Abstract
Human hookworm infections are distributed widely in tropical areas and have a significant impact on host morbidity and human health. In the present study, we investigated the cellular responsiveness and cytokine production in peripheral blood mononuclear cells (PBMC) from Necator americanus-infected schoolchildren who had recently received chemotherapy, and compared them with non-infected endemic controls. Hookworm patients and treated, egg-negative individuals showed a lower cellular reactivity against phytohaemagglutinin (PHA) and hookworm antigen when compared with egg-negative endemic controls. The baseline production of proinflammatory tumour necrosis factor-α (TNF-α) in PBMC from infected patients and treated, egg-negative individuals was elevated. On the other hand, PHA- or hookworm antigen-induced interleukin (IL)-12 and interferon (IFN)-γ secretion was higher in endemic controls than in hookworm patients, who either continued egg-positive or were egg-negative after treatment. Also, PBMC from endemic controls secreted more IL-5 and IL-13 than the other patient groups. Opposite to that, the spontaneous as well as the antigen-driven IL-10 secretion was lower in endemic controls when compared with the other groups. In summary, patently hookworm-infected as well as egg-negative treated patients disclosed an elevated spontaneous cellular secretion of proinflammatory TNF-α, a prominent secretion of regulatory Th2-type IL-10 and an impaired production of IL-12, IFN-γ, IL-5 and IL-13.
Keywords: cellular reactivity, cytokines, hookworm infection, Necator americanus, schoolchildren
INTRODUCTION
Human hookworm infection, caused by the species Ancylostoma duodenale and Necator americanus, affects approximately 1·3 billion people worldwide [1,2]. The biology of human hookworm infection has been studied extensively and the transmission of the parasite depends upon faecal contamination of the soil, adequate environmental conditions for larval development and intense contact of contaminated soil with human skin [3]. Furthermore, the transmission of the parasite is facilitated by poor sanitation or by the use of nightsoil as fertilizer.
Clinical symptoms of human hookworm infection are iron-deficiency anaemia [4] and protein-losing enteropathy [5], symptoms which are usually correlated with parasitic load. Even light infections can lead to adverse consequences and increased morbidity in chronically malnourished populations [6].
The intensity of intestinal helminth infections on a population level is characterized by an overdispersed distribution [7] and heavy infections in an individual might be dependent on genetic, ecological, behavioural and social factors [8–10]. Several studies have shown that highest intensity of infection can be observed in grown-ups and young adults [11] and a second peak in intensity has been observed in patients older than 60 years [12,13]. Human hookworm infection is acquired frequently in young children and, as such, may have a profound impact on their immune competence, but there is little information on the induced cellular immune responses in hookworm-infected as well as exposed individuals. In the present study, we investigated the cellular reactivity and cytokine production profiles of schoolchildren who, following chemotherapy, segregated in treated egg-negative patients (Cured) and those who remained egg-positive for N. americanus (Nec) even after two treatment rounds, plus a group of non-treated negative endemic controls (EC).
MATERIALS AND METHODS
Study population
The study was conducted in Arinos, a rural town 700 km north-west of Belo Horizonte, the capital of the state of Minas Gerais, Brazil. From a quarter of Arinos with insufficient access to clean water supplies and inappropriate sanitation, a total of 305 schoolchildren from the local primary and secondary schools were examined and occurrence of gastrointestinal helminth infections recorded. During the first examination and treatment patients positive for hookworms Ascaris lumbricoides, Trichuris trichiura, Enterobius vermicularis or Hymenolepis nana received a standard treatment of 6 × 100 mg mebendazole for 3 consecutive days. Patients with a Schistosoma mansoni infection received praziquantel treatment from the local health authority. Seven weeks later a randomly selected group of patients, participating in the immunological study, was examined again. This time, in order to increase efficacy of intestinal helminths elimination, egg-positive individuals received a single-dose albendazole treatment (400 mg). At 6 months after the first treatment, all individuals were re-examined and all patients positive for intestinal helminths were given treatment with albendazole (400 mg). Patients co-infected with other helminths were excluded from the immunological study.
This study was approved by the Ethical Committee of the Centro de Pesquisas René Rachou and was performed in collaboration with the local primary healthcare unit in Arinos. For participation in the study written consent was given by the parents or guardians of the children.
Subject sampling and parasitological examination
Individuals were examined for intestinal helminth infections using the Kato–Katz technique [14]. Mean egg counts per gram of faeces (epg) were calculated from at least two slides per sample. For quantitative assessment of hookworm eggs, slides were examined shortly after preparation to avoid drying of eggs. Egg-negative children were examined three times, with one stool sample each time, during 6 months before inclusion in this study and were considered as negative endemic controls (EC). Those individuals had no recent report of drug treatment and remained egg-negative during all examinations, with the last stool examination performed 1 week before the start of this study. Six months after the first treatment, 20 ml of blood were collected from randomly selected individuals in a heparinized Vacutainer® tube (Becton Dickinson, Franklin Lakes, NJ, USA). For the investigation of antibody responses, 5 ml blood samples were collected in a non-heparinized tube before the first treatment and 6 months after. Serum samples were stored at −70°C until further use.
Mitogen and antigen preparation
For polyclonal stimulation of peripheral blood mononuclear cells (PBMC) phytohaemagglutinin-L (PHA-L, Difco Laboratories, Detroit, MI, USA) was used. For the in vitro cell culture experiments, adult hookworms (Ancylostoma caninum) were obtained from infected dogs kept at the animal facilities of the prefecture of Belo Horizonte. Hookworms were also collected from stools of albendazole-treated patients from the endemic area during 1 week post-treatment. Hookworm antigen preparations were tested negative for endotoxin content by the Limulus lysate assay (sensitivity: 0·06 U/ml; Sigma, St Louis, MO, USA). About 10% of the adult worms obtained from human patients were preserved, examined under a stereomicroscope and identified as N. americanus. The remaining worms were used as soluble antigen in an enzyme-linked immunosorbent asay (ELISA) for investigation of the humoral immune response. In order to avoid bacterial contamination, all parasites were washed extensively in cold, sterile phosphate buffered saline (PBS), cut into pieces and transferred into a tissue grinder in a small volume of PBS. The hookworm antigen (ANC) was homogenized on ice and centrifuged subsequently at 20 000 g for 40 min at 4°C. S. mansoni parasitic stages were isolated from Swiss mice infected experimentally at the animal facilities of the Centro de Pesquisas René Rachou-Fiocruz in Belo Horizonte. Other, non-related worm antigen preparations were collected and prepared as described previously [15,16]. All PBS-soluble antigen fractions were passed through a 0·2 µm filter (Millipore, Bedford, MA, USA) and the protein concentration determined according to the method of Lowry et al. [17].
PBMC proliferation assays
Isolation of PBMC was performed as described previously [16]. Stimulation assays were performed in triplicates and mitogen and antigens were added at previously determined concentrations known to result in optimal proliferation, i.e. PHA (4 µg/ml), ANC (20 µg/ml), S. mansoni soluble egg antigen (SEA) (25 µg/ml) and S. mansoni adult worm antigen (SWAP) (25 µg/ml). For mitogenic stimulation, the cells were pulsed after 48 h of culture with tritiated thymidine (Amersham Pharmacia, Sao Paulo, Brazil; 0·5 µCi/well, specific activity = 6·7 Ci/mm) and harvested after a further 18 h of culture. Antigen-stimulated cell cultures were pulsed after 6 days with tritiated thymidine and harvested after an additional 6 h of culture. Incorporated activity was determined in a liquid scintillation counter and the data expressed as stimulation indices SI (SI = mean proliferation of stimulated culture/mean proliferation of unstimulated culture).
Cytokine production in PBMC supernatants
Determination of cytokine secretion patterns was performed as described elsewhere [16]. The following cytokine-specific ELISAs were used and performed according to the manufacturer's instructions (Opti-EIA Sets; Pharmingen, Heidelberg, Germany): interleukin (IL)-5, IL-10, IL-12 (p40), IL-13, interferon (IFN)-γ, and tumour necrosis factor (TNF)-α. Cytokine concentrations in supernatants were measured in duplicate and were calculated according to standard curves present in each plate. Mean cytokine concentrations are expressed in pg/ml and are expressed either as net production from which background cytokine secretion has been subtracted or baseline production of the cytokine is indicated separately. The detection limits of the different cytokine assays were below the standard curves as recommended by the manufacturers for IL-5 and IL-10 (7·8 pg/ml), IL-13 (3·1 pg/ml), IFN-γ (9·4 pg/ml) and TNF-α (15·6 pg/ml). For IL-12, the detection limit was assessed to be approximately 40 pg/ml.
Parasite-specific antibody reactivity in sera
Sera from infected patients before (n = 23) and 6 months after first treatment (n = 9), cured individuals (n = 12) and negative endemic controls (n = 17) were screened for N. americanus-specific antibodies in an indirect ELISA. Briefly, ELISA plates (Nunc, Maxisorp, Naperville, IL, USA) were coated overnight at 4°C with 100 µl of Necator antigen (Nec-Ag, 5 µg/ml) diluted in carbonate buffer (15 mm Na2CO3, 35 mm NaHCO3, 0·02% NaN3, pH 9·6). The plates were washed twice with PBS/0·05% Tween-20 and incubated with the individual sera diluted in PBS/10% fetal calf serum (FCS) (1 : 100 for IgG and IgA and 1 : 50 for IgG1, IgG4 and IgE) at 4°C overnight. On the following day, the plates were washed (5×) and 100 µl per well of alkaline phosphatase-conjugated detection antibody in PBS/10% FCS was added at the following dilutions: total IgG 1 : 1000 (Sigma-Aldrich, Steinheim, Germany); IgG1, IgG4, IgA 1 : 1000 (Pharmingen); IgE 1 : 2000 (Pharmingen). The plates were incubated for 2 h at RT and subsequently washed seven times with PBS/0·05% Tween-20. In a final step, 100 µl of p-nitrophenylphosphate substrate (Sigma) diluted in substrate buffer (0·5 mm MgCl2, 10% diethanolamine, pH 9·8) were added to the plates and the colorimetric reaction measured in an automated ELISA reader at 405 nm. Serum antibody reactivity for individual samples was performed in duplicate. Values from different plates were compared and standardized according to reference sera from infected patients and endemic controls, which were run in each plate. Values are expressed as mean optical densities and compared between the different patient groups.
Statistical analyses
Data were checked for normal distribution by the Shapiro–Wilk W-test. Differences between the patient groups were checked for significance by using the non-parametric Wilcoxon rank test. Bivariate analysis was performed by performing the non-linear Spearman's rank test. Significance levels were taken at the 5% level unless indicated otherwise. Due to multiple testing, a Bonferroni–Holm adaptation was performed in order to correct significance values in each experiment and adjusted P-values are indicated in the figures and tables.
Results
Patient groups and parasitological results
A total of 305 schoolchildren were examined for intestinal helminth infections. Hookworm infection, with 31·1% (n = 95 patients), was most prevalent in Arinos, while H. nana infection was diagnosed in 19 patients (6·2%). Cases with other intestinal helminths, i.e. S. mansoni, E. vermicularis, A. lumbricoides or T. trichiura, were less frequent and prevalences were around or below 1%.
Hookworm patients and egg-negative endemic controls were selected randomly to participate in the immunological study. Hookworm egg-positive patients (n = 23) were treated with mebendazole (6 × 100 mg), then re-examined after 7 weeks post-treatment to verify successful treatment and positive patients were treated again with a single dose of albendazole (400 mg). Six months after the first treatment, from patients and endemic controls PBMC and serum were collected.
For the 95 egg-positive schoolchildren from the primary and secondary school, no correlation between age and hookworm egg counts was found. In those individuals who were included in the immunological study the infection intensity before treatment was classified in six patients as moderate to high, whereas 17 patients harboured a light infection (range of all infected patients: 12–9·636 epg), according to Stoltzfus et al. [18]. Six months after the first treatment, 14 patients became egg-negative (Cured) and nine presented a light infection (Nec), with a geometric mean egg count of 357 epg (range: 48–1·272 epg). Negative endemic controls (EC; n = 7) were examined three times within 6 months and remained egg-negative in all stool preparations, although they were considered as exposed individuals, living in the same endemic area and under similar environmental and sanitation conditions. The mean age in patients who continued egg-positive (mean age: 10·3 years; range 8–13 years), in cured individuals (mean age: 9·9 years; range: 7–15 years) and in negative endemic controls (mean age: 8·9 years; range: 7–11 years) was not significantly different between the patient groups and was in a narrow age range.
In vitro lymphocyte proliferation assays
After stimulation of PBMC with the mitogen PHA, cellular proliferation was higher in EC than in egg-positive and treated, egg-negative hookworm patients; however, the differences were not statistically significant (Table 1). Similarly, after stimulation with hookworm antigen or other non-related worm antigens, the in vitro lymphocyte proliferation was highest in EC compared to Nec and Cured hookworm patients, but differences were not statistically significant. In all groups an elevated proliferation after stimulation with soluble hookworm antigen as well as against soluble egg antigen from S. mansoni (SEA) was measured, whereas reactivity to S. mansoni adult worm antigen (SWAP) remained low (Table 1).
Table 1.
Lymphocyte proliferation in hookworm egg-positive patients (Nec), hookworm treated, egg-negative patients (Cured) and egg-negative endemic controls (EC) after stimulation with mitogen or helminth antigens (ANC: A. caninum adult worm antigen, 20 µg/ml; SEA: S. mansoni soluble egg antigen, 25 µg/ml; SWAP: S. mansoni adult worm antigen, 25 µg/ml; ASC: A. lumbricoides adult worm antigen, 20 µg/ml; TRIC: T. trichiura adult worm antigen, 20 µg/ml). Values are indicated as mean stimulation indices ± s.d.
| Antigens | ||||||
|---|---|---|---|---|---|---|
| Mitogen PHA | ANC | SEA | SWAP | ASC | TRIC | |
| Nec, SI ± s.d. | 67·2 ± 31·3 | 12·2 ± 6·8 | 9·9 ± 6·1 | 3·3 ± 2·1 | 13·4 ± 11·3 | 5·0 ± 1·5 |
| Cured, SI ± s.d. | 66·4 ± 26·2 | 11·1 ± 11·3 | 9·6 ± 6·3 | 4·0 ± 5·2 | 7·3 ± 4·1 | 4·9 ± 4·4 |
| EC, SI ± s.d. | 90·0 ± 42·8 | 14·4 ± 10·0 | 12·0 ± 8·7 | 4·9 ± 3·3 | 9·9 ± 6·8 | 8·2 ± 4·3 |
PHA: phytohaemagglutinin; SI: stimulation index; s.d.: standard deviation.
In vitro cytokine secretion by PBMC
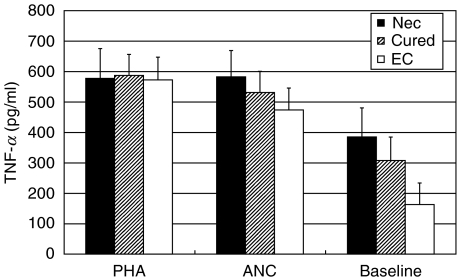
The mitogen- and antigen-induced TNF-α secretion was similar in all groups. Baseline TNF-α production was elevated in cured individuals and especially in hookworm patients, when compared to EC. The differences did not reach statistical significance (Fig. 1). For IL-12 and IFN-γ the baseline production in unstimulated cell cultures was low and without prominent differences between the patient groups, thus in Table 2 cytokine concentrations are indicated as net cytokine concentrations with the baseline values subtracted from stimulated cells (Table 2). After PHA stimulation, the IL-12 as well as the IFN-γ production was highest in PBMC cultures from EC; however, without reaching statistical significance. A similar result was obtained after stimulation with the hookworm antigen. Again, PBMC from EC secreted more IL-12 and IFN-γ than cured patients or infected individuals. For IFN-γ, the concentration in supernatants from negative endemic controls was significantly higher (P < 0·05) than in cured patients (Table 2).
Fig. 1.
PHA- and hookworm antigen-induced as well as baseline TNF-α production (PHA: 4 µg/ml; ANC: A. caninum adult worm antigen, 20 µg/ml) in PBMC cultures from hookworm patients (Nec; n = 9), hookworm egg-negative, cured patients (Cured; n = 14) and egg-negative endemic controls (EC; n = 7). Mean cytokine concentrations are indicated in pg/ml ± standard error of the mean. Baseline concentrations are separately indicated and are not subtracted from stimulated cells.
Table 2.
Cytokine production (IL-12, IFN-γ, IL-5, IL-13) of PBMC from hookworm egg-positive patients (Nec; n = 9), hookworm treated, egg-negative patients (Cured; n = 14) and egg-negative, endemic controls (EC; n = 7) after stimulation with PHA or hookworm antigen (ANC: A. caninum adult worm antigen). Cytokine concentrations are indicated in pg/ml ± s.d. with baseline values subtracted from those of stimulated cells and significant differences between patient groups are indicated with an asterisk
| Cytokine | Patient groups | PHA (4 µg/ml) | ANC (20 µg/ml) |
|---|---|---|---|
| IL-12 (pg/ml) | Nec | 95 ± 106 | 355 ± 332 |
| Cured | 215 ± 273 | 370 ± 277 | |
| EC | 299 ± 243 | 420 ± 266 | |
| IFN-γ (pg/ml) | Nec | 1·270 ± 791 | 118 ± 113 |
| Cured | 855 ± 824 | 76 ± 111** | |
| EC | 1·294 ± 719 | 229 ± 136** | |
| IL-5 (pg/ml) | Nec | 124 ± 228** | 83 ± 83** |
| Cured | 218 ± 368 | 83 ± 116** | |
| EC | 546 ± 514** | 290 ± 177** | |
| IL-13 (pg/ml) | Nec | 788 ± 337 | 244 ± 198 |
| Cured | 783 ± 530 | 167 ± 180** | |
| EC | 1·598 ± 696 | 434 ± 202** |
PHA: phytohaemagglutinin; s.d.: standard deviation
P ≤ 0·05)
The concentrations of IL-5 and IL-13 in supernatants also are indicated as net production in Table 2, which was the cytokine concentration in stimulated cell cultures subtracted by the cytokine concentration in unstimulated cultures. The IL-5 as well as the IL-13 production was higher in PBMC cultures from EC than in the other groups. After PHA stimulation, more IL-5 was produced by PBMC from EC than by infected patients (P < 0·05), and following hookworm antigen stimulation significantly elevated IL-5-concentrations (P < 0·05) were detected in supernatants from EC when compared to the other groups. Also, hookworm antigen-induced IL-13 production was significantly higher (P < 0·05) in EC than in treated, egg-negative patients (Cured) (Table 2).
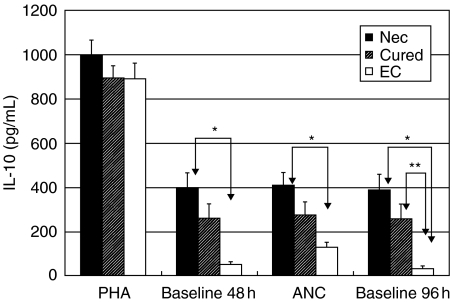
For IL-10, all patients produced comparable levels of IL-10 after PHA stimulation. Interestingly, the baseline production of IL-10 in unstimulated cultures was elevated in Nec and Cured individuals when compared to EC. For Nec and EC this difference was significant (P < 0·01)(Fig. 2). Strikingly, when PBMC from Nec and Cured patients were stimulated with parasite antigen, IL-10 production did not surpass unstimulated cultures. When compared between the patient groups, IL-10 secretion was higher (P < 0·01) in Nec than in EC. Spontaneous release of IL-10 by unstimulated PBMC was clearly elevated in Nec and Cured patients when compared to EC (P < 0·01 and P < 0·05, respectively) (Fig. 2).
Fig. 2.
Phytohaemagglutinin-induced (4 µg/ml; 48 h) and hookworm antigen-induced (ANC: A. caninum adult worm antigen, 20 µg/ml; 96 h) IL-10 production in PBMC cultures from hookworm patients (Nec; n = 9), hookworm egg-negative cured patients (Cured; n = 14) and egg-negative endemic controls (EC; n = 7). Mean cytokine concentrations are indicated in pg/ml ± standard error of the mean and significant differences between patient groups are indicated with an asterisk (*P ≤ 0·01, **P ≤ 0·05). Baseline concentrations are indicated separately and are not subtracted from stimulated cells.
Parasite-specific antibody reactivity in sera
Necator americanus-specific antibodies in sera from different patient groups were investigated with an indirect ELISA and significant differences between patients are shown in Table 3. In general, an elevated reactivity for IgG, IgG1, IgG4, IgA and IgE was measured in Nec patients before and 6 months after treatment. Treated, egg-negative patients (Cured) showed intermediate responses, whereas EC disclosed only low serological reactivity, especially for IgG4 and IgE (Table 3). If the IgG4 reactivity against hookworm antigen is evaluated in non-endemic individuals, Ascaris/Trichuris patients (n = 40; mean OD = 0·22 ± 0·01) and egg-negative, non-endemic controls (n = 10; mean OD = 0·05 ± 0·19) displayed significantly lower responses when compared with N. americanus-infected individuals before or after treatment (P = 0·01, for each group).
Table 3.
Necator americanus-specific antibody responses in hookworm patients before (Nec pretreatment, n = 23) and 6 months after first treatment (Nec post-treatment, n = 9), treated, egg-negative individuals (Cured, n = 12) and egg-negative endemic controls (EC, n = 17). Parasite-specific IgG, IgG1, IgG4, IgA, and IgE antibody reactivity is shown as optical densities ± s.d. Significant differences between groups are indicated
| Immunoglobulin | Nec pretreatment | Nec post-treatment | Cured | EC |
|---|---|---|---|---|
| IgG, OD ± s.d. | 1·01 ± 0·64a | 0·81 ± 0·82 | 0·65 ± 0·64 | 0·41 ± 0·51a* |
| IgG1, OD ± s.d. | 0·35 ± 0·26b | 0·23 ± 0·26 | 0·18 ± 0·22b** | 0·1 ± 0·15b* |
| IgG4, OD ± s.d. | 1·19 ± 0·77c | 1·14 ± 1·01d | 0·53 ± 0·38c** | 0·35 ± 0·28c*d** |
| IgA, OD ± s.d. | 0·93 ± 0·66e | 0·81 ± 0·88 | 0·35 ± 0·13e** | 0·36 ± 0·25e* |
| IgE, OD ± s.d. | 0·33 ± 0·49 | 0·37 ± 0·55 | 0·18 ± 0·18 | 0·11 ± 0·07 |
OD: optical density; s.d.: standard deviation.
P < 0·01;
P < 0·05). Due to multiple testing a Bonferroni–Holm adaptation for significance levels was performed and adjusted P-values are indicated in the table
DISCUSSION
Information on cellular immune responses and cytokine profiles in human hookworm infection remain scarce and first reports occurred only recently [19,20]. Even though prevalence and intensity of hookworm infections peak in adolescent children, young adults [21–23] and in middle-aged and elderly patients [11, 13, 24], infection is already acquired frequently during the first 5 years of life. Therefore, adult N. americanus, with an assumed mean parasite life span of 5 years, might have a profound impact on host immune responsiveness already in the young patient groups.
In the present study, we compared the cellular immune response in N. americanus infected children, who remained egg-positive even after two anthelminthic treatments (Nec), with treated, egg-negative patients (Cured) and exposed, egg-negative endemic controls (EC). The cellular reactivity in response to mitogen as well as to hookworm antigen was suppressed in Nec as well as in Cured patients when compared to EC, although these differences were not statistically significant. Such a suppressed cellular reactivity was reported for other helminthic infections as well and attributed to the immune-evasion strategy of the parasite [25,26].
The cytokine secretion pattern of stimulated PBMC cultures showed several clear differences between the patient groups. The baseline secretion of inflammatory TNF-α was highest in Nec, intermediate in Cured and lowest in EC, whereas after stimulation of PBMC with PHA or hookworm antigen an equally high production was detected in all patient groups. Furthermore, the spontaneous secretion of IL-10, as well as the production of IL-10 upon antigenic stimulation, was lowest in EC.
TNF-α was described originally as a factor which inhibits proliferation of certain tumour cells. Apart from that, it is a multi-potential, proinflammatory cytokine with various biological effects playing a key role during septic shock and some autoimmune diseases, including intestinal bowel disease [27–29]. Although experimental infection of SCID mice with S. mansoni showed that TNF-α is an important factor in the early granuloma formation [30], elevated levels of TNF-α in combination with IFN-γ correlated with hepatosplenic disease in human schistosomiasis [31].
It has been shown recently that IFN-γ plays a critical role in the regulation of epithelial cell proliferation during chronic intestinal inflammation in the course of a T. muris infection [32]. IFN-γ is known to be a potent inducer of TNF-α secretion and synergizes with its activities [33]. In our study, different from the TNF-α concentrations, IL-12 as well as IFN-γ secretion patterns were higher in EC than in Nec or Cured. The higher production of type 1 cytokines in EC may reflect the absence of a patent infection, or acute larval challenge, or else IL-12 and IFN-γ may be important for mediating protective immunity. On the other side, low IFN-γ secretion in hookworm patients and cured individuals might be down-modulated by the higher spontaneously produced IL-10. In contrast to our results, others reported an increased IFN-γ and TNF-α secretion in patients coinfected with N. americanus and Oesophagostomum bifurcum than in mono-infected Necator patients and attributed this to inflammatory responses and increased intestinal pathology [20]. However, in their study negative endemic controls were not investigated. Also, as our patients received two chemotherapies before the assessment of immunological parameters, the cytokine profile may have changed and may be different from before treatment.
For other experimental gastrointestinal nematode infections, it was shown that worm expulsion and immunity is dependent on a strong type 2 immune response [34–36]. In this respect, our experiments disclosed a markedly elevated IL-5 and IL-13 production in EC when compared to Nec, which was not yet restored in previously treated and cured patients. The down-regulatory capacity of IL-10 on both type 1 and type 2 cytokine responses was described in experimental schistosomiasis [37,38] and therefore the lower IL-5 and IL-13 as well as IL-12 and IFN-γ production in our hookworm patients and egg-negative cured patients supported these previous findings. One of these studies pointed out not only the central regulatory role of IL-10 in the pathogenesis of schistosomiasis, but also showed that excessive either type 1 or type 2 immune responses will exacerbate pathological processes [38]. The central immunomodulatory role of helminth-induced IL-10 secretion was demonstrated further in a murine model for allergy, where mice were protected against the development of allergy by a prior intestinal helminth infection. In that study, the helminth-induced IL-10 production together with the absence of allergic responses was reversed by the administration of anti-IL-10 antibodies [39].
The humoral immune responses against different stage-specific parasite antigens and excretory–secretory(ES) products of hookworms have been studied extensively and reviewed recently in a thorough manner [19]. Here, Nec and Cured patients presented mainly with elevated parasite-specific IgG4, IgA and IgE antibodies when compared with EC and the cross-reactivity with Ascaris or Trichuris patients was low. Antigen-specific IgG4 was confirmed especially to be a specific antibody for immunodiagnosis of hookworm infection, as proposed recently [40].
In summary, the present study disclosed an elevated production of type 1 (IL-12, IFN-γ) as well as type 2 (IL-5, IL-13) cytokines in negative endemic controls from a hookworm area. On the other hand, the spontaneous secretion of proinflammatory TNF-α and down-regulatory IL-10 was shown to be low in these individuals. Whether type 1 or type 2 immune responses are necessary for protective immunity in hookworm infections should be addressed further in longitudinal studies with a well-defined group of endemic controls. For hookworm patients and previously cured individuals elevated TNF-α levels may indicate ongoing intestinal inflammation and, on the other hand, elevated IL-10 production might serve to minimize cellular responsiveness and to down-regulate pathogenic processes.
Acknowledgments
This work was supported by grants from FAPEMIG and FIOCRUZ. S. M. Geiger received postdoctoral research fellowships from the institutions of higher education in the Federal Republic of Germany by the German Academic Exchange Service (DAAD) and from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). J. Bethony is supported by an International Scientists Research Development Award from the John C. Fogarty Center, NIH.
REFERENCES
- 1.Chan MS, Medley GF, Jamison D, Bundy DAP. The evaluation of potential global morbidity attributable to intestinal nematode infections. Parasitology. 1994;109:373–87. doi: 10.1017/s0031182000078410. [DOI] [PubMed] [Google Scholar]
- 2.Crompton DWT. How much human helminthiasis is there in the world? J Parasitol. 1999;85:397–403. [PubMed] [Google Scholar]
- 3.Gilles HM. Selective primary health care: strategies for control of disease in the developing world. XVII. Hookworm infection and anemia. Rev Infect Dis. 1985;7:111–8. doi: 10.1093/clinids/7.1.111. [DOI] [PubMed] [Google Scholar]
- 4.Fleming AF. Iron deficiency in the tropics. Clin Haematol. 1982;11:365–88. [PubMed] [Google Scholar]
- 5.Cooper ES, Whyte-Allen CAM, Finzi-Smith JS, MacDonald TT. Intestinal nematode infections in children: the pathophysiological price paid. Parasitology. 1992;104:S91–S103. doi: 10.1017/s0031182000075272. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Report of the WHO informal consultation on hookworm infection and anaemia in girls and women. Geneva: WHO, Division of Control of Tropical Diseases; 1996. WHO/CTD/SIP, 96.1. [Google Scholar]
- 7.Bundy DA, Hall A, Medley GF, Savioli L. Evaluating measures to control intestinal parasitic infections. World Health Stat Q. 1992;45:168–79. [PubMed] [Google Scholar]
- 8.Schad GA, Anderson RM. Predisposition to hookworm infection in humans. Science. 1985;228:1537–40. doi: 10.1126/science.4012307. [DOI] [PubMed] [Google Scholar]
- 9.Haswell-Elkins MR, Anderson RM. Evidence for predisposition in humans to infection with Ascaris, hookworm, Enterobius and Trichuris in a South Indian fishing community. Parasitology. 1987;95:323–37. doi: 10.1017/s0031182000057772. [DOI] [PubMed] [Google Scholar]
- 10.Upatham ES, Viyanant V, Brockelman WY, Kurathong S, Ardsungnoen P, Chindaphol U. Predisposition to reinfection by intestinal helminths after chemotherapy in South Thailand. Int J Parasitol. 1992;22:801–6. doi: 10.1016/0020-7519(92)90130-d. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi NS, Jizhang C, Khoshnood K, et al. Epidemiology of Necator americanus hookworm infections in Xiulongkan Village, Hainan Province, China: high prevalence and intensity among middle-aged and elderly residents. J Parasitol. 2001;87:739–43. doi: 10.1645/0022-3395(2001)087[0739:EONAHI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Humphries DL, Stephenson LS, Pearce EJ, The PH, Dan HT, Khanh LT. The use of human faeces for fertilizer is associated with increased intensity of hookworm infection in Vietnamese women. Trans R Soc Trop Med Hyg. 1997;91:518–20. doi: 10.1016/s0035-9203(97)90007-9. [DOI] [PubMed] [Google Scholar]
- 13.Changhua L, Xiaorong Z, Dongchuan Q, et al. Epidemiology of human hookworm infections among adult villagers in Hejiang and Santai Counties, Sichuan Province. China Acta Tropica. 1999;73:243–9. doi: 10.1016/s0001-706x(99)00035-2. [DOI] [PubMed] [Google Scholar]
- 14.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 15.Gazzinelli G, Katz N, Rocha RS, Colley DG. Immune responses during human Schistosomiasis mansoni X. Production and standardization of an antigen-induced mitogenic activity by peripheral blood mononuclear cells from treated but not active cases of schistosomiasis. J Immunol. 1983;130:2891–5. [PubMed] [Google Scholar]
- 16.Geiger SM, Massara CL, Bethony J, Soboslay PT, Carvalho OS, Corrêa-Oliveira R. Cellular responses and cytokine profiles in Ascaris lumbricoides and Trichuris trichiura infected patients. Parasite Immunol. 2002;24:499–509. doi: 10.1046/j.1365-3024.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- 17.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 18.Stoltzfus RJ, Albonico M, Chwaya HM, et al. Hemoquant determination of hookworm related blood loss and its role in iron deficiency in African children. Am J Trop Med Hyg. 1996;55:399–404. doi: 10.4269/ajtmh.1996.55.399. [DOI] [PubMed] [Google Scholar]
- 19.Loukas A, Prociv P. Immune responses in hookworm infections. Clin Microbiol Rev. 2001;14:689–703. doi: 10.1128/CMR.14.4.689-703.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pit DSS, Polderman AM, Baeta S, Schulz-Key H, Soboslay PT. Parasite-specific antibody and cellular immune responses in humans infected with Necator americanus and Oesophagostomum bifurcum. Parasitol Res. 2001;87:722–9. doi: 10.1007/s004360100419. [DOI] [PubMed] [Google Scholar]
- 21.Anderson RM. The population dynamics and control of hookworm and roundworm infections. In: Anderson RM, editor. Population dynamics of infectious diseases. London: Chapman & Hall; 1982. pp. 67–106. [Google Scholar]
- 22.Anderson RM. The population dynamics and epidemiology of intestinal nematode infections. Trans R Soc Trop Med Hyg. 1986;80:686–96. doi: 10.1016/0035-9203(86)90367-6. [DOI] [PubMed] [Google Scholar]
- 23.Crompton DWT. The public health importance of hookworm disease. Parasitology. 2000;121:S39–S50. doi: 10.1017/s0031182000006454. [DOI] [PubMed] [Google Scholar]
- 24.Bethony J, Chen J, Lin S, et al. Emerging patterns of hookworm infection: influence of aging on the intensity of Necator infection in Hainan Province, People's Republic of China. Clin Infect Dis. 2002;35:1336–44. doi: 10.1086/344268. [DOI] [PubMed] [Google Scholar]
- 25.Finkelman FD, Pearce EJ, Urban JF, Jr, Sher A. Regulation and biological function of helminth-induced cytokine responses. Immunoparasitology. 1991. pp. A62–A66. [DOI] [PubMed]
- 26.Riffkin M, Seow H-F, Jackson D, Brown L, Wood P. Defence against the immune barrage: helminth survival strategies. Immunol Cell Biol. 1996;74:564–74. doi: 10.1038/icb.1996.90. [DOI] [PubMed] [Google Scholar]
- 27.Dinarello CA. Cytokines as mediators in the pathogenesis of septic shock. Curr Top Microbiol Immunol. 1996;216:133–65. doi: 10.1007/978-3-642-80186-0_7. [DOI] [PubMed] [Google Scholar]
- 28.Dinarello CA. Proinflammatory and anti-inflammatory cytokines as mediators in the pathogenesis of septic shock. Chest. 1997;112:S321–S329. doi: 10.1378/chest.112.6_supplement.321s. [DOI] [PubMed] [Google Scholar]
- 29.Neurath MF, Fuss I, Pasparakis M, et al. Predominant pathogenic role of tumour necrosis factor in experimental colitis in mice. Eur J Immunol. 1997;27:1743–50. doi: 10.1002/eji.1830270722. [DOI] [PubMed] [Google Scholar]
- 30.Amiri P, Locksley RM, Parslow TG, et al. Tumour necrosis factor alpha restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature. 1992;356:604–6. doi: 10.1038/356604a0. [DOI] [PubMed] [Google Scholar]
- 31.Mwatha JK, Kimani G, Kamau T, et al. High levels of TNF, soluble TNF receptors, soluble ICAM-1, and IFN-γ, but low levels of IL-5, are associated with hepatosplenic disease in human Schistosomiasis mansoni. J Immunol. 1998;160:1992–9. [PubMed] [Google Scholar]
- 32.Artis D, Potten CS, Else KJ, Finkelman FD, Grencis RK. Trichuris muris: host intestinal cell hyperproliferation during chronic infection is regulated by interferon-γ. Exp Parasitol. 1999;92:144–53. doi: 10.1006/expr.1999.4407. [DOI] [PubMed] [Google Scholar]
- 33.Gray PW. Interferon-γ. In: Nicola NA, editor. Guidebook to cytokines and their receptors. New York: Sambrook and Tooze/Oxford University Press; 1994. pp. 118–19. [Google Scholar]
- 34.Finkelman FD, Shea-Donohue T, Goldhill J, et al. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–33. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 35.Finkelman FD, Wynn TA, Donaldson DD, Urban JF., Jr The role of IL-13 in helminth-induced inflammation and protective immunity against nematode infections. Curr Opin Immunol. 1999;11:420–6. doi: 10.1016/S0952-7915(99)80070-3. [DOI] [PubMed] [Google Scholar]
- 36.Grencis RK. Enteric helminth infection: immunopathology and resistance during intestinal nematode infection. Chem Immunol. 1997;66:41–61. doi: 10.1159/000058665. [DOI] [PubMed] [Google Scholar]
- 37.Wynn TA, Morawetz R, Scharton-Kersten T, et al. Analysis of granuloma formation in double cytokine deficient mice reveals a central role for IL-10 in polarizing both T helper cell 1- and T helper cell 2-type cytokine responses in vivo. J Immunol. 1997;159:5014–23. [PubMed] [Google Scholar]
- 38.Hoffmann KF, Cheever AW, Wynn TA. IL-10 and the dangers of immune polarization: excessive type1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol. 2000;164:6406–16. doi: 10.4049/jimmunol.164.12.6406. [DOI] [PubMed] [Google Scholar]
- 39.Bashir ME, Andersen P, Fuss IJ, Shi HN, Nagler-Anderson C. An enteric helminth infection protects against an allergic response to dietary antigen. J Immunol. 2002;169:3284–92. doi: 10.4049/jimmunol.169.6.3284. [DOI] [PubMed] [Google Scholar]
- 40.Palmer DR, Bradley M, Bundy DAP. IgG4 responses to antigens of adult Necator americanus: potential for use in large-scale epidemiological studies. Bull WHO. 1996;74:381–6. [PMC free article] [PubMed] [Google Scholar]