Abstract
Severe trauma can lead to a compromised immune response, thereby increasing susceptibility to infections. Here we will study to what extent these early changes in the immune status upon trauma affect a primary immune response to keyhole limpet haemocyanin (KLH). Because glutamine is the preferred respiratory substrate for immune competent cells and known to be depleted after trauma, we studied the immune status and the primary sensitization in relation to the glutamine plasma concentration in a group of severe trauma patients [injury severity score (ISS) >17]. Trauma patients (n = 31) were sensitized with KLH within 12 h after trauma; plasma glutamine concentrations and immune parameters were determined, after which KLH-specific immune responsiveness was evaluated on days 9 and 14. Low plasma glutamine concentrations were found after trauma. Significantly elevated numbers of granulocytes and CD14-positive leucocytes were found, whereas the HLA-DR expression on CD14-positive cells was significantly lower in trauma patients than in healthy controls. Trauma did not change the in vitro proliferative capacity of lymphocytes when cultured with glutamine; however, when lymphocytes were cultured without glutamine, trauma resulted in lower proliferation than healthy controls. Phytohaemagglutinin-(PHA)-induced interferon (IFN)-γ and interleukin (IL)-10 production was significantly lower after trauma, whereas IL-4 production was not affected. KLH sensitization following trauma resulted in poor skin test reactivity and low in vitro KLH-induced lymphocyte proliferation compared to controls. In contrast, the development of anti-KLH IgM, IgG, IgA, IgG1, IgG2, IgG3 and IgG4 production on days 9 and 14 following trauma was not different from that in healthy controls. Major trauma was associated with a reduced cell-mediated immune response, correlating with low plasma glutamine concentrations, while no effects of trauma were found on the development of a primary humoral immune response.
Keywords: glutamine, humoral immune response, interferon-γ, KLH, trauma
INTRODUCTION
Severely traumatized patients are at high risk for developing systemic infections, due to an injury-induced impaired immune system. The order of events and mechanisms underlying this depressed state of the immune system are not completely understood [1], but acute glutamine depletion, the main ‘fuel’ for lymphocytes [2], may play an important role.
In this study we characterized the post-traumatic immune status as well as the glutamine status of severely injured patients, and investigated to what extent these factors affect a primary immune response in the post-traumatic period.
Hence, the primary immune response towards the neoantigen keyhole limpet haemocyanin (KLH) was studied in a group of severely injured patients. These patients were immunized with KLH within 12 h after trauma [3] and KLH-specific antibody responses were measured on days 1, 9 and 14. The KLH-specific lymphocyte proliferation in vitro, as well as skin test responsiveness upon intradermal challenge, was assessed on day 14. Simultaneously, the occurrence of cutaneous anergy was tested by a skin test with phytohaemagglutinin (PHA) on day 14 [4–6].
Immune status on the day of KLH immunization, i.e. 1 day after the traumatic event, was evaluated by assessment of HLA-DR expression on monocytes, a parameter known to correlate with clinical outcome in diverse populations [7–12]. In addition, the proliferative capacity of T cells and cytokine production profile upon mitogenic stimulation were evaluated, because T cells are essential for a primary immune response and were reported to be functionally compromised in traumatized patients [13,14]. Severe trauma and major surgery result in a skewing towards a type II T cell (TH2) response [14–16]. For an effective antimicrobial response, however, a TH1 response with proinflammatory cytokines, leading to an appropriate antibody production and an adequate cell-mediated immune response, is thought to be required [14].
As suggested above, glutamine availability is a prerequisite for the functioning of immune cells [2,17]. Following severe trauma, the body will employ its stores of amino acids for healing, resulting in low plasma concentrations. Therefore, we tried to relate the trauma-induced decrease in plasma levels of glutamine, the body's most abundant amino acid, towards the immune status and in particular to the capacity to mount a primary immune response to KLH.
PATIENTS AND METHODS
The study was approved by the Council for Medical Research of the Netherlands Organization for Scientific Research and by the Institutional Review Board of our institute. Informed consent was obtained from each patient or closest relative prior to inclusion.
Patient population
Thirty-one multi-system trauma patients admitted to the intensive care unit of the VU University Medical Centre of Amsterdam, the Netherlands, were included if they were between the ages of 18 and 70 years, with an injury severity score (ISS) of ≥17 [18].
The exclusion criteria were: pregnancy or lactation; renal insufficiency; third-degree burns involving more than 15% body surface; use of investigational drugs, steroids or immunosuppressive medication; malignancy; genetic immune disorder; HIV infection; previous splenectomy; liver insufficiency; inflammatory bowel disease; and insulin-dependent diabetes.
Clinical assessments
Each patient with multiple traumatic injuries was evaluated clinically on admission by means of the following scores: ISS, Glascow Coma Scale (GCS) and Acute Physiology and Chronic Health Evaluation II score (APACHE II).
Blood sampling and analysis
Within 12 h after the initial traumatic event the trauma patients were sensitized with KLH by subcutaneous injection of 500 µg KLH in the deltoid region. Seventeen age-matched healthy controls were sensitized similarly with KLH [19].
Venous blood samples were drawn shortly after the traumatic event (before KLH immunization) and on days 9 and 14. From the samples of the first day after trauma the number of leucocytes was counted morphologically, and percentages of granulocytes, lymphocytes and monocytes were determined.
Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation (Lymphoprep, Nycomed Pharma AS, Oslo, Norway) after which the cells were stored in RPMI-1640 (BioWhittaker, Verviers, Belgium) containing 10% fetal calf serum (FCS) (Integro BV, Leuvenheim, the Netherlands) and 10% dimethylsulphoxide (DMSO) (Merck, Darmstadt, Germany). All PBMCs were cryopreserved and to eliminate the effect of interassay variation samples were run as a batch. Supernatants were stored at −80°C until batch analysis. Sera were stored at −20°C until batch determination. Healthy controls donated blood before KLH sensitization (day 1) and after 9 and 14 days.
Plasma glutamine concentrations were determined on day 1. Blood samples were processed, and analysed by high performance liquid chromatography as described previously [20]. Reference range of plasma glutamine concentrations was determined as 482–938 µmol/l.
Flowcytometric analyses
Within several hours after collection, the heparinized blood samples were incubated for 30 min with fluorescent monoclonal antibodies, after which the erythrocytes were lysed using the Coulter Q-prep method (Coulter Electronics, Mijdrecht, the Netherlands). The monoclonal antibodies that were used were CD14-PE, HLA-DR-FITC (Becton Dickinson) and CD64-FITC (FcγR1, Medarex, West Lebanon, CA, USA). A FACStar Plus flowcytometer (Becton Dickinson) was calibrated daily with fluorescent microspheres (Polysciences Europe GmbH, Eppelheim, Germany) prior to usage. CD14-positive cells were gated and the peak channel (PC) for fluoroscein isothyocyanate (FITC) was established within this gate. The ratio of the PC of the FITC-positive sample divided by the PC of the isotype control was used as a final parameter for HLA-DR and CD64 expression defined as ratio mode.
Mitogen-induced proliferation and cytokine production
To investigate the proliferative capacity of cryopreserved PBMCs of day 1, 0·25 × 106 thawed PBMC/ml were cultured for 72 h in 96-well plates (Costar Corporation, Cambridge, MA, USA) in Dulbecco's modified Eagle's medium (DMEM, BioWhittaker, Inc.,Verviers, Belgium) supplemented with 10% human pooled serum (HPS) (Central Laboratory Blood transfusion, Amsterdam, the Netherlands), 100 IU/ml penicillin and 100 µg/ml streptomycin in the absence or presence of five concentrations (0, 12, 24, 48, 96, 192 µg/ml) of purified PHA (Murex Diagnostics Ltd, Dartford, UK). As our intention was to evaluate the intrinsic lymphocyte responsiveness, it was important not to use autologous serum, but to challenge the cells in the same ‘pooled’ serum. Cells were cultured with or without 0·6 mm glutamine in the culture medium. The uptake of 20 µl [3H]-thymidine/well was measured after a 4-h incubation period in 37°C. Samples were counted in a beta counter, counts per minute (cpm). Cytokine production was determined by means of incubating 2·5 × 106 cells/ml PBMCs in a 24-well plate (Costar Corporation) in the presence of PHA alone or 10 µg/ml PHA with aCD28 (CLB) at a concentration of 1 µg/ml. After 72 h of incubation (37°C) the supernatants were harvested.
Cytokine measurements
Interleukin (IL)-10 was evaluated in the sera of patients on day 1 and compared with the mean IL-10 serum level of the healthy controls. Interferon (IFN)-γ, IL-4 and IL-10 were measured in the PHA culture supernatants. All cytokines were measured by the enzyme-linked immunosorbent assay (ELISA) technique using commercially available antibodies or kits. The detection levels were, respectively, 50 pg/ml for IFN-γ (Medgenix diagnostics SA, Fleurs, Belgium), 10 pg/ml for IL-4 (CLB) and 10 pg/ml for IL-10 (CLB).
Detection of KLH-antibodies
Sera were analysed on days 1, 9 and 14 by ELISA for KLH-antibodies of the immunoglobulin isotypes IgM, IgA and IgG and for the subclasses IgG1, IgG2, IgG3 and IgG4. Our assay was based on Korver's protocol [19]. In brief, the ELISAs were performed as follows: a 96-well flat-bottomed plate (Maxisorp, Nunc, Roskilde, Denmark) was coated at room temperature with 50 mg KLH (Calbiochem Corporation, San Diego, CA, USA) per well overnight and blocked subsequently with bovine serum albumin (BSA) 0·5% (Boehringer Mannheim, Mannheim, Germany). Serum samples were incubated for 1·5 h in a 25–200-fold dilution in BSA 0·5%. Bound antibodies were detected by peroxidase-conjugated isotype-specific polyclonal antibodies for IgM, IgA and IgG (Dakopatts, Glostrup, Denmark) or monoclonal antibodies for IgG1, IgG3 and IgG4 (Zymed, San Francisco, CA, USA), followed by an ortho phenylene diamine (OPD)-substrate reaction (Dakopatts, Denmark) and measurement of ODs at 492 nm. For the detection of IgG2 plates were incubated with 1 : 500 dilution of mouse 35 1-27-2 (a kind gift from Dr Radl, TNO, Rijswijk, the Netherlands) (2 h, 37°C) followed, after washing five times with PBS, by rabbit α-mouse IgG-HRP (1 : 50 Dako) (1 h, 37°C). Sera from several sensitized healthy donors (n = 5) were pooled. These findings were used to obtain a standard curve used for calibration, which was included in each experiment, enabling the investigators to compare the ELISA results from different test-runs. Arbitrary units (AU) were validated so that the mean value of presensitization samples of a group of healthy donors was 100 AU/ml for each subclass. IgE-antibodies to KLH were evaluated by Pharmacia (Sweden).
KLH skin test responsiveness and proliferation
KLH skin test responsiveness was detected on day 14 by intracutaneous injection of 0·1 ml saline containing 10 and 1 µg KLH in the lower arm. Delayed-type hypersensitivity was evaluated by measuring the induration and erythema after 24 h.
In a small group (n = 13) the KLH skin test was compared to a simultaneous injection of 0·5 µg PHA (Glaxo Wellcome, Raleigh, NC, USA) in 0·1 ml saline, which was also injected intradermally on the volar aspect of the forearm. The response was measured as the mean diameter of induration and erythema 24 h after injection.
The KLH-induced proliferation on day 14 was evaluated by incubating thawed PBMC for 6 days at a concentration of 1 × 106/ml culture medium (see the PHA cultures) in the presence of KLH at concentrations of 0, 5, 15 and 50 µg/ml. During the last 4 h [3H]-thymidine was added and the [3H]-thymidine uptake was used to calculate stimulation indices, by dividing the cpm of wells with PBMC in the presence of KLH by the cpm of the wells with cells in culture medium alone.
Statistical analysis
Differences between trauma patients and healthy controls were compared by either non-parametric Mann–Whitney U-test or Student's t-test, as considered appropriate. Two-way anova for repeated measures was performed for the antibody measurements on days 1, 9 and 14. Spearman's correlations (two-tailed) were performed. All data were analysed using the Statistical Package for the Social Sciences (SPSS 11·0™). Results are given as means ± s.e.m. or median ±range. P-values <0·05 were considered significant.
RESULTS
On admission the mean age of the trauma patients (n = 31) was 32·8 (range, 18–59), the mean ISS was 32·0 (17–59), APACHE II score of 13·5 (4–22) and mean GCS of 8·7 (3–15). There were 25 men and six women. Finally, for several reasons only 18 trauma patients [mean age, 31·9 (18–59)] were evaluable for the specific humoral immune response to KLH on days 9 and 14. The KLH-sensitized control group consisted of 17 age-matched healthy controls [31·2 years (22–52)].
Plasma glutamine concentrations
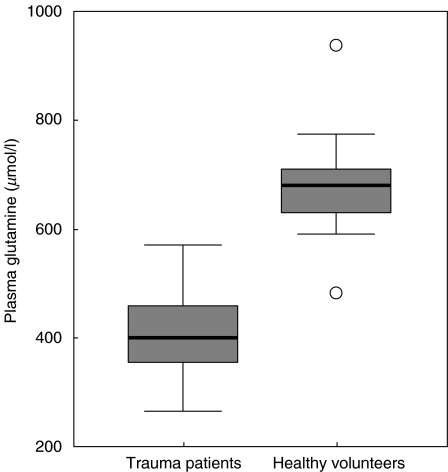
Trauma patients had lower plasma glutamine concentrations on day 1 (397·9 ± 16·0 µmol/l) than the healthy controls (see Fig. 1).
Fig. 1.
Plasma glutamine concentrations. Horizontal bars and boxes show median levels and interquartile range, respectively. Outlier values are indicated as circles. Glutamine concentrations are significantly different (P < 0·001) between the trauma patients versus healthy controls on day 1.
Leucocytes
Leucocytes were increased in trauma patients compared to healthy controls (see Table 1). In particular, the percentages of PMNs were significantly higher on day 1 after trauma compared to healthy controls. In the trauma patient group, a trend towards a significantly lower percentage of monocytes was seen (n.s., P = 0·074). The percentage of lymphocytes was lower on day 1 compared to healthy controls (P < 0·001). The absolute numbers of the different leucocytes resulted in similar significances as the given percentages.
Table 1.
Peripheral leucocyte characteristics
| Trauma patients day 1 (n = 31) | Healthy controls (n = 17) | ||
|---|---|---|---|
| WBC | |||
| Leucocytes (WBC × 106/ml) | 10·1 ± 0·7 | 7·3 ± 0·7 | P < 0·05 |
| PMNs (% of leucocytes) | 80·5 ± 2·3 | 53·1 ± 6·4 | P < 0·001 |
| Monocytes (% of leucocytes) | 2·2 ± 0·6 | 4·1 ± 0·8 | P = 0·074 |
| Lymphocytes (% of leucocytes) | 15·3 ± 2·0 | 36·9 ± 3·4 | P < 0·001 |
| FACS analysis | |||
| CD14+ cells (% of leucocytes) | 5·4 ± 0·4 | 3·6 ± 0·4 | P < 0·005 |
| HLA-DR on CD14+ cells (ratio mode) | 3·6 ± 0·3 | 14·4 ± 1·6 | P < 0·001 |
| CD64 (FcγRI) on CD14+ cells (ratio mode) | 9·84 ± 1·4 | 6·99 ± 1·1 | P = 0·066 |
FACS-analyses were performed on peripheral blood samples to evaluate numbers and phenotype of CD14 positive cells. Significantly higher frequencies of these cells (% of leucocytes) were found in the trauma patient group compared to healthy controls (P < 0·005). As expected, and as shown in Table 1, the HLA-DR expression on monocytes on day 1 after trauma was significantly lower in the trauma patients compared to healthy controls (P < 0·001). On the contrary, the CD64 expression was not significantly different.
Serum IL-10
Serum IL-10 was detectable in the serum of most of the trauma patients with a range of 33–474 pg/ml, whereas no IL-10 (levels below 20 pg/ml) could be detected in the sera of healthy controls.
In vitro proliferative capacity and cytokine production (PHA)
No difference was found between the in vitro proliferative capacity of PBMCs upon stimulation with different concentrations of PHA of trauma patients and healthy controls cultured with glutamine in the medium (data not shown). As expected, the proliferative capacity of PBMCs cultured without glutamine added to the cultures was significantly lower irrespective of stimulation (PHA). In the latter cultures, however, a significantly lower thymidine uptake was measured in the presence of 96 µg/ml PHA in the trauma patient group compared to the healthy controls (Mann–Whitney U, P = 0·047); a trend was seen for a lower proliferation when stimulated with 48 and 192 µg/ml PHA without glutamine in the trauma group (n.s.; both P = 0·088).
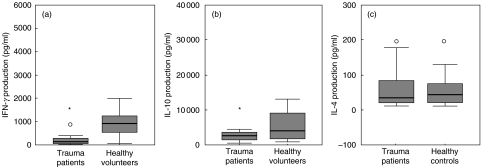
Assessment of IFN-γ, IL-4 and IL-10 production upon stimulation with PHA was performed in parallel cultures. The ability to produce IFN-γ and IL-10 upon stimulation (PHA) was significantly lower after trauma compared to the healthy controls (IFN-γ P < 0·001 and IL-10 P < 0·05) (Fig. 2a, b). Addition of CD28 to these cultures could not overcome the impaired IFN-γ or IL-10 production (data not shown). No effect of trauma on the in vitro IL-4 production was measured (Fig. 2c).
Fig. 2.
Cytokine production in supernatant of stimulated PBMCs (10 µg PHA/ml) on day 1 after trauma and of healthy controls. The in vitro productions of IFN-γ (a), IL-10 (b) and IL-4 (c) are expressed in pg/ml. Horizontal bars and boxes show median levels and interquartile range, respectively. Outlier values are indicated as circles. Significant differences (P < 0·05) between the trauma patients versus healthy controls are indicated by an asterisk (anova, Student's t-test).
Correlations
Plasma glutamine concentration on day 1 correlated positively with the percentage of monocytes of the white blood cells (WBC, whole blood) (r = 0·5, P < 0·05), HLA-DR expression on CD14+ monocytes (r = 0·5, P < 0·05) and in vitro IFN-γ production in the supernatant of PHA-stimulated PBMCs (r = 0·5, P < 0·05) on day 1. HLA-DR expression on CD14+ monocytes correlated with the IFN-γ production of PBMCs in vitro (r = 0·5, P < 0·05). In vitro IL-4 and IL-10 productions as well as the proliferation of stimulated PBMCs were not correlated with plasma glutamine concentrations.
The in vivo primary humoral response, measured as the specific KLH antibodies on days 1, 9 and 14 in serum, did not correlate with plasma glutamine concentration of day 1. HLA-DR expression on day 1 correlated with anti-KLH IgM (r = 0·4, P < 0·05) on day 9.
Cellular immune response towards the primary antigen KLH on day 14
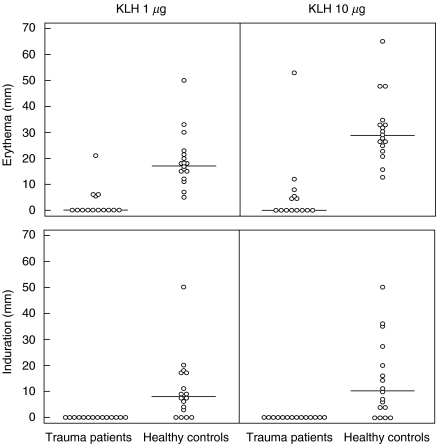
Patients were immunized with KLH within 12 h of the traumatic event and KLH skin test responsiveness was evaluated on day 14 (see Fig. 3). Skin tests could be evaluated in 14 patients. Only six patients showed some erythema and none of the patients had detectable induration. All 17 healthy controls developed erythematous reactions and 13 of them had detectable induration after 24 h.
Fig. 3.
In vivo cellular immune responses to KLH on day 14 by means of delayed-type hypersensitivity skin testing. The induration of the skin is shown, as measured 24 h after intradermal injection with 1 or 10 µg KLH.
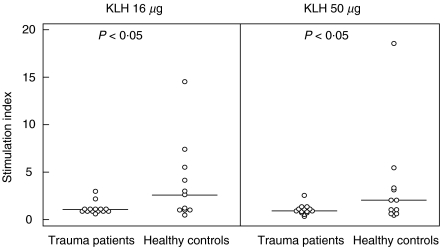
To check whether this impaired skin reactivity was due to a lack of KLH sensitization or to a general anergy of the skin, PHA skin reactivity was also assessed in 13 patients. The results show that PHA-induced skin reactions were still impaired on day 14. In order to test whether lack of skin reactivity to KLH was also due to reduced lymphocyte responsiveness, the cell-mediated immune response to KLH was evaluated additionally by in vitro lymphocyte stimulation in patients (n = 14) and healthy controls (n = 11). The results of these KLH-induced proliferations are shown in Fig. 4. Two of 14 patients and six of 11 controls showed a KLH-specific stimulation index of >2 for at least two KLH concentrations tested (P < 0·05).
Fig. 4.
In vitro cellular immune response to KLH on day 14; the stimulation indices of PBMCs of trauma patients and healthy controls after 6 days of in vitro stimulation with 15 or 50 µg KLH/ml are shown. Median values and significant differences (P < 0·05) (Mann–Whitney U-test) between trauma patients versus healthy controls are indicated.
KLH-specific antibodies
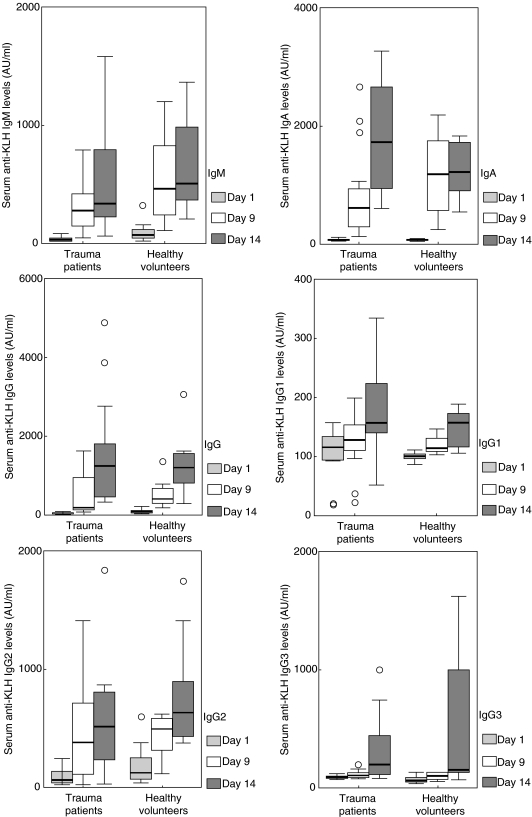
The humoral response towards KLH was studied by measuring KLH-specific antibodies of immunoglobulin classes IgM, IgA and IgG on days 1, 9 and 14 in serum (Fig. 5). By using a two-way anova for repeated measures, no significant differences were found with regard to IgM, IgA or IgG anti-KLH between the trauma patients and healthy controls. As it is known that the IgG subclass switch depends on the T cell cytokine profile, it was decided to analyse the different IgG subclasses induced by KLH immunization. The IgG1, IgG2 and IgG3 concentrations were not significantly different between trauma patients and healthy controls (Fig. 5). IgG4 anti-KLH was not detectable in any of the patients or the healthy controls. Some of the healthy controls showed an immediate-type skin reactivity to KLH; IgE antibodies to KLH were evaluated by Pharmacia (Sweden). However, neither in the patients nor in the healthy controls was anti-KLH of the IgE-isotype detectable.
Fig. 5.
Humoral immune responses to KLH. IgM, IgA, IgG and the IgG-subclasses, IgG1, IgG2, IgG3 and IgG4 specific antibodies to KLH are expressed in arbitrary units per ml serum on days 1, 9 and 14. Horizontal bars and boxes show median antibody levels and interquartile range, respectively. Outlier values are indicated as a circle if present per group of values. No significant differences were measured (GLM repeated measures, factorial).
DISCUSSION
In this study the effects of immune alterations, as observed early after a severe trauma, on the development of a primary immune response to KLH were evaluated. In addition, because depletion of glutamine stores is thought to be a major factor in the early events after trauma, we studied the immune status as well as the primary immune response to KLH in relation to the plasma glutamine concentrations.
Directly after trauma, the percentage of CD14+ cells in peripheral blood was elevated and their phenotype promoted phagocytic function (normal or increased CD64) rather than antigen-presenting capacity, as the HLA-DR expression was much lower compared to the values of healthy controls. It is still not known fully what causes the loss of HLA-DR expression on these peripheral monocytes following trauma. Although hypothetical, it is very possible that the cells with normal HLA-DR expressions have all migrated to the tissues and that the cells present in the peripheral blood are predominantly immature monocytes derived from bone marrow. It is known that immature monocytes have a constitutively lower HLA-DR expression [21], and that in vitro maturation of the monocytes is associated with a loss of CD64 [22]. Based upon the characteristics of these two cell surface markers, the findings might confirm the presence of immature monocytes in peripheral blood.
On the other hand, it is known that several factors such as stress hormones, IL-10 and glutamine depletion may affect HLA-DR expression on monocytes in vitro[23–27]. IL-10 serum levels were significantly elevated in our patients compared to healthy controls and could have contributed to the decline of HLA-DR expression on monocytes. In addition, we found that the low plasma glutamine concentrations upon trauma correlated with the low HLA-DR expression on monocytes. A causal relation between the lack of peripheral glutamine availability and HLA-DR expression would be in line with the literature, as Spittler et al. showed that expression of HLA-DR antigen and FcγRI/CD64 on monocyte-derived macrophages of healthy volunteers in vitro was dependent on the availability of glutamine in the medium [24]. Moreover, it was demonstrated that administering a glutamine-enriched nutrition increased the HLA-DR on monocytes in surgical and trauma patients [28,29].
Another requirement for the development of a primary immune response is proliferation of the T cells. Because several authors [13–15] described a decreased proliferative capacity of T cells after a traumatic event, it was decided to investigate the mitogen-induced proliferation of the PBMC of our trauma patients on day 1. In contrast to previously published data [30], no effect of trauma on lymphocyte proliferation could be observed in the cultures with glutamine added to the medium when stimulated with different concentrations of PHA. However, in the absence of extra glutamine, we measured lower in vitro proliferation of PBMCs stimulated with one of the PHA concentrations after trauma. The intrinsic T cell proliferative capacity after trauma was considered to be unchanged, while substrate depletion can reduce this, and it is probably still adequate for a primary immune response.
In addition, we investigated to what extent T cells, following severe trauma, were able to produce the cytokine profile required for cell-mediated immune responses. Measuring the IFN-γ production capacity of PHA-stimulated PBMCs was chosen as a representative cytokine of the TH1 response, and IL-4 as representative of the TH2 response. Our results showed a shifted cytokine pattern towards the TH2 type, as the production of IFN-γ upon PHA stimulation was impaired, whereas IL-4 production was normal. Our finding was consistent with the decreased IFN-γ production after stimulation of fresh PBMCs in severe thermal injury, as described by O’Sullivan et al. [31], or following major surgery [15,32]. In contrast to our findings, O’Sullivan and several other authors reported an increase in production of IL-4 by PBMCs of severely injured patients [15, 31, 33, 34]. Consistent with our profile were the findings of Brune et al. [32] and Wick et al. [35], who also presented normal IL-4 production in severe trauma patients. An important finding is that the low plasma glutamine concentrations correlated with low IFN-γ production, suggesting an association between the extracellular release of type I T-lymphocyte cytokines and glutamine availability. No correlations were found between in vitro IL-4 and IL-10 production capacity and plasma glutamine. Chang et al. have already concluded that TH1 cells in vitro are more dependent than TH2 cells on sufficient glutamine concentrations [36].
With regard to the low in vitro IL-10 production by PHA-stimulated PBMCs, high IL-10 serum levels were found after trauma when compared to healthy controls. A possible explanation for the low in vitro IL-10 production in our trauma patients could be that hyperactivation of the PBMCs in vivo leads to an increased IL-10 release, as reflected by the higher serum IL-10 levels, but to a decreased in vitro IL-10 production, because the PBMCs were already exhausted [37].
Because impaired TH1 cytokine responsiveness could affect the induction of cell-mediated immunity, the cellular immune response towards primary antigen KLH was evaluated in vivo and in vitro. In our KLH-sensitized trauma patients, virtually no skin reaction to KLH could be detected. PHA skin reactivity was also impaired, due probably to anergy of the skin. Such skin anergy is associated strongly with sepsis-related mortality in trauma patients [6] and has been described previously in surgical patients by Puyana et al. [38]. Therefore, the in vitro proliferative response to KLH was used additionally as a measurement for the cell-mediated immunity. Of the patients tested, most patients did not respond to KLH. Unexpectedly, five of 10 (45%) of the healthy controls also had no detectable in vitro response to KLH on day 14. This observation confirmed that healthy controls can also be non-responsive at this time-point [38].
Because adequate antibody profiles, being crucial for the specific elimination of infectious agents, are dependent on T cell cytokine production, all (sub)classes of antibodies towards neoantigen KLH were evaluated. Several studies indicated that following thermal, surgical or mechanical trauma defects in the humoral immune response might also occur [39–42]. On the contrary, our trauma patients appeared to have antibody levels that were not affected compared to healthy controls. Remarkably, the IgG subclass profile of the anti-KLH antibodies was also comparable to that of the healthy controls, despite impaired proinflammatory T cell reactivity in the period of KLH sensitization. Our findings are in contrast with the findings of Puyana et al., who described lower KLH-specific IgG and IgM levels after surgical trauma [38]. This might be due to differences in KLH-sensitization doses (500 µg in our study and 200 µg in their study) or differences in the detection techniques used or to the severity of the exposed injury. Nohr et al. also described a normal in vivo antibody response to a relatively T cell-independent bacterial polysaccharide antigen in surgical patients and found, moreover, a good correlation of in vivo- to in vitro-antibody responses [43]. Our study shows that severe trauma leads to an impaired TH1 immune response related partially to low plasma glutamine concentrations. Moreover, after trauma the induction of a primary immune response, in this study towards the antigen KLH, results despite normal antibody levels in a poor cell-mediated immune response.
Acknowledgments
We wish to thank the input of Dr J. C. Puyana, Department of Surgery, Surgical ICU, University of Pittsburgh Medical Center, Pittsburgh, PA, USA. We also thank Renate Wünsche for skillful technical assistance. P.G. Boelens and A.P.J. Houdijk are recipients of a fellowship of the Dutch Council for Medical Research of the Netherlands Organization of Scientific Research.
REFERENCES
- 1.Bone RC. Immunologic dissonance: a continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS) Ann Intern Med. 1996;125:680–7. doi: 10.7326/0003-4819-125-8-199610150-00009. [DOI] [PubMed] [Google Scholar]
- 2.Ardawi MS. Glutamine and glucose metabolism in human peripheral lymphocytes. Metabolism. 1988;37:99–103. doi: 10.1016/0026-0495(88)90036-4. [DOI] [PubMed] [Google Scholar]
- 3.Harris JR, Markl J. Keyhole limpet hemocyanin (KLH): a biomedical review. Micron. 1999;30:597–623. doi: 10.1016/s0968-4328(99)00036-0. [DOI] [PubMed] [Google Scholar]
- 4.Meakins JL, McLean AP, Kelly R, Bubenik O, Pietsch JB, MacLean LD. Delayed hypersensitivity and neutrophil chemotaxis: effect of trauma. J Trauma. 1978;18:240–7. doi: 10.1097/00005373-197804000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Wolfe JH, Wu AV, O'Connor NE, Saporoschetz I, Mannick JA. Anergy, immunosuppressive serum, and impaired lymphocyte blastogenesis in burn patients. Arch Surg. 1982;117:1266–71. doi: 10.1001/archsurg.1982.01380340002002. [DOI] [PubMed] [Google Scholar]
- 6.Christou NV, Meakins JL, Gordon J, et al. The delayed hypersensitivity response and host resistance in surgical patients: 20 years later. Ann Surg. 1995;222:534–46. doi: 10.1097/00000658-199522240-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ditschkowski M, Kreuzfelder E, Rebmann V, et al. HLA-DR expression and soluble HLA-DR levels in septic patients after trauma. Ann Surg. 1999;229:246–54. doi: 10.1097/00000658-199902000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hershman MJ, Cheadle WG, Wellhausen SR, Davidson PF, Polk HC., Jr Monocyte HLA-DR antigen expression characterizes clinical outcome in the trauma patient. Br J Surg. 1990;77:204–7. doi: 10.1002/bjs.1800770225. [DOI] [PubMed] [Google Scholar]
- 9.Guillou PJ. Biological variation in the development of sepsis after surgery or trauma. Lancet. 1993;342:217–20. doi: 10.1016/0140-6736(93)92303-b. [DOI] [PubMed] [Google Scholar]
- 10.Lin RY, Astiz ME, Saxon JC, Rackow EC. Altered leukocyte immunophenotypes in septic shock. Studies of HLA-DR, Cd11b, Cd14, and IL-2R expression. Chest. 1993;104:847–53. doi: 10.1378/chest.104.3.847. [DOI] [PubMed] [Google Scholar]
- 11.Lin RY, Astiz ME, Saxon JC, Saha DC, Rackow EC. Relationships between plasma cytokine concentrations and leukocyte functional antigen expression in patients with sepsis. Crit Care Med. 1994;22:1595–602. [PubMed] [Google Scholar]
- 12.Wakefield CH, Carey PD, Foulds S, Monson JR, Guillou PJ. Changes in major histocompatibility complex class II expression in monocytes and T cells of patients developing infection after surgery. Br J Surg. 1993;80:205–9. doi: 10.1002/bjs.1800800224. [DOI] [PubMed] [Google Scholar]
- 13.De AK, Kodys K, Puyana JC, Fudem G, Pellegrini J, Miller-Graziano CL. Only a subset of trauma patients with depressed mitogen responses have true T cell dysfunctions. Clin Immunol Immunopathol. 1997;82:73–82. doi: 10.1006/clin.1996.4289. [DOI] [PubMed] [Google Scholar]
- 14.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 15.Decker D, Schondorf M, Bidlingmaier F, Hirner A, von Ruecker AA. Surgical stress induces a shift in the type-1/type-2 T-helper cell balance, suggesting down-regulation of cell-mediated and up-regulation of antibody-mediated immunity commensurate to the trauma. Surgery. 1996;119:316–25. doi: 10.1016/s0039-6060(96)80118-8. [DOI] [PubMed] [Google Scholar]
- 16.Mack VE, McCarter MD, Naama HA, Calvano SE, Daly JM. Dominance of T-helper 2-type cytokines after severe injury. Arch Surg. 1996;131:1303–8. doi: 10.1001/archsurg.1996.01430240057007. [DOI] [PubMed] [Google Scholar]
- 17.Newsholme EA, Newsholme P, Curi R. The role of the citric acid cycle in cells of the immune system and its importance in sepsis, trauma and burns. Biochem Soc Symp. 1987;54:145–62. [PubMed] [Google Scholar]
- 18.Baker SP, O'Neill B, Haddon W, Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–96. [PubMed] [Google Scholar]
- 19.Korver K, Zeijlemaker WP, Schellekens PT, Vossen JM. Measurement of primary in vivo IgM- and IgG-antibody response to KLH in humans: implications of pre-immune IgM binding in antigen-specific ELISA. J Immunol Meth. 1984;74:241–51. doi: 10.1016/0022-1759(84)90291-6. [DOI] [PubMed] [Google Scholar]
- 20.Teerlink T, van Leeuwen PA, Houdijk A. Plasma amino acids determined by liquid chromatography within 17 minutes. Clin Chem. 1994;40:245–9. [PubMed] [Google Scholar]
- 21.Volenec FJ, Wood GW, Mani MM, Robinson DW, Humphrey LJ. Mononuclear cell analysis of peripheral blood from burn patients. J Trauma. 1979;19:86–93. doi: 10.1097/00005373-197902000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Grage-Griebenow E, Lorenzen D, Fetting R, Flad HD, Ernst M. Phenotypical and functional characterization of Fc gamma receptor I (CD64)-negative monocytes, a minor human monocyte subpopulation with high accessory and antiviral activity. Eur J Immunol. 1993;23:3126–35. doi: 10.1002/eji.1830231213. [DOI] [PubMed] [Google Scholar]
- 23.Schwiebert LM, Schleimer RP, Radka SF, Ono SJ. Modulation of MHC class II expression in human cells by dexamethasone. Cell Immunol. 1995;165:12–9. doi: 10.1006/cimm.1995.1181. [DOI] [PubMed] [Google Scholar]
- 24.Spittler A, Winkler S, Gotzinger P, et al. Influence of glutamine on the phenotype and function of human monocytes. Blood. 1995;86:1564–9. [PubMed] [Google Scholar]
- 25.de Waal MR, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spittler A, Schiller C, Willheim M, Tempfer C, Winkler S, Boltz-Nitulescu G. IL-10 augments CD23 expression on U937 cells and down-regulates IL-4-driven CD23 expression on cultured human blood monocytes: effects of IL-10 and other cytokines on cell phenotype and phagocytosis. Immunology. 1995;85:311–7. [PMC free article] [PubMed] [Google Scholar]
- 27.Klava A, Windsor AC, Farmery SM, et al. Interleukin-10. A role in the development of postoperative immunosuppression. Arch Surg. 1997;132:425–9. doi: 10.1001/archsurg.1997.01430280099016. [DOI] [PubMed] [Google Scholar]
- 28.Spittler A, Sautner T, Gornikiewicz A, et al. Postoperative glycyl-glutamine infusion reduces immunosuppression: partial prevention of the surgery induced decrease in HLA-DR expression on monocytes. Clin Nutr. 2001;20:37–42. doi: 10.1054/clnu.2000.0153. [DOI] [PubMed] [Google Scholar]
- 29.Boelens PG, Houdijk AP, Fonk JC, et al. Glutamine-enriched enteral nutrition increases HLA-DR expression on monocytes of trauma patients. J Nutr. 2002;20:37–42. doi: 10.1093/jn/132.9.2580. [DOI] [PubMed] [Google Scholar]
- 30.Puyana JC, DE Pellegrini JDAK, Kodys K, Silva WE, Miller CL. Both T-helper-1- and T-helper-2-type lymphokines are depressed in posttrauma anergy. J Trauma. 1998;44:1037–45. doi: 10.1097/00005373-199806000-00017. [DOI] [PubMed] [Google Scholar]
- 31.O'Sullivan ST, Lederer JA, Horgan AF, Chin DH, Mannick JA, Rodrick ML. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg. 1995;222:482–90. doi: 10.1097/00000658-199522240-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brune IB, Wilke W, Hensler T, Holzmann B, Siewert JR. Downregulation of T helper type 1 immune response and altered pro-inflammatory and anti-inflammatory T cell cytokine balance following conventional but not laparoscopic surgery. Am J Surg. 1999;177:55–60. doi: 10.1016/s0002-9610(98)00299-2. [DOI] [PubMed] [Google Scholar]
- 33.Zedler S, Faist E, Ostermeier B, von Donnersmarck GH, Schildberg FW. Postburn constitutional changes in T-cell reactivity occur in CD8+ rather than in CD4+ cells. J Trauma. 1997;42:872–80. doi: 10.1097/00005373-199705000-00018. [DOI] [PubMed] [Google Scholar]
- 34.Zedler S, Bone RC, Baue AE, von Donnersmarck GH, Faist E. T-cell reactivity and its predictive role in immunosuppression after burns. Crit Care Med. 1999;27:66–72. doi: 10.1097/00003246-199901000-00028. [DOI] [PubMed] [Google Scholar]
- 35.Wick M, Kollig E, Muhr G, Koller M. The potential pattern of circulating lymphocytes TH1/TH2 is not altered after multiple injuries. Arch Surg. 2000;135:1309–14. doi: 10.1001/archsurg.135.11.1309. [DOI] [PubMed] [Google Scholar]
- 36.Chang WK, Yang KD, Shaio MF. Effect of glutamine on Th1 and Th2 cytokine responses of human peripheral blood mononuclear cells. Clin Immunol. 1999;93:294–301. doi: 10.1006/clim.1999.4788. [DOI] [PubMed] [Google Scholar]
- 37.Faist E, Schinkel C, Zimmer S. Update on the mechanisms of immune suppression of injury and immune modulation. World J Surg. 1996;20:454–9. doi: 10.1007/s002689900071. [DOI] [PubMed] [Google Scholar]
- 38.Puyana JC, Rode HN, Christou NV, Meakins JL, Gordon J. Induction of an immune response to keyhole-limpet hemocyanin in surgical patients with anergy. Surgery. 1990;107:442–8. [PubMed] [Google Scholar]
- 39.Ertel W, Faist E, Nestle C, Schuebel I, Storck M, Schildberg FW. Dynamics of immunoglobulin synthesis after major trauma. Influence of recombinant lymphokines. Arch Surg. 1989;124:1437–41. doi: 10.1001/archsurg.1989.01410120087017. [DOI] [PubMed] [Google Scholar]
- 40.Richter M, Jodouin CA, Moher D, Barron P. Immunologic defects following trauma: a delay in immunoglobulin synthesis by cultured B cells following traumatic accidents but not elective surgery. J Trauma. 1990;30:590–6. [PubMed] [Google Scholar]
- 41.McRitchie DI, Girotti MJ, Rotstein OD, Teodorczyk-Injeyan JA. Impaired antibody production in blunt trauma. Possible role for T cell dysfunction. Arch Surg. 1990;125:91–6. doi: 10.1001/archsurg.1990.01410130097013. [DOI] [PubMed] [Google Scholar]
- 42.Schneider RP, Christou NV, Meakins JL, Nohr C. Humoral immunity in surgical patients with and without trauma. Arch Surg. 1991;126:143–8. doi: 10.1001/archsurg.1991.01410260027004. [DOI] [PubMed] [Google Scholar]
- 43.Nohr CW, Latter DA, Meakins JL, Christou NV. In vivo and in vitro humoral immunity in surgical patients: antibody response to pneumococcal polysaccharide. Surgery. 1986;100:229–38. [PubMed] [Google Scholar]