/adc.86.6.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lane PJ, MacLennan ICM. Impaired IgG 2 anti-pneumococcal antibody responses in patients with recurrent infection and normal IgG2 levels but no IgA. Clin Exp Immunol. 1986;65:427–33. [PMC free article] [PubMed] [Google Scholar]
- 18.Arkwright PD, Patel L, Moran A, Haeney MR, Ewing CI, David TJ. Atopic eczema is associated with delayed maturation of the antibody response to pneumococcal vaccine. Clin Exp Immunol. 2000;122:16–9. doi: 10.1046/j.1365-2249.2000.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dworkin MS, Ward JW, Hanson DL, Jones JL, Kaplan JE. Pneumococcal disease among human immunodeficiency virus-infected persons: incidence, risk factors and impact of vaccination. Adult Adolescent Spectrum HIV Disease Project. Clin Infect Dis. 2001;32:794–800. doi: 10.1086/319218. [DOI] [PubMed] [Google Scholar]
- 20.Aucoturier P, Barra A, Intrator L, et al. Long lasting IgG subclasses and antibacterial polysaccharide antibody deficiency after allogeneic bone marrow transplantation. Blood. 1987;70:779–85. [PubMed] [Google Scholar]
- 21.Go ES, Ballas ZK. Anti-pneumococcal antibody response in normal subjects: a meta-analysis. J Allergy Clin Immunol. 1996;98:205–15. doi: 10.1016/s0091-6749(96)70244-0. [DOI] [PubMed] [Google Scholar]
- 22.Acharya IL, Lowe CU, Thapa R, et al. Prevention of typhoid fever in Nepal with the Vi capsular polysaccharide of Salmonella typhi. A preliminary report. N Engl J Med. 1987;317:1101–4. doi: 10.1056/NEJM198710293171801. [DOI] [PubMed] [Google Scholar]