Abstract
Blood samples were collected over a 4-year period from 335 children (aged 1–16 years) suffering from recurrent respiratory infections and 78 controls. The patients were subdivided into four groups: I, children with no immune system defects detected (n = 101); II, children with allergies (n = 94); III, children with humoral response defects (n = 93); and IV, children with disturbances of cellular immunity (n = 66). Nineteen patients had both humoral and cellular abnormalities. All patients and controls were investigated to determine the exon 1 and promoter region variants of the mbl-2 gene. MBL serum concentrations were also determined in samples from 291 patients and 75 controls. The proportion of O (B, D or C) alleles was significantly higher in the patient group compared to controls, and this association was strongest for subgroup III. The promoter LX variant frequency was also commoner in the patients as a whole, and significantly so in subgroups II and IV. Genotypes markedly influenced MBL concentrations in all groups, and correlated with ability to activate the lectin pathway of complement activation. The strongest and most significant inverse correlations between serum MBL and respiratory disease were found in patient group III and in 17 patients with multiple humoral and/or cellular abnormalities. Among nine patients with unexpectedly low LP activity in view of their MBL concentrations, one person was found to be MASP-2 deficient. Our results indicate that mannan-binding lectin insufficiency, with or without a coexisting immune defect, is associated with the occurrence of recurrent respiratory infections in childhood, and this relationship is particularly strong and statistically significant in children with concomitant impairments of humoral immunity.
Keywords: mannan-binding lectin (MBL), infection, respiratory system, innate immunity
INTRODUCTION
Mannan-binding lectin (MBL) is thought to be one of the key molecules in the innate immune system. It belongs to the collectin family: a group of Ca2+-dependent lectins possessing a collagen-like helical domain. MBL recognizes pathogen-associated molecular patterns (PAMPs) [1,2]. The C-terminal carbohydrate recognition domain (CRD) binds microbial surface glycoconjugates rich in mannose, N-acetylglucosamine and fucose. MBL protects the host from infection by lysis of microorganisms involving complement activation via the lectin pathway, in which MBL-associated serine proteases (MASPs) take part [3,4]. Anaphylatoxins released during this process help to limit the infection through their chemotactic activities. In parallel, MBL may enhance phagocytosis by direct opsonization involving MBL receptors on phagocytic cells. Thus MBL insufficiency, believed to be the most common human immunodeficiency, may contribute to increased susceptibility to numerous infectious diseases. The apparent serum concentration and complement-activating activity of MBL markedly depends on mbl-2 gene point mutations in codons 52, 54, and 57 of exon 1. These variants, giving dominant alleles D, B, and C, respectively (commonly designated collectively as O; the normal wild-type is designated A) lead to disruption of the collagen domain structure. This, in consequence, prevents oligomerization of the basic triplet polypeptide subunit and therefore normal interaction with MASPs resulting in diminished complement activation and opsonic activity. A shortened biological half-life of the protein is a reflection of an increased sensitivity to serum metalloproteases. As well as the afore-mentioned mutations, polymorphisms in the promoter region (at positions −550 and −221; variants H/L and X/Y, respectively) and the exon 1 untranslated region (at position 4, variants P/Q) have been described. These influence gene expression and, in consequence, the serum concentration of the protein. The highest MBL level occurs in association with the genotype, HYP/HYP, and the lowest with LXP/LXP homozygotes [5, 6, 7, 8, 9, 10, 11].
Respiratory diseases in children, including infections, are among the most important public health problems. Although infant mortality rates are falling in Poland, respiratory disorders of childhood are amongst the commonest reasons for medical consultations in primary medical care and one of the main indications for hospitalization.
Primary and acquired immunodisorders are considered to play an important role in recurrent and chronic respiratory system infections [12]. Here we present results of an investigation of the heterogeneity of the mbl-2 gene as well as serum MBL concentrations and activity in selected groups of children suffering from recurrent infective diseases. Some of our paediatric patients had no known immunological abnormalities other than MBL insufficiency, some were children with allergies, and some had established defects of humoral and/or cellular immunity. Our data indicate that MBL insufficiency contributes to increased susceptibility to infections of the respiratory system.
PATIENTS AND METHODS
Blood and serum samples
Blood samples for DNA preparation were taken to tubes containing sodium citrate and stored at −70°C. Serum samples were obtained from blood taken into tubes with no anticoagulant, from the same group of children. They were also stored at −70°C until tested.
Patients and controls
The patients were Polish children (mean age 7·6 ± 3·5; range 1–16 years) suffering from recurrent infections of the respiratory system (two or more pneumonias or serious sinus infections within one year; eight or more new upper respiratory tract infections within the same year), attending the Unit of Immunodisorders, Institute-Polish Mothers’ Memorial Hospital, Lodz, Poland. Samples were collected between 1999 and 2003. Blood was taken when no symptoms of infection were observed by physicians. All children had been previously investigated for immune abnormalities other than MBL insufficiency. On the basis of evaluation of immune status (see below), the patients were classified into four groups (Table 1): I, children with no known immune system abnormalities (n = 101; mean age 7·1 ± 3·3 years); II, children with allergies/high IgE (n = 94; mean age 8·3 ± 3·5 years); III, children with humoral response defects (n = 93; mean age 7·2 ± 3·9 years); IV, children with cellular response defects (n = 66; mean age 7·8 ± 3·1) in which HIV infection was excluded. The total number of patients (groups I-IV) was 335, as 19 children had both humoral and cellular response defects. This small group with multiple immune defects is denoted in Table 1 as group X. Serum samples were obtained from 291 patients.
Table 1.
Patients classified by principal diagnosis and immune status
| Group | Immunodefects | Number of DNA (serum) samples |
|---|---|---|
| I | None | 101 (90) |
| II | Asthma | 39 (35) |
| Allergic rhinitis (pollen allergy) | 18 (16) | |
| Allergic rhinitis (dust allergy) | 11 (8) | |
| Allergic rhinitis (pollen and dust allergy) | 6 (5) | |
| Allergic rhinitis (animal hair allergy) | 5 (5) | |
| Food allergy | 3 (3) | |
| Atopic dermatitis | 4 (3) | |
| High IgE only, without atopic manifestations | 8 (7) | |
| III | Hypogammaglobulinemia | 12 (11) |
| Agammaglobulinemia | 2 (2) | |
| IgA deficiency | 15 (11) | |
| Low IgA level | 8 (7) | |
| Low IgG level | 14 (13) | |
| Low IgA and IgG levels | 5 (4) | |
| Low IgG and IgM levels | 3 (2) | |
| Low CH50 value | 5 (2) | |
| IgA deficiency and low CH50 value | 2 (1) | |
| CVID | 6 (5) | |
| Nijmegen syndrome | 2 (2) | |
| IV | Low CD3 lymphocyte count | 12 (12) |
| Low CD4/CD8 lymphocyte count | 19 (15) | |
| Low CD3 and CD4/CD8 lymphocyte counts | 14 (13) | |
| Low CD3 lymphocyte count and defect of phagocytosis | 1 (1) | |
| Defect of phagocytosis | 1 (1) | |
| X | Hypogammaglobulinemia and low CD3 lymphocyte count | 1 (1) |
| Hypogammaglobulinemia and low CD4/CD8 lymphocyte count | 1 (1) | |
| Hypogammaglobulinemia and low NK cell count | 1 (1) | |
| IgA deficiency and low CD4/CD8 lymphocyte count | 3 (3) | |
| CVID and low CD4/CD8 lymphocyte count | 2 (2) | |
| CVID, low CD3 and CD4/CD8 lymphocyte counts | 1 (0) | |
| IgA deficiency and low CD4/CD8 lymphocyte and NK cell counts | 1 (1) | |
| Low IgA level and low CD3 lymphocyte count | 1 (1) | |
| Low CH50 value and defect of phagocytosis | 1 (0) | |
| Low CH50 value and low CD3 lymphocyte count | 1 (1) | |
| Low IgG and IgM levels and low CD3 lymphocyte count | 1 (1) | |
| Low IgG level and low CD3 and CD4/CD8 lymphocyte count | 2 (2) | |
| Low IgG level and low CD3 lymphocyte count | 1 (1) | |
| Low IgA and IgG levels and low CD3 lymphocyte count | 2 (2) |
The control group consisting of 78 healthy children (mean age 7·4 ± 3·1 years), receiving no medication, attending hospital for reasons unconnected with infections (e.g. delayed vaccination). Serum samples were obtained from 75 children.
Approval of the local ethical commission and informed parental consent was received. Parents were informed about results of the investigation.
Evaluation of immune status
In patients with recurrent infections or other indications of possible immunodeficiency disease (recommendations of the European Society for Immunodeficiencies), cystis fibrosis, local obstructive problems, bronchial hyperreactivity and allergy were excluded first. A multistage laboratory protocol for the diagnosis of immunodeficiency was used. A low threshold for the performance of simple screening tests (total blood count and differential, zone-electrophoresis, Ig levels, CH50, CD3/CD19 lymphocyte subsets and HIV-status) allows early exclusion or identification of potential immunodeficiency, whereas more elaborate tests (IgG-subclasses, specific antibody responses, lymphocyte subsets, lymphocyte function tests, individual complement components, neutrophil function tests, in vitro cytokine/interleukin production, chromosomal analysis, determination of genetic defect) leading to diagnosis and definitive classification are reserved for those patients in whom an immunodeficiency is more probable [12].
Enumeration of T and B lymphocyte subsets, NK cells, and the phagocytosis test were performed on peripheral blood cells by standard immunofluorescence techniques with the use of monoclonal antibodies and flow cytometry (FACSCalibur, Becton-Dickinson, San Jose, CA, USA). The response of lymphocytes to stimulation in vitro was determined by the uptake of 3H-thymidine after stimulation with mitogens and monoclonal antibodies. Levels of serum immunoglobulins and complement components was measured by automated immunoturbidometric methods or ELISA techniques. Total haemolytic activity of the complement system (CH50) was evaluated by Mayer's method.
Age-matched reference ranges for lymphocyte subpopulations and NK cells, lymphocyte proliferation tests, values of complement components, immunoglobulins and IgG-subclasses in serum, complement activation tests and neutrophil function tests have been published for healthy Polish children [12].
Mbl-2 gene analysis
Genomic DNA was extracted from blood samples according to the GTC method [13]. Exon 1 mbl-2 gene was amplified by PCR (685 bp) using the following primers:
5′-AGTCGACCCAGATTGTAGGACAGAG-3′;
5′-AGTTGTTGTTCTCCTGTCCAG-3′[5]
The PCR products were digested by BanI and MboII restriction enzymes and separated on a 6% polyacrylamide gel.
The D allele was detected by RFLP method performed on PCR products of 125 bp using primers:
5′-CATCAACGGCTTCCCAGGCAAAGACGCG-3′
5′-AGGATCCAGGCAGTTTCCTCGGAAGG-3′[5]
The PCR product was digested by MnlI and HpaI restriction enzymes and electrophoresed on a 6% polyacrylamide gel in TAE buffer.
The cis-trans promoter/exon 1 untranslated region variants H, L, X, Y, P, Q relative to the structural variants A, B, C, D were determined by PCR using the following primers:
5′-GGAGGCTTAGACCTATGGGGCTAGG-3′
5′-GGGACATGGTCCTCACCTTGGTG-3′[5]
The appropriate PCR products of 866 bp were separated on Wizard columns (Promega, Madison, WI, USA) and sequenced by the dideoxy chain termination method (Epicentre Technologies, Madison, WI, USA) starting from the following primers specific for:
H/L: 5′-CCAACGTAGTAAGAAATTTCC-3′
X/Y: 5′-GGCATAAGCCAGCTGGCAATGC-3′
P/Q: 5′-GGGATGGGTCATCTATTTCTATATAGCC-3′[5]
Isolation of MBL from plasma
MBL was isolated from pooled plasma from healthy blood donors essentially as described in [14,15]. The MBL concentration was determined using human recombinant MBL as a standard (originally from Dr R. A. Ezekowitz, Harvard Medical School, Boston, USA, and kindly given to us by Prof B. Rozalska, Institute of Microbiology and Immunology, University of Lodz).
Determination of MBL concentration in sera
Determination of serum MBL concentration was performed using ELISA, according to the procedure described by Aittoniemi et al. [16], modified. Microtitre plates (MaxiSorp U96, NUNC, Denmark) were coated with mannan from Saccharomyces cerevisiae (Sigma) solution in carbonate buffer (pH 9·6) and incubated at 4°C, overnight. After that, wells were blocked with 5% BSA (Sigma) in phosphate buffered saline (PBS), pH 7·2. Next, sera to be tested serially diluted in imidazole buffer supplemented with NaCl and CaCl2 containing 5% BSA, pH 7·8, were added. Samples were incubated for 2 h at 37°C and at 4°C overnight with gentle shaking. The primary antibody was mouse anti-human MBL mAb (clone 131–01, AntibodyShop/Statens Serum Institut, Denmark at 0·5 µg/ml in Tris buffered saline supplemented with CaCl2 and 5% BSA); secondary antibody was peroxidase conjugated rabbit anti-mouse Ig (Sigma) in the same buffer. As substrate for peroxidase, 2,2′-azino-bis(3-ethylbenz-thiazoline-6-sulphonic) acid (ABTS; Sigma, St. Louis, MO, USA) was employed. Absorbance values (λ = 405 nm) were determined with the help of EIA Multiscan reader (Labsystems, Helsinki, Finland). MBL purified from plasma was used as a standard.
All washing steps were done with PBS containing 0·05% Tween 20 (Bio-Rad, Hercules, CA, USA).
Complement lectin pathway activity of sera
Determination of serum LP activity (C4b deposition capacity) was performed using ELISA, as described by Petersen et al. [17], modified. Microtiter plates (MaxiSorp U96, NUNC, Roskilde, Denmark) were coated with mannan from Saccharomyces cerevisiae (Sigma) and incubated at 4°C overnight. Next, they were blocked with BSA (Sigma) for 2 h at 37°C. After that, the sera to be tested were serially diluted in MBL-binding buffer [17] (high ionic strength, to exclude the classical pathway). Samples were incubated at 4°C, overnight with gentle shaking. Then complement component C4 (Sigma) was added and plates incubated for 2 h at 37°C. The primary antibody (rabbit anti-human C4 serum from Sigma) was added, followed by the secondary antibody (peroxidase conjugated goat anti-rabbit Ig from DAKO). As substrate for peroxidase, 2,2′-azino-bis(3-ethylbenz-thiazoline-6-sulphonic) acid (ABTS, Sigma) was employed. Absorbance values were determined with the help of EIA Multiscan reader (Labsystems). As a standard, the serum of healthy blood donor of HYPA/HYPA genotype, containing 2000 ng/ml MBL was used, for which LP activity was arbitrarily determined as 1 U/ml.
Masp-2 gene sequencing
Mutations in the gene encoding the CUB1 domain of MASP-2 were detected by the method of Stengaard-Pedersen et al. [18], using DNA extracted from citrated blood.
Statistical analysis
Frequencies of O and LX alleles were compared by Fisher's exact test (2-sided). Median serum MBL concentrations among different groups were compared with the Mann–Whitney U-test. P-values < 0·05 were considered statistically significant.
RESULTS
Analysis of exon 1 and promoter regions
Among 78 children in the control group, 62 (79·5%) were A/A homozygotes, 14 (18%) were A/B heterozygotes, one (1·3%) was an A/C heterozygote and one (1·3%) was an A/D heterozygote. Two hundred and fifteen (64%) of 335 children suffering from recurrent infections of the respiratory system had the A/A genotype, 102 (30·5%) were A/B heterozygotes, and one (0·3%) was an A/C heterozygote. Seventeen patients were homozygous for variant alleles (13 in codon 54 and 4 in codon 52). The frequency of O alleles in the patient group was 0·204 and 0·102 in the control group (Table 2). Relative to A/A homozygotes, the patients had a significantly increased proportion of O/O homozygotes (P = 0·03) and of variant alleles (O/O or O/A; P = 0·01) compared to controls.
Table 2.
The frequency of exon 1 alleles and promoter haplotypes in patients and controls
| Group | Frequency of exon 1 alleles | Frequency of promoter region haplotypes |
|---|---|---|
| Control | A − 0·898 | HY – 0·513 |
| B − 0·090 | LY – 0·359 | |
| C − 0·006 | LX – 0·128 | |
| D − 0·006 | ||
| I | A − 0·812 | HY – 0·406 |
| B − 0·178 | LY – 0·416 | |
| C − 0·000 | LX – 0·178 | |
| D − 0·010 | ||
| II | A − 0·819 | HY – 0·356 |
| B − 0·165 | LY – 0·420 | |
| C − 0·005 | LX – 0·223 | |
| D − 0·011 | ||
| III | A − 0·729 | HY – 0·280 |
| B − 0·250 | LY – 0·506 | |
| C − 0·000 | LX – 0·215 | |
| D − 0·021 | ||
| IV | A − 0·811 | HY – 0·303 |
| B − 0·189 | LY – 0·416 | |
| C − 0·000 | LX – 0·280 | |
| D − 0·000 | ||
| Total I-IV | A − 0·796 | HY – 0·340 |
| B − 0·191 | LY – 0·448 | |
| C − 0·001 | LX – 0·212 | |
| D − 0·012 |
The frequency of the insufficiency-associated LX haplotype in the control group was 0·128 and 0·212 in the patient group (Table 2). Two (2·6%) of the control children were LX/LX homozygotes compared to 29 (8·7%) in the patients. The trends towards increased presence of the LX allele (P = 0·08) and homozygosity for LX (P = 0·06) in the patient group failed to reach statistical significance.
Detailed mbl-2 genotype data for patients and controls are summarized in Table 3. These data were analysed separately for the four clinical subgroups.
Table 3.
Genotypes of mbl-2 gene exon 1 and promoter region represented in patients and controls
| Group | ||||||
|---|---|---|---|---|---|---|
| Genotype | Control | I | II | III | IV | Total I-IV* |
| HYPA/HYPA | 29 | 21 | 14 | 11 | 9 | 53 |
| LYPA/LYPA | 2 | 2 | 6 | 0 | 1 | 9 |
| LYQA/LYQA | 9 | 8 | 9 | 9 | 5 | 31 |
| LXPA/LXPA | 2 | 7 | 6 | 9 | 10 | 29 |
| HYPA/LYPA | 2 | 1 | 1 | 1 | 2 | 5 |
| HYPA/LYQA | 3 | 9 | 2 | 5 | 2 | 18 |
| HYPA/LXPA | 7 | 11 | 16 | 8 | 6 | 39 |
| LYPA/LXPA | 6 | 1 | 2 | 1 | 1 | 4 |
| LYQA/LXPA | 2 | 7 | 6 | 7 | 8 | 27 |
| LYPA/LYPB | 0 | 5 | 1 | 3 | 1 | 10 |
| LYQA/LYPB | 5 | 4 | 4 | 8 | 4 | 18 |
| HYPA/LYPB | 8 | 17 | 17 | 16 | 12 | 57 |
| LXPA/LYPB | 1 | 4 | 7 | 6 | 2 | 17 |
| HYPA/LYQC | 1 | 0 | 1 | 0 | 0 | 1 |
| HYPA/HYPD | 1 | 0 | 0 | 0 | 0 | 0 |
| LYPB/LYPB | 0 | 3 | 1 | 7 | 3 | 13 |
| HYPD/HYPD | 0 | 0 | 1 | 0 | 0 | 1 |
| LYPD/LYPD | 0 | 1 | 0 | 2 | 0 | 3 |
Numbers presented as ‘total’ do not equal the sum of values from columns corresponding to groups I-IV since 19 patients, as was mentioned in Patients and Methods section, were included in two groups.
Sixty-seven (66·3%) of group I (no known immune defect) patients were A/A homozygotes, 30 (29·7%) were A/B heterozygotes, while 3 (3%) and 1 (1%) had homozygotic B and D type mutations, respectively. The proportion of genotypes containing variant alleles (O/O or A/O) relative to the wild-type (A/A) was higher in the patients but just failed to reach statistical significance (P = 0·065). The increased frequency of LX haplotypes in this subgroup was not significant (P = 0·5), although 7% of the children were LX/LX homozygotes.
Sixty-two (66%) of group II (atopy/allergy) patients were A/A homozygotes, 29 children (31%) were A/B heterozygotes, and 1 (1·1%) was an A/C heterozygote. Two children were O/O homozygotes (1 B/B and 1 D/D). Again, the higher proportion of variant alleles relative to A/A homozygotes in the patients failed to reach statistical significance (P = 0·06). However, there was a significantly increased proportion of LX haplotypes in this subgroup (P = 0·047). Six (6·4%) children were LX/LX homozygotes.
Fifty-one (54·8%) of group III (humoral defects) children were A/A homozygotes, 33 (35·5%) were A/B heterozygotes, 7 (7·5%) were B/B and 2 (2·15%) were D/D homozygotes. In this group the highest proportion of structural gene mutations was found and this was highly significant (P = 0·001). Although 8 patients (8·5%) were LX/LX homozygotes, the LX haplotype was not significantly over-represented in this subgroup (P = 0·13).
Forty-four (66·7%) of the 66 group IV (cellular abnormality) patients were A/A homozygotes, 19 (28·8%) were A/B heterozygotes; and 3 (4·6%) were B/B homozygotes. Neither C nor D alleles were found in this group. The proportion of the B variant alleles was not significantly higher in these patients (P = 0·09), but 10 (15%) of those children were LX/LX homozygotes and the increased presence of the LX haplotype in this subgroup did reach statistical significance (P = 0·03).
When the proportions of variant alleles in the patient groups with additional immune abnormalities (groups II to IV) were compared, as individual groups or collectively, with the patients with no known immune abnormalities (group I), no statistically significant differences were found (data not shown). On the other hand, it is remarkable that all but 2 of the 19 patients with multiple immune defects (group X) had at least one variant allele (P = 0·0002, compared with group I).
MBL concentration in sera
MBL concentrations were determined in serum samples from 291 patients with respiratory infections and 75 healthy controls (Table 4; Fig. 1). In general, patient values were significantly lower than those of controls (medians 1082 versus 1444 ng/ml; P = 0·002). When the patients were divided into clinical subgroups, each subgroup had a median value significantly lower than that of the control group (Table 4). When similar comparisons were made with group I, only group III approached statistical significance (P < 0·06). However, the median value of the 17 sera from patients with two or more immune abnormalities common to groups III and IV was significantly different from that of group I (P = 0·02).
Table 4.
Serum MBL concentrations in patients and controls
| Group | n | Median | Range | Significance |
|---|---|---|---|---|
| Control | 75 | 1444 | 117–6160 | |
| I | 90 | 1201 | 51–5244 | P = 0·035 |
| II | 82 | 1016 | 69–3462 | P = 0·01 |
| III | 77 | 715 | 0–4984 | P = 0·0003 |
| IV | 59 | 1082 | 8–4105 | P = 0·005 |
MBL concentrations are expressed in ng/ml. Significance values refer to comparisons of the medians with that of the controls.
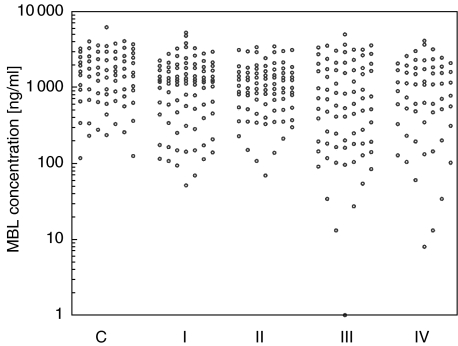
Fig. 1.
Individual values of MBL concentration in sera of children with recurrent infections of the respiratory system and in control group. C, controls; I, children with no other immunodefects; II, children with allergies; III, children with humoral response defects; IV, children with cellular response defects.
The lowest concentration in the control group was 117 ng/ml. This concentration approximately corresponds to the originally described opsonic defect found in about 10% of the apparently healthy population. Patients with MBL concentrations < 117 ng/ml were therefore deemed MBL deficient for the purpose of the following analyses. Using this cut-off, 21 (7%) of patients overall were MBL deficient (P = 0·01). Significant corresponding relationships were found for clinical subgroups I (6·7%; P = 0·03), III (13%; P = 0·001) and IV (10%; P = 0·006). The increased level of deficiency so defined, however, was not significant in the allergy-associated subgroup II (2·4%; P = 0·5). Any cut-off level, however, is to some extent arbitrary. It is therefore noteworthy that any cut-off level chosen at random up to 600 ng/ml yields a statistically significant distinction between patient group III and the controls, while that is not true for the other patient groups (verifiable from Fig. 1).
It has been argued that 600 ng/ml is the most suitable cut-off level to denote ‘insufficiency’, as 90% of healthy individuals who are homozygous for the wild-type structural gene (A/A) have MBL concentrations >600 ng/ml, while the majority of A/O and O/O individuals have MBL concentrations below 600 ng/ml. Twelve children (16%) from the control group and 96 patients (33%) had MBL concentrations lower than 600 ng/ml (P = 0·004). Significant differences were apparent in patient groups III (46·8%; P < 0·0001) and IV (35·6%; P = 0·015), while the relationship did not reach statistical significance in groups I (28·9%; P = 0·06) or II (28%; P = 0·085). A majority (10/17) of the subgroup with multiple immune abnormalities had <600 ng/ml MBL (P = 0·0006).
When comparisons were made between patients with immune abnormalities (groups II to IV) and those without (group I) at the 600 ng/ml level, a significant difference was found (35%versus 29%; P = 0·03). This was entirely due to the contribution of group III patients (47%; P = 0·02). Moreover, a similar relationship was found with the subgroup with multiple immune abnormalities (P = 0·02).
The genotype/phenotype relationship
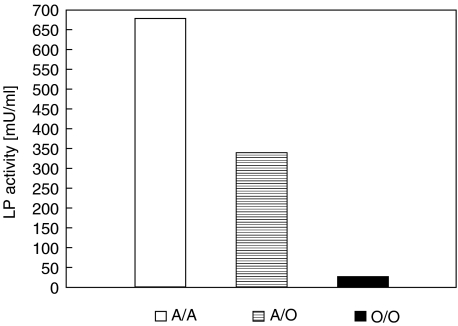
As expected, both exon 1 and promoter region genotypes influenced MBL concentrations (Table 5). The influence of exon 1 mutations on the lectin pathway (LP) of complement activation was also investigated. Median LP activity (C4b deposition) in wild type homozygotes was 675 mU. The corresponding value determined in A/O heterozygotes was approximately 50% lower (340 mU; P = 0·0001), while among patients with two variant alleles (O/O), it was nearly 30 times lower (24 mU) (Fig. 2).
Table 5.
MBL concentrations [ng/ml] in sera according to genotype
| Control group | Children with recurrent infections (groups I-IV) | |||||
|---|---|---|---|---|---|---|
| Genotype | Median | Range | n | Median | Range | n |
| HYA/HYA | 2236 | 257–6160 | 28 | 2226 | 439–5244 | 47 |
| LYA/LYA | 1212 | 340–2799 | 10 | 1350 | 60–3509 | 33 |
| LXA/LXA | 226 | 124–327 | 2 | 230 | 8–954 | 25 |
| HYA/LYA | 2231 | 1756–2570 | 4 | 1542 | 392–3569 | 18 |
| HYA/LXA | 1440 | 275–3261 | 7 | 1548 | 117–3391 | 33 |
| LYA/LXA | 1141 | 805–3745 | 8 | 1093 | 644–2605 | 28 |
| HYA/LYB | 944 | 236–2555 | 8 | 948 | 107–4105 | 53 |
| LYA/LYB | 638 | 229–2440 | 5 | 371 | 131–3791 | 24 |
| LXA/LYB | 117 | 117 | 1 | 144 | 0–1536 | 16 |
| HYA/HYD | 936 | 936 | 1 | – | – | – |
| HYA/LYC | 640 | 640 | 1 | 682 | 682 | 1 |
| LYB/LYB | – | – | – | 172 | 13–522 | 11 |
| HYD/HYD | – | – | – | 355 | 355 | 1 |
| LYD/LYD | – | – | – | 282 | 261–391 | 3 |
Fig. 2.
Complement lectin pathway activity (median) of sera of children with no mutation (A/A) and with heterozygous (A/O) as well as homozygous (O/O) mutations in mbl-2 gene exon 1. Results are given in arbitrary milliunits.
Nine blood samples from patients with unexpectedly low LP activity in view of their MBL concentrations were further analysed for determination of masp-2 gene mutations. One person from patient group I (mbl-2 genotype: LYPA/LXPA; MBL concentration 1398 ng/ml; no detectable LP activity) was found to be homozygous for a mutation in the CUB-1 domain (GGC codon instead of GAC causing substitution of Gly for Asp at position 105 of mature protein). Another patient (group III, mbl-2 genotype: HYPA/LYPA; MBL concentration 968 ng/ml; LP activity 38 mU/ml) was a heterozygote GAC/GGC.
In sera from A/A genotype individuals, highly oligomerized MBL fractions (corresponding to hexa- and tetramers) were observed in SDS-PAGE under nonreducing conditions followed by Western-blot analysis, while sera from A/O and O/O individuals were dominated by low-molecular weight species (data not shown).
DISCUSSION
Various functional disorders of the immune system reflect innate, genetically determined defects that lead to the temporary or permanent impairment of immunity [12]. MBL is considered to be particularly important for protection against infection in 5- to 18-month-old children whose immune system is not able to produce specific immunoglobulins sufficiently, after maternal antibodies have been catabolized (‘window of vulnerability’). Therefore, MBL is sometimes called an ‘ante-antibody’, acting before the adaptive immune system has time to function [19–21]. MBL deficit/dysfunction has been associated with an increased susceptibility to numerous infectious diseases (childhood diarrhoea, pneumococcal and fungal pulmonary infections, meningitis, otitis media, HIV, HBV and HCV infections and others) [22, 23, 24, 25, 26, 27]. Moreover, an association between MBL deficiency, poor lung function and shortened life span in cystic fibrosis patients was reported, and presumed to be connected with severity of pulmonary infections [28,29].
This study adds considerably to the rather slim literature relating full MBL genotypes to circulating protein concentrations. Our results confirm in general terms previously reported relationships between variant alleles (structural and promoter) and functional (opsonic) MBL protein with the capacity to initiate the lectin pathway of complement activation. Recently, Stengaard-Pedersen et al. [18] reported an abnormality of the MASP-2 CUB-1 domain associated with a functional deficit of the MBL/MASP-2 complex. These authors found no effect of CCP-2 domain polymorphism on protein concentration or activity. Among nine selected patients with unexpectedly low LP activity we found one mutant CUB-1 homozygote and one heterozygote.
Koch et al. [21] suggested XA/O and O/O genotype-carrying individuals be considered MBL insufficient. Our data indicate that approximately 70% of LXPA homozygotes have low (<600 ng/ml) MBL levels and similarly might be considered MBL-insufficient. Madsen et al. [5] and Steffensen et al. [30] reported also low MBL levels in persons of the same genotype. On the other hand, Garred et al. [28] found that the mean MBL concentration in LXA homozygotes reached approximately 1000 ng/ml. We also found several low-MBL individuals among HYPA homozygotes which might be a result of another, unknown polymorphism. Recently, Garred et al. [31], using another monoclonal antibody, found MBL levels in sera of O/O homozygotes to be similar to those of A/O heterozygotes. This observation alerts us to the importance of assaying serum MBL with a method that detects functional protein containing higher oligomeric forms that can activate complement.
There are some discrepancies in the literature regarding the relative importance of homozygosity and heterozygosity for variant alleles. Garred et al.[32] suggested that only homozygosity for MBL mutant alleles predisposes to recurrent infections. However, Hibberd et al. [33] demonstrated that heterozygosity as well as homozygosity increased susceptibility to meningococcal disease. Summerfield et al. [34] also found that heterogeneity for exon 1 mutations were significantly more prevalent in children (age 0–18 years) hospitalized for various infective diseases than in patients admitted with other diagnoses, but homozygotes presented with strikingly severe infections. Koch et al. [21] investigated the effect of MBL insufficiency on the incidence of various acute respiratory tract infections (ARI) in children younger than 2 years. They found that both heterozygosity and homozygosity for MBL mutations are important risk factors for ARI during this vulnerable period of infancy. These authors suggested that in older paediatric patients, MBL variants modify the course of infection rather than alter susceptibility.
It is also controversial whether MBL deficiency or insufficiency is clinically significant in the absence of another defined immune defect. For example, Ten et al. [35] found that symptomatic MBL deficiency was associated with neutrophil chemotactic unresponsiveness to C5a. Aittoniemi et al. [36] suggested that low MBL levels are clinically significant only in association with another defect of humoral immunity affecting opsonization, but later found no association between IgA and MBL deficiency in adults [37]. (In our study, among 21 IgA-deficient patients, 11 A/B and 1 B/B genotypes were observed).
In conclusion, this study is consistent with previous reports that mbl-2 gene mutations occur about twice as frequently in paediatric patients with recurrent infections of the respiratory system, and extends this field of investigation to subgroups with different immunological characteristics. Uniquely, this study contributes the observation that MBL insufficiency is a risk factor with or without a coexisting immune defect. Nevertheless, the data presented are perhaps the most convincing yet that children with low MBL in combination with established humoral response defects are particularly vulnerable. It may also be notable that extremely low (<117 ng/ml) MBL concentrations were less commonly found in the allergy (group II) patients, a subgroup in which low circulating MBL was significantly associated with the promoter group haplotype, LX.
Acknowledgments
This study was supported by Polish State Committee for Scientific Research (KBN), grant 4 P05E 127 19. Teams represented by authors belong to the Polish Research Network ‘The molecular basis of immunity’.
REFERENCES
- 1.Fraser IP, Koziel H, Ezekowitz RAB. The serum mannose-binding protein and the macrophage mannose receptor are pattern recognition molecules that link innate and adaptive immunity. Semin Immunol. 1998;10:363–72. doi: 10.1006/smim.1998.0141. [DOI] [PubMed] [Google Scholar]
- 2.Janeway CA., Jr How the immune system works to protect the host from infection: a personal view. Proc Natl Acad Sci USA. 2001;98:7461–8. doi: 10.1073/pnas.131202998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neth O, Jack DL, Johnson M, Klein NJ, Turner MW. Enhancement of complement activation and opsonophagocytosis by complexes of mannose-binding lectin with mannose-binding lectin-associated serine protease after binding to Staphylococcus aureus. J Immunol. 2002;169:4430–6. doi: 10.4049/jimmunol.169.8.4430. [DOI] [PubMed] [Google Scholar]
- 4.Matsushita M. The lectin pathway of the complement system. Microbiol Immunol. 1996;40:887–93. doi: 10.1111/j.1348-0421.1996.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 5.Madsen HO, Garred P, Thiel S, et al. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J Immunol. 1995;155:3013–20. [PubMed] [Google Scholar]
- 6.Lipscombe RJ, Sumiya M, Summerfield JA, Turner MW. Distinct physicochemical characteristics of human mannose binding protein expressed by individuals of differing genotype. Immunology. 1995;85:660–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Matsushita M, Ezekowitz RA, Fujita T. The Gly-54 (→) Asp allelic form of human mannose-binding protein fails to bind MBP-associated serine protease. Biochem J. 1995;311:1021–3. doi: 10.1042/bj3111021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butler GS, Sim D, Tam E, Devine D, Overall CM. Mannose binding lectin (MBL) mutants are susceptible to matrix metalloproteinase proteolysis: potential role in human MBL deficiency. J Biol Chem. 2002;277:17511–9. doi: 10.1074/jbc.M201461200. [DOI] [PubMed] [Google Scholar]
- 9.Wallis R. Dominant effects of mutations in the collagenous domain of mannose-binding protein. J Immunol. 2002;168:4553–8. doi: 10.4049/jimmunol.168.9.4553. [DOI] [PubMed] [Google Scholar]
- 10.Yokota Y, Arai T, Kawasaki T. Oligomeric structures required for complement activation of serum mannan-binding protein. J Biochem. 1995;117:414–9. doi: 10.1093/jb/117.2.414. [DOI] [PubMed] [Google Scholar]
- 11.Madsen HO, Satz ML, Hogh B, Svejgaard A, Garred P. Different molecular events result in low protein levels of mannan-binding lectin in populations from southeast Africa and South America. J Immunol. 1998;161:3169–75. [PubMed] [Google Scholar]
- 12.Zeman K. Immunodeficiency diseases in children(in Polish) 2. Warsaw: PZWL; 2003. [Google Scholar]
- 13.Chomczynski P. A reagent for single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;3:532–7. [PubMed] [Google Scholar]
- 14.Ji YH, Matsushita M, Okada H, Fujita T, Kawakami M. The C4 and C2 but not C1 component are responsible for the complement activation triggered by the Ra-reactive factor. J Immunol. 1988;141:4271–5. [PubMed] [Google Scholar]
- 15.Swierzko AS, Cedzynski M, Kirikae T, et al. Role of the complement lectin pathway in anaphylactoid reaction induced with lipopolysaccharide in mice. Eur J Immunol. 2003;33:2842–52. doi: 10.1002/eji.200323949. [DOI] [PubMed] [Google Scholar]
- 16.Aittoniemi J, Miettinen A, Laippala P, et al. Age-dependent variation in the serum concentration of mannan-binding protein. Acta Paediatr. 1996;85:906–9. doi: 10.1111/j.1651-2227.1996.tb14182.x. [DOI] [PubMed] [Google Scholar]
- 17.Petersen SV, Thiel S, Jensen L, Steffensen R, Jensenius JC. An assay for the mannan-binding lectin pathway of complement activation. J Immunol Meth. 2001;257:107–16. doi: 10.1016/s0022-1759(01)00453-7. [DOI] [PubMed] [Google Scholar]
- 18.Stengaard-Pedersen K, Thiel S, Gadjeva M, et al. Inherited deficiency of mannan-binding lectin-associated serine protease 2. N Engl J Med. 2003;349:554–60. doi: 10.1056/NEJMoa022836. [DOI] [PubMed] [Google Scholar]
- 19.Turner MW. Mannose-binding lectin (MBL) in health and disease. Immunobiology. 1998;199:327–39. doi: 10.1016/S0171-2985(98)80037-5. [DOI] [PubMed] [Google Scholar]
- 20.Turner MW. Mannose-binding lectin: the pluripotent molecule of the innate immune system. Immunol Today. 1996;17:532–40. doi: 10.1016/0167-5699(96)10062-1. [DOI] [PubMed] [Google Scholar]
- 21.Koch A, Melbye M, Sørensen P, et al. Acute respiratory tract infections and mannose-binding lectin insufficiency during early childhood. J Am Med Assoc. 2001;10:1316–21. doi: 10.1001/jama.285.10.1316. [DOI] [PubMed] [Google Scholar]
- 22.Kilpatrick DC. Mannan-binding lectin. clinical significance and applications. Biochim Biophys Acta. 2002;1572:401–13. doi: 10.1016/s0304-4165(02)00321-5. [DOI] [PubMed] [Google Scholar]
- 23.Matsushita M, Hijikata M, Ohta Y, et al. Hepatitis C virus infection and mutations of mannose-binding gene MBL. Arch Virol. 1998;143:645–51. doi: 10.1007/s007050050320. [DOI] [PubMed] [Google Scholar]
- 24.Pastinen T, Liitsola K, Niini P, Salminen M, Syvanen AC. Contribution of the CCR5 and MBL genes to susceptibility to HIV type 1 infection in Finnish population. AIDS Res Hum Retroviruses. 1998;14:695–8. doi: 10.1089/aid.1998.14.695. [DOI] [PubMed] [Google Scholar]
- 25.Thomas HC, Foster GR, Sumiya M, et al. Mutation of gene for mannose-binding protein associated with chronic hepatitis B viral infection. Lancet. 1996;348:1417–9. doi: 10.1016/s0140-6736(96)05409-8. [DOI] [PubMed] [Google Scholar]
- 26.Tezcan J, Yilmaz Y, Oner F, et al. Defective serum opsonization activity in children aged 6–48 months having acute purulent otitis media. Turkish J Pediatr. 1997;39:453–7. [PubMed] [Google Scholar]
- 27.Kilpatrick DC. Mannan-binding lectin and its role in innate immunity. Transfusion Med. 2002;12:335–51. doi: 10.1046/j.1365-3148.2002.00408.x. [DOI] [PubMed] [Google Scholar]
- 28.Garred P, Pressler T, Madsen HO, et al. Association of mannose-binding lectin gene heterogeneity with severity of lung disease and survival in cystic fibrosis. J Clin Invest. 1999;104:431–7. doi: 10.1172/JCI6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabolde M, Guilloud-Bataille M, Feingold J, Besmond C. Association of variant alleles of mannose binding lectin with severity of pulmonary disease in cystic fibrosis: cohort study. Br Med J. 1999;319:1166–7. doi: 10.1136/bmj.319.7218.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steffensen R, Thiel S, Varming K, Jersild C, Jensenius JC. Detection of structural gene mutations and promoter polymorphisms in the mannan-binding lectin (MBL) gene by polymerase chain reaction with sequence-specific primers. J Immunol Meth. 2000;241:33–42. doi: 10.1016/s0022-1759(00)00198-8. [DOI] [PubMed] [Google Scholar]
- 31.Garred P, Larsen F, Madsen HO, Koch C. Mannose-binding lectin deficiency – revisited. Mol Immunol. 2003;40:73–84. doi: 10.1016/s0161-5890(03)00104-4. [DOI] [PubMed] [Google Scholar]
- 32.Garred P, Madsen HO, Hoffman B, Svejgaard A. Increased frequency of homozygosity of abnormal mannan-binding-protein alleles in patients with suspected immunodeficiency. Lancet. 1995;346:941–3. doi: 10.1016/s0140-6736(95)91559-1. [DOI] [PubMed] [Google Scholar]
- 33.Hibberd ML, Sumiya M, Summerfield JA, Booy R, Levin M the Meningococcal Research Group. Association of variants of the gene for mannose-binding lectin with susceptibility to meningococcal disease. Lancet. 1999;353:1049–53. doi: 10.1016/s0140-6736(98)08350-0. [DOI] [PubMed] [Google Scholar]
- 34.Summerfield JA, Sumiya M, Levin M, Turner MW. Association of mutations in mannose-binding protein gene with childhood infection in consecutive hospital series. Br Med J. 1997;314:1229–32. doi: 10.1136/bmj.314.7089.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ten RM, Carmona EM, Babovic-Vuksanovic D, Katzmann JA. Mannose-binding lectin deficiency associated with neutrophil chemotactic unresponsiveness to C5a. J Allergy Clin Immunol. 1999;104:419–24. doi: 10.1016/s0091-6749(99)70387-8. [DOI] [PubMed] [Google Scholar]
- 36.Aittoniemi J, Baer M, Soppi E, Vesikari T, Miettinen A. Mannan binding lectin deficiency and concomitant immunodefects. Arch Dis Child. 1998;78:245–8. doi: 10.1136/adc.78.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aittoniemi J, Koskinen S, Laippala P, Laine S, Miettinen A. The significance of IgG subclasses and mannan-binding lectin (MBL) for susceptibility to infection in apparently health adults with IgA deficiency. Clin Exp Immunol. 1999;116:505–8. doi: 10.1046/j.1365-2249.1999.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]