Abstract
Recruitment of polymorphonuclear leucocytes (PMN) across the intestinal epithelium is dependent on specific adhesion molecules and chemoattractants diffusing from the intestinal lumen. The present understanding is that in response to fMLP, PMN migration across a T84 colon carcinoma monolayer is dependent on the β2 integrin, Mac-1 (CD11b/CD18). To further understand PMN transepithelial migration, we sought to determine whether migration to C5a, IL-8 and LTB4 was similarly Mac-1-, or even CD18-dependent. T84 epithelial cell monolayers growing on Transwell filters were used in combination with radiolabelled peripheral blood PMN. The number of migrated PMN was established by the amount of radioactivity recovered from the well after the migration period. Monoclonal antibodies were used to block integrin function. Whereas essentially all migration to fMLP across T84 monolayers was prevented by anti-CD18 antibody, significant migration to C5a, IL-8 or LTB4 persisted despite anti-CD18 antibody, indicating PMN are capable of β2 integrin-independent transepithelial migration. An antibody to CD11b but not CD11a blocked migration to an extent similar as with anti-CD18. CD18-independent PMN migration to C5a occurred only in the basolateral-to-apical direction across epithelial cells. Co-stimulation of PMN with C5a and fMLP or IL-8 plus LTB4 and fMLP still resulted in CD18-independent migration. Thus CD18 use during PMN migration across this model epithelium is a function of the chemoattractant inducing migration. The finding of CD18-independent migration mechanisms needs to be considered when developing antiadhesion molecule strategies to reduce or reverse intestinal inflammation.
Keywords: transepithelial migration, neutrophil, intestinal epithelial cell, adhesion molecule, colitis, inflammatory bowel disease
INTRODUCTION
Many intestinal inflammatory illnesses are marked by infiltration by polymorphonuclear leucocytes (PMN) including into the lumen. Indeed, PMN have been implicated in the damage to the epithelium during these diseases since the numbers of infiltrating cells is positively associated with disease activity, for example during inflammatory bowel disease [1]. PMN may injure epithelial cells through secretion of inflammatory products [2,3] and transepithelial migration can affect epithelial permeability, potentially exposing the mucosa to bacteria [4–6]. Otherwise migration of PMN through the epithelium may directly stimulate epithelial cytokine production [7] and large numbers of migrating PMN provoke epithelial cell apoptosis [8]. Once in the lumen, PMN release additional products which elicit secretory diarrhoea [9]. Thus one strategy to reduce and possibly reverse inflammation is to specifically impede the influx of PMN into the intestinal lumen.
Migration of PMN across cellular barriers such as vascular endothelium and epithelium is the result of the combined effect of chemoattractants and specific cell adhesion molecule interactions. Precisely which chemoattractants are active in the inflamed human intestinal lumen are unknown but IL-8, LTB4 and complement split products have all been found in the stool of IBD patients [10–13], and McCormick et al. [14] described a novel chemoattractant secreted apically following infection of T84 cells with S. typhimurium. In regards to the adhesion interactions during transepithelial migration, the principal adhesion molecule is reportedly Mac-1 (CD11b/CD18) because almost all migration to fMLP across a model intestinal epithelial monolayer can be blocked with anti-CD11b or anti-CD18 (β2 integrin) antibodies [15,16]. However, multiple lines of evidence suggest CD18-independent PMN migration into or across the epithelium likely occurs. CD18 blockade failed to prevent damage to the ileum in a phorbal myristate acetate treated cat ileitis model [17], and blockade failed to entirely prevent PMN infiltration of the small intestinal epithelium during T. spiralis infection in rats [7] or increased myeloperoxidase (MPO) in the intestine following ischaemia/reperfusion [18]. Finally, freshly passaged HT-29 colon carcinoma cells supported CD18-independent adhesion to PMN [19].
In our experiments we examined whether PMN migration to chemoattractants other than fMLP also employed CD18. We report here that PMN may utilize CD18-independent mechanisms to cross the intestinal epithelium depending on the chemoattractant. This discovery has implications in our effort to target adhesion mechanisms as a means of controlling intestinal inflammation.
MATERIALS AND METHODS
T84 epithelial cell culture on Transwells
T84 cells (ATCC, Bethesda, MD, USA) were cultured in medium consisting of a 1 : 1 mix of DMEM and Ham F12 which was then supplemented with 15 mm HEPES, 50 U/ml penicillin, 50µg/ml streptomycin and 5% newborn calf serum (Life Technologies, Burlington, Canada). Polystyrene 0·33 cm2 Transwell filters (Fisher Scientific, Nepean, Canada) with 3µm pore size were used. In order to grow the T84 on the filter bottom the Transwell cup was inverted and fitted with a tight plastic collar then placed in a bath of DMEM for 4 h at room temperature with 200 µl of 0·3% type 1 collagen (ICN, Montreal, QC, Canada) diluted 1 : 50 with DMEM added into the collar. T84 cells were harvested and 5 × 105 cells added to the collagen-coated surface of each filter which was then left to incubate at 37°C in 5% CO2. The collars were subsequently removed and the Transwells righted and placed into a 24 well plate with fresh media with final volumes of 600 µl in the well below and 100 µl in the cup above the filter. Monolayers were used 8 days after applying the T84 cells.
Prior to use the integrity of the monolayers was tested by adding 30µl 125I-human serum albumin (HSA, approximately 100 000 cpm) into the cup, generating a 1 mm positive water pressure. After 30 min a 30µl sample was taken from the well and the amount of 125I was determined in a γ-counter (LKB 1282 Compugamma, Wallac). Monolayers were considered suitable for use if less than 2% of the 125I-HSA diffused into the well. For comparison, typically 35% of the labelled albumin diffused across bare filters and 30% across collagen-coated filters.
Human PMN isolation
PMN were isolated from adult donors, with the approval of the IWK Health Centre Research Ethics Board, as described previously [20]. Briefly, blood was drawn into acid citrate dextrose (Travenol, Milton, Canada) and red cells sedimented (1 ×g) with 6% dextran-saline (Travenol). The leucocyte rich plasma (LRP) was harvested, the cells pelleted by centrifugation, then resuspended in Ca2+ Mg2+ free Tyrode's solution with 5% autologous platelet poor plasma (PPP). The leucocytes were then labelled with Na251CrO4 (25 µCi/ml, Amersham Corp, Oakville, Canada) at 37°C for 30 min followed by PMN enrichment using a discontinuous 58%/72% Percoll (Pharmacia, Uppsala, Sweden). PMN recovered from the 58%/72% interface were washed then counted in crystal violet and resuspended to 1 × 106 cells/ml in DMEM containing 5 mg/ml pyrogen-free HSA (Canadian Blood Services, Toronto, Canada) and 15 mm HEPES. Purity of PMN was routinely >95% with >95% viability.
Chemoattractants and monoclonal (mAb) antibodies
Recombinant human C5a was a gift from CIBA-Geigy (Summit, NJ, USA), recombinant human IL-8 (72 amino acids) was purchased from Peprotech Inc. (Rocky Hill, NJ, USA), fMLP from Sigma Chemical Company (Oakville, Canada) and LTB4 from Cayman Chemical Company (Cedarlane, Hornby, Canada).
The following adhesion blocking mAbs were used as purified immunoglobulin: 60·3 (anti-CD18, IgG1, a gift from Bristol-Myers Squibb, Seattle, WA, USA) [21]; IB4 (anti-CD18, IgG2a, ATCC, Bethesda, MD, USA); 9D4 (anti-β3 integrin, Genetech, South San Francisco, CA, USA). Monoclonal clone TS1/22 (anti-CD11a, IgG1, a gift from Dr T. Springer, Boston, MA, USA) was used as ascites and clone 2LPm19C (anti-CD11b, IgG1, a gift from Dr K Pulford, Oxford, UK) was used as culture supernatant. Antibody clones W6/32 (anti-MHC class I, IgG2a, ATCC) and 543 (anti-CR1 or CD35, IgG1, ATCC) were used in some experiments as PMN binding control antibodies.
For E-cadherin studies, mAb clone SHE78-7 was purchased from R & D Systems (Minneapolis, MN, USA). Recombinant E-cadherin-Fc and mAb clone E4·6 (inhibits E-cadherin binding to αEβ7) were kindly provided by Drs J. Higgins and M. Brenner (Harvard Medical School, Boston, MA, USA) [22], respectively. Anti-fractalkine mAb 81506 and anti-uPAR mAb were both from R & D Systems.
PMN transmigration assay
The media was removed from the Transwells, the monolayers washed with serum free DMEM, then 1 × 105 PMN containing approximately 1500 cpm of 51Cr in 100µl were placed in the Transwell cups. The cups were then placed in wells containing 600µl of DMEM-HSA-HEPES and chemoattractant as indicated in the Figures. Anti-CD18 antibodies were used at 30 µg/ml, a concentration shown previously by flow cytometry to be at least 5 fold greater than required for saturation of binding this number of PMN. PMN were preincubated with antibodies for 20 min at room temperature prior to addition to the cup and all mAbs remained present during the migration period. Migration across monolayers occurred over 2 h, then the medium and PMN remaining in the cup were removed by washing. The T84 monolayers were washed with media to dissociate loosely adherent PMN which were then pooled with the ‘migrated’ fraction in the well. TritonX100 (1% final concentration) was added to the well to lyse the migrated PMN. Finally, the filters were placed in 0·2 m NaOH to lyse the monolayers and any remaining PMN. Cells recovered from the monolayer and filter (adherent PMN) and the contents of the well plus monolayer wash (migrated PMN) were analysed for 51Cr content. Aliquots of stock cells from each different PMN treatment group (e.g. ± mAb) served as a standard for determining the total (100%) radioactivity added to the Transwell.
Data analysis
The numbers of migrating or adherent PMN are reported as a percentage of the number of cells added into the Transwell cup. The data for percent migrated PMN was found suitable for parametric analysis using the Levene test for homogeneity-of-variance. One Way anova was then used and if significant (P < 0·05), post hoc testing was conducted using Tukey's test. All statistical analyses were performed using SPSS Version 10·1.
RESULTS
fMLP, C5a, LTB4 and IL-8 induced PMN migration across T84 monolayers
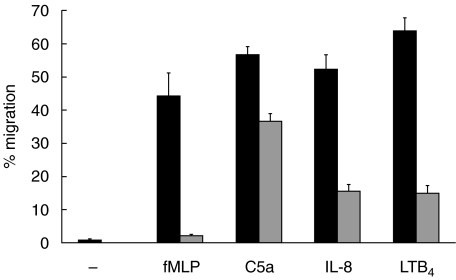
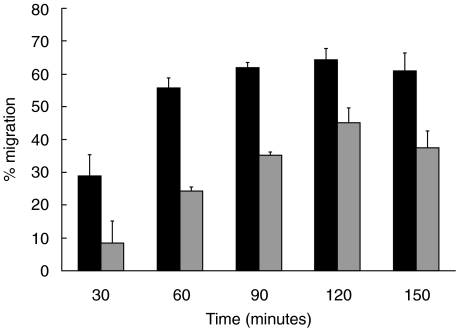
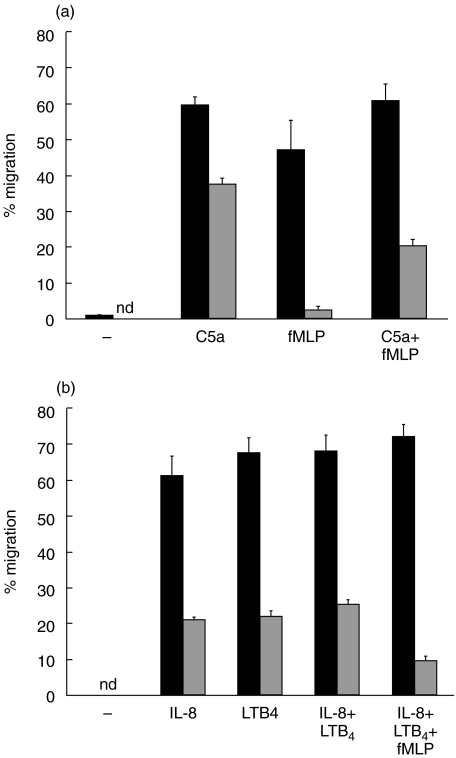
PMN migration across T84 monolayers in the absence of chemoattractant was less than 1% (Fig. 1). Figure 1 shows the extent of migration using the optimal concentration of each chemoattractant determined for 105 PMN, a number of cells which reportedly does not alter the monolayer electrical resistance [23] and is below the limit of detection using myeloperoxidase to enumerate migrated cells [15]. Inclusion of 5 mg/ml HSA in the media resulted in greater migration than assays run without added protein (not shown). Neither the type of filter, polystyrene or polycarbonate, nor substituting laminin for the collagen coating on the filters made any difference on migration (not shown). Also shown in Fig. 1 is the potency of blocking mAb to CD18 to inhibit PMN migration. There was almost complete inhibition of migration to fMLP while considerable migration persisted to C5a (65% of control migration), IL-8 (30% control migration) and LTB4 (23% of control migration). The extent of inhibition was similar using either anti-CD18 mAb and when we increased the concentration to 50µg/ml. To confirm that 2 h was sufficient time to expect PMN migration to C5a in the presence of anti-CD18 mAb, we conducted time course experiments and observed that the numbers of PMN recovered in the well plateau by 1·5 h, whether mAb was present or not (Fig. 2). The sum of these results led us to conclude that PMN can migrate across the T84 monolayer to C5a, IL-8 and LTB4 using CD18-independent mechanisms.
Fig. 1.
PMN migrate using CD18-independent mechanisms across T84 monolayers growing on the bottom of Transwell filters. Migration to fMLP (10−7m), C5a (10−8m), IL-8 (10−8m) and LTB4 (10−6m) of PMN without added mAb (▪) or anti-CD18 mAb pretreated PMN ( ). The data is from between six and 11 experiments and the mean of means ± standard error of the mean are shown. Values with control mAb treatment were similar to migration in absence of antibody for each chemoattractant (not shown).
). The data is from between six and 11 experiments and the mean of means ± standard error of the mean are shown. Values with control mAb treatment were similar to migration in absence of antibody for each chemoattractant (not shown).
Fig. 2.
Time course of PMN migration to C5a, in the absence of mAb (▪) or with anti-CD18 mAb ( ) across T84 monolayers growing on the bottom of Transwell filters. PMN were incubated with the mAb prior to adding to the Transwell and the migrated fraction was harvested at the time indicated and the 51Cr content determined. Shown is the mean ± standard deviation of three Transwells of a representative experiment.
) across T84 monolayers growing on the bottom of Transwell filters. PMN were incubated with the mAb prior to adding to the Transwell and the migrated fraction was harvested at the time indicated and the 51Cr content determined. Shown is the mean ± standard deviation of three Transwells of a representative experiment.
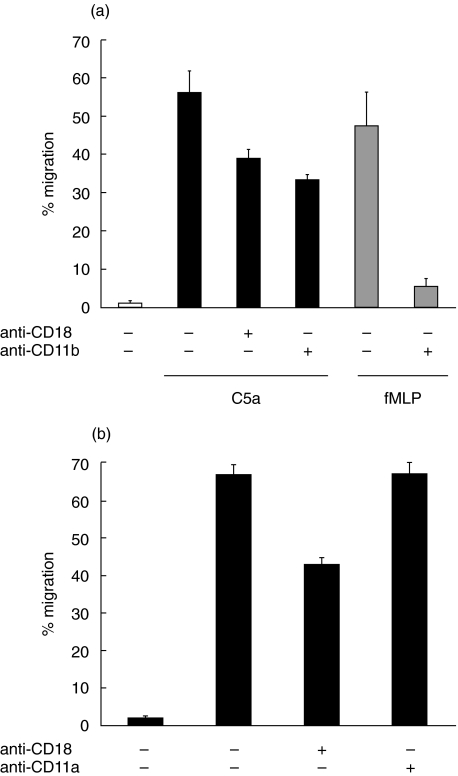
Since PMN migration to fMLP across T84 monolayers is reportedly Mac-1 (CD11b/CD18) dependent we used function blocking anti-CD11b (Fig. 3a) and anti-CD11a (Fig. 3b) mAbs to determine whether the CD18-dependent fraction of migration to C5a was also CD11b dependent. Indeed, migration was blocked by anti-CD11b but not by anti-CD11a mAb and furthermore, anti-CD11b was as effective as anti-CD18 mAb at blocking PMN migration to C5a or fMLP.
Fig. 3.
PMN CD18-dependent migration across T84 monolayers is CD11b-dependent. PMN were incubated with mAb to (a) CD18, CD11b or (b) CD11a prior to adding to the well, then after 2 h incubation the migrated fraction of cells was recovered. All the treatment groups in (b) were migrated in response to C5a.
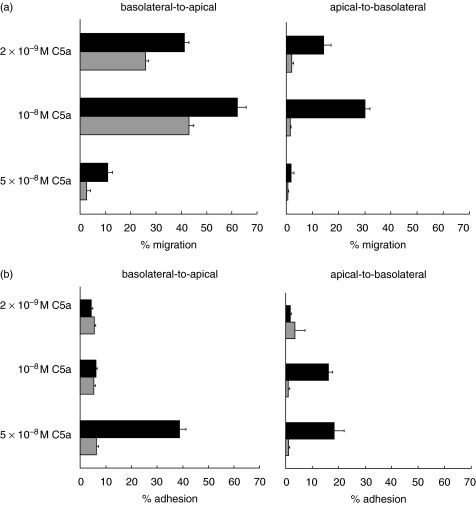
Migration in the apical-to-basolateral direction to C5a is CD18-dependent
We next tested whether the adhesion molecules supporting CD18-independent migration to C5a showed cell polarization. In order for PMN to first encounter the epithelial apical surface T84 were grown on the top of collagen coated Transwell filters. Shown in Fig. 4a, total PMN migration was lower in the apical to basolateral direction (30%versus 62% in the basolateral to apical direction using 10–8 M C5a). Moreover, migration across T84 cells to C5a in the apical to basolateral direction was completely blocked by anti-CD18 mAb. At the highest dose of C5a the numbers of monolayer adherent PMN was roughly inversely proportional to the number of cells migrating through the monolayer and adhesion to the apical T84 surface was entirely CD18-dependent (Fig. 4b). These results led us to conclude that the ligands used for CD18-independent migration are expressed only on the basolateral surface of the T84 cells.
Fig. 4.
Migration of PMN across T84 monolayers in the apical-to-basolateral direction is CD18-dependent. (a) Comparison of migration in the basolateral-to-apical (T84 grown on the filter bottom) versus apical-to-basolateral (T84 grown on the filter top) directions to multiple C5a concentrations. ▪ migration across T84 monolayers in absence of antibody; ( ) migration in presence of mAb to anti-CD18. (b) Fraction of PMN remaining adherent to T84 monolayers following a two hour migration period in response to C5a; the data is collected from the same experiments as (a). ▪ adherent PMN in absence of antibody; (
) migration in presence of mAb to anti-CD18. (b) Fraction of PMN remaining adherent to T84 monolayers following a two hour migration period in response to C5a; the data is collected from the same experiments as (a). ▪ adherent PMN in absence of antibody; ( ) adherent PMN in the presence of anti-CD18 mAb. Shown are the means ± standard deviation of three Transwells from one experiment. The experiment was repeated twice more using only the 10−8 C5a concentration, with similar results.
) adherent PMN in the presence of anti-CD18 mAb. Shown are the means ± standard deviation of three Transwells from one experiment. The experiment was repeated twice more using only the 10−8 C5a concentration, with similar results.
Influence of multiple chemoattractants on CD18-independent migration
It is likely that PMN migratingin vivo to and across the intestinal epithelium encounter multiple chemoattractants or at least sequential exposures to several chemoattractants. Indeed, Kitayama et al.[24] showed that one chemoattractant could de-sensitize PMN to a second chemoattractant during transendothelial migration. We therefore sought to determine whether C5a, IL-8 or LTB4 could elicit CD18-independent migration in the presence of fMLP. When fMLP was combined with C5a in the well, the CD18-independent migration was reduced yet remained significantly higher than due to fMLP alone (Fig. 5a). In similar experiments examining the use of IL-8 and LTB4; first, combining both chemoattractants resulted in a similar extent of CD18-independent migration as either chemoattractant used alone, and secondly, when fMLP was included the CD18-independent migration persisted albeit at a lower level than when fMLP was excluded (Fig. 5b).
Fig. 5.
CD18-independent PMN migration induced by C5a or IL-8 plus LTB4 persists in the presence of fMLP. (a) The effect 10−8 M C5a and 10−7 M fMLP combined in the bottom chamber on PMN migration. Pooled data of four experiments ± standard error of the mean are shown. The difference between the three nonmAb treated groups (▪) in which a chemoattractant was used were not significant; however, each of the anti-CD18 treated groups ( ) were significantly (P < 0·001) different from each other. (b) CD18-independent migration persists when IL-8 and LTB4 are combined with fMLP in the bottom chamber of the Transwell. No mAb (▪); PMN pretreated with anti-CD18 mAb (
) were significantly (P < 0·001) different from each other. (b) CD18-independent migration persists when IL-8 and LTB4 are combined with fMLP in the bottom chamber of the Transwell. No mAb (▪); PMN pretreated with anti-CD18 mAb ( ). nd = not done. Shown is the mean ± standard deviation of three wells from a single donor; one of two experiments.
). nd = not done. Shown is the mean ± standard deviation of three wells from a single donor; one of two experiments.
Mechanism of CD18-independent PMN migration
To begin to identify the CD18-independent transmigration mechanisms we first focused on E-cadherin since it is expressed laterally and mediates adhesion with lymphocytes [25]. However, in comparison to PMN migration to C5a alone (54·6 ± 1·3% migration), neither mAb E4·6 (52·7 ± 3·2% migration), nor the soluble E-cadherin-Fc (52·9 ± 6·8% migration) affected migration. There also was no significant effect on C5a induced migration by E4·6 or E-cadherin-Fc in the presence of anti-CD18 (30·2 ± 14·7%, 29·1 ± 2·2% and 28·4 ± 1·5% migration, respectively). Finally, mAb SHE78-7 had no effect on the numbers of PMN migrating to C5a, including in the presence of mAb to CD18 and thus E-cadherin does not appear to be involved in PMN transepithelial migration. In similar mAb blocking experiments we have ruled-out fractalkine, a membrane-bound chemoattractant, uPAR, a coreceptor for CD18 [26], and β3 integrins (not shown).
DISCUSSION
PMN feature prominently in the cellular infiltrate, including in the lumen, in various intestinal inflammatory diseases but how the cells get through the epithelium is incompletely understood. Here we contribute to this understanding with the discovery of CD18-(β2 integrin) independent transepithelial migration. That multiple chemoattractants support CD18-independent migration implies this mechanism may be widely employed. Indeed it remains possible that critical CD18-independent adhesion events even follow PMN CD11b/CD18 engagement of the T84 cells migrating in the presence of fMLP. Using C5a we further demonstrate that the CD18-independent mechanisms apply to migration in the physiological, basolateral to apical direction only. The most likely explanation is that the epithelial ligand(s) that support CD18-independent migration are not available on the apical surface of the T84 cells. This is consistent with the results of Meenan et al. [19] who showed CD18-independent PMN adhesion only to recently (within 18 h) passaged HT29 colon carcinoma cells, presumably because basolateral molecules were still exposed. As for the CD11b/CD18-dependent ligand on the T84 apical surface, sulphated fucose moieties on T84 reportedly bind to CD11b/CD18 [27] and these proteoglycans are likely abundantly expressed in the apical brushborder.
Despite evidence that intestinal epithelial cells are a potent source of multiple chemokines [28] and the abundance of other PMN chemoattractants, precisely which molecules draw PMN into the lumen has not yet been determined. IL-8 [29] and LTB4[30], both of which support CD18-independent migration, have been detected in lumen dialysates recovered from humans. McCormick et al. [14] described a PMN chemoattractant secreted apically following infection of T84 with S. typhimurium; it is not known whether this factor mediates CD18-independent migration. In rabbits with shigellosis, in which PMN migrate into the intestinal lumen, animals treated with blocking IL-8 mAb showed reduced numbers of PMN infiltrating the lamina propria and none in the epithelium, inferring that IL-8 was recruiting cells across the epithelium [31]. A nonintestinal example of chemokine-dependent PMN transepithelial migration used a model murine urinary tract infection. In those studies PMN reached but failed to cross the uroepithelium in anti-MIP-2 treated [32] or CXCR1 gene knockout mice [33]. We interpret all this evidence to suggest that PMN recruitment is likely a product of the epithelial cell response, rather than leakage of bacterial products across the epithelium. In regards to the relevance of C5a to migration into the lumen, intestinal epithelial cells make and secrete complement components C3 and C4 apically but C5 has not been measured [12,34]. Complement activation has been reported on the intestinal epithelial surface of IBD patients [12,13] and combined with the fact that epithelial cells express decay accelerating factor [35], split complement components could establish a gradient from the lumen, a hypothesis possibly testable using gene knockout animal models.
The inflamed bowel likely contains multiple chemoattractants, for example different molecules may recruit cells out of the blood versus across the epithelium, and thus it is important to understand how multiple stimuli may affect PMN migration. Others had shown a hierarchy in the potency of chemoattractants to ‘desensitize’ PMN during transendothelial migration to a second chemoattractant [24] but whether CD18-independent migration was also affected was not examined. In our experiments PMN were exposed to both C5a and fMLP and while CD18-independent migration was reduced it remained significantly greater than in the presence of fMLP alone (Fig. 5a). This was likewise the case using a combination of IL-8, LTB4 and fMLP, indicating that the CD18-independent mechanisms are preserved. Thus even if bacterial products such as fMLP were to mediate some transepithelial migration the CD18-independent mechanisms are likely to remain active.
Finally, it is important to identify the molecules involved in the CD18-independent migration, not only to consider these as a means of inhibiting migration but also because of collateral activities such molecules may have in the inflammatory response. In identifying these adhesion events it is noteworthy that the lessons from transendothelial migration have provided only limited insights into transepithelial migration, presumably because the two cell types have quite different surface molecules. Another limitation is the lack of availability of normal human intestinal epithelial cells for these studies and most information on transepithelial migration is derived using T84 cells. For example, while ICAM-1 is the principal ligand for CD11b/CD18 during transendothelial migration it was ruled-out as an adhesion molecule in migration across T84 cells [36]. In view of the apparent discord between T84 versus endothelial adhesion mechanisms and the basolateral bias of the CD18-independent mechanism we chose to first test whether E-cadherin was involved as there is a precedent for this molecule to bind to leucocytes [25]. However our results indicate this is not the case; neither blocking homophilic interactions, the use of an E-cadherin antagonist, nor blocking binding to αEβ7 prevented PMN migration in response to C5a. We subsequently also ruled-out epithelial membrane-bound fractalkine [37] and PMN uPAR, a coreceptor for Mac-1-dependent adhesion [26]. We also determined that the β3 integrins are not involved although this integrin has been implicated in PMN locomotion in other systems. PMN expression of β1 integrins is increased after transendothelial migration [38] and presumably this would be relevant to cells arriving at the epithelium. In our early experiments we have observed up to a 25% reduction in migration to C5a using a mAb to β1 integrin but only in the presence of anti-CD18 mAb. This result needs to be confirmed with additional antibody clones. Clearly more investigation is required to identify the ligands supporting CD18-independent migration. Meanwhile our new evidence for CD18-independent PMN migration across intestinal epithelium will need to be considered in any strategy to block PMN migration to reduce inflammation.
Acknowledgments
This study was supported by grants from the Crohn's and Colitis Foundation of Canada and the Natural Sciences and Engineering Research Council of Canada (to AWS) and grant MOP-7684 from the Canadian Institutes of Health Research (to ACI). K. Blake was supported by a scholarship from the Dalhousie Inflammation Group and S. Carrigan by the Robbie Thompson Scholarship of the IWK Health Centre.
REFERENCES
- 1.Saverymuttu SH, Camilleri M, Rees H, Lavender JP, Hodgson HJF, Chadwick VS. Indium 111-granulocyte scanning in the assessment of disease extent and disease activity in inflammatory bowel disease. a comparison with colonoscopy, histology and fecal indium 111-granulocyte excretion. Gastroenterology. 1986;90:1121–8. doi: 10.1016/0016-5085(86)90376-8. [DOI] [PubMed] [Google Scholar]
- 2.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–76. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 3.Kubes P, McCafferty DM. Nitric oxide and intestinal inflammation. Am J Med. 2000;109:150–8. doi: 10.1016/s0002-9343(00)00480-0. [DOI] [PubMed] [Google Scholar]
- 4.Perdomo JJ, Cavaillon J-M, Huerre M, Ohayon H, Sansonetti PJ. Acute inflammation causes epithelial invasion and mucosal destruction in experimental Shigellosis. J Exp Med. 2001;180:1307–19. doi: 10.1084/jem.180.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nusrat A, Parkos CA, Liang TW, Carnes DK, Madara JL. Neutrophil migration across model intestinal epithelia: Monolayer disruption and subsequent events in epithelial repair. Gastroenterology. 1997;113:1489–500. doi: 10.1053/gast.1997.v113.pm9352851. [DOI] [PubMed] [Google Scholar]
- 6.Higa A, McKnight GW, Wallace JL. Attenuation of epithelial injury in acute experimental colitis by immunomodulators. Eur J Pharmacol. 1993;239:171–6. doi: 10.1016/0014-2999(93)90990-y. [DOI] [PubMed] [Google Scholar]
- 7.Stadnyk AW, Dollard CD, Issekutz AC. Neutrophil migration stimulates epithelial cell cytokines during T. spiralis infection of the rat. J Leukoc Biol. 2000;68:821–7. [PubMed] [Google Scholar]
- 8.Le’Negrate G, Selva E, Auberger P, Rossi B, Hofman P. Sustained polymorphonuclear leukocyte transmigration induces apoptosis in T84 intestinal epithelial cells. J Cell Biol. 2000;150:1479–88. doi: 10.1083/jcb.150.6.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madara JL, Parkos C, Colgan SP, et al. Cl– secretion in a model intestinal epithelium induced by a neutrophil-derived secretagogue. J Clin Invest. 1992;89:1938–44. doi: 10.1172/JCI115800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keshavarzian A, Fusunyan RD, Jacyno R, Winship D, MacDermott RP, Sanderson IR. Increased interleukin-8 (IL-8) in rectal dialysate from patients with ulcerative colitis: Evidence for a biological role for IL-8 in inflammation of the colon. Am J Gastroenterol. 1999;94:704–12. doi: 10.1111/j.1572-0241.1999.00940.x. [DOI] [PubMed] [Google Scholar]
- 11.Hommes DW, Meenan J, de Haas M, et al. Soluble Fc gamma receptor III (CD16) and ecosanoid concentrations in gut lavage fluid from patients with inflammatory bowel disease: reflection of mucosal inflammation. Gut. 1996;38:564–7. doi: 10.1136/gut.38.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laufer J, Oren R, Goldberg I, et al. Cellular localization of complement C3 and C4 transcripts in intestinal specimens from patients with Crohn's disease. Clin Exp Immunol. 2000;120:30–7. doi: 10.1046/j.1365-2249.2000.01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halstensen TS, Mollnes TE, Garred P, Fausa O, Brandtzaeg P. Surface epithelium related activation of complement differs in Crohn's disease and ulcerative colitis. Gut. 1992;33:902–8. doi: 10.1136/gut.33.7.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCormick BA, Parkos CA, Colgan SP, Carnes DK, Madara JL. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J Immunol. 1998;160:455–66. [PubMed] [Google Scholar]
- 15.Parkos CA, Delp C, Arnaout MA, Madara JL. Neutrophil migration across a cultured intestinal epithelium. Dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. J Clin Invest. 1991;88:1605–12. doi: 10.1172/JCI115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parkos CA, Colgan SP, Bacarra AE, et al. Intestinal epithelia (T84) possess basolateral ligands for CD11b/CD18-mediated neutrophil adherence. Am J Physiol. 1995;268:C472–C479. doi: 10.1152/ajpcell.1995.268.2.C472. [DOI] [PubMed] [Google Scholar]
- 17.Overdahl MC, Julian MW, Weisbrode SE, Dorinsky PM. Anti-CD18 antibody does not block ileal injury induced by phorbol myristate acetate. Am J Respir Crit Care Med. 1995;152:1331–6. doi: 10.1164/ajrccm.152.4.7551391. [DOI] [PubMed] [Google Scholar]
- 18.Hill J, Lindsay T, Rusche J, Valeri CR, Shepro D, Hechtman HB. A Mac-1 antibody reduces liver and lung injury but not neutrophil sequestration after intestinal ischemia-reperfusion. Surgery. 1992;112:166–72. [PubMed] [Google Scholar]
- 19.Meenan J, Mevissen M, Monajemi H, et al. Mechanisms underlying neutrophil adhesion to apical epithelial membranes. Gut. 1996;38:201–5. doi: 10.1136/gut.38.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shang XZ, Issekutz AC. β2 (CD18) and β1 (CD29) integrin mechanisms in migration of human polymorphonuclear leucocytes and monocytes through lung fibroblast barriers: shared and distinct mechanisms. Immunology. 1997;92:527–35. doi: 10.1046/j.1365-2567.1997.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beatty P, Ledbetter JA, Martin PJ, Price TH, Hansen JA. Definition of a common leukocyte cell-surface antigen (GP95-150) associated with diverse cell-mediated immune functions. J Immunol. 1983;131:2913–9. [PubMed] [Google Scholar]
- 22.Higgins JMG, Mandlebrot DA, Shaw SK, et al. Direct and regulated interaction of integrin αEβ7 with E-cadherin. J Cell Biol. 1998;140:197–210. doi: 10.1083/jcb.140.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nash S, Stafford J, Madara JL. Effects of polymorphonuclear leukocyte transmigration on the barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1987;80:1113. doi: 10.1172/JCI113167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitayama J, Carr MW, Roth SJ, Buccola J, Springer TA. Contrasting responses to multiple chemotactic stimuli in transendothelial migration. Heterologous desensitization in neutrophils and augmentation of migration in eosinophils. J Immunol. 1997;158:2340–9. [PubMed] [Google Scholar]
- 25.Cepek KL, Shaw SK, Parker CM, et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the αEβ7 integrin. Nature. 1994;372:190–3. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 26.Ross GD. Regulation of the adhesion versus cytotoxic functions of the Mac-1/CR3/alphaMbeta2-integrin glycoprotein. Crit Rev Immunol. 2000;20:197–222. [PubMed] [Google Scholar]
- 27.Zen K, Liu Y, Cairo D, Parkos CA. CD11b/CD18–dependent interactions of neutrophils with intestinal epithelium are mediated by fucosylated proteoglycans. J Immunol. 2002;169:5270–8. doi: 10.4049/jimmunol.169.9.5270. [DOI] [PubMed] [Google Scholar]
- 28.Stadnyk AW. Intestinal epithelial cells as a source of inflammatory cytokines and chemokines. Can J Gastroenterol. 2002;16:241–6. doi: 10.1155/2002/941087. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen OH, Gionchetti P, Ainsworth M, et al. Rectal dialysate and fecal concentrations of neutrophil gelatinase-associated lipocalin, interleukin-8, and tumor necrosis factor-α in ulcerative colitis. Am J Gastroenterol. 1999;94:2923–8. doi: 10.1111/j.1572-0241.1999.01439.x. [DOI] [PubMed] [Google Scholar]
- 30.Lauisten K, Laursen LS, Bukhave K, Rask-Madsen J. Effects of topical 5-aminosalicylic acid and prednisolone on prostaglandin E2 and leukotriene B4 levels determined by equilibrium in vivo dialysis of rectum in relapsing ulcerative colitis. Gastroenterology. 1986;91:837–44. doi: 10.1016/0016-5085(86)90684-0. [DOI] [PubMed] [Google Scholar]
- 31.Sansonetti PJ, Arondel J, Huerre M, Harada A, Matsushima K. Interleukin-8 controls bacterial transepithelial translocation at the cost of epithelial destruction in experimental shigellosis. Infect Immun. 1999;67:1471–80. doi: 10.1128/iai.67.3.1471-1480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hang L, Haraoka M, Agace WW, et al. Macrophage inflammatory protein-2 is required for neutrophil passage across the epithelial barrier of the infected urinary tract. J Immunol. 1999;162:3037–44. [PubMed] [Google Scholar]
- 33.Godaly G, Hang L, Frendéus B, Svanborg C. Transepithelial neutrophil migration is CXCR1 dependent in vitro and is defective in IL-8 receptor knockout mice. J Immunol. 2000;165:5287–94. doi: 10.4049/jimmunol.165.9.5287. [DOI] [PubMed] [Google Scholar]
- 34.Ahrenstedt O, Knutson L, Nilsson B, Nilsson-Ekdahl K, Odlind B, Hallgren R. Enhanced local production of complement components in the small intestines of patients with Crohn's disease. N Engl J Med. 1990;322:1345–9. doi: 10.1056/NEJM199005103221903. [DOI] [PubMed] [Google Scholar]
- 35.Andoh A, Fujiyama Y, Sumiyoshi K-I, Sakumoto H, Bamba T. Interleukin 4 acts as an inducer of Decay-Accelerating Factor gene expression in human intestinal epithelial cells. Gastroenterology. 1996;111:911–8. doi: 10.1016/s0016-5085(96)70058-6. [DOI] [PubMed] [Google Scholar]
- 36.Parkos CA. Cell adhesion and migration. 1. Neutrophil adhesive interactions with intestinal epithelium. Am J Physiol. 1997;273:G763–G768. doi: 10.1152/ajpgi.1997.273.4.G763. [DOI] [PubMed] [Google Scholar]
- 37.Lucas AD, Chadwick N, Warren BF, et al. The transmembrane form of the CX3CL1 chemokine fractalkine is expressed predominantly by epithelial cells in vivo. Am J Pathol. 2002;158:855–66. doi: 10.1016/S0002-9440(10)64034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kubes P, Niu X-F, Smith CW, Kehrli ME, Reinhardt PH, Woodman RC. A novel β1-dependent adhesion pathway on neutrophils: a mechanism invoked by dehydrocytochalasin B or endothelial transmigration. FASEB J. 1995;9:1103–11. [PubMed] [Google Scholar]