Abstract
Intestinal epithelial cell (IEC)-derived cytokines, such as stem cell factor (SCF), interleukin (IL)-7 and IL-15 are known to be required for the development of intestinal intraepithelial lymphocytes (IELs). A newly described cytokine, IL-18, has also been shown to be produced by intestinal epithelial cells. To demonstrate the functional effects of IL-18 on human IELs, we assessed IL-18/IL-18 receptor expression in IEC/IEL and proliferation following stimulation of intestinal IELs by IL-18. IL-18 transcripts were detected both in freshly isolated human colonic epithelial cells and in various colonic epithelial cell lines. IL-18 protein was also detected by ELISA and flow cytometric analysis using antihuman IL-18-specific monoclonal antibody (MoAb). Furthermore, IELs constitutively expressed the IL-18 receptor in addition to the IL-2 and IL-7 receptors. More importantly, IL-18 augmented significant proliferative responses of IEL in combination with IL-2, IL-7 and IL-15 both in the presence and in absence of anti-CD3 MoAb. These results suggest that IL-18 might play a crucial role in the proliferation and maintenance of intestinal IELs.
Keywords: human intestinal epithelial cell, IL-18, intraepithelial lymphocytes, proliferation
INTRODUCTION
Unique T cells, the intestinal intraepithelial lymphocytes (IELs), are interspersed between the epithelial cells of both the small and large intestines[1–3]. IELs have higher numbers of T cell receptors (TCR)γδ+ T cells than are found in murine or human circulation. Several lines of evidence suggest that TCRγδ+ IELs exclusively express CD8αα homodimers and that a significant fraction of TCRγδ+ IELs develop extrathymically [4].
IELs are located at the basolateral side of the epithelial layer of the intestines where they are likely to be exposed to a diverse range of food and bacterial antigens. Given the immense surface area of epithelia and that there is an average of 10–20 IELs per 100 villus enterocytes in the small intestines in humans, resident IELs can comprise a substantial fraction of the total T cells [4]. Accordingly, IELs represent the important first line of defence against external environmental challenges; they may monitor intestinal enterocytes and respond to non-polymorphic molecules such as CD1d expressed at the surface of epithelial cells [5,6]. Although a great deal has been learned about the phenotypic characteristics of these lymphocytes, their exact immunological role remains to be understood.
Interleukin-18 (IL-18) is a multi-functional cytokine with structural similarities to the IL-1 cytokine family [7]. The receptor for IL-18 was characterized and identified as an IL-1 receptor-related protein (IL-1Rrp) with an unknown ligand [8]. IL-18 production has been detected in diverse type of cells including activated macrophages, keratinocytes, osteoblasts and airway epithelial cells [9]. In addition, pro- (immature) and mature forms of IL-18 are also present in the colonic mucosa [10,11]. Interestingly, highly elevated protein or mRNA levels of IL-18 have been detected in lamina propria mononuclear cells [10, 12, 13] and also in colonic epithelial cells from Crohn's disease patients [10,11], one of the major inflammatory bowel diseases in humans. However, the physiological role of epithelial cell-derived IL-18 remains unclear.
To investigate the role of intestinal epithelial cell-derived IL-18 in the development of IELs, we examine the proliferation of IL-18 to IELs in humans and assess the synergistic effects of IL-2, IL-7, IL-15 and stem cell factor (SCF) with IL-18.
MATERIALS AND METHODS
Cell lines
We used the following human cell lines from the American Type Culture Collection (ATCC; Rockville, MD, USA): HT29, Caco-2, ACM, colo 205, THP-1 and Jurkat. The cell cultures were maintained in Dulbecco's modified Eagle medium (DMEM) medium supplemented with 10% heat-inactivated fetal bovine serum at 37°C in the presence of 5% CO2.
Reagents, cytokines and monoclonal antibodies (MoAbs)
TNBS (2, 4, 6-trinitrobenzene sulphonic acid) was obtained from Wako Chemical Co. (Tokyo, Japan), brefeldin A, phorbol 12-myristate 13-acetate (PMA), Ca ionophore (A23187), lipopolysaccharide (LPS), phenylmethylsulphonyl fluoride (PMSF), leupeptin, pepstatin, aprotinin and saponin were purchased from Sigma Chemical Co. (St Louis, MO, USA). Recombinant human IL-18 was purchased from MBL Co. Ltd (Nagoya, Japan). Recombinant human interferon-γ (IFN-γ) and recombinant human IL-2 were kindly provided by Shionogi Pharmaceutical Co. (Osaka, Japan). Recombinant human IL-7 was kindly provided by Dr Sudo (Toray Industries, Inc., Tokyo, Japan). Recombinant human IL-12, IL-15 and SCF were purchased from Pepro Tech, Inc. (Rocky Hill, NJ, USA). Fluorescein isothiocyanate (FITC)-conjugated goat polyclonal antimouse IgG and FITC-conjugated goat polyclonal antimouse IgM were purchased from Tago (Burlingame, CA, USA). Isotype-matched mouse IgG1 MoAb (107·3), isotype-matched mouse IgM MoAb (G155-228), purified and phycoerythrin (PE)-conjugated antihuman CD3 MoAb (UCHT1, mouse IgG1), PE-conjugated mouse antihuman IL-2 receptor (M-A251, mouse IgG1) and mouse antihuman IL-12 MoAb (mouse IgG1, G161-566) were purchased from BD-Pharmingen (San Diego, CA, USA). PE-conjugated mouse antihuman IL-7 receptor (M29, mouse IgG1) was purchased from Genzyme (Cambridge, MA, USA). Mouse antihuman IL-18 MoAb (mouse IgG1, no. 125–2H) and PE-conjugated mouse antihuman IL-18 receptor (mouse IgM, 117–10C) were prepared at Hayashibara Biochemical Laboratories (Okayama, Japan) [8].
Samples
All experiments were approved by the Keio University Hospital Committee on Human Subjects, Tokyo, Japan. After obtaining informed consent, mucosal biopsies of 2–3 mm in size were obtained from unaffected areas of 17 colon and ileal specimens from colon cancer patients during surgery. Macroscopic and microscopic histopathological examination revealed no malignancy or inflammation. The mucosa was prepared immediately after stripping from the underlying submucosa by blunt dissection.
Active IL-18 enzyme-linked immunosorbent assay (ELISA)
Concentrations of IL-18 in cell lysates and supernatants were determined using ELISA specific for the active form of human IL-18 as described previously [14]. Briefly, HT-29 cells were cultured in six-well plates in DMEM supplemented with 10% (v/v) fetal bovine serum, 100 U/ml penicillin and 100 U/ml streptomycin. After confluence, fresh medium with the presence or absence of stimuli [LPS (10 µg/ml), Ca ionophore (100 ng/ml) + PMA (10 ng/ml) and TNBS (0·1 mm)] was added. After culture for an additional 48 h, supernatants and cells were harvested. Preliminary experiments demonstrated that the various treatments did not influence cell viability or cell number. Cells were washed three times with phosphate buffered saline (PBS), and lysates were prepared by resuspending the cells in 500 µl lysis buffer [50 mm Tris-HCl (pH 7·6), 150 mm NaCl, 0·5% Triton X-100, 1 mm PMSF, 10 mm iodoacetamide, 10 µg/ml leupeptin, 10 µg/ml pepstatin and 10 µg/ml aprotinin]. After incubation on ice for 30 min, the lysates were centrifuged at 15 000 g for 20 min. Protein concentrations of the resulting supernatant were adjusted to 1 mg/ml following protein assay by the Bradford method (Protein Assay Kit; Sigma). The presence of human active IL-18 was detected by two-site sandwich ELISA as described previously. In brief, a maxisorp plate was coated with MoAb 125–2H (20 µg/ml in PBS) at room temperature (RT) for 3 h and blocked with PBS containing 1% BSA at 4°C overnight. After the wells were washed with 250 µl of washing buffer (PBS containing 0·05% Tween 20), 50 µl of assay buffer (PBS containing 1% BSA, 5% fteal calf serum (FCS) and 1 m NaCl) was dispensed to each well followed by 50 µl of sample or standard human IL-18. The plate was shaken on a microplate mixer throughout the 2 h incubation at RT. After washing three times, 100 µl of PBS containing 1% BSA, 5% FCS, 0·1% CHAPS (Dojin Chemicals, Tokyo, Japan), 0·3 m NaCl, and supplemented 0·5 µg/ml with peroxidase-conjugated no. 159–12B was added to each well and the plate was incubated at RT for 2 h. The wells were washed three times and 100 µl substrate solution (0·1 m sodium phosphate–citrate buffer containing 0·5 mg/ml o-phenylene diamine and 0·003% H2O2, pH 5·0) was added. The reaction was stopped with 100 µl of 2 N H2SO4 and the absorbance at 490 nm (reference at 650 nm) was measured. The assay was generally performed in duplicate.
Immunohistochemistry
Tissue sections were stained by a well-described method [12]. Intestinal specimens were placed in ice-cold calcium and magnesium-free PBS (CMF = PBS; Gibco-BRL, Gaithersburg, MD, USA) at the time of surgery or colonoscopy examination, and transported immediately to the laboratory. They were then orientated, embedded in an optimal cutting temparature compound, snap-frozen and stored at −80°C until staining. Indirect staining with antihuman IL-18 MoAb was performed using an avidin–biotin–peroxidase technique (ABC-Vectastain Kit; Vector Laboratories, Inc., Burlingame, CA).
Preparation of mucosal intraepithelial lymphocytes and epithelial cells
IELs and epithelial cells were isolated from surgically resected intestinal specimens using previously described enzymatic techniques [15]. Briefly, the dissected mucosa was incubated in calcium and magnesium-free Hank's buffered saline solution containing 2·5% fetal bovine serum and 1 mm dithiothreitol (Sigma) to remove mucus. The mucosa was then incubated in 0·75 mm EDTA (Sigma) for 60 min at 37°C to release IELs and epithelial cells from the tissue. The IEL fraction was pelleted twice using a 40% isotonic Percoll solution and a Ficoll-Hypaque density gradient. The CD3+ cells of the resulting IELs cell preparations were confirmed to be 32–76%α/β T cells and 21–56%γ/δ T cells by flow cytometry analysis. Cell preparations were adequately pure, and were used for further experiments immediately or after culture in DMEM medium.
Flow cytometry
Flow cytometry with two-colour analysis was carried out as described previously [12]. For staining, freshly isolated IELs expressing the IL-18 receptor (R)α, IL-2Rα and IL-7Rα, 1 × 106 cells were incubated with 20 µl of 1 µg/ml PE-conjugated antihuman IL-18R MoAb, antihuman IL-2R MoAb or antihuman IL-7R MoAb, or PE-conjugated isomatched mouse IgG or IgM MoAb and FITC-conjugated goat antihuman αβ or γδ MoAb for 30 min on ice. After washing three times with PBS, the fluorescence intensity on the surface of the cells was analysed with a FACScan (Becton Dickinson, Mountain View, CA, USA).
To investigate IL-18 at the protein level, HT-29 cells (2 × 106/ml) were cultured in 24-well plates for 24 h at 37°C under 5% CO2 in the presence of 10 µg/ml brefeldin A. Cytoplasmic IL-18 was stained and analysed using a modified procedure [16]. Briefly, cells were fixed in 4% paraformaldehyde in Dulbecco's phosphate-buffered saline for 20 min at RT and permeabilized with PBS containing 0·1% saponin and 10% human pooled serum for 10 min. All subsequent washing and incubation steps were carried out with PBS containing 0·1% saponin and 0·5% BSA. Cytoplasm was stained with antihuman IL-18 MoAb (5 µg/ml, 30 min) or with antihuman IL-12 MoAb (5 µg/ml, 30 min) as a control. After washing and incubating with FITC-conjugated goat antimouse IgG, the cells were suspended at 1 × 106 cells/ml and the fluorescence intensity on the surface of the cells was analysed with a FACScan.
Reverse-transcription polymerase chain reaction (RT-PCR) assay
RT-PCR for IL-18 and IL-1β-converting enzyme (ICE, also termed caspase 1) was performed as previously described [14]. Total RNA was extracted from isolated colonic epithelial cells and human colonic cell lines and reverse transcribed using RNA zol (Cinna/Biotech Laboratory, Houston, TX, USA) and 100 ng of total RNA in the presence of RAV 2 reverse transcriptase (BRL, Bethesda, MD, USA). Total RNA (4 ng/ml) was prepared for cDNA synthesis by heating to 65°C for 5 min and cooling rapidly. Reverse transcription reactions of 1 µl of 10× PCR buffer (500 mm KCl; 100 mm Tris-HCl buffer, pH 8·4; 15 mm MgCl2; and 0·01% gelatin), 1 µl of 25 mm dNTPs (Takara, Kyoto, Japan), 1 µl of 10× hexanucleotide mixture (Boehringer Mannheim, Mannheim, Germany), 1 µl of 100 mm dithiothreitol (Boehringer Mannheim), 20 U of ribonuclease inhibitor (TaKaRa) and 3 U of RNA-2 reverse transcriptase, along with 5 µl of heat-treated total cellular RNA were incubated at 42°C for 60 min, heated at 94°C for 5 min, and quick-chilled on ice. The cDNAs of human IL-18, ICE and G3PDH were amplified using equal amounts of cDNA (10 µl), 5 µl of 10× PCR buffer, 8 µl of 1·25 mm dNTPs, 207 µl of dH20, 5 µl of 20 pm 5′-and 3′ primers and 1·5 U of Taq polymerase (Perkin Elmer Cetus, Norfolk, CT, USA) with 40 cycles of amplifications at 94°C for 1 min, 60°C for 2 min and 72°C for 3 min. The following forward and reverse primers, synthesized by the phosphoramine method using a DNA synthesizer (model 392 PCR-MATA; Applied Biosystems, Inc., Foster City, CA, USA), were purchased from Sawady Tech (Tokyo, Japan): human IL-18, 5′ primer, GCT TGA ATC TAA ATT ATC AGT C and 3′ primer, CAA ATT GCA TCT TAT TAT CAT G; human ICE, 5′ primer, TGA TTG ACT CCG TTA TTC CGA and 3′ primer, TCT GCC GAC TTT TGT TTC CAT; G3PDH, 5′ primer, TTA TGA CTC CAG AAG GCA AAT G and 3′ primer, GGC CTG GGA TAT CAG CCA T (Mapping Amplimers, Clonotech). The PCR products were electrophoresed on an agarose gel, photographed, and the negatives were analysed by scanning densitometry.
Proliferation assay
Proliferation assays were performed by culturing purified ileum-derived IELs (5 × 104 per well) in 96-well round microplates for 72 h as described previously [12]. After incubation, cultures were pulsed for 12 h with [3H]-thymidine (0·5 µCi/well) (New England Nuclear, Boston, MA, USA), collected on glass fibre filters, and assessed for radioactivity (in counts per min) in a liquid scintillation system. Recombinant human IL-18 (0–200 ng/ml), IL-2 (100 U/ml), IL-7 (10 ng/ml), IL-12 (10 ng/ml), IL-15 (10 ng/ml) or antihuman CD3 MoAb (5 ng/ml) was added to cultures as a stimulant.
Co-culture assay
Freshly isolated intestinal epithelial cells (2 × 104) and IELs (1 × 105) were co-cultured in 96-well round microplates in the presence or absence of anti-IL-18 MoAb (5 µg/ml or 0·5 µg/ml) and incubated for 72 h. The recovered cells were harvested and counted.
Statistical analysis
Results were expressed as mean ± s.d. Groups of data were compared by Student's t-test. Differences were considered to be statistically significant at P < 0·05.
RESULTS
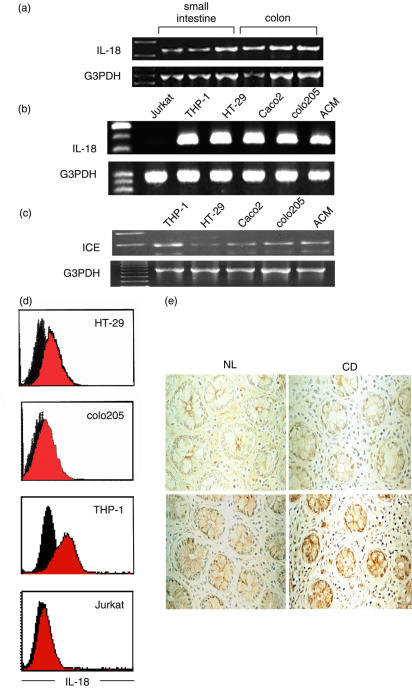
To investigate the possible contribution of IL-18 to the development of IELs in humans, we first quantified the level of IL-18 transcripts in freshly isolated normal human intestinal epithelial cells and various human intestinal epithelial cell lines. RT-PCR products with the predicted size of 335 base pairs (bp) demonstrated IL-18 mRNA expression in freshly isolated human intestinal epithelial cells from normal individuals and various human colonic epithelial cell lines as well as positive control cells, THP-1 cells, but not in negative control cells, Jurkat cells (T cell line) as shown in Fig. 1a and b. To assess whether bioactive IL-18 can be produced in epithelial cells, we next examined the expression of ICE mRNA in colonic epithelial cell lines. As shown in Fig. 1c, all colonic cell lines as well as THP-1 cells expressed ICE, indicating that mature IL-18 can be produced after the cleavage of pro-IL-18 by ICE.
Fig. 1.
IL-18 expression in human intestinal epithelial cells. (a) RT-PCR detection of IL-18 mRNA in freshly isolated human intestinal epithelial cells (small intestine and colon). (b) RT-PCR detection of IL-18 mRNA in human colonic epithelial cell lines (HT-29, Caco2, colo205, and ACM): Jurkat (negative control), THP 1 (positive control). (c) RT-PCR detection of ICE mRNA in human cell lines. All the lanes (a, b and c) are products from single RT-PCR reactions using one representative preparation of cell RNA. The specificity of amplified IL-18 and ICE bands were identified by their predicted size (335 bp and 283 bp, respectively). The results are representative of three similar experiments. (d) Flow cytometric analysis of cytoplasmic IL-18 protein expression in HT-29, colo205, THP-1 and Jurkat cells using intracellular staining method as described in Materials and methods. Cells were stained with anti-IL-18 MoAb (red) or antihuman IL-12 MoAb (black, as a negative control), and FITC-conjugated goat antimouse IgG1. A minimum of 10 000 cells was analysed for each histogram. The results are representative of three similar experiments. (e) Immunohistochemical analysis using anti-IL-18 monoclonal antibody confirmed IL-18 protein expression in human intestinal epithelial cells (left; colon, right; ileum; upper; iso-matched IgG control; lower; anti-IL-18 MoAb). IL-18 was expressed mainly in intestinal epithelial cells and in only a few mononuclear cells in the lamina propria. This figure is representative of samples from four separate subjects (original magnifications: ×200).
Next, IL-18 expression at the post-transcriptional level was analysed in two human colonic cell lines, HT-29 and colo205 cells, using antihuman IL-18-specific MoAb intracellular IL-18 protein staining. As shown in Fig. 1d, both HT-29 and colo205 cells, as well as THP-1 cells, but not Jurkat cells, showed intracellular expression of IL-18 protein. Immunohistochemical analysis using an anti-IL-18 MoAb also confirmed IL-18 protein expression in both human colonic and small intestinal epithelial cells (Fig. 1e).
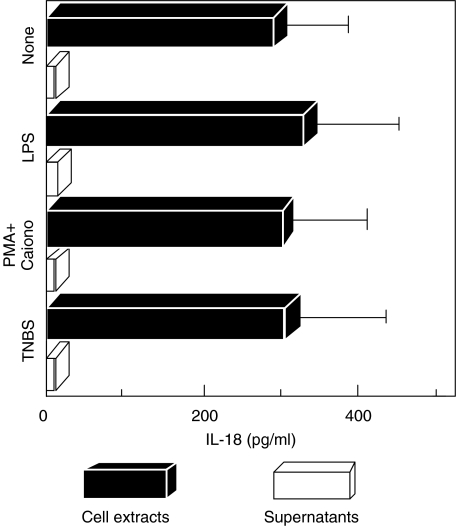
To evaluate IL-18 secretion, the amount of IL-18 in cell extracts and culture supernatants of HT-29 cells was quantified using IL-18-specific ELISA. In our previous study, IL-18 activities measured by ELISA were very well correlated with the appearance of mature IL-18 (p18) and IFN-γ-inducing bioactivity [14]. As shown in Fig. 2, HT-29 cell extracts contained 289·0 ± 91·1 pg/ml IL-18 protein, and the levels did not change with stimulation by LPS (327·3 ± 116·7 pg/ml), PMA + Ca ionophore (298·0 ± 106·9 pg/ml) or TNBS (303·3 ± 124·7 pg/ml). In contrast, IL-18 protein was not detected in the culture supernatant of HT-29 by ELISA, suggesting that IL-18 secretion by epithelial cells should be very low (Fig. 2). In addition, IL-18 protein in the culture supernatants was not increased by TNBS, PMA + Ca ionophore or LPS stimulation. Flow cytometric analysis using intracellular staining showed that cytoplasmic expression of IL-18 was also unaffected by TNBS, PMA or LPS stimulation (Fig. 2).
Fig. 2.
Quantification of IL-18 protein level in cell lysates and culture supernatants of HT-29 by IL-18-specific ELISA assay. HT-29 cells were cultured in the presence or absence of stimuli [LPS (10 µg/ml), Ca ionophore (100 ng/ml) + PMA (10 ng/ml), TNBS (0·1 mm)]. After culturing for 48 h, supernatants and cells were harvested and analysed. Results are averages of three replicates.
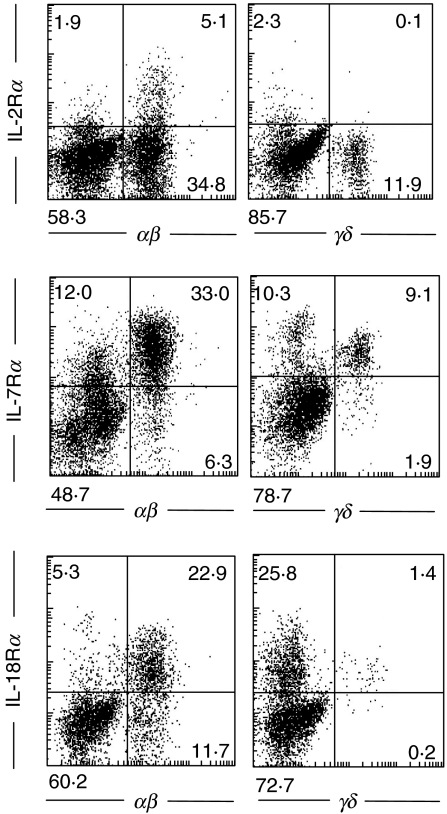
IL-18 production in human intestinal epithelial cells has been confirmed, and we sought to determine whether locally produced IL-18 in the intestinal epithelial cells affects the properties of mucosal lymphocytes, especially IELs. First, quantification of IL-18Rα expression for freshly isolated IELs by flow cytometric analysis demonstrated that both αβ and γδ IELs in the normal intestinal mucosa expressed IL-18Rα(Fig. 3). In addition, both αβ and γδ IELs express both IL-2Rα and IL-7Rα (Fig. 3).
Fig. 3.
Flow-cytometric analysis of IL-2 receptor α, IL-7 receptor α and IL-18 receptor α with freshly isolated IELs. Cells were stained with PE-conjugated anti-IL-2Rα, IL-7Rα or IL-18Rα MoAb and FITC-conjugated antihuman TCRαβ or γδ MoAb. After washing, a minimum of 10 000 cells was analysed by FACScan. The results are representative of three similar experiments.
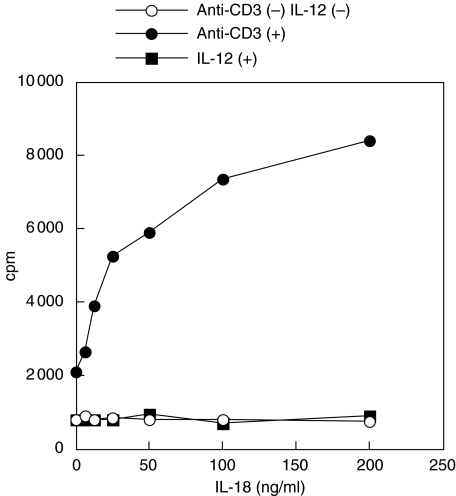
Having confirmed that IL-18 protein production by intestinal epithelial cells and IL-18Rα expression on IELs, the IEL response to IL-18 was tested. The functional activity of the IL-18 receptor on intestinal IELs was assessed using exogenous recombinant IL-18 as a growth factor. As shown in Fig. 4, no proliferation of IELs was observed after stimulation with various concentrations of IL-18 alone (0–200 ng/ml). Previously, we reported that the combined stimulation of IL-12 and IL-18 induced significant proliferation of IELs in intestinal lamina propria lymphocytes from normal subjects [12]. Therefore, we then measured IEL proliferation with combined stimulation of IL-12 and IL-18, and unexpectedly found no significant increases in proliferation in response to IL-12 (10 ng/ml) in the presence of various concentrations of IL-18 (1·0–200 ng/ml) (Fig. 4). IL-12, unlike lamina propria mononuclear T cells, might not be a proliferation factor for IELs [12]. In contrast, IL-18 in the presence of soluble anti-CD3 MoAb (5 ng/ml) induced significant dose-dependent increases in proliferation (Fig. 4).
Fig. 4.
Effect of IL-18 on proliferation of freshly isolated IELs. IEL were cultured for 72 h with various concentrations of recombinant human IL-18 (0–200 ng/ml) in the presence or absence of soluble anti-CD3 MoAb (5 ng/ml) or recombinant human IL-12 (10 ng/ml). Recombinant human IL-18 in the presence of anti-CD3 MoAb stimulated significant increases in DNA synthesis in freshly isolated IELs in a dose-dependent manner. By contrast, various concentrations of IL-18 in combination with IL-12 stimulation did not induce a significant response in proliferation. The results are representative of three similar experiments.
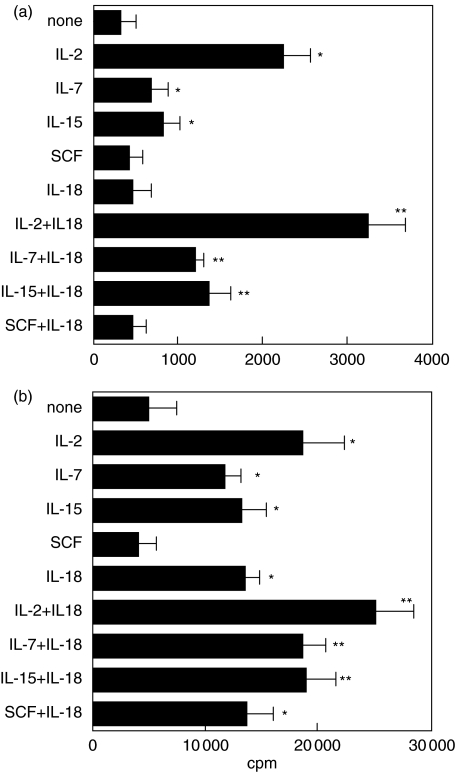
Consistent with previous reports [3], IL-2, IL-7 and IL-15 supplied individually and in the absence of anti-CD3 MoAb induced significant proliferation of IELs compared with no stimuli (Fig. 5a). However, combinations of IL-18 + IL-2, IL-18 + IL-7, and IL-18 + IL-15 in the absence of anti-CD3 MoAb were far more effective in inducing significant proliferative responses of IELs compared with stimuli with IL-2, IL-7 or IL-15 alone (Fig. 5a). In contrast, SCF did not induce any response in the presence or absence of IL-18 (Fig. 5a). The synergistic effects of IL-2 + IL-18, IL-7 + IL-18 and IL-15 + IL-18 were also observed in the presence of anti-CD3 MoAb (Fig. 5b). In addition, the combination of SCF + IL-18, but not SCF alone, induced significant responses in proliferation in the presence of anti-CD3 MoAb (Fig. 5b).
Fig. 5.
Synergistic effects of IL-18 with IL-2, IL-7 or IL-15 in the proliferative responses of IEL were assessed in the absence (a) or presence (b) of 5 ng/ml anti-CD3 MoAb. IL-18 alone did not induce IEL proliferation, but with the combination of stimuli IL-18 with IL-2, IL-7 or IL-15 increased the proliferative responses of IELs compared with the stimulation of IL-2, IL-7 or IL-15 alone. *P < 0·05, treatment versus medium alone (none), **P < 0·05, treatment versus IL-18 alone. The results are representative of three experiments.
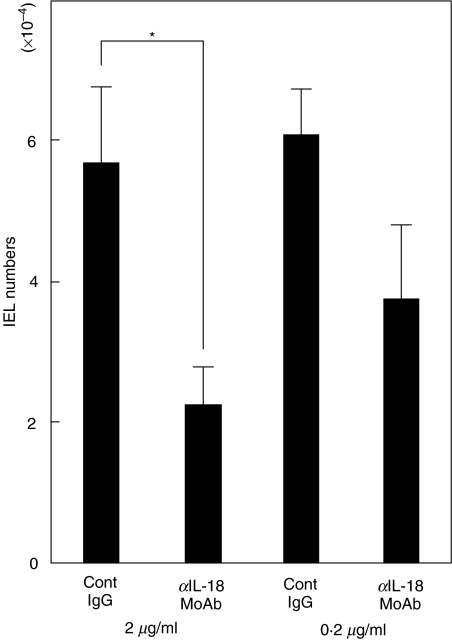
To evaluate directly the role of intestinal epithelial cell-derived IL-18 in the survival of IELs, we cultured both freshly isolated IELs and intestinal epithelial cells both obtained from small intestines in the presence or absence of neutralizing IL-18 MoAb, and counted IEL cell numbers recovered 72 h after the co-culture. As shown in Fig. 6, the recovered cell number of IELs in the presence of anti-IL-18 MoAb (5 µg/ml) was significantly decreased compared to that in control IgG (5 µg/ml).
Fig. 6.
Neutralizing anti-IL-18 MoAb resulted in the significantly decreased IEL cell recovery in co-culture of isolated intestinal epithelial cells and IELs. Freshly isolated intestinal epithelial cells (2 × 104) and IELs (1 × 105) were co-cultured in 96-well round microplates in the presence or absence of anti-IL-18 MoAb (5 µg/ml or 0·5 µg/ml). 72 h after the co-culture, the recovered cells were harvested and counted. The results are representative of three experiments. *P < 0·05.
DISCUSSION
IECs have been shown to produce a wide variety of cytokines, some of which have autocrine function in the maintenance of growth, differentiation and functional status of the epithelium itself. Furthermore, some cytokines, such as IL-7 and IL-15, are involved in regulating the development and propagation of IELs [3,4]. IL-7 may have particular importance in the intestines as an extrathymic regulator of γδ IEL differentiation and growth [17–21], in addition to being a modulator of lamina propria T cell regulation of luminal antigens. Mice lacking IL-7 show severely decreased γδ IEL numbers [22]. γδ IEL are absent in mice lacking the common gamma (cγ) chain, a common component of the receptor for IL-2, IL-4, IL-7, IL-9 and IL-15 [23]. Furthermore, mice lacking Jak3 kinase associated with the γc chain for the cytokines have no γδ IEL [24]. This indicates that the presence of IL-7 is essential for the development of γδ IEL. Recently, Suzuki et al. [25] have also reported that mice lacking the IL-2Rβ chain have low numbers of γδ IELs, suggesting that cytokines using IL-2Rβ as a receptor are also critical in the development of γδ IELs. Inagaki-Ohara et al. have reported that IL-15 also promotes the growth of γδ IEL [26]. Taken together, it is certain that cytokines containing the γc chain and/or the IL-2Rβ chain for signalling, such as IL-2, IL-7 and IL-15, are involved in the proliferation of γδ IELs.
Whereas activated macrophages were the first to be shown to express high levels of IL-18, it is also clearly detectable in murine and human intestinal epithelial cells [11, 27, 28]. However, the role of large amounts of epithelium-derived IL-18 in the mucosa is still unclear. IL-18 was characterized initially as a novel IFN-γ-inducing factor. Previous studies have shown that the IFN-γ receptor is expressed widely [29], and IFN-γ is also expressed in many tissues of fetal mice. Therefore, a factor such as IL-18, capable of inducing IFN-γ production by IELs, may not only enhance the proliferation of IELs, but also have protective antimicrobial effects. However, we must consider that most IL-18 in normal intestinal mucosa would be in the pro-IL-18 form (24 kDa), corresponding to the immature IL-18 [14,30]. Previous studies by Monteleone et al. [10] and Pizarro et al. [11] have been shown that the 18 kDa mature form of IL-18 is more abundant in intestinal mucosal biopsies from inflammatory bowel disease patients but is absent in normal controls. In our previous study, we measured human IL-18 activity by the IL-18-specific ELISA used in this study and compared those activities with the appearance of the p18 molecule by immunoblotting and IFN-γ-inducing assay on KG-1 cells [14]. IL-18 activities measured by our ELISA method were quite well correlated with the appearance of the p18-bioactive IL-18 and IFN-γ-inducing bioactivity. Moreover, p24, p16 and p15 were barely detected by our ELISA method. Therefore, we believe that our ELISA method mainly detects bioactive p18 IL-18. As expected, ICE mRNA were also detected in colonic epithelial cell lines. In addition, the neutralization of anti-IL-18 MoAb resulted in significantly decreased IEL cell recovery in co-culture with isolated intestinal epithelial cells and IELs should support not only the possible existence of bioactive IL-18 in intestinal epithelial cells and the functional contribution for IEL development. Further studies using Western blot analysis are needed to investigate the ratio of pro-IL-18 and bioactive IL-18 in epithelial cells. Interestingly, Salivati et al. reported recently that bioactive IL-18 was increased significantly in mucosal samples from coeliac disease patients [31]. Coeliac disease is unique among small intestinal disorders in that these patients manifest a striking expansion of particularly γδ IELs. This evidence, taken together with the present study, suggests that IL-18 might be involved, at least in part, in IELs development. However, because no evidence that IL-18-deficient mice show defective IEL development has been presented to date, IL-18 may play a less significant role in the development of IELs; alternatively, the requirement of IL-18 for IELs might be different between mice and humans. In particular, our results agree with the finding that IELs are possibly involved in early immune responses occurring independently of TCR engagement, and also suggest that IELs may use IL-18 as a growth factor synergistically with IL-2, IL-7 or IL-15 for their proliferation and maintenance in both innate and acquired immunity.
In conclusion, our present study suggests that IL-18 produced by IECs, in collaboration with other cytokines such as IL-7 and IL-15, may play an important role in the development of IELs.
Acknowledgments
This work was supported in part by grants-in-aid from the Japan Ministry of Education, Culture and Science, the Japan Ministry of Health and Welfare, Chiyoda Mutual Life Foundation, Japan Health Sciences Foundation, Keio University and Keio University Medical Fund. The authors would like to express special thanks to Dr Ryoichi Iiyama for technical assistance.
REFERENCES
- 1.Janeway CA, Jones JB, Hayday A. Specificity and function of T cells bearing γδ receptors. Immunol Today. 1988;9:73–6. doi: 10.1016/0167-5699(88)91267-4. [DOI] [PubMed] [Google Scholar]
- 2.Barrett TA, Gajewski TF, Danielpour D, et al. Differential function of intestinal intraepithelial lymphocyte subsets. J Immunol. 1992;149:1124–30. [PubMed] [Google Scholar]
- 3.Aranda R, Sydora BC, Kronenberg M. Intraepithelial lymphocytes: function. In: Ogara PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, editors. Mucosa immunology. San Diego: Academic Press; 1999. pp. 429–37. [Google Scholar]
- 4.Hayday A, Theodoridis E, Ramsburg E, et al. Intraepithelial lymphocytes: exploring the third way in immunology. Nat Immunol. 2001;2:997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 5.Kerckhove CV, Russell GJ, Deusch K, et al. Oligoclonality of human intestinal intraepithelial T cells. J Exp Med. 1992;175:57–63. doi: 10.1084/jem.175.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balk SP, Ebert EC, Blumenthal RL, et al. Oligoclonal expansion and CD1 recognition by human intestinal intraepithelial lymphocytes. Science. 1991;253:1411–5. doi: 10.1126/science.1716785. [DOI] [PubMed] [Google Scholar]
- 7.Okamura H, Tsutsui H, Komatsu M, et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 8.Torigoe K, Ushio S, Okura T, et al. Purification and characterization of the human interleukin-18 receptor. J Biol Chem. 1997;272:25737–42. doi: 10.1074/jbc.272.41.25737. [DOI] [PubMed] [Google Scholar]
- 9.Dinarello CA. Interleukin-18. Methods. 1999;19:121–32. doi: 10.1006/meth.1999.0837. [DOI] [PubMed] [Google Scholar]
- 10.Monteleone G, Trapasso F, Parello T, et al. Bioactive IL-18 expression is up-regulated in Crohn's disease. J Immunol. 1999;163:143–7. [PubMed] [Google Scholar]
- 11.Pizarro TT, Michie MH, Bentz M, et al. IL-18, a novel immunoloregulatory cytokine, is upregulated in Crohn's disease. J Immunol. 1999;162:6829–35. [PubMed] [Google Scholar]
- 12.Kanai T, Watanabe M, Okazawa A, et al. Interleukin 18 is a potent proliferative factor for intestinal mucosal lymphocytes in Crohn's disease. Gastroenterology. 2000;119:1514–23. doi: 10.1053/gast.2000.20260. [DOI] [PubMed] [Google Scholar]
- 13.Ushio S, Namba M, Okura T, et al. Cloning of the cDNA for human IFN-γ-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J Immunol. 1996;156:4274–9. [PubMed] [Google Scholar]
- 14.Akita K, Ohtsuki T, Nukada Y, et al. Involvement of caspase-1 and caspase-3 in the production and processing of mature human interleukin 18 in monocytic THP.1 cells. J Biol Chem. 1997;42:26595–603. doi: 10.1074/jbc.272.42.26595. [DOI] [PubMed] [Google Scholar]
- 15.Bull DM, Bookman MA. Isolation and functional characterization of intestinal mucosal lymphoid cells. J Clin Invest. 1977;59:966–74. doi: 10.1172/JCI108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanai T, Watanabe M, Okazawa A, et al. Macrophage-derived IL-18-mediated intestinal inflammation in themurine model of Crohn's disease. Gastroenterology. 2001;121:875–88. doi: 10.1053/gast.2001.28021. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe M, Ueno Y, Yajima T, et al. Interleukin-7 is produced by human intestinal epithelial cells and regulates the proliferation of intestinal mucosal lymphocytes. J Clin Invest. 1995;95:2945–53. doi: 10.1172/JCI118002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maki K, Sunaga S, Komagane Y, et al. Interleukin 7 receptor-deficient mice lack gamma delta T cells. Proc Natl Acad Sci USA. 1996;93:7172–7. doi: 10.1073/pnas.93.14.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He YH, Malek TR. Interleukin-7 receptor alpha is essential for the development of gamma delta + T cells, but not natural killer cells. J Exp Med. 1996;184:289–93. doi: 10.1084/jem.184.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas W, Pereira P, Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637–85. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- 21.Klein JR. Advances in intestinal T-cell development and function. Immunol Today. 1995;16:322–4. doi: 10.1016/0167-5699(95)80145-6. [DOI] [PubMed] [Google Scholar]
- 22.Laky K, Lafrancois L, von Freeden-Geffry U, et al. The role of IL-7 in thymic and extrathymic development of TCR gamma delta cells. J Immunol. 1998;161:707–13. [PubMed] [Google Scholar]
- 23.Cao X, Shores EW, Hu-Li J, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–38. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 24.Park SY, Saijo K, Takahashi T, et al. Development defects of lymphoid cells in Jak3 kinase-deficient mice. Immunity. 1995;3:771–82. doi: 10.1016/1074-7613(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki H, Duncan GS, Takimoto H, et al. Abnormal development of intestinal intraepithelial lymphocytes and peripheral natural killer cells in mice lacking the IL-2 receptor beta chain. J Exp Med. 1997;185:499–505. doi: 10.1084/jem.185.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inagaki-Ohara K, Nishimura H, Mitani A, Yoshikai Y. Interleukin-15 preferentially promotes the growth of intestinal intraepithelial lymphocytes bearing gamma delta T cell receptor in mice. Eur J Immunol. 1997;27:2885–91. doi: 10.1002/eji.1830271121. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi M, Nishizaki Y, Sano O, et al. Immunohistochemical and immuno-electron-microscopic detection of interferon-gamma-inducing factor (interleukin-18) in mouse intestinal epithelial cells. Cell Tissue Res. 1997;289:499–503. doi: 10.1007/s004410050895. [DOI] [PubMed] [Google Scholar]
- 28.Siegmund B, Fantuzzi G, Rieder F, et al. Neutralization of interleukin-18 reduces severity in murine colitis and intestinal IFN-γ and TNF-α production. Am J Physiol. 2001;281:1264–73. doi: 10.1152/ajpregu.2001.281.4.R1264. [DOI] [PubMed] [Google Scholar]
- 29.Vallente G, Ozmen L, Novelli F, et al. Distribution of interferon-γ receptor in human tissues. Eur J Immunol. 1992;22:2403–12. doi: 10.1002/eji.1830220933. [DOI] [PubMed] [Google Scholar]
- 30.Gu Y, Kuida K, Tsutsui H, et al. Activation of interferon-γ inducing factor mediated by interleukin-1β converting enzyme. Science. 1997;275:206–9. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 31.Salvati VM, macDonald TT, Bajaj-Elliott M, et al. Interleukin 18 and associated markers of T helper cell type 1 activity in celiac disease. Gut. 2002;50:186–90. doi: 10.1136/gut.50.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]