Abstract
MRL/Mp mice bearing the Fas deletion mutant gene, lpr (MRL/lpr), spontaneously develop polyarthritis, sialoadenitis and dacryoadenitis, resembling rheumatoid arthritis (RA), and also corneal involvement such as keratopathy and scleritis, which is a major complication in RA patients. In this study, we found that the expression levels of IL-1β and MMP-1 mRNAs in cornea were high in both MRL/lpr and MRL/Mp-+/+ strains of mice at an age younger than when they develop any inflammatory lesions. This was not true of other inbred strains, even those bearing the lpr gene, and also not of (NZB × NZW) F1 lupus mice. There was no significant difference in the expression of IL-1α and TGFβ in cornea in these strains. Using crosses between MRL/lpr and C3H/HeJ-lpr/lpr (C3H/lpr) mice, at least the expression of IL-1β was found to be under the control of the MRL genetic background, likely with a recessive mode of inheritance. Considering that IL-1β in cornea was detected particularly in the epithelial layer, the high expression of IL-1β in cornea is most likely involved in the genetic predisposition for corneal involvement and possibly also for arthritis in an MRL strain of mice.
Keywords: rheumatoid arthritis, corneal ulcer, IL-1α, MMP-1, TGFβ, genetic
INTRODUCTION
Rheumatoid arthritis (RA) is a systemic autoimmune disease involving polyarthritis, rheumatoid vasculitis, sialoadenitis and dacryoadenitis [1]. Corneal involvement such as scleritis, kerato-conjunctivitis sicca and sclerosing keratitis is a major complication in RA patients [2], who frequently have corneal ulceration [3]. The lesion seems to occur rapidly, even in sterile conditions and without trauma [4], and is possibly associated with destruction of collagen matrices of the cornea [2–6].
The major component of corneal stroma is type I collagen [7]. It is well known that MMP-1 and MMP-8 degrade type I collagen, and IL-1β is a possible inducer of MMP-1 [8–10]. It is likely that IL-1β is a major factor involved in the destruction of corneal matrices in RA since it is produced significantly in the synovial tissues and other inflammatory lesions in the patients [11,12]. On the other hand, IL-1β production might occur in the cornea uniquely in RA patients and be followed by corneal ulceration. These possibilities are still controversial.
MRL/Mp-lpr/lpr (MRL/lpr) is a unique strain of mice which spontaneously develop polyarthritis, sialoadenitis and dacryoadenitis, and coincidentally corneal lesions such as keratopathy and scleritis [13–15], associated with cytokine abnormalities involving an increased of level of IL-1β in serum [16,17]. In general, MRL/lpr, MRL/+, NZB/WF1, NZB, BXSB and LG/J strains of mice are considered to be multiple murine models of autoimmunity. Among these strins, only those with an MRL background are arthritis-prone and develop corneal lesions, while both MRL/lpr and NZB/WF1 strains are considered useful murine models for Sjogren's syndrome. Thus, this strain of mice may be a model of corneal involvement in rheumatoid arthritis.
In the present study, we found that the production of IL-1β in cornea is increased in MRL/lpr mice, and we studied its genetic basis. We will present evidence that IL-1β production in cornea is under the control of the MRL genetic background.
MATERIALS AND METHODS
Animals
MRL/lpr, MRL/Mp-+/+(MRL/+), C3H/HeJ-lpr/lpr (C3H/lpr) mice, all of which have an H-2k haplotype, were obtained from SLC (Sizuoka, Japan). Using MRL/lpr and C3H/lpr mice, we prepared F1 intercross and N2 backcross progenies, (MRL/lpr × C3H/lpr) F1 and MRL/lpr × (MRL/lpr × C3H/lpr) F1, respectively. BALB/c (H-2d), C57BL/6 (H-2b), DBA/2 (H-2d), C3H/HeJ (H-2 k), DBA/1 (H-2q), NZB (H-2d), and (NZB × NZW) F1 (NZB/W) (H-2d/z) mice were obtained from Japan Charles River (Kanagawa, Japan).
All mice were housed in specific pathogen-free conditions and were used when they were 5–7 weeks old. At this age, MRL/lpr and MRL/+mice did not have any characteristic histopathological manifestations of inflammatory lesions involving polyarthritis, sialoadenitis, dacryoadenitis and corneal lesions.
Measurements of cytokines
Total RNA was extracted from the corneas of each strain of mice by a single-step procedure [18] using RNAzolTM (Biotex Laboratory; Houston, TX, USA) and cDNAs were prepared using random-hexamer-primed M-MLV reverse transcriptase (SuperScriptTMII RNase H–Reverse Transcriptase, GibcoBRL, Gainthersburg, MD, USA). Specific cDNAs were amplified by polymerase chain reaction (PCR) using primer pairs specific for the mouse nucleotide sequences of IL-1α, IL-1β, TGFβ, G3PDH (Clontech Laboratory, Palo Alto, CA, USA) and MMP-1 (newly synthesized in our laboratory). A hot start amplification procedure was used whereby the reaction mixture without primers and DNA polymerase was overlaid with mineral oil and heated to 94°C for 4 min. After addition of primers and Taq DNA polymerase, incubation proceeded in a Program Temp Control System (Astec; Fukuoka, Japan) for 30 cycles of denuturation, for 45s at 94°C, annealing for 45s at 60°C and extension for 2 min at 72°C.
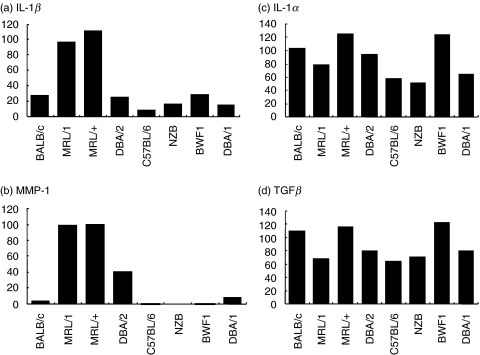
After separation by electrophoresis through 2% agarose gels, PCR products were detected by ethidium bromide staining. Digital images of fluorescent bands were quantified using Quantity One image-analysing soft ware (Toyobo, Tokyo, Japan). As shown in Fig. 2, the relative expression units of each sample (from the corneas of 10 mice) were calculated by defining the highest value in each electrophoresis series as ‘100’. In Fig. 4, the relative expression units of each sample were calculated by defining the value of the sample from MRL/lpr, which was used in the experiment of Fig. 2. The relative expression units were standardized according to the values of G3PDH for each sample.
Fig. 4.
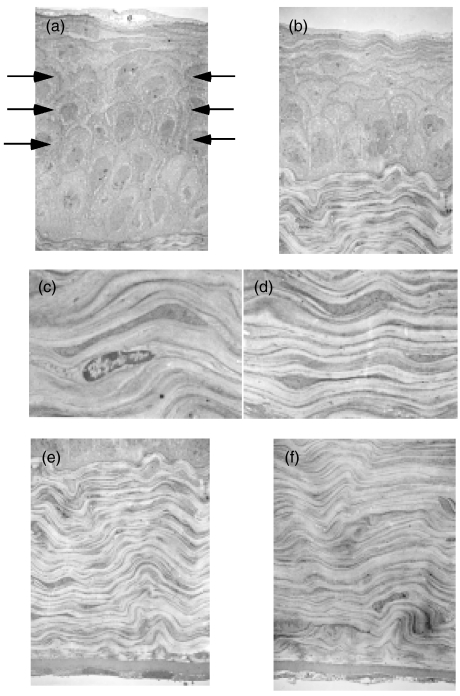
Transmission electron microscopy of the corneas of BALB/c and MRL/lpr mice (Original magnification × 2700). No inflammatory cells were seen in any layers of the corneas of both strains. The wing cells in (b) the corneal epithelia of MRL/lpr mice were decreased compared with (a) those of BALB/c (arrows). No differences were seen in the structure or dimensions of the corneal stroma or endothelial cells between MRL/lpr (c,e) and BALB/c (d,f) mice.
Fig. 2.
Semi-quantitative analyses under ‘Night Hawk’ of PCR products following the procedures described in Fig. 1.
Immunohistochemical staining of IL-1β
Mouse corneas were fixed with PLP solution (2% para formaldehyde, 0·1 m lysine, 0·05 m phosphate buffer (PB) pH 7·4, 10 mm NaIO4). Endogenous peroxidase was quenched by incubating with 40 mm gelatin and 20 ml 30% H2O2 in 40 ml 0·1 m PB for 20 min. Non-specific protein binding was blocked by covering the sections overnight with blocking solution which consisted of 5% BSA, 5% normal sheep serum and 0·1% NaN3 in 0·1 m PB, pH 7·4.
Immunohistochemical staining was performed with 1 mg/ml anti-IL-1β rabbit polyclonal antibody (Genzyme, Cambridge, MA, USA) and biotin-labelled antirabbit sheep IgG (Vector Laboratories, Burlingane, CA, USA). The sections were washed in 0·1 m PB containing 0·5% BSA, and 0·1% Triton-X 100, and the reacted for 1 h at 4°C with streptoavidin-peroxdase solution (Vector Laboratories). Then, the sections were treated at room temperature for 10 min with 0·5% cobalt acetate in 0·1 m Tris buffer (TB) after washing sequently in 0·1 m PB twice, 0·05 m TB and 0·1 m TB. After washing in 0·1 m TB and 0·1 m PB, antigens were visualized by the DAB reaction.
Transmission electron microscopy
Corneal tissues isolated from mouse eye globes were fixed in an improved fixative containing 2·5% glutaraldehyde, 0·1 m cacodylate buffer(pH 7·3), 50 mm l-lysine, and 1% tannicacid, followed by postfixation with 1% OsO4. En bloc staining was performed with a 2·5% aqueous solution of uranyl acetate, and embedding was done with a Spurr mixture of vinylcyclohexane dioxide. Thin sections were cut with a glass knife and poststained with uranyl acetate and Sorenson's lead citrate. Micrographs were taken on a JEOL 100CX Electron Microscope (JEOL, Peabody, MA, USA).
RESULTS
High expression of IL-1β and MMP-1 mRNAs in corneas of MRL mice
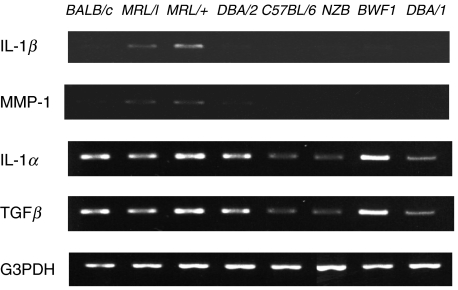
IL-1β and MMP-1 mRNAs were highly expressed in the corneas of both MRL/lpr and MRL/+mice (Fig. 1). However, the corneas from BALB/c and other strains of mice, even NZB/W, which are known to develop autoimmune diseases involving glomerulonephritis, expressed low levels IL-1β and MMP-1 mRNAs (Fig. 1). Remarkably IL-1α and TGFβ seemed to be expressed in the corneas of all the mouse strains examined.
Fig. 1.
RT-PCR analyses of IL-1β and MMP-1 expression in the corneas of various strains of mice. The PCR products from 0·5 mg of total RNA extracted from corneas pooled from 10 eyes of each strain were electrophoresed through 2% agarose gels and detected by ethidium bromide staining. Amplified PCR fragment sizes with specific primers for IL-1β, TGF-β, and G3PDH were confirmed with 563, 525, and 983 bp fragments, respectively, of φX174/Hae III.
Figure 2 shows the results of semiquantitative analyses of these results. The expression levels of IL-1β and MMP-1 mRNAs were almost the same in MRL/lpr and MRL/+mice, the latter having the same genetic background as MRL/lpr mice except for the lpr mutation. This indicates that the lpr mutation is not associated with the levels of expression of these cytokines. These values were considerably lower in other strains. However, IL-1α and TGFβ were not particularly highly expressed in both MRL strains compared with other strains examined.
High expression of IL-1β and MMP-1 mRNAs in the corneas of the MRL strains seemed not to be related to the H-2k haplotype itself since their expression levels in corneas of C3H/HeJ mice, whose H-2 haplotype is k, the same as that of MRL strains, were much lower and almost the same as those of BALB/c mice (data not shown).
IL-1β expression localized in the corneal epithelial layer
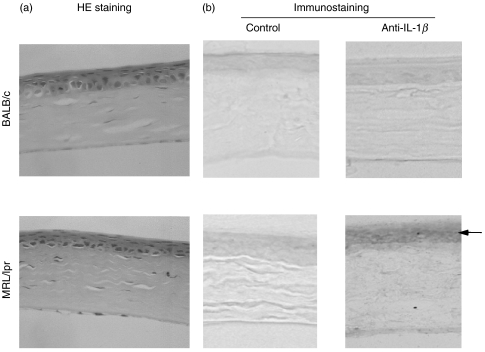
We employed immunohistochemistry to examine the localization of IL-1β expression in cornea. As shown in Fig. 3, IL-1β was expressed in the corneal epithelial layer of MRL/lpr mice although there was no inflammatory cell infiltration into the cornea or degenerative changes of the epithelial cells. Transmission electron microscopy indicated that there were no inflammatory cells in any layer of the cornea of MRL/lpr mice (Fig. 4). Another interesting transmission electron microscopy finding was the decrease in corneal epithelial thickness along with disarrangement of basal epithelial cells in the MRL strains. The corneal epithelium of BALB/c mice consisted of 6–7 cell layers, while that of MRL/lpr mice consisted of only 4–5 cell layers possibly due to the decreased wing cell layers. In addition to the decreased cell density in the basal epithelial layer, the basal cells were spherical in shape (BALB/c mice have a single layer of columnar basal cells). There were no differences in the structure or dimensions of corneal stroma or endothelium between MRL/lpr and BALB/c mice.
Fig. 3.
Histological and immunohistochemical examinations of corneas from MRL/lpr and BALB/c mice. (a) Representative histological pictures of corneas showing no significant differences between the two strains. (H&E staining, × 400) (b) Representative pictures of IL-1β expression in corneas: left; without specific antibodies, right; stained with anti-IL-1β antibodies. IL-1β is specifically localized in the corneal epithelial layer and was observed only in an MRL/lpr strain. (×400)
Genetic control of IL-1β expression in cornea
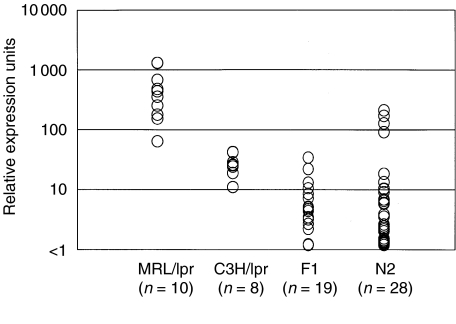
The results described above lead us to speculate that the expression of IL-1β in cornea may be under the control of the MRL genetic background. To explore this, we examined IL-1β expression levels in corneas from the F1 and N2 progenies prepared from MRL/lpr and C3H/lpr strains. As shown in Fig. 5, IL-1β expression levels in the corneas of all (MRL/lpr × C3H/lpr) F1 mice were significantly lower (less than 80 units) than in MRL/lpr mice (more than 90 units) and almost the same level as in C3H/lpr mice. In MRL/lpr × (MRL/lpr × C3H/lpr) F1 mice, there were regular variations in the expression levels as a few mice showed high expression at the same level as some MRL/lpr mice and the majority expressed less than 10 units, the same as the F1 progeny. These findings indicate that IL-1β expression in cornea is under the control of the MRL genetic background and that is likely has a recessive mode of inheritance.
Fig. 5.
Relative expression of IL-1β in corneas of MRL/lpr and C3H/lpr mice and their intercrosses.
DISCUSSION
In the present study, we found that IL-1β is highly expressed in the corneas of MRL strains of mice under the control of the MRL genetic background. Such IL-1β expression was not observed in any other strains of mice including NZB/W mice, which spontaneously develop a variety of autoimmune disorders, but not arthritis [19]. With the same PCR amplification conditions, we previously observed a transient elevation of IL-1β expression after wounding the cornea of BALB/c mice with photoblation, indicating the validity of the comparison of IL-1β expression in mouse strains [20].
In the past decade, extensive studies of the genetic basis of arthritis in an MRL strain of mice [21,22] have indicated that arthritis might be under the control of multiple genes in an additive fashion. Those studies did not identify the polymorphism at the IL-1 promoter loci and did not shown an association of IL-1 expression with any polymorphism. Since the overproduction of IL-1β in the synovial cells of joints has been shown to play an essential role in the development of arthritis [11,23], some gene loci controlling the development of arthritis may also be involved in the high expression of IL-1β in the corneas of these particular strains. Our results from crosses between MRL/lpr and C3H/lpr mice showed that only less than 15% (4/28) of MRL/lpr × (MRL/lpr × C3H/lpr) F1 mice had high expression of IL-1β in their corneas, suggesting that two or more gene loci might affect IL-1β production in the corneas of MRL mice. Although we perfomed sequence analysis of the region spanning −500 bp upstream of the transcription start site, which includes IL-1β promoter[42], there was no polymorphism between MRL and C3H strains. An association study of IL-1β expression with polymorphic microsatellite markers is needed to further investigate the genetic mechanisms.
Although the mechanism responsible for the high expression of IL-1β remains unclear, our finding may partly explain the predisposition to corneal pathology common to MRL/lpr mice and RA patients. It is well known that IL-1β stimulates the production of MMP-1 by chondrocytes [9, 10, 24, 25] or keratocytes [26], which is known to be involved in the degradation of type I collagen, the major structural protein of the corneal stroma [8]. In this study, the expression of MMP-1 was also found to be high in the corneas of MRL strains, while TGFβ was not expressed as much as IL-1β. TGFβ is known to suppress collagenase production [27] as well as to promote collagen production by fibroblasts [28,29] and TIMP-1 production by keratinocytes [26] or chondrocytes [30]. Thus, the homeostasis of collagen turnover in the corneal stroma may become imbalanced in MRL strains of mice, leading to an elevation of collagenase activity in the corneal stroma [31,32] or the induction of IL-1-associated keratocyte apoptosis as shown by the study of Wilson et al. [33].
It was clearly demonstrated by immunohistochemistry that IL-1β was intensely expressed in the corneal epithelial layer of an MRL strain of mice. This is consistent with the report by Stephen et al. [34] who showed IL-1 expression in cultured human corneal epithelial cells, but not in cultured stromal cells. It is unlikely that IL-1β was derived from infiltrating inflammatory cells in the cornea or dendritic cells at the limbus because MRL/lpr and MRL/+mice did not show any inflammatory lesions at those sites. It is still unclear as to what kinds of factors actually promote IL-1β production in corneal epithelial cells. Extrinsic factors, such as proinflammatory cytokines [17], which might be released from the surrounding tissue, or mouse tear proteins [35], may exhibit MRL allelic polymorphism and/or be regulated by the MRL genetic background. Lemay et al. [36] reported that IL-1 expression depended on the onset of disease and the lpr mutation in the kidneys, liver, lymph node and spleen of MRL strain mice. They also reported that lymphocytes highly expressed IL-1 in the MRL/lpr strain mice [37]. To the best of our knowledge, this is the first report investigating the expression of IL-1β in an organ that is isolated from blood cells and blood vessels.
The decreased thickness of the corneal epithelial layer in the MRL background mice, characterized by the loss of wing cell layers and the disarrangement of basal epithelial cells, is another interesting observation. These changes in the corneal epithelium were not related to the lpr mutation, because similar pathological changes were also seen in MRL/+mice. The decreased number of wing cell layers probably reflects disturbed cell supply or accelerated desquamation of the corneal epithelium. Several investigators reported that IL-1β induces apoptotic cell death of fibroblasts [38], anterior pituitary cells [39], islets of Langerhans [40], macrophages [41] and keratocytes [33] through nitric oxide production. Thus, one explanation of our finding is that overproduction of IL-1β from corneal epithelial cells may lead to abnormalities in corneal epithelial structure.
Based on the results of this study, we speculate that the excessive production of IL-1β by the cornea may be associated with genetic susceptibility and corneal involvement in RA through the acceleration of MMP-1 production. However, there is no report concerning penetrating keratoplasty due to the corneal perforation in this mouse model, although other ocular manifestations in RA patients, such as keratopathy, scleritis and dry eye have been investigated. Our preliminary study disclosed that corneal epithelial healing was significantly delayed in MRL/lpr mice compared with BALB/c mice following excimer laser photoablation. This animal model could be a valuable tool for elucidating the significance of high IL-1β expression in the corneal pathology of RA patients and its genetic basis.
Acknowledgments
This study was supported in part by a Grant-in-aid from the Ministry of Education, Science and Culture, Japan (♯09671802) and the research fund from the Japan Owner's Association (♯302) and by Grant-in Aid for Scientific Research Funds of the Ministry of Education, Science and Culture of Japan (♯11557019). The authors are indebted to Dr Herbert M. Schulman for reviewing the manuscript.
REFERENCES
- 1.Lems WF, Dijkmans AC. Rheumatoid arthritis: clinical picture and its variants,Rheumatoid ArthritisNew Frontiers in Pathogenesis and Treatment. In: Firestein GS, Panayi GS, Wollheim FA, editors. New York: Oxford University Press; 2000. pp. 213–25. [Google Scholar]
- 2.Fuerst DJ, Schanzlin DJ, Smith RE. Rheumatoid Disease. In: Smolin G, Thoft RA, editors. The Cornea. Boston: Little Brown; 1994. pp. 364–84. [Google Scholar]
- 3.Jyson MIV, Easty DL. Ulceration of the cornea in rheumatoid arthritis. Ann Rheum Dis. 1977;36:428. doi: 10.1136/ard.36.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watson PG, Hayreh SS. Scleritis and episcleritis. Br J Ophthalmol. 1976;60:163–91. doi: 10.1136/bjo.60.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGavin DDM, Williamson J, Forrester JV, et al. Episcleritis and scleritis: a study of their clinical manifestations and associations with rheumatoid arthritis. Br J Ophthalmol. 1976;60:192–226. doi: 10.1136/bjo.60.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfister RR, Murphy GE. Corneal ulceration and perforation associated with Sjogren's syndrome. Arch Ophthalomol. 1980;98:89–94. doi: 10.1001/archopht.1980.01020030091006. [DOI] [PubMed] [Google Scholar]
- 7.Marshall GE, Konstas AGP, Lee WR. Collagens in ocular tissues. Br J Ophthalmol. 1993;77:515–24. doi: 10.1136/bjo.77.8.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matrisian LM. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990;6:121–5. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- 9.Mizel SB, Dayer JM, Krane SM, Mergenhagen SE. Stimulation of rheumatoid synovial cell collagenase and prostaglandin production by partially purified lymphocyte activating factor (interleukin-1) Proc Natl Acad Sci USA. 1981;78:2474–7. doi: 10.1073/pnas.78.4.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postlethwaite AE, Lachmann L, Mainardi CL, Kang AH. Interleukin 1 stimulation of collagenase production by cultured fibroblasts. J Exp Med. 1983;157:801–6. doi: 10.1084/jem.157.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 12.Buchan G, Barrett K, Turner M, Chantry D, Maini RN, Feldmann M. Interleukin-1 and tumour necrosis factor mRNA expression in rheumatoid arthritis. prolonged production of IL-1 alpha. Clin Exp Immunol. 1988;73:449–55. [PMC free article] [PubMed] [Google Scholar]
- 13.Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17:584–9. doi: 10.1097/00003226-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Jabs DA, Alexander EL, Green WR. Ocular inflammation in autoimmune MRL/Mp mice. Invest Ophthalmol Vis Sci. 1985 September;26:1223–9. [PubMed] [Google Scholar]
- 15.Hoffman RW, Yang HK, Waggie KS, Durham JB, Burge JR, Walker SE. Band keratopathy in MRL/1 and MRL/n mice. Arthritis Rheum. 1983;26:645–52. doi: 10.1002/art.1780260511. [DOI] [PubMed] [Google Scholar]
- 16.Schorlemmer HU, Kanzy EJ, Langner KD, Kurrle R. Immunomodulatory activity of recombinant IL-1 receptor (IL-1-R) on models of experimental rheumatoid arthritis. Agents Actions. 1993;39(Spec No):C113–6. doi: 10.1007/BF01972739. [DOI] [PubMed] [Google Scholar]
- 17.Hamano H, Saito I, Haneji N, Mitsuhashi Y, Miyasaka N, Hayashi Y. Expressions of cytokine genes during development of autoimmune sialadenitis in MRL/lpr mice. Eur J Immunol. 1993;23:2387–91. doi: 10.1002/eji.1830231002. [DOI] [PubMed] [Google Scholar]
- 18.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 19.Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, Murphy ED, Dixon FJ. Spontaneous murine lupus–like syndromes: clinical and immunopathological manifestations in several strains. J Exp Med. 1978;148:1198. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joko T, Okamoto S, Okamoto M, Ohashi Y. Close association of interleukin-1β and metalloproteinase 1 (MMP-1) mRNA during corneal wound healing. Invest Ophthal Vis Sci. 1998;39(Suppl.):S756. [Google Scholar]
- 21.Nakatsuru S, Terada M, Nishihara M, et al. Genetic dissection of the complex pathological manifestations of collagen disease in MRL/lpr mice. Pathol Int. 1999;49:974–82. doi: 10.1046/j.1440-1827.1999.00979.x. [DOI] [PubMed] [Google Scholar]
- 22.Nose M, Nishihara M, Kamogawa J, Terada M, Nakatsuru S. Genetic basis of autoimmune disease in MRL/lpr mice: dissection of the complex pathological manifestations and their susceptibility loci. Rev Immunogenet. 2000;2:154–64. [PubMed] [Google Scholar]
- 23.Ivashkiv LB. Cytokine expression and cell activation in inflammatory arthritis. Adv Immunol. 1996;63:337–76. doi: 10.1016/s0065-2776(08)60859-7. [DOI] [PubMed] [Google Scholar]
- 24.Gowen M, Wood DD, Ihrie EJ, Meats JE, Russell RGG. Stimulation by human interleukin 1 of cartilage breakdown and production of collagenase and proteoglycans by human chondrocytes but not by human osteoblasts in vitro. Biochm Biophys Acta. 1984;797:186–98. doi: 10.1016/0304-4165(84)90121-1. [DOI] [PubMed] [Google Scholar]
- 25.MacNaul KL, Chartrain N, Lark M, Tocci MJ, Hutchinson NI. Discoordinate expression of stromelysin, collagenase and tissue inhibitor of metalloproteinase-1 in rheumatoid human synovial fibroblasts: synergistic effects of interleukin-1 and tumor necrosis factor-alpha on stromelysin expression. J Biol Chem. 1990;265:17238–45. [PubMed] [Google Scholar]
- 26.Strissel KJ, Rinehart WB, Fini ME. A corneal epithelial inhibitor of stromal cell collagenase synthesis identified as TGF-b2. Invest Ophthalmol Vis Sci. 1995;36:151–62. [PubMed] [Google Scholar]
- 27.Overall CM, Wrana JL, Sodek J. Independent regulation of collagenase, 72-kDa progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor-β. J Biol Chem. 1989;264:1860–9. [PubMed] [Google Scholar]
- 28.Fine AP, Godstein RH. The effect of transforming growth factor-β on cell proliferation and collagen formation by lung fibroblasts. J Biol Chem. 1987;262:3897–902. [PubMed] [Google Scholar]
- 29.Rajendra R, Postlethwaite AE, Keski-Oja HL, Kang AH. Transforming growth factor-β increases steady state levels of type I procollagen and fibronectin messenger RNAs posttranscriptionally in cultured human dermal fibroblasts. J Clin Invest. 1987;79:1285–8. doi: 10.1172/JCI112950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolfe GC, NacNaul KL, Buechel FF, McDonnell J, Hoerrner LA, Lark MW, Moore VL, Hutchinson NI. Differential in vivo expression of collagenase messenger RNA in synovium and cartilage: Quantitative comparison with stromelysin messenger RNA levels in human rheumatoid arthritis and osteoarthritis patients and two animal models of acute inflammatory arthritis. Arthritis Rheum. 1993;36:1540–993. doi: 10.1002/art.1780361108. [DOI] [PubMed] [Google Scholar]
- 31.Johnson-Wint B. Regulation of stromal cell collagenase production in adult rabbit cornea: In vitro stimulation and inhibition by epithelial cell products. Proc Natl Acad Sci USA. 1980;77:5331–5. doi: 10.1073/pnas.77.9.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Girard MT, Mastubara M, Kublin C, Tessier MJ, Clinton C, Fini ME. Stromal fibroblasts synthesized collagenase and stromelysin during long-term tissue remodeling. J Cell Sci. 1993;104:1001–11. doi: 10.1242/jcs.104.4.1001. [DOI] [PubMed] [Google Scholar]
- 33.Wilson SE, He YG, Weng J, Li Q, McDowall AW, Vital M, Chwang EL. Epithelial injury induces keratocyte apoptosis: hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp Eye Res. 1996;62:325–7. doi: 10.1006/exer.1996.0038. [DOI] [PubMed] [Google Scholar]
- 34.Stephen RP, Huang XN, Robertson JE, Rosenbaum JT. Cytokine mRNA levels in rat ocular tissues after systemic endotoxin treatment. Invest Ophthal Vis Sci. 1992;33:924–30. [PubMed] [Google Scholar]
- 35.Nishimura M, Hirayama N, Serikawa T, et al. The SMXA a new set of recombinant inbred strain of mice consisting of 26 substrains and their genetic profile. Mamm Genome. 1995;6:850–7. doi: 10.1007/BF00292434. [DOI] [PubMed] [Google Scholar]
- 36.Lemay S, Mao C, Singh AK. Cytokine gene expression in the MRL/lpr model of lupus nephritis. Kidney Int. 1996;50:85–93. doi: 10.1038/ki.1996.290. [DOI] [PubMed] [Google Scholar]
- 37.Mao C, Singh AK. IL-1 beta gene expression in B cells derived from the murine MRL/lpr model of lupus. Autoimmunity. 1996;24:71–9. doi: 10.3109/08916939609001949. [DOI] [PubMed] [Google Scholar]
- 38.Zhang HY, Gharaee-Kermani M, Phan SH. Regulation of lung fibroblast alpha-smooth muscle expression, contractile phenotype, and apoptosis by IL-1beta. J Immunol. 1997;158:1392–9. [PubMed] [Google Scholar]
- 39.Chuvet N, Mouihate A, Verrier D, Lestage J. Apoptosis occurs indepently of the release of interleukin-1 beta in the anterior pituitary of end-lactating rats. Neuroreport. 1996;7:2593–6. doi: 10.1097/00001756-199611040-00037. [DOI] [PubMed] [Google Scholar]
- 40.Dunger A, Augstein P, Schmidt S, Fischer U. Identification of interleukin 1-induced apoptosis in rat islets using in situ specific labelling of fragmented DNA. J Autoimmun. 1996;9:309–13. doi: 10.1006/jaut.1996.0042. [DOI] [PubMed] [Google Scholar]
- 41.Wingren AG, Bjökdahl O, Labuda T, et al. Fusion of a signal seguence to the interleukin-1 beta gene directs the protein from cytoplasmic accumulation to extracellular release. Cell Immunol. 1996;169:226–37. doi: 10.1006/cimm.1996.0113. [DOI] [PubMed] [Google Scholar]
- 42.Lebedeva TV, Singh AK. Consitutive activity of the murine IL-1 beta promoter is regulated by a transcriptional repressor. Biochim Biophys Acta. 1997;1353:32–8. doi: 10.1016/s0167-4781(97)00040-7. [DOI] [PubMed] [Google Scholar]