Abstract
Sensitization to cockroach allergens (CRA) has been implicated as a major cause of asthma, especially among inner-city populations. Endotoxin from Gram-negative bacteria has also been investigated for its role in attenuating or exacerbating the asthmatic response. We have created a novel model utilizing house dust extract (HDE) containing high levels of both CRA and endotoxin to induce pulmonary inflammation (PI) and airway hyperresponsiveness (AHR). A potential drawback of this model is that the HDE is in limited supply and preparation of new HDE will not contain the exact components of the HDE used to define our model system. The present study involved testing HDEs collected from various homes for their ability to cause PI and AHR. Dust collected from five homes was extracted in phosphate buffered saline overnight. The levels of CRA and endotoxin in the supernatants varied from 7·1 to 49·5 mg/ml of CRA and 1·7–6 µg/ml of endotoxin in the HDEs. Following immunization and two pulmonary exposures to HDE all five HDEs induced AHR, PI and plasma IgE levels substantially higher than normal mice. This study shows that HDE containing high levels of cockroach allergens and endotoxin collected from different sources can induce an asthma-like response in our murine model.
Keywords: Asthma, endotoxin, cockroach allergens
INTRODUCTION
The ability of cockroach allergens to cause bronchial hyperresponsiveness was first investigated 30 years ago [1]. Since this initial study it has been well established that sensitivity to cockroach allergens is associated with the severity of asthma. Clinically, it has been shown that the rate of hospitalization, the number of days missed from school due to asthma and the number of days of wheezing were all significantly higher in asthmatic children sensitive to cockroach allergen compared to asthmatic children who are not [2]. Experimental models comparing the responses of guinea-pigs sensitized to ovalbumin or cockroach allergen showed that animals sensitized to cockroach allergen had increased airway resistance that persisted far longer than those sensitized to ovalbumin and the cockroach allergen sensitized animals had significantly higher pulmonary cellular influx compared to ovalbumin sensitized animals [3]. Environmentally, asthmatic subjects are exposed to an infinite number of allergens and particulates which may affect the morbidity of disease. Epidemiological studies have focused intensively on the role of bacterial components in the development and severity of asthma.
The role of endotoxin from Gram-negative bacteria in allergic asthma remains a topic of debate. Epidemiological studies have correlated bacterial infection and endotoxin exposure with reduced risk for allergy and asthma. Children raised on farms and those entering day care at an early age have been shown to have a decreased incidence of asthma [4,5]. It is proposed that this is a result of shifting the immune response to a Th1-type response at an early age. Further studies have shown that antibiotic use during infancy affects the intestinal microflora and interferes with Th1 cell development [6].
Conversely, there is increasing evidence that endotoxin exposure may compound bronchial inflammation and hyperreactivity in asthmatic subjects. Studies conducted by Michel et al. have shown that endotoxin levels in house dust correlated with the severity of asthma [7]. Asthmatic individuals have also been shown to be sensitive to endotoxin challenge [8]; in this study a dose of 20 µg of lipopolysaccharide (LPS) led to broncho-obstruction and histamine hyperresponsiveness in asthmatic patients which was not seen in healthy individuals. Eldridge et al. have shown that atopic asthmatics exposed to dust mite antigen followed by LPS exposure exhibited increased neutrophilia and eosinophilia compared to those administered dust mite antigen followed by saline [9]. Increased levels of soluble CD14 and lipopolysaccharide binding protein (LPB) in the bronchoalveolar compartment of asthmatic subjects [10] may make them sensitive to lower concentrations of endotoxin than a normal individual by enhancing the ability of LPS to activate macrophages, neutrophils and endothelial cells [11–13].
The lack of a good animal model has made it difficult for researchers to study the relationship between endotoxin, allergen and the asthmatic response. While some researchers have attempted to resolve this problem by challenging mice with aerosolized LPS either concurrently, prior to or following allergen challenge [14,15], the clinical relevance of this model is not clear. Studies by Eisenbarth et al. have shown that while sensitizing mice with OVA containing a high level of 100 µg endotoxin or a low level of 0·1 µg endotoxin resulted in pulmonary responses, OVA depleted of endotoxin to levels < 0·001 µg did not induce any response compared to a phosphate buffered saline (PBS) control group [16]. This brings to question the usefulness of OVA as a model of allergic asthma. The role of cockroach allergens in asthma has been established clinically and studies using clinical grade cockroach allergen in a mouse model of allergic asthma have established that this allergen is capable of inducing a significant pulmonary response that mimics the human disease [17]. Previous studies in our laboratory have characterized a novel murine model using house dust extract containing high levels of cockroach allergen and endotoxin prepared from dust collected from the home of an asthmatic patient in Detroit, MI. This house dust extract (HDE) is able to induce an acute asthma-like response characterized by leucocyte infiltration in the lung, increased sensitivity to methacholine challenge and increased chemokine levels [18]. One possible drawback to this model is that our extract is a heterogeneous solution that will differ in content and allergen concentration, even if dust is collected from the same home at a later date. In order to show that this model is a reproducible model of allergic airway dysfunction, HDE was prepared from dust collected from five different homes and leucocyte infiltration, airway hyperreactivity and chemokine levels were measured following HDE challenge.
MATERIALS AND METHODS
House dust collection and extraction
Dust was collected from a 1-m2 area of the kitchen from five homes of asthmatic children in Detroit, MI using an electric vacuum cleaner with a dust collector (Indoor Biotechnologies, Charlottesville, VA, USA) for preliminary screening. Dust was weighed and a volume of PBS was added to achieve an equivalent weight/volume ratio for all five samples. Samples were mixed overnight at 4°C on a rotator, then centrifuged for 10 min at 1000 g, 4°C, the supernatant was collected, recentrifuged and the supernatant collected for analysis of allergen and endotoxin content. Once it was established that all homes had high levels of allergen and endotoxin a second dust collection was performed to obtain larger volumes of dust; the dust was processed as before, with the weight/volume concentration equivalent to 350 mg/ml of dust in PBS for all five samples. Allergen and endotoxin levels were again assessed and the dust samples from this final screen were used in all experiments at various dilutions.
Allergen enzyme-linked immunosorbent assays (ELISAs)
House dust extracts were tested for content of various indoor allergens including: cockroach allergens (Bla g1 and Bla g2), dust mite allergens (Der p1 and Der f1), cat allergen (Fel d1) and dog allergen (Can f1). ELISAs were performed as before [18]. Briefly, 96-well plates (Nunc Immunoplate Maxisorb; Nunc, Neptune, NJ, USA) were coated with monoclonal antibody (MoAb) diluted in 1× PBS and incubated at 4° overnight. All antibodies and standards were purchased from Indoor Biotechnologies. Plates were washed 5× in wash buffer containing 0·05% Tween 20 (FisherBiotech, Fair Lawn, NJ, USA) in PBS and blocked with Blocker Casein (Pierce, Rockford, IL, USA) at room temperature for 1- h. Plates were washed as before, standards and samples were added, and plates were incubated at room temperature for 1 h. Standards and samples were diluted in dilution buffer containing 10% casein, 0·05% Tween 20 and 0·0001% bovine serum albumin (BSA) in 1× PBS. Secondary MoAbs were diluted in the same dilution buffer used for samples and standards and added to plates after washing. Plates were then incubated for 1 h at room temperature. After washing, HRP-conjugated goat antirabbit IgG was added (for Bla g1, Bla g2 and Can f1; BioSource International, Camarillo, CA, USA) or HRP-conjugated streptavidin (for Der p1, Der f1 and Fel d1; Jackson ImmunoReseach Laboratories, West Grove, PA, USA) for 1 h at room temperature. Colour was developed with 3,3′, 5,5′-tetramethylbenzidine (Genzyme Diagnostics, San Carlos, CA, USA) and the reaction was stopped with 1·5 N H2SO4. Plates were read at 465 and 590 nm in a microplate reader (Bio-Tek Instruments, Winooski, VT, USA).
Endotoxin measurement
The endotoxin content of the house dust was measured using a limulus amebocyte lysate assay kit (Bio-Whittaker, Walkersville, MD, USA). Ninety-six-well microtitre plates (Costar, Corning, NY, USA) and substrate solution were warmed to 37°C, 50 µl of sample and standard were added in duplicate rows and 50 µl of freshly prepared LAL was mixed in each well. The plate was incubated at 37°C for 20 min. One hundred µl of preheated substrate solution was added and mixed and the plate was incubated at 37°C for 6 min. The reaction was stopped with 50 µl of 25% acetic acid and read at 405 nm.
Animals and induction of asthma-like response
The house dust extract (HDE) was diluted in sterile PBS (for specific dilutions see Results). The diluted house dust extract was mixed 1 : 1 with TiterMax Gold adjuvant. Pipetting with a 15-gauge needle attached to a glass syringe emulsified the mixture. One hundred µl of HDE : adjuvant was administered i.p. per mouse on day 0. On days 14 and 21 female BALB/c mice were anaesthetized using methoxyflurane (Metofane; Shering-Plough, NJ, USA) and challenged i.t. with HDE diluted in sterile PBS. The anaesthetized mouse was suspended from the front incisors on an inclined board and the base of the tail was taped to support its weight. While holding the jaw open the tongue was extended using forceps and two 25 µl aliquots of house dust were pipetted at the base of the oropharynx. On day 22 airway hyperresponsiveness (AHR) was measured. Mice were sacrificed by cervical dislocation, plasma, brochoalveolar lavage (BAL) fluid and lungs were collected for further examination on day 23. Non-immunized mice were challenged without prior i.p. sensitization, airway hyperresponsiveness was assessed 24 h post-challenge and mice were sacrificed 48 h post-challenge.
AHR
Previous studies in our laboratory have shown airway hyperresponsiveness (AHR) to peak at 24 h after the last airway challenge [18], therefore this time-point was used to assess AHR using a whole-body plesthysmography system (Buxco, Troy, NY, USA). Mice were placed into the main chamber and allowed to acclimate for 10 min. Baseline values were then recorded for 5 min. Mice were subsequently challenged with aerosolized PBS or increasing doses of aerosolized β-methacholine (Sigma, St Louis, MO, USA) for a 2-min period. The response was recorded for 5 min after each dose by measuring the pressure differences between the main chamber containing the mouse and a reference chamber. Differences in signal quantified during respiratory cycle are reflected by enhanced pause (Penh). Penh is used widely in models of pulmonary dysfunction and has been shown to correlate with airway resistance [19].
Penh values are normalized as a percentahe increase of average Penh for each acetyl β-methacholine (Mch) dose over the average Penh for PBS challenge. Calculating the change in Penh will account for pressure changes resulting from heating and humidification of air travelling between the chamber and lungs, termed gas conditioning. If gas conditioning is present, then an absolute Penh may not be indicative of bronchial constriction [20]. Penh, as measured by unrestrained plethysmography, is a commonly measured parameter for the assessment of pulmonary hyperreactivity in mice [21–25]. A recent study by McGraw et al. has shown that Penh assessed using whole body plethysmography, in accordance with our studies, did correlate well with invasive measures of airway resistance and tracheal ring contractility as a measure of airway hyperreactivity [26].
BAL fluid analysis
Following exsanguination, the trachea was exposed and intubated with a polyethylene catheter. Lungs were lavaged with HBSS (Life Technologies, Grand Island, NY, USA); two 1-ml fractions were collected. The two washes were centrifuged at 1000 g for 5 min. The supernatant of the first wash was saved for later cytokine analysis while the supernatant of the second 1-ml fraction was discarded. The cell pellets from both washes were combined and total cells were counted using a Coulter counter (Coulter Electronics, Hialeah, FL, USA). Cytospins of 100 000 cells were prepared and slides were stained with Diff-Quick (Baxter, Detroit, MI, USA) and differentials calculated from counting 300 cells.
Eotaxin, MCP and IgE ELISAs
Ninety-six-well plates (Nunc Immunoplate Maxisorb) were coated with antibody diluted in PBS overnight at 4°C (Eotaxin and MCP goat antibodies: R&D Systems, Minneapolis, MN, USA; IgE rat antibody: BD Pharmingen, San Diego, CA, USA). Plates were washed 5× in wash buffer containing 0·05% Tween 20 (FisherBiotech, Fair Lawn, NJ, USA) in PBS and blocked with Blotto blocking solution (Pierce) on a shaker for 1 h at room temperature. Standards and sample were diluted in dilution buffer containing 10% Blotto, 0·05% Tween 20 and 0·0001% BSA in 1× PBS, added to plates and incubated at room temperature for 2 h on a shaker. Plates were washed and secondary antibodies diluted in dilution buffer were added for 2 h at room temperature (eotaxin goat polyclonal antibody: R&D Systems; MCP goat polyclonal antibody: R&D Systems; IgE rat polyclonal antibody: BioSource International). HRP-conjugated streptavidin (Jackson ImmunoReseach Laboratories, West Grove, PA, USA) was added after washing and plates were incubated for 30 min at room temperature. Colour was developed with 3,3′,5,5′-tetramethylbenzidine (Genzyme Diagnostics, San Carlos, CA, USA) and the reaction was stopped with 1·5 N H2SO4. Plates were read at 465 and 590 nm in a microplate reader (Bio-Tek Instruments, Winooski, VT, USA).
Statistics
Values are expressed as mean ± s.e.m. One-way anova with Newman–Keuls post-test or t-tests were performed using GraphPad Prism version 4·0 (GraphPad Software, San Diego, CA, USA).
RESULTS
Composition of house dust extracts
House dust was collected from five homes in Detroit, MI. The dust was weighed and a volume of PBS added such that all five homes had equivalent weight : volume concentrations of 350 mg/ml; this concentration was chosen based on the smallest sample. The house dust extract was analysed for levels of German cockroach (Blatella germanica, Bla g 1 and Bla g 2), house dust mite (Dermatophagoides pteronyssinus, Der p 1 and D. farinae, Der f 1), cat (Felix domesticus, Fel D 1) and dog (Canis familiarizes, Can f 1), as well as endotoxin.
As shown in Table 1 all five homes contained high levels of the cockroach allergens Bla g 1 and Bla g 2. Feline antigen, Fel d 1 was detectable at low levels in the extract from home no. 1 (1·19 mU/ml) but not the other extracts and other allergens assayed for were below the limits of detection in all five homes. The endotoxin concentration, as assayed by Limulus assay, was very high in all five homes. Although slightly variable all five extracts had endotoxin levels in the µg/ml range. Total cockroach allergen levels (µg/ml) were calculated by adding the Bla g 1 concentration to the Bla g 2 concentration of each sample. House dust extract no. 2 (HDE 2) contained the lowest level of total cockroach allergen, nearly 7× less than that of HDE 4, which shows the highest total cockroach allergen concentration.
Table 1.
Allergen and endotoxin levels present in HDE. Indoor allergen levels were measured by ELISA following laboratory protocol. House dust extract samples were diluted serially from 1 : 10 to 1 : 10 000 for ELISAs. Dilutions falling within the linear portion of the standard curve were used to determine concentration. Total Bla g as reported here is the sum total of Bla g1 and 2; endotoxin was measured by LAL
| HDE no. | Bla g 1 (µg/ml) | Bla g 2 (µg/ml) | Total Bla g (µg/ml) | Endotoxin (µg/ml) |
|---|---|---|---|---|
| 1 | 15344 | 541 | 15890 | 2·07 |
| 2 | 6102 | 1000 | 7102 | 2·33 |
| 3 | 15340 | 6067 | 46007 | 1·76 |
| 4 | 39940 | 9622 | 49562 | 4·35 |
| 5 | 31780 | 7910 | 39690 | 5·99 |
Initially the house dust extracts were diluted 1 : 10 in sterile PBS and used for immunization and challenge. In this first experiment animals receiving HDE 4 and HDE 5 showed signs of peritonitis and were sensitive to methoxyflurane anaesthesia on day 14. Data from this experiment is not presented here and subsequently all five HDEs were heat inactivated at 56°C for 30 min prior to dilution and administration. Following the heat inactivation, no further signs of illness were observed.
Induction of asthma-like phenotype with HDEs is similar despite differences in allergen and endotoxin content
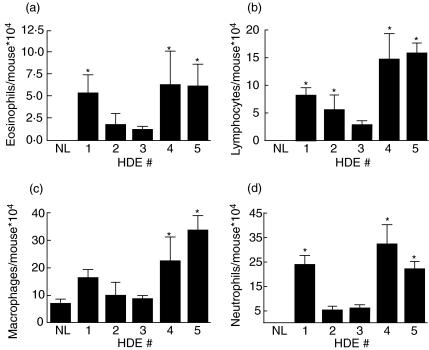
Immunizing and challenging mice with 1 : 10 diluted house dust extract resulted in cellular infiltration to the lungs as measured at 48 h after the last pulmonary challenge. All five HDE samples were able to induce pulmonary leucocyte recruitment with HDEs 1, 4 and 5 exhibiting significantly greater BAL fluid cell levels than normal mice (data not shown). Normal mice sacrificed without prior manipulation had alveolar macrophages in the BAL fluid without significant numbers of eosinophils, lymphocytes or neutrophils present. Mice immunized and challenged with HDE showed heterogeneous cell recruitment to the lungs. BAL fluid eosinophil levels ranged from 11 000 to 63 000 eosinophils/mouse for mice immunized and challenged, while normal mice only had on average 90 eosinophils/mouse (Fig. 1a). Similarly, lymphocyte levels in the BAL of normal mice were 540 lymphocytes/mouse compared to mice immunized and challenged with the various HDEs which resulted in recruitment of lymphocytes at levels ranging from 28 000 to 157 000 lymphoctyes/mouse (Fig. 1b). Pulmonary macrophage levels did not differ significantly from normal mice to experimental animals with the exception of HDEs 4 and 5 which showed higher macrophage levels in the lavage fluid compared to normal mice (Fig. 1c). Neutrophil recruitment to the lung in response to HDE was anticipated considering the high levels of endotoxin present in the dust. Lung lavage neutrophil levels in normal control animals were 1000 neutrophils/mouse compared to experimental animals’ lavage neutrophil levels of 50 000–320 000 neutrophils/mouse (Fig. 1d).
Fig. 1.
Differential counting of the BAL fluid. Mice were immunized i.p. on day 0 with 1 : 10 diluted HDE mixed with TiterMax adjuvant. Mice were challenged intratracheally with 1 : 10 diluted HDE on days 14 and 21; 48 h following the last challenge mice were sacrificed and BAL fluid was collected. Differential counts were performed by counting 300 cells. *P < 0·05 compared to normal mice (NL). Each value is the mean ± s.e.m. for n = 7, except HDE 4, n = 4 due to limited supply.
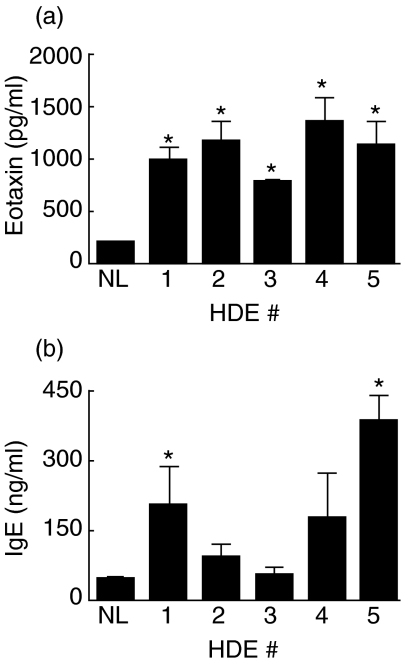
Eotaxin has been described as a key mediator in the inflammatory responses elicited in asthma and allergy. Normal control animals had eotaxin levels below the lower limit of detection in our assay, 200 pg/ml. Plasma levels of eotaxin were significantly elevated (P < 0·05 compared to normal controls) for all experimental animals with levels ranging from 790 to 1470 pg/ml (Fig. 2a). BAL fluid levels of eotaxin were below the lower limit of detection for all animals at this time point. Plasma levels of total IgE were also measured by ELISA. The IgE response was more variable with normal controls averaging 46 ng/ml total IgE and experimental animals ranging from 56 to 388 ng/ml total plasma IgE (Fig. 2b).
Fig. 2.
Plasma eotaxin and IgE levels. Mice were immunized once and challenged twice with 1 : 10 diluted HDE and sacrificed 48 h after the second challenge. Plasma was diluted 1 : 10 for ELISAs. *P < 0·05 compared to normal mice (NL). Each value is the mean ± s.e.m. for n = 7, except HDE 4, n = 4 due to limited supply.
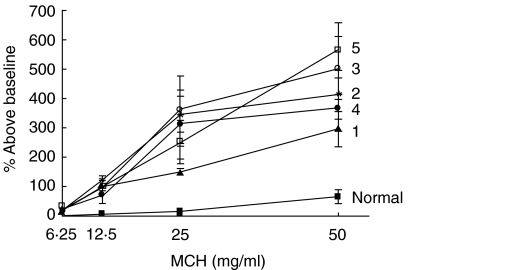
Airway hyperresponsiveness was measured by whole body plethysmography 24 h following the last challenge. Mice were allowed to acclimate in chambers and sterile PBS and increasing doses of β-acetylmethacholine were nebulized into the chambers for 2 min followed by a 5-min recording period. Data is normalized such that the average Penh resulting from each MCh dose is expressed as a percentage increase in the average Penh seen after PBS exposure. Mice receiving all extracts exhibited substantial increases in Penh in response to MCh challenge at all doses compared to normal controls. All immunized and challenged groups were significantly more reactive at the 50 mg/ml dose compared to normal control animals (P < 0·05, Fig. 3).
Fig. 3.
Bronchial hyperreactivity. Airway hyperresponsiveness to methylcholine challenge was measured in conscious animals in a whole body plethsymography system 24 h after the last airway challenge. Data are reported as a percentage increase in the average Penh for each dose of methylcholine compared to the average Penh of a PBS challenge. All mice challenged with HDE had significantly higher percentage increase at the highest methacholine dose compared to normal mice, P < 0·05. Each value is the mean ± s.e.m. for n = 7, except HDE 4, n = 4 due to limited supply.
Sensitization prior to challenge is required for chemokine production, eosinophil recruitment and IgE response
Mice were challenged with 1 : 10 diluted HDE (with the exception of HDE 4 which was diluted 1 : 50 due to limited sample) without prior immunization to assess the requirement for sensitization in the responses elicited in our model. Immunization prior to challenge did not significantly alter total BAL cell numbers in mice receiving HDEs 1, 2 or 3 (data not shown, P > 0·05), but did change the differential levels of recruited cells. HDE 5 showed increased total BAL cells when administered without prior immunization (127·5 × 104 cells/mouse compared to 77·6 × 104 cells/mouse in immunized and challenged mice, P < 0·05). This increase in total cells for HDE 5 can be accounted for primarily by an increase in lavage neutrophil levels (100·5 × 104 neutrophils/mouse in non-immunized animals compared to 22·2 neutrophils/mouse in immunized and challenged mice, P < 0·0001). Neutrophil levels were not significantly altered in mice challenged with the other HDEs with or without prior immunization. The number of eosinophils and lymphocytes were markedly lower in mice not immunized prior to challenge, while macrophage counts were not as affected (data not shown). Total plasma IgE levels were below the lower limit of detection (98 ng/ml, data not shown) in mice receiving a pulmonary challenge without prior immuziation regardless of the HDE used.
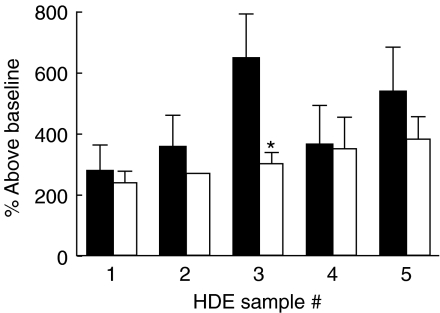
Airway hyperresponsiveness was measured 24 h after pulmonary challenge as before. With the exception of mice challenged with HDE 3 the percentage increase above baseline Penh was comparable between mice immunized prior to challenge and those challenged without prior immunization suggesting sensitization is not required for the development of airway hyperresponsiveness (Fig. 4).
Fig. 4.
Bronchial hyperreactivity. Mice were challenged with 1 : 10 diluted HDE without prior immunization (thatched bars) and airway hyperresponsiveness to methylcholine challenge was measured 24 h later in a whole body plethysmography. The percentage increase above baseline for the 50 mg/ml methylcholine dose is reported. *P < 0·05 compared to mice immunized and challenged (solid bars) with the same HDE. Each value is the mean ± s.e.m. for n = 6, non-immunized groups, n = 7 immunized and challenged, except HDE 4 immunized, n = 4 due to limited supply.
Highly diluted HDE containing equivalent levels of cockroach allergen
In order to determine if highly diluted HDE still resulted in pulmonary inflammation and airway hyperresponsiveness we diluted the extracts in sterile PBS such that they would contain equivalent levels of total cockroach allergen. Previous studies in our laboratory have used HDE containing ∼13·8 µg/ml of total cockroach allergen, therefore the five extracts studied here were all diluted to produce this concentration of total cockroach allergen (1 : 515–1 : 3594 dilution range). Total cockroach allergen as referred to in these studies is the sum total of Bla g 1 and Bla g 2 which we have the ability to measure by ELISA. The resultant endotoxin concentrations were 1·8, 4·5, 0·5, 1·2, and 2·1 ng/ml for HDEs 1–5, respectively.
Immunizing and challenging the mice with HDE highly diluted to equivalent Bla g concentrations resulted in heterogeneous pulmonary cellular influx at 48 h after the last challenge although to a lesser degree than seen with 1 : 10 diluted HDE. Experimental animals receiving Bla g equivalently diluted HDE exhibited pulmonary inflammation consisting of eosinophils, lymphocytes and neutrophils (Table 2). Interestingly, total plasma IgE levels were not significantly altered in animals sensitized and challenged with highly dilute HDE compared to those receiving 1 : 10 diluted extract with the exception of animals receiving HDE 5 which had a significant decrease in IgE when highly diluted (Table 2).
Table 2.
Pulmonary inflammatory and IgE responses from HDE with equivalent cockroach allergen levels. Mice were immunized and challenged with HDE diluted 1 : 10, Imm (1 : 10), or containing equivalent Bla g levels, Imm (equiv.); 48 h after the last pulmonary challenge mice were sacrificed and BAL fluid was collected. Differential counts were performed by counting 300 cells. Total plasma IgE was measured by ELISA as before
| Total cells/mouse × 104 | Eos./mouse × 104 | Lymphs./mouse × 104 | Macs./mouse × 104 | PMNs/mouse × 104 | IgE (ng/ml) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Imm (1 : 10) | Imm (equiv.) | Imm (1 : 10) | Imm (equiv.) | Imm (1 : 10) | Imm (equiv.) | Imm (1 : 10) | Imm (equiv.) | Imm (1 : 10) | Imm (equiv.) | Imm (1 : 10) | Imm (equiv.) | |
| 1 | 53·9 | 12·2* | 5·3 | 0·1 | 8·0 | 0·4* | 16·5 | 10·4 | 24·1 | 1·3* | 203 | 90 |
| 2 | 21·0 | 6·8 | 1·8 | 0·0 | 5·4 | 0·1 | 10·0 | 6·4 | 5·0 | 0·3 | 94 | 85 |
| 3 | 18·7 | 6·7* | 1·1 | 0·3 | 2·8 | 0·4* | 8·7 | 5·3 | 6·0 | 0·7* | 56 | 46 |
| 4 | 77·0 | 15·2* | 6·3 | 0·1 | 14·5 | 1·4* | 22·5 | 10·7 | 32·0 | 2·9* | 175 | 66 |
| 5 | 77·6 | 18·1* | 6·1 | 0·2 | 15·7 | 0·4* | 33·6 | 13·6* | 22·2 | 3·8* | 389 | 73* |
P < 0·05 compared to mice immunized and challenged with the same HDE diluted 1 : 10. Each value is the mean for n = 4, equivalent bla g groups, n = 7 HDE (1 : 10), except HDE 4 (1 : 10), n = 4 due to limited supply. PMN: polymorphonuclear cell.
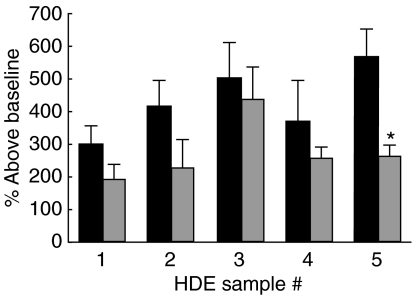
Airway hyperresponsiveness was similar between groups of mice receiving Bla g equivalently diluted house dust and compared back to those receiving the same extract diluted 1 : 10 (Fig. 5) suggesting pulmonary inflammation and airway hyperresponsiveness are differentially regulated in our model.
Fig. 5.
Bronchial hyperreactivity. Mice were immunized and challenged with HDE containing equivalent Bla g levels (grey bars) and airway hyperresponsiveness to methylcholine challenge was measured 24 h after the last pulmonary challenge. The percentage increase above baseline for the 50 mg/ml methylcholine dose is reported. *P < 0·05 compared to mice immunized and challenged with the same HDE diluted 1 : 10 (solid bars). Each value is the mean ± s.e.m. for n = 4, equivalent bla g groups, n = 7 HDE (1 : 10), except HDE 4 (1 : 10), n = 4 due to limited supply.
DISCUSSION
The model system developed by this laboratory [18] is a clinically relevant animal model utilizing house dust extract containing high levels of both cockroach allergen and endotoxin to elicit an asthma-like response characterized by increased levels of chemotactic cytokines, pulmonary cellular infiltration and increased Penh. In the present study house dust was collected from five different homes of asthmatic children, extracted in sterile PBS and analysed for levels of common allergens and endotoxin. While it would not be possible to directly compare results from studies using HDEs collected at different times or from different sources we are encouraged that our model system is reproducible, in that a number of HDEs tested were able to promote pulmonary inflammation and increase Penh and chemotactic cytokine levels following immunization and pulmonary challenge. With this knowledge we may collect house dust from the homes of asthmatics, assess the levels of allergen and endotoxin and test the extract in this model in an attempt to determine what environmental factors contribute to the development and morbidity of asthma and have a clinically relevant model to test the mechanism of physiological responses seen in asthma.
Presently, mice immunized and challenged with HDE exhibited leucocyte infiltration in the lung consisting primarily of neutrophils with significant numbers of eosinophils and lymphocytes also present. Challenging mice without prior immunization altered the cellular composition of BAL fluid such that eosinophil and lymphocyte recruitment were reduced significantly without affecting the neutrophil pulmonary infiltration. The extensive neutrophil response is not surprising due to the high endotoxin levels of the house dust. Studies have suggested neutrophils may be important in acute asthma as patients show a prominent neutrophil response during asthma exacerbations [27] and patients with severe asthma have a higher percentage of neutrophils than mild asthmatics [28]. It has also been shown recently that non-eosinophilic asthma is far more common even in mild and moderate asthma than previously thought and that patients with non-eosinophilic asthma have a marked increase in pulmonary neutrophils [29].
Slight variations in the BAL fluid differential cell counts led us to question if the allergen content of the extracts correlated with these findings. HDE were therefore diluted such that they would contain equivalent levels of cockroach allergens; this, however, did not alter the variations seen when 1 : 10 diluted extracts were used. Others have correlated the severity of asthma to endotoxin levels present in house dust [30], although this study did not assess pulmonary cellular infiltration. We have not diluted the HDE to equivalent endotoxin levels in this model due to limited sample; however, the endotoxin levels did not correlate to pulmonary neutrophil influx in any of these studies (data not shown).
The heterogeneity of our extracts makes this model unique while mimicking the human response with only two challenges post-immunization. Despite the heterogeneity of our extracts all five resulted in similar concentrations of the chemotactic cytokine eotaxin. While eotaxin levels were below the lower limit of detection in the BAL fluid in all experiments plasma levels were substantial. Plasma eotaxin levels have been shown to be increased in patients during disease exacerbations and plasma levels correlate with disease severity [31]. IgE levels were significantly increased in mice receiving HDEs 1 and 5; this response was dependent on sensitization prior to challenge. Interestingly, when house dust extracts were highly diluted to equivalent cockroach allergen levels the levels of total plasma IgE were not significantly altered compared to the 1 : 10 diluted extract. It should be noted that although there have, to date, been six cockroach allergens cloned from the German cockroach we are capable of testing only Bla g 1 and Bla g 2. It is possible that the severity of asthma-like responses seen in our model correlate to one particular cockroach allergen or the sum total; at this time we cannot draw any such conclusions.
It has been suggested that exposure to low levels of endotoxin may exacerbate an asthmatic response while exposure to high levels will prove protective [32], this is based on two animal studies with differential findings. Studies by Wan et al. [33] showed mice exposed to 40 ng/ml aerosolized endotoxin prior to ova challenge resulted in higher IgE levels and inhibited tolerization compared to mice exposed to saline prior to ova challenge. Contrary to this, Tulic et al. found LPS exposure of 50 µg/ml prior to ova challenge or up to 6 days after ova challenge was protective and prevented the IgE response in rats [34]. In our study the 1 : 10 diluted HDE contains endotoxin levels between 176 ng/ml and 600 ng/ml, while the highly diluted HDE contains equivalent cockroach allergen levels and endotoxin levels between 0·5 ng/ml and 4·5 ng/ml. Although we failed to see an exacerbated response when the highly diluted HDEs were used, airway hyperresponsiveness to MCh and total plasma IgE levels were not reduced significantly despite diluting the extracts in a range from 1 : 515 to 1 : 3594. Our results may differ due to the nature of our model where allergen and endotoxin exposure occurs concomitantly mimicking what is seen in the environment, while many studies will pre-expose animals to nebulized endotoxin prior to ever encountering allergen.
To evaluate further the pulmonary response to house dust extract airway hyperresponsiveness to various doses of methacholine was determined at 24 h after the last challenge. Immunizing and challenging mice with 1 : 10 diluted HDE, Bla g equivalently diluted HDE or challenging mice with 1 : 10 diluted HDE without prior immunization resulted in comparable hyperreactivity. The mechanism of antigen-induced AHR remains unclear although previous studies have shown that AHR may occur independently of eosinophilia and IgE [35,36]. Studies by MacLean et al. [37] further show that B-cell deficient mice are capable of developing AHR in response to an ova challenge. While the mechanism of AHR is not clarified in the present study, it has been shown that AHR is independent of IgE and eosinophilia in this model; the finding that mice challenged without prior immunization develop hyperresponsiveness without eosinophilia or serum IgE leads to this conclusion. Previous studies in our laboratory have also shown that AHR peaks at 24 h following the last pulmonary challenge while eosinophil and lymphocyte recruitment does not occur until 36–48 h [18].
The data in this publication show that house dust extracts containing high levels of cockroach allergen and endotoxin are capable of inducing pulmonary inflammation, IgE and eotaxin expression and AHR. The extracts collected from different homes induced comparable responses, making this a clinically relevant, reproducible model for studying airway allergic responses.
Acknowledgments
This work was supported in part by National Institute of Environmental Health Sciences Grant ES 09589 and US Environmental Protection Agency Grant R826710-01. The Michigan Center for the Environment and Children's Health (MCECH) is a community-based participatory research initiative investigating the influence of environmental factors on childhood asthma. MCECH involves collaboration among the University of Michigan Schools of Public Health and Medicine, the Detroit Health Department, the Michigan Department of Agriculture, Plant and Pest Management Division and nine community-based organizations in Detroit (Butzel Family Center, Community Health and Social Services Center, Detroiters Working for Environmental Justice, Detroit Hispanic Development Corporation, Friends of Parkside, Kettering/Butzel Health Initiative, Latino Family Services, United Community Housing Coalition and Warren/Conner Development Coalition) and the Henry Ford Health System.
REFERENCES
- 1.Bernton HS, McMahon TF, Brown H. Cockroach asthma. Br J Dis Chest. 1972;66:61–6. [PubMed] [Google Scholar]
- 2.Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma [see comments] N Engl J Med. 1997;336:1356–63. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 3.Chen G, Zhou D, Kang BC. A comparative study on cockroach and ovalbumin sensitizations and challenge responses in Hartley guinea-pigs. Respir Physiol. 2001;125:239–47. doi: 10.1016/s0034-5687(00)00222-x. [DOI] [PubMed] [Google Scholar]
- 4.Ernst P, Cormier Y. Relative scarcity of asthma and atopy among rural adolescents raised on a farm. Am J Respir Crit Care Med. 2000;161:1563–6. doi: 10.1164/ajrccm.161.5.9908119. [DOI] [PubMed] [Google Scholar]
- 5.Kramer U, Heinrich J, Wjst M, Wichmann HE. Age of entry to day nursery and allergy in later childhood. Lancet. 1999;353:450–4. doi: 10.1016/S0140-6736(98)06329-6. [DOI] [PubMed] [Google Scholar]
- 6.Oyama N, Sudo N, Sogawa H, Kubo C. Antibiotic use during infancy promotes a shift in the T(H)1/T(H)2 balance toward T(H)2-dominant immunity in mice. J Allergy Clin Immunol. 2001;107:153–9. doi: 10.1067/mai.2001.111142. [DOI] [PubMed] [Google Scholar]
- 7.Michel O, Ginanni R, Duchateau J, Vertongen F, Le Bon B, Sergysels R. Domestic endotoxin exposure and clinical severity of asthma. Clin Exp Allergy. 1991;21:441–8. doi: 10.1111/j.1365-2222.1991.tb01684.x. [DOI] [PubMed] [Google Scholar]
- 8.Michel O, Duchateau J, Sergysels R. Effect of inhaled endotoxin on bronchial reactivity in asthmatic and normal subjects. J Appl Physiol. 1989;66:1059–64. doi: 10.1152/jappl.1989.66.3.1059. [DOI] [PubMed] [Google Scholar]
- 9.Eldridge MW, Peden DB. Allergen provocation augments endotoxin-induced nasal inflammation in subjects with atopic asthma. J Allergy Clin Immunol. 2000;105:475–81. doi: 10.1067/mai.2000.104552. [DOI] [PubMed] [Google Scholar]
- 10.Dubin W, Martin TR, Swoveland P, et al. Asthma and endotoxin: lipopolysaccharide-binding protein and soluble CD14 in bronchoalveolar compartment. Am J Physiol. 1996;270:L736–44. doi: 10.1152/ajplung.1996.270.5.L736. [DOI] [PubMed] [Google Scholar]
- 11.Haziot A, Rong GW, Silver J, Goyert SM. Recombinant soluble CD14 mediates the activation of endothelial cells by lipopolysaccharide. J Immunol. 1993;151:1500–7. [PubMed] [Google Scholar]
- 12.Martin TR, Mathison JC, Tobias PS, et al. Lipopolysaccharide binding protein enhances the responsiveness of alveolar macrophages to bacterial lipopolysaccharide. Implications for cytokine production in normal and injured lungs. J Clin Invest. 1992;90:2209–19. doi: 10.1172/JCI116106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright SD, Ramos RA, Hermanowski-Vosatka A, Rockwell P, Detmers PA. Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J Exp Med. 1991;173:1281–6. doi: 10.1084/jem.173.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerhold K, Bluemchen K, Franke A, Stock P, Hamelmann E. Exposure to endotoxin and allergen in early life and its effect on allergen sensitization in mice. J Allergy Clin Immunol. 2003;112:389–96. doi: 10.1067/mai.2003.1646. [DOI] [PubMed] [Google Scholar]
- 15.Tulic MK, Holt PG, Sly PD. Modification of acute and late-phase allergic responses to ovalbumin with lipopolysaccharide. Int Arch Allergy Immunol. 2002;129:119–28. doi: 10.1159/000065881. [DOI] [PubMed] [Google Scholar]
- 16.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–51. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell EM, Kunkel SL, Strieter RM, Lukacs NW. Temporal role of chemokines in a murine model of cockroach allergen-induced airway hyperreactivity and eosinophilia. J Immunol. 1998;161:7047–53. [PubMed] [Google Scholar]
- 18.Kim J, Merry AC, Nemzek JA, Bolgos GL, Siddiqui J, Remick DG. Eotaxin represents the principal eosinophil chemoattractant in a novel murine asthma model induced by house dust containing cockroach allergens. J Immunol. 2001;167:2808–15. doi: 10.4049/jimmunol.167.5.2808. [DOI] [PubMed] [Google Scholar]
- 19.Hamelmann E, Schwarze J, Takeda K, et al. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography [see comments] Am J Respir Crit Care Med. 1997;156:766–75. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- 20.Lundblad LK, Irvin CG, Adler A, Bates JH. A reevaluation of the validity of unrestrained plethysmography in mice. J Appl Physiol. 2002;93:1198–207. doi: 10.1152/japplphysiol.00080.2002. [DOI] [PubMed] [Google Scholar]
- 21.McMillan SJ, Kearley J, Campbell JD, et al. Matrix metalloproteinase-9 deficiency results in enhanced allergen-induced airway inflammation. J Immunol. 2004;172:2586–94. doi: 10.4049/jimmunol.172.4.2586. [DOI] [PubMed] [Google Scholar]
- 22.Maezawa Y, Nakajima H, Seto Y, et al. IgE-dependent enhancement of Th2 cell-mediated allergic inflammation in the airways. Clin Exp Immunol. 2004;135:12–8. doi: 10.1111/j.1365-2249.2004.02337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawicka E, Zuany-Amorim C, Manlius C, et al. Inhibition of th1- and th2-mediated airway inflammation by the sphingosine 1-phosphate receptor agonist FTY720. J Immunol. 2003;171:6206–14. doi: 10.4049/jimmunol.171.11.6206. [DOI] [PubMed] [Google Scholar]
- 24.Strong P, Townsend P, Mackay R, Reid KB, Clark HW. A recombinant fragment of human SP-D reduces allergic responses in mice sensitized to house dust mite allergens. Clin Exp Immunol. 2003;134:181–7. doi: 10.1046/j.1365-2249.2003.02281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blaeser F, Bryce PJ, Ho N, et al. Targeted inactivation of the IL-4 receptor alpha chain I4R motif promotes allergic airway inflammation. J Exp Med. 2003;198:1189–200. doi: 10.1084/jem.20030471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGraw DW, Almoosa KF, Paul RJ, Kobilka BK, Liggett SB. Antithetic regulation by beta-adrenergic receptors of Gq receptor signaling via phospholipase C underlies the airway beta-agonist paradox. J Clin Invest. 2003;112:619–26. doi: 10.1172/JCI18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fahy JV, Kim KW, Liu J, Boushey HA. Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J Allergy Clin Immunol. 1995;95:843–52. doi: 10.1016/s0091-6749(95)70128-1. [DOI] [PubMed] [Google Scholar]
- 28.Jatakanon A, Uasuf C, Maziak W, Lim S, Chung KF, Barnes PJ. Neutrophilic inflammation in severe persistent asthma. Am J Respir Crit Care Med. 1999;160:1532–9. doi: 10.1164/ajrccm.160.5.9806170. [DOI] [PubMed] [Google Scholar]
- 29.Douwes J, Gibson P, Pekkanen J, Pearce N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. 2002;57:643–8. doi: 10.1136/thorax.57.7.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michel O, Kips J, Duchateau J, et al. Severity of asthma is related to endotoxin in house dust. Am J Respir Crit Care Med. 1996;154:1641–6. doi: 10.1164/ajrccm.154.6.8970348. [DOI] [PubMed] [Google Scholar]
- 31.Lilly CM, Woodruff PG, Camargo CA, et al. Elevated plasma eotaxin levels in patients with acute asthma. J Allergy Clin Immunol. 1999;104:786–90. doi: 10.1016/s0091-6749(99)70288-5. [DOI] [PubMed] [Google Scholar]
- 32.Reed CE, Milton DK. Endotoxin-stimulated innate immunity: a contributing factor for asthma. J Allergy Clin Immunol. 2001;108:157–66. doi: 10.1067/mai.2001.116862. [DOI] [PubMed] [Google Scholar]
- 33.Wan GH, Li CS, Lin RH. Airborne endotoxin exposure and the development of airway antigen-specific allergic responses. Clin Exp Allergy. 2000;30:426–32. doi: 10.1046/j.1365-2222.2000.00730.x. [DOI] [PubMed] [Google Scholar]
- 34.Tulic MK, Wale JL, Holt PG, Sly PD. Modification of the inflammatory response to allergen challenge after exposure to bacterial lipopolysaccharide. Am J Respir Cell Mol Biol. 2000;22:604–12. doi: 10.1165/ajrcmb.22.5.3710. [DOI] [PubMed] [Google Scholar]
- 35.Wilder JA, Collie DD, Wilson BS, Bice DE, Lyons CR, Lipscomb MF. Dissociation of airway hyperresponsiveness from immunoglobulin E and airway eosinophilia in a murine model of allergic asthma. Am J Respir Cell Mol Biol. 1999;20:1326–34. doi: 10.1165/ajrcmb.20.6.3561. [DOI] [PubMed] [Google Scholar]
- 36.Tournoy KG, Kips JC, Schou C, Pauwels RA. Airway eosinophilia is not a requirement for allergen-induced airway hyperresponsiveness. Clin Exp Allergy. 2000;30:79–85. doi: 10.1046/j.1365-2222.2000.00772.x. [DOI] [PubMed] [Google Scholar]
- 37.MacLean JA, Sauty A, Luster AD, Drazen JM, De Sanctis GT. Antigen-induced airway hyperresponsiveness, pulmonary eosinophilia, and chemokine expression in B cell-deficient mice. Am J Respir Cell Mol Biol. 1999;20:379–87. doi: 10.1165/ajrcmb.20.3.3291. [DOI] [PubMed] [Google Scholar]