Abstract
Problems of logistics, compliance and drug resistance point to an urgent need for immunotherapeutic strategies capable of shortening the current six month antibiotic regimens used to treat tuberculosis. One potential immunotherapeutic agent is transfer factors. Transfer factors (TF) are low molecular weight dialysable products from immune cells which transmit the ability to express delayed-type hypersensitivity (DTH) and cell mediated immunity from sensitized donors to nonimmune recipients. In this study we determined the efficiency of TF as immunotherapy to treat experimental tuberculosis. When BALB/c mice are infected via the trachea with Mycobacterium tuberculosis H37Rv there is an initial phase of partial resistance dominated by Th-1 type cytokines plus tumour necrosis factor-alpha (TNFα) and the inducible isoform of nitric oxide synthase (iNOS), followed by a phase of progressive disease characterized by increasing expression of IL-4, diminished expression of TNFα and iNOS, and low DTH. Animals in this late progressive phase of the disease (day 60) were treated with different doses of TF (one injection per week) obtained from spleen cells when the peak of immune protection in this animal model is reached (day 21), or with different doses of TF from peripheral leucocytes of PPD + healthy subjects. We show here that the treatment with murine or human TF restored the expression of Th-1 cytokines, TNFα and iNOS provoking inhibition of bacterial proliferation and significant increase of DTH and survival. This beneficial effect was dose dependent. Interestingly, murine TF in combination with conventional chemotherapy had a synergistic effect producing significant faster elimination of lung bacteria loads than chemotherapy alone.
Keywords: transfer factor, immunotherapy, experimental tuberculosis
INTRODUCTION
Transfer factors (TF) or leucocyte dialysates are low molecular weight dialysable products from immune cells that are able to transmit the ability to express delayed-type hypersensitivity (DTH) and cell mediated immunity (CMI) from sensitized donors to nonimmune recipients [1]. At present, neither the precise chemical nature nor the exact molecular mechanisms of action of TF have been defined. However, several studies have shown that TF are low molecular weight proteins (<5 kD) that can be purified to a high degree of homogeneity [2]. TF have high levels of tyrosine and glycine with a great similarity to the N-terminal regions of some neuropeptides, such as those of the encephalin family [3]. In general, all TF tested are very efficient factors to correct or enhance CMI responses, and are antigen-specific [2,4].
Since their discovery by Sherwood Lawrence, almost 50 years ago [1], the therapeutic and prophylactic applications have been the most important and interesting aspects of TF [4]. In fact, TF have been demonstrated to be very effective in those diseases in which CMI plays a relevant role in protection and control of the disease, such as viral infections (herpes simplex, varicella zoster), intracellular bacterial diseases (tuberculosis, leprosy) and parasite infections (leishmaniasis, toxoplasmosis) [5–9], as well as in primary immunodeficiencies (chronic granulomatosis, Wiskott Aldrich syndrome) [10] and some types of cancer [11].
Tuberculous infection is mainly controlled by CMI [12]. Th1 type cytokines like interferon gamma (IFNγ) and proinflammatory cytokines like tumour necrosis factor alpha (TNFα) have a central role in this process, by inducing macrophage activation and nitric oxide synthase (iNOS) expression. The nitric oxide (NO) produced is essential – at least for mice – to kill intracellular mycobacteria [13,14]. This protective activity fails if there is a marked release of Th2 type cytokines [15,16]. This interplay of immune cells and cytokines is clearly depicted in a BALB/c model of pulmonary tuberculosis following intratracheal inoculation [17–19]. In this model, an initial phase is dominated by high production of Th1 cell cytokines that together with high levels of TNFα and iNOS, temporarily controls the infection. Granulomas develop in this phase. One month after infection the expression of Th1 cell cytokines, TNFα and iNOS start to decline. This impairment of protective CMI during late tuberculosis infection is manifested by low DTH against mycobacterial antigens [20], and progressive pneumonia which prevails over granulomas. Pneumonia, in coexistence with high burden of bacteria, causes death [17,18].
Despite the fact that efficient chemotherapy was established many years ago, tuberculosis remains an expanding global health crisis, causing three million deaths and eight million new cases annually [21]. One of the main reasons for the current failure to control tuberculosis is that, even using the best chemotherapy regimen, treatment must be continued for at least six months. This treatment regimen is not a realistic proposition in most developing countries, because patients feel well after a few weeks and stop taking the drugs, provoking disease recurrences and multidrug resistance. One possible alternative to resolve this problem is the design of ultra-short chemotherapy regimens, using conventional antibiotics supplemented with immunotherapy that would provide realistic tuberculosis control particularly in the developing world. In this study, we first used the above-mentioned model of pulmonary tuberculosis to evaluate the immunotherapeutic effect of TF during advanced pulmonary tuberculosis. Secondly, we studied the efficiency of TF to shorten conventional chemotherapy.
MATERIALS AND METHODS
Experimental model of progressive pulmonary tuberculosis
The experimental model of pulmonary tuberculosis has been described previously [17–20]. Briefly, the virulent strain H37Rv of M. tuberculosis was cultured in Proskauer and Beck medium as modified by Youmans. After 1 month of culture, mycobacteria were harvested and adjusted to 2·5 × 105 cells in 100 µl of phosphate-buffered saline (PBS), aliquoted and maintained at −70°C until they were used. Before use, bacteria were recounted and viability checked as described [22]. Male BALB/c mice at 6–8 weeks of age were anaesthetized with 56 mg/kg of intraperitoneal pentothal, the trachea was surgically exposed and 2·5 × 105 viable bacteria resuspended in 100 µl of PBS were injected. The incision was then sutured with sterile silk. Infected mice were maintained in cages fitted with microisolators connected to negative pressure. All procedures were performed in a laminar flow cabinet in a biosafety level III facility. The protocol was approved by the Ethics Committee for Experimentation in Animals of the National Institute of Medical Sciences and Nutrition in Mexico.
Preparation of transfer factors (TF)
Specific murine TF were obtained from tuberculous BALB/c mice after 21 days of intratracheal infection. We have previously shown that this is the time when pulmonary granulomas reach maturation and the peak of protection is highest [17,18]. Ten infected mice were sacrificed and their spleens were aseptically removed. Single cell suspensions from the spleens were disrupted by 10 cycles of freezing and thawing (78°C and 37°C). The lysate was dialysed against water using a Spectra/Por® dialysis membrane (Spectrum Laboratories Inc, Rancho Dgz., CA, USA) with a nominal ‘cut-off’ of 12 kD. Then, the dialysates were mixed with 4·8% glycine and 2·4% sucrose as cryoprotectors.We considered one unit of murine specific TF (mTF) as the dialysate obtained from 1 × 106 spleen cells from mice intratracheally infected 21 days before. In order to check the antigen specificity of TF, we treated tuberculous mice with TF obtained from spleens of mice after 3 weeks of intraperitoneal infection with Listeria monocytogenesis (1 × 106) using the same procedure described above.
Specific human TF (hTF) was elaborated from packed blood peripheral leucocytes obtained from PPD + healthy donors following the same procedure described above. One unit of human TF is the dialysate obtained from 5 × 108 cells. Human and murine TF were checked for mycobacterial products by wetern blotting using commercial polyclonal antibodies against BCG (Dako).
Determining the efficiency and the best dose of TF
At 60 days postinfection, surviving mice were randomly allotted to six experimental groups to determine the effect, specificity, and dose efficiency of TF from human peripheral blood leucocytes and murine spleen cells. Groups of 20 tuberculous mice were treated with hTF at doses of 0·01, 0·001, and 0·0001 U. Other groups with the same number of tuberculous mice were treated with mTF at doses of 1 and 0·1 U. The control group received only the cryoprotector solution. The corresponding dose of mTF was administered subcutaneously in a total volume of 100 µl, three doses the first week, then one dose per week. Groups of 10 mice per group were killed by exsanguination under terminal anaesthesia at 3 and 6 months after TF administration. Ten mice in each group were left undisturbed and their survival was recorded. Two experiments were performed and the data pooled. In order to check TF antigen specificity, another experimental group of tuberculous mice was treated with 0·1 U of TF obtained from mice infected with Listeria.
Preparation of tissue samples for histopathology and immunohistochemistry
For histological study, the lungs from four mice per time interval were fixed by intratracheal perfusion with absolute ethanol, embedded in paraffin, sectioned and stained with haematoxylin and eosin [17,18]. In these slides the area of granuloma and the percentage of lung area affected by pneumonia were determined using a Zidas Zeiss image analyser (Carl Zeiss Ltd, Herts, UK). For immunohistochemistry, lung sections were mounted on silane-covered slides, deparaffinized, and the endogenous peroxidase quenched with 0·03% H2O2 in absolute methanol. Lung sections were incubated overnight at room temperature with rabbit–specific polyclonal antibodies against mouse TNFα and IL-1α (Genzyme, Boston, NE, USA) diluted to 1/300 and 1/200 in PBS, respectively. Bound antibodies were detected with goat anti-rabbit IgG labelled with peroxidase (Dako, Carpinteria, CA, USA) and diaminobenzidine. The slides were counterstained with haematoxylin [17–19]. The percentage of positive cells located in pneumonic areas were determined with a computerized image analyser (Qwin Leica, Leica imaging systems, Cambridge, UK) as previously described [23]. The negative control consisted of performing the whole procedure using normal rabbit sera or isotype nonrelevant antibody instead of the primary antibody.
Determination of colony-forming units (CFU) in infected lungs
Right or left lung from four mice per each sacrifice time point were used in two different experiments. Lungs were homogenized with a Polytron homogenizer (Kinematica, Luzern, Switzerland) in sterile tubes containing 3 ml of isotonic saline. Four dilutions of each homogenate were spread onto duplicate plates containing Bacto Middlebrook 7H11 agar (Difco Laboratories, Detroit, MI, USA) enriched with OADC. The number of colonies was counted 21 days postinoculation [23].
RT-PCR analysis of cytokines and iNOS in lung homogenates
Four lungs, right or left, were used to isolate RNA from the different mice groups in each sacrifice time point, using Trizol (Gibco BRL, Gaithersburgh, MD, USA) as described previously [17–19]. cDNA was synthesized by using Maloney murine leukaemia virus reverse transcriptase (Gibco BRL) and oligo dT priming. The expression of IFNγ, IL-4, and iNOS was determined by reverse transcription PCR (RT-PCR) as described previously [17–19]. The PCR products were electrophoresed on 6% polyacrylamide gels, running molecular weight standards with known DNA mass concentrations (Low DNA mass ladder, Gibco BRL), and analysed with an image analysis densitometer coupled to a computer program (ID image analysis software (Kodak Digital Science, Rochester, NY, USA). To determine the quantity of PCR product for each cytokine in nanograms, the computer program compared the optical densities from the experimental samples with the molecular marker bands for which the DNA content was provided by the manufacturer. The densitometer reading of the glyceraldehyde 3-phosphate dehydrogenase PCR product was used to correct for errors in the quantity of starting material.
Quantification of cytokines in lung homogenates by ELISA
The lung homogenates used for RNA purification were also used to quantify cytokines by ELISA [23]. After homogenization and centrifugation, the protein phase was extensively dialysed in sodium dodecylsulphate (SDS), and quantified with Bradford reagent (Bio-Rad) using a bovine serum albumin (BSA) standard curve. To quantify IL-2, IFNγ, and IL-4 a capture ELISA using monoclonal antibody pairs and recombinant standards (Pharmingen, CA, USA) was performed. Briefly, 96-well plates were coated with 0·5 µg/ml of monoclonal rat anti-mouse cytokine dissolved in 100 µl of 0·05 m carbonate buffer, pH 9·5, overnight at 4°C. After washing with PBS-Tween 0·05% (PBS-T), wells were blocked with 1% BSA in PBS-T for 2 h at room temperature. Lung homogenate proteins at 0·5 µg/ml concentration resuspended in 100 µl PBS were incubated for 3 h at 37°C. After washing, biotinylated polyclonal rabbit anti-mouse IL-2, IFN or IL-4 at 1 µg/ml in PBS-T were incubated with streptoavidin peroxidase diluted 1/1000 in PBS-T for 1 h at room temperature. To reveal the peroxidase, orthophenylendiamine and H2O2 were used.
Measurement of cutaneous delayed type hypersensitivity
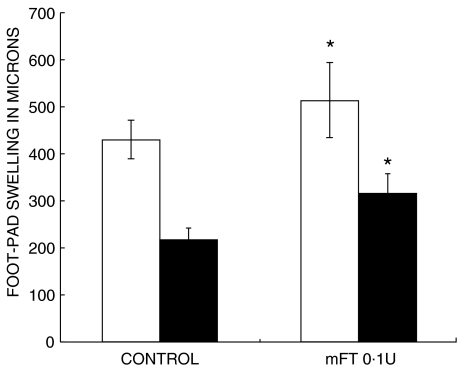
Culture filtrate was harvested by filtration from M. tuberculosis H37Rv grown as described above for 4–5 weeks. Then, culture filtrate antigens were precipitated with 45% (w/v) ammonium sulphate, washed and redissolved in PBS. For delayed type hypersensitivity (DTH) measurement, each mouse received an injection of 20 µg of antigen in 40 µl of PBS into the hind footpad. The footpad was measured with an engineer's micrometer before and 24 h after the antigen injection as previously described [20]. Each data point represents the mean of eight mice, four from each time point and experiment comparing the different groups.
Using TF as supplement in conventional chemotherapy
The experiments designed to study the efficiency and best dose of TF to treat experimental pulmonary tuberculosis showed that 0·1 U of mTF was highly efficient to control disease progression. Thus, we used this dose of mTF alone or combined with conventional chemotherapy at 60 days postinfection, in order to analyse whether mTF could have any effect on the conventional antibiotic treatment. For this, 60 days after M. tuberculosis infection, surviving mice were randomly allotted to four experimental groups, each with 20 mice. The first group of 20 tuberculous mice was treated with conventional chemotherapy: rifampicin (10 mg/kg), isoniazid (10 mg/kg), and pyrazinamide (30 mg/kg), administered daily through an intragastric cannula, plus 0·1 U of mTF administered subcutaneously once per week. The second group was treated only with the same mTF dose. The third group was treated only with antibiotics. The control group exclusively received the cryopreserving solution. Groups of 10 mice per group were killed by exsanguination under terminal anaesthesia at 7, 15, 21, 30, 60, and 90 days after TF administration. Lungs were used to determine CFU; and the percentage of lung affected by pneumonia was determined by automated morphometry. Two independent experiments were performed and the data pooled.
Statistics
A one-way analysis of variance (anova) was used to compare possible differences between the control nontreated group and animals treated with TF, in cytokine gene expression (RT-PCR) and cytokine quantification (ELISA), as well as in lung bacillary loads, delayed type hypersensitivity, the size of granulomas and the percentage of lung affected by pneumonia. A difference of P < 0·05 was considered significant. CFU data in chemotherapy alone and chemotherapy plus TF treated groups was analysed using the Kruskal–Wallis test, and the Kaplan Meier test was used for the survival curves analysis.
RESULTS
Effect of the administration of murine or human TF on survival, pathology and bacillary load
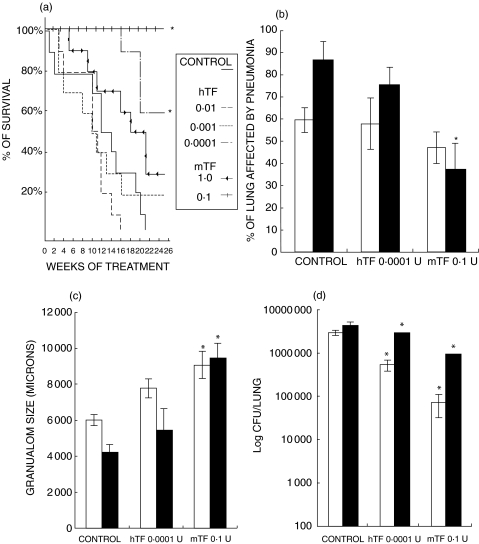
The efficiency of TF was highly depended on the dose and, in a lesser degree, to its source (murine or human). After 26 weeks of treatment, the best result was observed in animals treated with 0·1 U of mTF, which showed a significant 95% survival when compared with the control group, which exhibited 100% mortality after 21 weeks (P< 0·0001), followed by the dose of 0·0001 of hTF which exhibited 60% survival. Interestingly, the group that received the highest dose of mTF (1 U) produced 30% survival, whereas the highest human TF dose (0·01 U) killed all the animals after 16 weeks of treatment, 5 weeks before the control group (Fig. 1a). These survival curves correlated with lung histopathology. As shown in Figs 1 and 2, control mice showed 85% ± 10% of lung surface affected by pneumonia at the end of the experiment, whereas animals treated during six months with 0·1 U of mTF showed a significant (50%) reduction of pneumonic area and a two-fold increment of granuloma size (Fig. 1b,c). The highest human TF dose, which induced higher mortality than the control nontreated group, produced massive pneumonia (95 ± 3% of lung surface) with large areas of necrosis (Fig. 2c).
Fig. 1.
The effects on survival, pathology and bacilli loads, after three (□) and six (▪) months of treatment with different doses of Transfer Factor from spleen mouse (mTF) or human blood (hTF) when used as immunotherapy in mice suffering progressive pulmonary tuberculosis. (a) Survival curves after treatment with different doses of mTF and hTF, control group only received the diluent. (b) The percentage of the lung occupied by pneumonia, and (c) the granuloma size in the selected groups. (d) The comparison of colony-forming units/lung in tuberculous mice treated with the best dose of mTF (0·1 U), hTF (0·0001 U) and control nontreated mice. CFU and morphometry data are from four different animals in each time point in two different experiments. Asterisks represent statistical significance (P< 0·05).
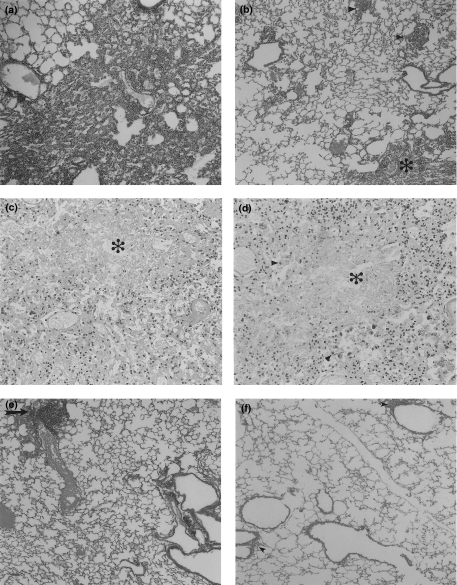
Fig. 2.
Representative lung histopathology and immunohistochemistry from control mice and animals treated with murine Transfer Factor (mTF) or mTF plus antibiotics. (a) Massive pneumonia in the lung of control animal after 6 months of infection. (b) In contrast, the lung of mice after 6 months of mTF treatment (dose 0·1 U) shows milder pneumonia (*) and bigger granulomas (arrow heads). (c) Animals treated with the highest dose of hTF (0·01 U) exhibited extensive pneumonia with large necrotic patches (*). (d) These necrotic patches (*) have numerous macrophages with strong TNFα immunostaining (arrow heads). (e) Small areas of pneumonia (arrow) are seen in tuberculous animals after one month of antibiotic treatment. (F) No pneumonia is seen when infected mice are treated with antibiotics plus mTF for one month, only lymphocyte cuffs around bronchial and vascular walls are apparent (arrow heads).
In comparison with control mice, all the doses of murine or human TF produced a significant decrease of bacillary loads. Tuberculous mice treated during six months with the most efficient dose, 0·1 U of mTF, had a mean of 1 million CFU, whereas control untreated animals had 4·3 millions (Fig. 1d). We also tested disease reactivation in five mice after six month of mTF treatment, by administrating corticosterone in drinking water (1% in saline solution during one month). All these animals suffered disease reactivation (data not shown). Thus, as has been reported after chemotherapy, TF treatment can not completely eliminate mycobacteria.
Tuberculous mice treated with TF obtained from mice infected with L monocytogenes showed 20% lesser CFU and the same percentage of pneumonia than control nontreated mice (data not shown). Thus, slight and nonsignificant improvement of clearing lung bacilli was obtained with nonspecific TF. Western blot analysis for mycobacterial products in murine and human TF did not show any bacterial moieties (data not shown).
The effects of TF administration on cytokine expression and DTH responses
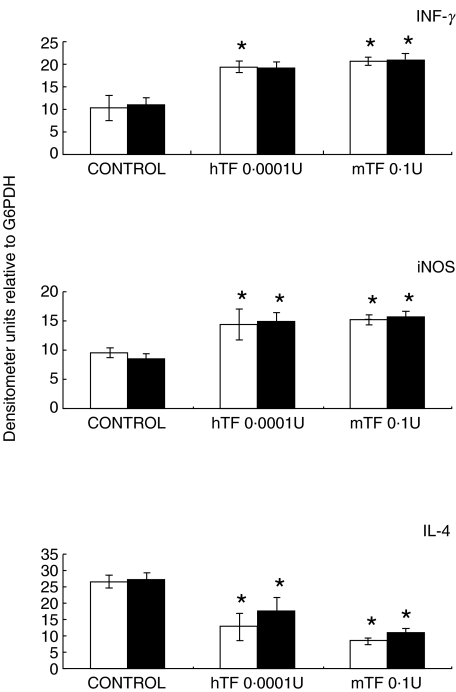
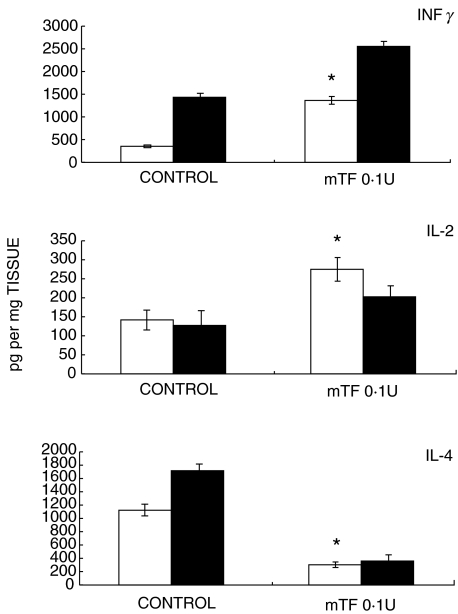
Gene expression and quantification of cytokines was done by RT-PCR and ELISA, respectively. Quantitative automated morphometry was done in mice treated with the best immunotherapeutic regimen (0·1 U mTF, 0·0001 hTF) and in untreated control mice. As we have previously reported, when BALB/c mice are infected intratracheally with M. tuberculosis H37Rv, a progressive phase of the disease is produced after one month postinfection, which is characterized by low expression of IFNγ, IL-2, TNFα, and iNOS, and high IL-4 production [17–19]. This cytokine pattern analysed by RT-PCR (Fig. 3) and ELISA (Fig. 4) was completely reversed when 0·1 U of mTF or 0·0001 U of hTF was administered during six months, together with a significant increment in DTH (Fig. 5). Interestingly, chronic infected mice treated with 0·01 U of hTF, which produced very high mortality and massive pneumonia, exhibited the highest percentage of TNFα immunostained macrophages (14 ± 3%) located in large necrotic patches embedded into the pneumonic areas (Fig. 2d). The lung of mice treated with 0·1 U of mTF showed non-necrotic pneumonia with more TNFα immunostained macrophages (12 ± 2%), than control animals that exhibited larger pneumonic areas with the lowest percentage of TNFα immunostained macrophages (7 ± 2%). Similar percentages were observed in the IL-1α immunohistochemistry analysis (data not shown).
Fig. 3.
Expression of mRNA encoding cytokines and nitric oxide synthase after three (□) and six (▪) months of immunotherapy, with specific 0·0001 U of human Transfer Factor (hTF) or 0·1 U of murine Transfer Factor (mTF) in comparison with control nontreated tuberculous mice. Animals treated with murine or human TF showed higher IFNγ and iNOS and lesser IL-4 mRNA concentrations than control mice. Bars represent the means and standard deviation from four different animals at each time point. Asterisks represent statistical significance when compared with the control group.
Fig. 4.
ELISA quantification of IFNγ, IL-2, and IL-4 cytokines in lung homogenates from tuberculous control nontreated mice and animals treated during three (□) and six (▪) months with 0·1 U of murine Transfer Factor (mTF). In comparison with the control group, animals treated with mTF showed higher concentrations of IFNγ and IL-2, and lower IL-4 production. Asterisks mean significant statistical differences (P < 0·05).
Fig. 5.
Delayed hypersensitivity responses to soluble antigens of Mycobacterium tuberculosis in tuberculous animals treated with 0·1 U of murine Transfer Factor (mTF) after three (□) and six (▪) months, in comparison with the control nontreated group. Animals treated with mTF show higher responses than control animals. *P< 0·05.
The effects of TF as supplement of conventional chemotherapy
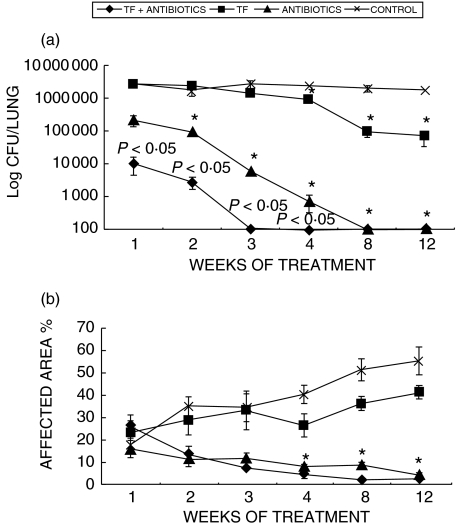
The experiments described above clearly showed that 0·1 U of mTF was the best dose and source of TF to control the progression of pulmonary tuberculosis. Thus, we chose this to supplement conventional chemotherapy in an effort to shorten the antibiotic treatment. In comparison with control animals (which did not received any treatment), the antibiotic treatment with rifampicin, isionazid and pyrazinamide produced a rapid drop of lung bacilli counts, since one week after treatment (10 times lesser CFU than in control mice), and after two months there were no detectable CFU in lung homogenates (Fig. 6a). Interestingly, the fastest clearance of bacteria was produced when chemotherapy was supplemented with mTF. As shown in Fig. 6a, this combined treatment after one week produced 200 times lesser CFU than in control mice and, after three weeks, no bacterial growth was detected in lung homogenates. Thus, administering mTF in addition to conventional chemotherapy from day 60 resulted in significantly accelerated reduction in bacterial counts in the lungs compared to chemotherapy alone (P< 0·05).
Fig. 6.
The effects of the conventional antibiotic treatment and combined treatment (conventional chemotherapy plus 0·1 U of murine Transfer Factor (mTF)) on bacterial counts and pathology. (a) Colony forming units at intervals after mTF treatment, chemotherapy, and chemotherapy plus TF in comparison with control nontreated mice. (b) The percentage of the lung affected by pneumonia. In comparison with the control group, all the different treatments produced significant decrease of CFU and pneumonic areas, being the combined treatment the most efficient and fastest treatment. Combined therapy resulted in significantly reduced CFU when compared to chemotherapy alone (P< 0·05), these P-values are indicated on the figure. Data are mean and standard deviations from five lungs per time interval. Asterisks means statistical significance when compared with the control group.
Both groups, chemotherapy alone and chemotherapy plus mTF, showed a progressive reduction of pneumonic areas, which was more pronounced in the latter (Figs 2 and 6B). Animals treated only with murine TF experienced a progressively slower decrease of CFU and pneumonia (Fig. 6).
DISCUSSION
Immunity to M. tuberculosis requires a Th-1 pattern of cytokine release, accompanied by expression of TNFα[12]. This response can be compromised by excessive Th-2 activity [12, 15, 16]. Impairment of CMI is a constant characteristic of patients with advanced pulmonary tuberculosis, which is manifested by low production of IFNγ and TNFα with 35% lower PPD responses or lack of them [24,25]. Correction of these immune defects producing patient benefit is the objective of immunotherapy. Our mouse model of progressive pulmonary tuberculosis is suitable to explore the efficiency of different immunotherapeutic agents. First, it is based on an aerogenic infection, which is the usual route of tuberculosis in humans. Second, the rate of bacterial multiplication in the lungs correlates with the extent of tissue damage (pneumonia) and mortality. Third, the infection is successfully controlled as long as a strong Th1 cell response is sustained [17,18].
Using this model of pulmonary tuberculosis, we demonstrated that the administration of human or murine TF evoked an efficient reconstitution of CMI. Thus, the immunotherapeutic effect is not species specific, although murine TF was more efficient permitting a 95% survival with a significant decrease in lung bacillary loads and tissue damage after six months of treatment. These results using an experimental model of pulmonary tuberculosis, are in agreement with several clinical studies that have reported dramatic benefits in patients treated with TF suffering cutaneous tuberculosis (lupus vulgaris) [4], pulmonary tuberculosis refractory to all antibiotics [26], tuberculous osteomyelitis [27], and infections by Mycobacterium fortuitum or Mycobacterium xenopi [8,28]. Our results extend these clinical observations, showing that TF treatment induced a significant production of IFNγ, IL-2, and iNOS concomitantly with a significant decrease in IL-4 expression. These immune protective profile of cytokine production in experimental tuberculosis is very similar to the reported cytokine pattern induced by TF in other experimental models in mice and human diseases [4,29]. However, this TF treatment did not prevent disease reactivation, due that infected animals treated during six months with TF showed pneumonia and high CFU counts after one month of corticosterone administration in drinking water.
Although the source of TF, human or murine, was not critically important to produce benefits, our results clearly showed that the dose of TF is absolutely crucial to get a beneficial result. In fact, 0·1 U of mTF evoked 95% survival, whereas 0·01 U of hTF induced 100% mortality together with large pneumonic patches surrounded by numerous TNFα positive macrophages after 14 weeks of treatment. We have shown that TNFα is a significant cytokine related to necrosis induction in advanced tuberculosis when a mixed Th1/Th2 cytokine balance is produced [30,31]. Thus, it is possible that the high TF dose induced very high TNFα production inducing excessive inflammation, extensive lung consolidation, and necrosis leading to death. This striking difference has been observed also in human recipients [4]. Thus, TF treatment responses vary according to several factors, the most important are: the dose and potency of the TF preparation, the severity of the disease, and the amount of the antigen burden [4]. The fact that tuberculous mice treated with TF from animals infected with Listeria monocytogenes showed limited ability to decrease lung bacillary proliferation corroborated the antigen specificity of TF. Thus, TF is not species specific but antigen specific [4].
In the present study, we used spleen cells from BALB/c mice after 21 days of intratracheal M. tuberculosis infection as a source of TF, because the peak of the protective immunity is produced at this time point [17,18] In fact, this mTF induced the best response when compared with hTF obtained from peripheral leucocytes from healthy PPD + individuals. However, these experimental conditions are difficult to get in the clinical practice limiting its use in the human disease. Thus, it is clear that an appropriate in-vitro laboratory evaluation of each TF batch and of its destined recipient is essential in order to define the best TF dose and administration frequency. In the past, several leucocytes migration inhibition test were used [32,33]. Considering the crucial role of IFNγ in the control of tuberculosis, and the very efficient induction of this cytokine by TF, it is plausible to propose that a new useful determination could be the in vitro IFNγ production by peripheral leucocytes from the patient after stimulation with different TF concentrations.
Since the introduction of an effective chemotherapy 50 years ago, the standard four and two drugs dosage combination must still be taken for six to nine months. With such a long regimen, there is a real challenge to control the rapid spread of tuberculous infection, the rise of drug-resistant strains, and the dangerous coinfection with HIV. Thus, it is quite important to implement new therapeutic schemes based on short chemotherapy courses. To this regard, our results clearly showed that one potential alternative is the use of TF as supplement of conventional chemotherapy. In fact, this combination induced a very rapid clearance of lung bacilli with quick elimination of pneumonia, so in only two weeks it was possible to induce bacterial sterilization of the lungs, whereas conventional chemotherapy alone needed two months. It seems that the direct toxic effect of the antibiotics against the bacilli, plus the strong stimulation of CMI by TF, produced a very efficient and synergistic therapeutic activity. Considering that TF is, in general innocuous due that its administration can produce transitory hyperpyrexia, but it is free of hypersensitivity and long lasting side-effects (only one reported case with transitory cerebral white matter lesions [34]), and that its production is relatively inexpensive, we consider this therapeutic modality a real proposition for ultra-short chemotherapy treatment, particularly in the developing world where low economic resources are an important factor in the control of this significant disease.
Many years ago, Lawrence [1] demonstrated that DTH responsiveness to tuberculin could be transferred by soluble extracts of leucocytes from 20 ml of blood and termed the factor responsible for this phenomenon ‘transfer factor’. For many years this observation was ignored, until 1970 when the use of TF started in primary immunodeficiencies [10]. Since then, TF has been used in many clinical trials monitoring its effects by tests of CMI, and several clinical and experimental studies have informed usefulness of TF in different diseases [4]. Unfortunately, because TF is a complex group of many low molecular weight proteins (more than 200), its precise molecular structure and mechanisms of action have not been elucidated yet, producing deep skepticism for its use in clinical practice. However, we believe that the use of specific TF as immunotherapy in the treatment of pulmonary tuberculosis is quite valuable, but before its administration it is important to evaluate the specificity, potency, and the best dose, trying to individualize the treatment for each patient.
Acknowledgments
This work was partially supported by CONACyT and CGPI. AF is a scholarship recipient from CONACyT. IEG and SEP are scholarship recipients of COFAA/EDD/EDI.
REFERENCES
- 1.Lawrence HS. The transfer in humans of delayed skin sensitivity to Streptococcial M substances and to tuberculin with disrupted leukocytes. J Clin Invest. 1955;34:219–30. doi: 10.1172/JCI103075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rozzo SJ, Kirkpatrick CH. Purification of transfer factors. Molec Immunol. 1992;29:167–82. doi: 10.1016/0161-5890(92)90098-i. [DOI] [PubMed] [Google Scholar]
- 3.Sudhir KS, Sizemore RS, Gottlieb AA. Immunomodulatory components present in IMREG-1, an experimental immunosupportive biologic. Biotechnol. 1988;6:810–5. [Google Scholar]
- 4.Fudenberg HH, Pizza G. Transfer factor 1993. New frontiers. Prog Drug Res. 1993;42:309–400. doi: 10.1007/978-3-0348-7153-2_7. [DOI] [PubMed] [Google Scholar]
- 5.Estrada Parra S, Chavez Sanchez R, Ondarza Aguilera R, Correa MB, Serrano MA, Monges NA, Calva PC. Immunotherapy with transfer factor of recurrent herpes simplex type1. Arch Med Res. 1995;26:S87–S92. [PubMed] [Google Scholar]
- 6.Estrada Parra S, Nagaya A, Serrano E, et al. Comparative study of transfer factor and acyclovir in the treatment of herpes zoster. Int J Immunopharm. 1998;20:521–31. doi: 10.1016/s0192-0561(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 7.Bullock WE, Fields JP, Bandvias MW. An evaluation of transfer factor as immunotherapy for patients with lepromatous leprosy. New Eng J Med. 1972;287:10–53. doi: 10.1056/NEJM197211232872101. [DOI] [PubMed] [Google Scholar]
- 8.Wilson GB, Metcalf JF, Fudenberg HH. Treatment of Mycobacterium fortuitum infection with’transfer factor′. New methodology for evaluating TF potency and predicting clinical response. Clin Immunol Immunopathol. 1982;23:478–91. doi: 10.1016/0090-1229(82)90132-5. [DOI] [PubMed] [Google Scholar]
- 9.Delgado O, Romano EL, Belfort E, Pifano F, Scorza JV, Rojas Z. Dialyzable leukocyte extract therapy in immunodepressed patients with cutaneous leishmaniasis. Clin Immunol Immunopathol. 1981;19:351–9. doi: 10.1016/0090-1229(81)90078-7. [DOI] [PubMed] [Google Scholar]
- 10.Levin AS, Spitler LE, Stites DP, Fudenberg HH. Wiscott Aldrich syndrome, a genetically determined cellular immunologic deficiency: clinical and laboratory responses to therapy with transfer factor. Proc Natl Acad Sci USA. 1970;67:821–8. doi: 10.1073/pnas.67.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whyte RI, Schork MA, Sloan H, Orringer MB, Kirsh MM. Adjuvant treatment using transfer factor for bronchogenic carcinoma: long term follow up. Ann Thorac Surg. 1992;53:391–6. doi: 10.1016/0003-4975(92)90256-4. [DOI] [PubMed] [Google Scholar]
- 12.Rook GAW, Hernandez Pando R. The pathogenesis of tuberculosis. Annu Rev Microbiol. 1996;50:259–84. doi: 10.1146/annurev.micro.50.1.259. [DOI] [PubMed] [Google Scholar]
- 13.Chan X, Xing Y, Magliozzo RS, Bloom BR. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated macrophages. J Exp Med. 1992;175:1111–22. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 15.Seah GT, Scott GM, Rook GAW. Type 2 cytokine gene activation and its relationship to extent of disease in patients with tuberculosis. J Infect Dis. 2000;181:385–9. doi: 10.1086/315200. [DOI] [PubMed] [Google Scholar]
- 16.Wangoo A, Sparer T, Brown IN, et al. Contribution of Th1 and Th2 cells to protection and pathology in experimental models of granulomatous disease. J Immunol. 2001;166:3432–9. doi: 10.4049/jimmunol.166.5.3432. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Pando R, Orozco EH, Sampieri A, Pavón L, Velasquillo C, Larriva-Sahd L, Madrid MW. Correlation between kinetics of Th1/Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology. 1996;89:26–33. [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez-Pando R, Orozco EH, Arriaga AK, Sampieri A, Larriva-Sahd J, Madrid Marina V. Analysis of the local kinetics and localization of interleukin 1α, tumor necrosis factor α and transforming growth factor β during the course of experimental pulmonary tuberculosis. Immunology. 1997;90:607–17. doi: 10.1046/j.1365-2567.1997.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez-Pando R, Schön T, Orozco EH, Serafín J, Estrada-Garcia I. Expression of nitric oxide synthase and nitrotyrosine during the evolution of experimental pulmonary tuberculosis. Exp Toxicol Pathol. 2001;53:257–65. doi: 10.1078/0940-2993-00182. [DOI] [PubMed] [Google Scholar]
- 20.Hernàndez Pando R, Orozco H, Honour J, Silva P, Leyva R, Rook GAW. Adrenal changes in murine pulmonary tuberculosis; a clue to pathogenesis? FEMS Immunol Med Microbiol. 1995;12:63–72. doi: 10.1111/j.1574-695X.1995.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. TB – a Global Emergency. Geneva: WHO; 1994. WHO report on the TB endemics. [Google Scholar]
- 22.Jarnagin JL, Luschinger DW. The use of fluorescein diacetate and ethidium bromide as a stain for evaluating viability of mycobacteria. Stain Technol. 1980;55:253–8. doi: 10.3109/10520298009067249. [DOI] [PubMed] [Google Scholar]
- 23.Hernández Pando R, Pavón L, Orozco EH, Rangel J, Rook GAW. Interactions between hormone-mediated and vaccine-mediated immunotherapy for pulmonary tuberculosis in BALB/c mice. Immunology. 2000;100:391–8. doi: 10.1046/j.1365-2567.2000.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirsh CS, Hussain R, Toossi Z, Dawood G, Shahid F, Ellner JJ. Crossmodulatory role for transforming growth factor b in tuberculosis; suppression of antigen driven interferon γ production. Proc Natl Acad Sci USA. 1996;93:3193–8. doi: 10.1073/pnas.93.8.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takashima T, Ueta Ch, Tsuyuguchi I, Kishimoto S. Production of tumor necrosis factor alpha by monocytes from patients with pulmonary tuberculosis. Infect Immun. 1990;58:3286–92. doi: 10.1128/iai.58.10.3286-3292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kind CH, Gartmann JC, Grob PJ. Transfer factor therapy in a patient with anergic pulmonary tuberculosis. Schweiz Med Wochenschr. 1977;107:1742–3. [PubMed] [Google Scholar]
- 27.Zielinski CC, Savoini E, Ciotti M, Orani R, Konigswieser H, Eibl MM. Dialyzable leukocyte extract (transfer factor) in the treatment of superinfected fistulating tuberculosis of the bone. Cell Immunol. 1984;84:200–5. doi: 10.1016/0008-8749(84)90091-1. [DOI] [PubMed] [Google Scholar]
- 28.Dwyer JM, Gerstenhaber BJ, Dobuler KJ. Clinical and immunologic response to antigen-specific transfer factor in drug-resistant infection with Mycobacterium xenopi. Am J Med. 1983;74:161–8. doi: 10.1016/0002-9343(83)91136-1. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez Thull L, Kirkpatrick CH. Profiles of cytokine production in recipients of transfer factors. Biotherapy. 1996;9:55–9. doi: 10.1007/BF02628657. [DOI] [PubMed] [Google Scholar]
- 30.Hernàndez Pando R, Rook GAW. The role of TNFalpha in T cell-mediated inflammation depends on the Th1/Th2 cytokine balance. Immunology. 1994;82:591–5. [PMC free article] [PubMed] [Google Scholar]
- 31.Hernández Pando R, Pavón L, Arriaga K, Orozco EH, Madrid MV, Rook GAW. Pathogenesis of tuberculosis in mice exposed to low and high doses of an environmental mycobacterial saprophyte before infection. Infect Immun. 1997;6:84–90. doi: 10.1128/iai.65.8.3317-3327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson GB, Fudenberg HH, Horsemanheimo M. Effects of dialyzable leukocyte extracts (DEL) with transfer factor (TF) activity on leukocyte migration in-vitro. I antigen dependent inhibition and antigen independent inhibition and enhacement of migration. J Laboratory Clin Med. 1979;93:800–18. [PubMed] [Google Scholar]
- 33.Gallin JI, Kirkpatrick JH. Chemotactic activity in dialyzable transfer factor. Proc Natl Acad Sci USA. 1974;71:498–502. doi: 10.1073/pnas.71.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foschi FG, Marsigli L, Bernardi M, Salvi F, Mascalchi M, Gasbarrini G, Stefanini G. Acute multifocal cerebral white matter lesions during transfer factor therapy. J Neurol Neurosurg Psych. 2000;68:114–5. doi: 10.1136/jnnp.68.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]