Abstract
Because abnormalities in redox balance cluster in type I diabetes families and the intracellular thiol redox status seems to modulate immune function, we aimed to investigate the relationship between oxidative stress and immunological features. We measured oxidative markers, serum proinflammatory cytokines, soluble cytokine receptors and subsets of peripheral blood lymphocytes (by varying combinations of CD4, CD8, CD23 or low-affinity IgE receptor, and CD25 or IL-2 receptor) from 38 type I patients, 76 low-risk (i.e. without underlying islet autoimmunity) non-diabetic first-degree relatives of diabetic patients, and 95 healthy subjects. In type I diabetes families, protein and lipid oxidation was confirmed by the presence of reduced sulphhydryl groups, increased advanced oxidation protein products, and increased plasma and erythrocyte malondialdehyde. Relatives had decreased counts of monocytes, of cells co-expressing CD23 and CD25 and of CD25+ cells in peripheral blood. Patients with TIDM had similar defects and, in addition, showed decreased counts of peripheral CD4+CD8+ lymphocytes and increased serum levels of soluble receptors for interleukin (IL)-6 and IL-2. Abnormal indicators of oxidative stress were related in part to immune abnormalities. In the whole study group, we found a correlation (multiple R 0·5, P < 0·001) of CD23+CD25+ cells with blood counts of monocytes, CD4+CD8+ cells, CD25+ cells, basal haemolysis and plasma levels of thiols. In type I diabetics, anti-GAD65 antibody levels were associated (multiple R 0·6, P = 0·01) positively with sIL-6R, negatively with duration of diabetes and CD23+CD25+ counts; plasma creatinine correlated positively (multiple R 0·6, P < 0·001) with both sIL-2R and tumour necrosis factor (TNF)-α concentration. Our study reports the first evidence that the oxidative stress observed in type I families is related to immunological hallmarks (decreased peripheral numbers of monocytes as well as cells bearing a CD4+CD8+, CD23+CD25+ and CD25+ phenotype) from which the involvement of some immunoregulatory mechanisms could be suspected. It remains to be elucidated the course of events culminating in the loss of physiological immune homeostasis and disease pathology.
Keywords: CD23, CD25, cytokines, oxidative stress, type I diabetes families
INTRODUCTION
Immune-mediated type I diabetes mellitus (TIDM) is an organ-specific autoimmune disease characterized by a mononuclear infiltrate within Langerhans islets, immunocyte activation and autoantibody production [1]. Combined analysis of autoantibodies against islet cell antigens may predict overt disease [2]. Furthermore, evidence is accumulating to implicate cytokine-induced free-radical formation in beta cell damage [3]. An abnormal redox status clusters in T1Dm families and even precedes diabetes mellitus [4,5]. Markers of inflammation and oxidative stress are abnormal in non-diabetic relatives of type I diabetics, apart from the presence of soluble markers of autoimmunity and despite seemingly intact antioxidant defences [4]. Therefore, redox imbalance could be ascribed to an overproduction of radicals whose origin remained to be elucidated. The present study aimed to investigate further the eventual contribution of immunological elements in the familial status of oxidative stress.
We measured oxidative markers, proinflammatory cytokines, soluble cytokine receptors and subsets of peripheral blood lymphocytes from low-risk (i.e. without underlying islet autoimmunity), non-diabetic first-degree relatives of patients affected by TIDM, and compared them with age- and sex-matched controls. Lymphocyte subsets were identified by varying combinations of CD4, CD8, CD23 (or low-affinity IgE receptor) and CD25 [or interleukin (IL)-2 receptor]. Low-affinity IgE receptor is expressed on B cells, activated macrophages and follicular dendritic cells (FDC). Ligands for CD23 are IgE, CD11b and CD11c. In turn, CD23 is a ligand for CD19 : CD21 : CD81 B cell co-receptor. CD23 binding may affect not only IgE, IgM, and IgG production by B cells [6] but also the Th1/Th2 balance [7]. IL-2 receptor alpha chain is expressed on stimulated T cells, B cells and monocytes/macrophages. CD25 knock-out mice develop autoimmune disease [8].
MATERIALS AND METHODS
Subjects
The study was carried out in the following groups of subjects:
Thirty-eight TIDM patients with duration of disease ranging from 4 months to 41 years (mean 18 ± 11 years). The group consisted of 19 patients without diabetic complications, 10 patients with retinopathy (background or proliferative, determined by fluorescein angiography following fundus examination) and nine patients with nephropathy. All patients with nephropathy had also diabetic retinopathy. All patients had been treated from the time of diagnosis with at least two daily insulin injections and were now receiving at least four daily insulin injections. None received medical treatment except insulin (0·6 ± 0·2 U/kg) and possibly antihypertensive drugs (seven of the patients with nephropathy were taking drugs including angiotensin-converting enzyme inhibitors, calcium antagonists, and/or vasodilators).
Seventy-six first-degree relatives of TIDM patients, 44 parents and 32 siblings. None of the relatives had clinical evidence of illness or was taking any drugs. If fasting plasma glucose was ≥7 mmol/l, a 75-g oral glucose tolerance test (OGTT) was performed.
Ninety-five healthy subjects were recruited from the local community to achieve a similar distribution of age and sex to the families. They had no family history of TIDM, were tacking no drugs and had no clinical signs or symptoms of illness.
All subjects gave informed consent and the ethical committee of the hospital approved the study proposal.
Collection of blood samples
Fasting venous blood samples and 24-h urine collections were obtained on a single occasion during the same period. Measurements were performed in freshly obtained material immediately after withdrawal, except insulin and autoantibodies that have been measured on samples frozen at −20°C. Sera to determine cytokine and their soluble receptor levels were kept frozen at −80°C until used.
Laboratory methods
A complete blood count profile and differential count was performed on each specimen and the percentage and absolute count of leucocytes, lymphocytes and monocytes in the peripheral blood determined using Celldyn 4000 (Abbott Divisione Diagnostici, Rome, Italy) [9]. Creatinine, glucose, albumin, C-reactive protein (CRP), total cholesterol and triglycerides were measured by Modular Analytics SWA and reagents from Roche Diagnostics (Milan, Italy). HDL cholesterol was measured after precipitation with phosphotungstic acid. HbA1c was evaluated using a Bio-Rad Diamat™ fully automated glycosylated haemoglobin analyser system. Plasma fibrinogen concentration was assayed by Sysmex CA-7000 automated coagulation analyser (Sysmex Corporation, Kobe, Japan). Immunoreactive insulin (IRI) and anti-GAD65 antibodies (GADA) were measured by commercial radioimmunoassay (Medgenix Diagnostics, Fleurus, Belgium, and Anti-GAD, Biochem Immuno Systems, Milan, Italia, respectively). Islet-cell antibodies (ICA) were determined by indirect immunofluorescence with doubling dilutions of serum on frozen sections of pancreas from a blood group 0 donor, and results were calibrated to Juvenile Diabetes Foundation (JDF) units against a standard serum. The lower limit of detection of our ICA was 5 JDF units, with values of 80, 91 and 88% for validity, consistency and specificity according to the 12th International Proficiency Program for the Standardization of ICA [4].
Insulin resistance was estimated by homeostasis model assessment (HOMAIR) [10]. Erythrocyte glutathione (RBC GSH) was determined by the method of Beutler [11]. The peroxidation of plasma (plasma malondialdehyde, MDA) and membrane (RBC MDA) lipids was measured according to Esterbauer and Cheeseman [12]. Haemolysis was assessed as the percentage of haemoglobin released from incubated cells, relative to the total erythrocyte haemoglobin content. Plasma advanced oxidation protein products (AOPP) were measured according to Witko-Sarsat et al. [13] and expressed in chloramine-T equivalents. Plasma sulphydryl groups (SH groups) were measured using the colourimetric assay described by Ellman [14] and fresh plasma. The intra- and interassay coefficients of variation resulted: GSH 2 and 4%, respectively; MDA 6 and 9%; AOPP 1 and 5%; haemolysis (intra-assay only) 2%; SH groups 2 and 7%.
Serum interleukin-1β (IL-1β; minimum detectable dose 0·1 pg/ml, intra-assay CV 6·4–10·2%, interassay CV 8·2–19·2%), IL-6 (0·09, 3·8–11·1, 9·9–16·5) and tumour necrosis factor [(TNF)-α; 0·3 pg/ml, 5·3–8·8, 10·8–16·7] were assayed by the quantitative sandwich enzyme immunoassay technique using an amplification system (Quantikine HS, R&D Systems, Abingdon, UK). Interferon [(IFN)-γ; 8 pg/ml, 2·6–4·7%, 3·7–7·8%] soluble cytokine receptors sIL-2R (10 pg/ml, 4·6–6·1, 6–7·2) and sIL-6R (15·1 pg/ml, 2·3–8·6, 4·2–6·4) were determined by the quantitative sandwich enzyme immunoassay technique (Quantikine, R & D Systems).
Flow cytometric analysis
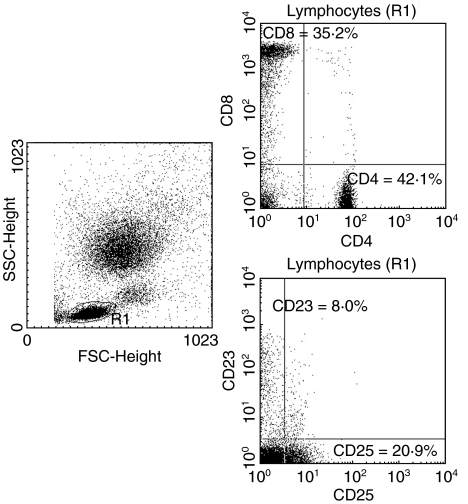
Peripheral blood lymphocytes of all subjects were stained with the following combinations of monoclonal antibodies conjugated with fluorescein isothiocyanate (FITC) and phycoerythrin (PE): CD4 FITC/CD8 PE, CD25 FITC/CD23 PE. The tests were performed according to the manufacturer's instructions (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA). Briefly, 100 µl of peripheral blood were incubated with 20 µl of fluorochrome-conjugated monoclonal antibody reagent for 30 min at 4°C in the dark. Following erythrocyte lysis with FACS Lysing Solution for 10 min at room temperature, cells were washed twice with phosphate-buffered saline and analysed. With appropriate analysis gate, the percentages of lymphocyte subsets were determined using a BD FACScan cytometer (equipped with laser at 488 nm excitation) and BD lysys ii software. The instrument was calibrated for two-colour cytometry before each experiment using BD Calibrite Beads to minimize spectral overlap between fluorochromes and facilitate compensation (coefficients of variation less than 2%). An example of FACS erythrocyte-lysed whole blood (LWB) sample from a patient with TIDM is in Fig. 1.
Fig. 1.
Example of FACS erythrocyte-lysed whole blood (LWB) sample from a patient with type I diabetes. The left panel depicts a forward versus side scatter (FSC/SSC) dot plot of all acquired cells with lymphocyte gating. Right panels depict CD4/CD8 (upper panel) and CD25/CD23 (lower panel) dot plots.
Statistical analysis
The results are presented as mean ± standard deviation or median (haemolysis). The statistical significance of the differences between groups was estimated by the Mann–Whitney U-test in combination with Bonferroni corrections for multiple hypothesis testing (26 paired comparisons). Regression analyses were performed using Spearman's rank correlation. Multiple regression analysis was used following logarithmic transformation of the variables that showed a skewed distribution (Statview Package, Abacus Concepts, Berkeley, CA, USA).
RESULTS
Principal clinical characteristics of the study groups are summarized in Table 1. Of the TIDM patients 12 (31·6%) were ICA positive (30 ± 10 JDFU, range from 5 to > 80), 18 (47·4%) had GADA levels above 1 U/ml (range from 1·3 to 275). Relatives were at low risk for TIDM: one of them (1·3%) was weakly ICA positive (5 JDFU, mother), three (3·9%; two mothers, one father) had GADA levels ranging from 1·1 to 2·4 U/ml. HOMAIR was 2·3 ± 1·6 in relatives versus 2·1 ± 1·2 in control subjects (n.s.). Inflammatory biomarkers, such as levels of fibrinogen and CRP, resulted in the normal range.
Table 1.
Subjects’ characteristics, oxidative markers, serum cytokines and soluble cytokine receptors
| Characteristic | Controls | Type I | P < | Controls | Relatives | P < |
|---|---|---|---|---|---|---|
| F/M | 22/16 | 21/17 | 43/33 | 43/33 | ||
| Age (years) | 38 ± 11 | 37 ± 12 | 48 ± 12 | 50 ± 13 | ||
| BMI (kg/m2) | 24 ± 3 | 25 ± 3 | 24 ± 4 | 26 ± 4 | ||
| MBP (mmHg) | 88 ± 9 | 88 ± 10 | 93 ± 12 | 95 ± 12 | ||
| HbA1c (%) | 5·2 ± 0·4 | 8·4 ± 1·5 | 0·001 | 5·4 ± 0·4 | 5·3 ± 0·7 | |
| GADA (U/ml) | 0·6 ± 0·6 | 40·2 ± 82·6 | 0·01 | 0·5 ± 0·7 | 0·3 ± 0·4 | |
| CRP (mg/l) | 2·8 ± 1·7 | 3·6 ± 2·6 | 3·4 ± 3·1 | 3·2 ± 3·7 | ||
| Fibrinogen (mg/dl) | 301 ± 55 | 311 ± 65 | 315 ± 60 | 311 ± 67 | ||
| AOPP (µmol/l) | 61 ± 21 | 85 ± 38 | 0·001 | 65 ± 32 | 116 ± 69 | 0·001 |
| P SH (µmol/l) | 431 ± 47 | 377 ± 69 | 0·001 | 426 ± 66 | 395 ± 53 | 0·01 |
| RBC GSH (mg/ml) | 0·83 ± 0·13 | 0·73 ± 0·15 | 0·01 | 0·80 ± 0·12 | 0·78 ± 0·14 | |
| Haemolysis (%) | (0·54) | (0·61) | 0·05 | (0·56) | (0·53) | |
| TNF-α (pg/ml) | 2·7 ± 1·8 | 2·8 ± 2·2 | 2·4 ± 1·8 | 2·4 ± 1·6 | ||
| IL-6 (pg/ml) | 3·2 ± 3·5 | 4·9 ± 11·1 | 3·1 ± 2·9 | 3·2 ± 3·0 | ||
| IL-6 sR (ng/ml) | 29·1 ± 7·6 | 33·4 ± 10·6 | 0·05 | 30·7 ± 8·1 | 32·8 ± 9·9 | |
| IL-2 sR (pg/ml) | 935 ± 573 | 1309 ± 604 | 0·01 | 1001 ± 556 | 1168 ± 693 |
Data are mean ± s.d. (or median) and were compared by the Mann–Whitney U-test.
Circulating levels of AOPP were increased, whereas plasma thiols were decreased in patients with TIDM and in first-degree relatives, notwithstanding their low immunological risk as assessed by two islet antibodies. Erythrocyte GSH was lower than normal only in TIDM patients who showed increased erythrocyte fragility as assessed by haemolysis percentage (Table 1). Both P MDA and RBC MDA were increased in TIDM families, as reported previously [4], and data have not been shown in Table 1.
IFN-γ was not detectable in serum of all subjects but one. Circulating levels of IL-1β remained below the minimum detectable concentration in many subjects. It was measured in 21 controls (22·1%; 0·30 ± 0·16 pg/ml), 15 TIDM (39·5%; 0·35 ± 0·21) and 27 relatives (35·5%; 0·31 ± 0·21) without significant differences among groups. Serum IL-6 and TNF-α did not differ in TIDM and relatives in comparison with age- and sex-matched control subjects. Serum soluble receptors of IL-2 and IL-6 were increased in TIDM than in controls (Table 1). In detail, 19 diabetics without known complications differed from 19 age- and sex-matched healthy controls in the higher serum levels of sIL-2 R (1238 ± 406 versus 907 ± 518 pg/ml, P < 0·05). Patients with nephropathy had the highest levels of IL-6 (14 ± 21 pg/ml, P < 0·05) and sIL-2R (1849 ± 845 pg/ml, P < 0·05) in comparison with matched control subjects (IL-6 3·7 ± 4·8, sIL-2R 1024 ± 352) and patients with retinopathy (IL-6 2·1 ± 1·0, sIL-2R 959 ± 312). Levels of sIL-6R, increased in TIDM, did not change as a function of diabetic complications (data not shown).
Monocyte percentage and absolute count were lower both in TIDM and their relatives in comparison with control groups (Table 2). Moreover, lymphocyte phenotypes from patients with TIDM highlighted decreased counts of CD4+CD8+ and CD23+CD25+ cells. A significant decrease in CD25+ and CD23+CD25+ cells was also observed in TIDM relatives versus control subjects (Table 2).
Table 2.
Absolute counts of leucocytes and subsets of peripheral blood lymphocytes
| Characteristic | Controls | Type I | P < | Controls | Relatives | P < |
|---|---|---|---|---|---|---|
| WBC (103/cu mm) | 6·18 ± 1·47 | 6·39 ± 1·50 | 6·07 ± 1·33 | 6·25 ± 1·50 | ||
| N (103/cu mm) | 3·60 ± 1·24 | 3·85 ± 1·33 | 3·53 ± 1·14 | 3·70 ± 1·19 | ||
| L (103/cu mm) | 1·93 ± 0·53 | 1·91 ± 0·55 | 1·92 ± 0·51 | 1·89 ± 0·56 | ||
| M (103/cu mm) | 0·40 ± 0·15 | 0·34 ± 0·12 | 0·05 | 0·42 ± 0·14 | 0·34 ± 0·13 | 0·001 |
| CD4+ (/cu mm) | 810 ± 298 | 857 ± 308 | 847 ± 281 | 829 ± 317 | ||
| CD8+ (/cu mm) | 579 ± 200 | 553 ± 217 | 558 ± 211 | 547 ± 269 | ||
| CD4+ 8+ (/cu mm) | 32 ± 24 | 22 ± 17 | 0·05 | 36 ± 44 | 34 ± 30 | |
| CD4-8- (/cu mm) | 510 ± 195 | 479 ± 166 | 483 ± 169 | 481 ± 141 | ||
| CD23+ (/cu mm) | 139 ± 90 | 141 ± 84 | 140 ± 76 | 128 ± 73 | ||
| CD25+ (/cu mm) | 443 ± 188 | 417 ± 133 | 483 ± 179 | 449 ± 221 | 0·05 | |
| CD23+ 25+ (/cu mm) | 29 ± 21 | 21 ± 16 | 0·05 | 33 ± 33 | 19 ± 13 | 0·001 |
| CD23-25- (/cu mm) | 1324 ± 405 | 1330 ± 138 | 1268 ± 421 | 1297 ± 435 |
Data are mean ± s.d. and were compared by the Mann–Whitney U-test.
In TIDM, GADA levels were associated (multiple R 0·6, P = 0·01) positively with sIL-6R (coefficient 4, t-value 3), negatively with duration of diabetes (− 0·03, −2) and absolute count of CD23+CD25+ cells (− 0·6, −2). Plasma creatinine correlated positively (multiple R 0·6, P < 0·001) with both sIL-2R (0·1, 2) and TNF-α (0·1, 2). In TIDM IL-6 levels correlated with the levels of HbA1c (Rho 0·5, P < 0·01) and plasma fibrinogen (Rho 0·5, P < 0·01).
In the whole study group, we found a positive correlation (multiple R 0·6, P < 0·001) of CD23+CD25+ cells with blood counts of monocytes, CD4+CD8+ cells, CD25+ cells, basal haemolysis and plasma levels of thiols (Table 3).
Table 3.
Multivariate measures of association between CD23+CD25+ counts (dependent variable) versus multiple listed independent variables
| CD23+CD25+ cells versus | Coefficient | Multiple R 0·6 Standard error | P < 0·0001 t-value |
|---|---|---|---|
| P SH | 1·5 | 0·3 | 4·4 |
| Monocytes | 0·6 | 0·2 | 3·6 |
| Haemolysis | 0·3 | 0·1 | 3·2 |
| CD25+ cells | 0·5 | 0·2 | 2·7 |
| CD4+CD8+ cells | 0·2 | 0·1 | 2·7 |
DISCUSSION
This is the first demonstration of leucocyte abnormalities in low-risk TIDM relatives still presenting signs of oxidative stress. Moreover, markers of oxidation and immunological abnormalities seem to be correlated.
First, our data confirm that free radical net balance is shifted to oxidation in members of TIDM families, even in the absence of metabolic or immune risk markers. Recruited relatives represented a status of ‘seemingly’ maintained self-tolerance. These relatives, without underlying autoimmunity or metabolic dysfunction of diabetes, had decreased counts of monocytes, of cells co-expressing CD23 and CD25 and of CD25+ cells in peripheral blood. Patients with TIDM had similar defects, and in addition showed decreased counts of peripheral CD4+CD8+ lymphocytes and increased serum levels of soluble receptors for IL-6 and IL-2.
Previous studies have reported either mild lymphopenia [15] or normal absolute number of total leucocytes, lymphocytes and monocytes [16–18] in the peripheral blood of TIDM families. Conceivably, the size of study groups was too small to reach statistical significance. Differently from Kuwaiti diabetics [15], our type I diabetics and their relatives showed no difference in percentage and absolute count of total, CD4+ or CD8+ lymphocytes.
Concerning CD23 in TIDM, the only antecedent report in literature is from Kretowsky et al. [16]: high-risk TIDM subjects (with a significant depletion release of the first phase of insulin) showed a lower percentage of CD20+CD23+ lymphocytes independently of islet antibodies. The median serum concentration of sCD23 was also lower than in the control group [16]. Differences in the stage of the autoimmune process cannot contribute to contrasting results from Avanzini et al. [19], as speculated [16]. Indeed, the duration of disease in our study covers a very wide range, yet we found no association with CD23 expression.
Monocytes are thought to home to tissues and differentiate into immature dendritic cells [20]. At-risk TIDM relatives with underlying islet autoimmunity but without overt metabolic dysfunction of diabetes had impaired yield, phenotype and function of monocyte-derived dendritic cells generated from peripheral blood [21] as well as a Th1/Th2 imbalance [22,23]. So far, no study has investigated humans at low risk for TIDM but for being first-degree relatives of TIDM patients. The homeostasis of antigen-presenting cells (APC, involving macrophages, dendritic cells and B cells) is regulated by apoptosis in vivo. Both high- and low-affinity IgE receptors have been identified on monocytes and involved in initiating signals that regulate differentiation and/or auto-apoptosis and influence inflammatory reactions [24–26], especially in allergic disorders. Furthermore, the low-affinity receptors for IgE should exert not only a limiting role in the development of allergic sensitization [7], but also opposite regulatory effects on antibody responses [6]. It has also been reported that prototypical models for Th1 cell-mediated autoimmunity could be contributed by Th2 cells [27].
With regard to CD25, a major indispensable role of IL-2 signalling is to limit the number of activated T cells in the periphery after exposure to self- or environmental antigens. The autoimmune phenotype associated with IL-2 deficiency results from the dysregulated activity of thymus-derived CD4+ and/or CD8+T cells [8]. A thymus-derived CD4+CD25+ regulatory T cell subset has been described to be absent in IL-2-deficient mice [8]. Indeed, CD4+CD8+ (thymocyte-like) T lymphocytes in the peripheral blood have been postulated to represent regulatory T cells [28–30]; a high proportion of these peripheral double-positive cells also express CD25. Thus, IL-2 signalling is required for the development of a regulatory T cell subset that serves to terminate antigen-induced responses of TCR αβ T cells. Cells co-expressing CD23 and CD25 could provide a cell contact-mediated suppression signal in the periphery. The negative correlation between CD23+CD25+ cells and GADA levels reinforces this hypothesis. At present, CD23, CD25 and CD45RO are considered to be activation markers of B cells. However, the expression of CD23 also on human T cells has been definitely proven [31,32]. CD23 mRNA was detected in RNA prepared from human peripheral blood T lymphocytes and confirmed by in situ hybridization [33]. Thus, CD23+CD25+ cells could plausibly be a heterogeneous cell population including both B and T cells. Distinct modulation by IFN-γ of CD23 expression on B and T lymphocytes of atopic subjects have been reported [34]. In atopic individuals, the high expression of CD23 on T cells would favour the interaction between T and B cells by means of CD23 expressed on T cells and its receptor CD21 on B cells. This would lead to IgE production. Combined with the report of Pedotti R et al. [27], this observation makes one think that the modulation of CD23 may be involved in the complex regulation of Th1/Th2 interplay.
A limitation of the current study is that the use of two-colour analysis makes the findings regarding the expression of cell markers more inferential than if multicolour techniques were used. However, for consistent characterization of cell heterogeneity, standardized cluster pattern recognition protocols should have been planned (yet results were unknown in advance). Moreover, such an extensive immunophenotyping was beyond the purpose of this study, which was a primary screening of some aspects of immune and redox status in TIDM families.
Secondly, we have found final evidence that abnormal indicators of oxidative stress in TIDM families may be, at least in part, related to immune abnormalities. The inseparable relationship of oxidative stress to inflammation has become well known along with the recognition that reactive oxygen species (ROS) at low levels can function as signalling intermediates in regulation of cell activities, whereas at higher concentrations ROS can cause cell injury and death [35]. Moreover, substantial evidence suggests that the intracellular thiol redox status regulates immune function, in particular intracellular GSH levels in antigen-presenting cells, influence the development of either Th1 or Th2 immunity [36]. Thus, mild oxidative changes in the thiol pool are likely to play a role in the normal immune response under physiological conditions [37,38].
A third outcome of our study was the evaluation of serum levels of cytokines and their soluble receptors in healthy humans and TIDM families. With respect to this topic, literature remains inconclusive [39–42], due probably to multiple factors such as incomparable assay procedures, the complexity and redundancy of cytokine networks, the cytokine nature of acting in an autocrine or paracrine manner and the confounding effect of underlying undiagnosed inflammatory processes. In our experience, IFN-γ and IL-1β were not readily measurable in serum, whereas detectable circulating IL-6 and TNF-α resulted in the normal range both in TIDM probands and in their relatives. High serum concentrations of sIL-2R and sIL-6R could be found only in TIDM. As in type II diabetes, IL-6 levels correlated with the levels of HbA1c, a marker of long-term glucose concentrations, and plasma fibrinogen, a risk factor for atherosclerosis [43].
Soluble cytokine receptors appear in serum concomitant with their increased expression on cells. However, most of the mechanisms controlling the shedding the soluble receptors are largely unknown. In our patients, overexpression of sIL-2R seems to characterize recent-onset TIDM, whereas both sIL-2R and IL-6 were high in long-standing disease with diabetic nephropathy. Cytokines have been implicated previously in idiopathic nephrotic syndrome as vascular permeability factors [43]. Our results confirm the linkage between renal damage (plasma creatinine) and circulating levels of both IL-2R (Th1 involvement?) and TNF-α (mononuclear cell contribution to glomerular dysfunction?). The IL-6R is present on hepatocytes, monocytes, neutrophils and some T and B cells. However, the soluble form is still able to bind IL-6 and the complex activates the target cells expressing gp130 [44]. As in Crohn's disease, the sIL-6R may entertain inflammatory processes causing disease activity. Indeed, GADA levels were associated positively with sIL-6R.
In conclusion, our study reports first evidence that the oxidative stress observed in TIDM families is correlated to immunological hallmarks (decreased peripheral numbers of monocytes as well as cells bearing a CD4+CD8+, CD23+CD25+ and CD25+ phenotype) suggestive of different immunoregulatory mechanisms. A crucial subject remains to be explored: the proper timing of events triggering disease pathology in TIDM families. Does the alteration in immune functions follow the altered intracellular redox status or vice versa?
Acknowledgments
We are obliged to Professor Giuliano Mariani for the radioimmunoassay of plasma insulin and to our patients for encouragement and support. We thank Dr Rossana Testi and Simone Pacini for flow cytometric analyses.
References
- 1.Bach JF. Insulin-dependent diabetes mellitus as an autoimmune disease. Endocrine Rev. 1994;15:516–42. doi: 10.1210/edrv-15-4-516. [DOI] [PubMed] [Google Scholar]
- 2.Bingley PJ, Bonifacio E, Williams AJK, Genovese S, Bottazzo GF, Gale EAM. Prediction of IDDM in the general population: strategies based on combinations of autoantibodiy markers. Diabetes. 1997;46:1701–10. doi: 10.2337/diab.46.11.1701. [DOI] [PubMed] [Google Scholar]
- 3.Mandrup-Poulsen T. The role of interleukin-1 in the pathogenesis of IDDM. Diabetologia. 1996;39:1005–29. doi: 10.1007/BF00400649. [DOI] [PubMed] [Google Scholar]
- 4.Matteucci E, Giampietro O. Oxidative stress in families of type I diabetic patients. Diabetes Care. 2000;23:1182–6. doi: 10.2337/diacare.23.8.1182. [DOI] [PubMed] [Google Scholar]
- 5.Matteucci E, Giampietro O. Oxidative stress in families of type I diabetic patients: further evidence. Diabetes Care. 2001;24:167–8. doi: 10.2337/diacare.24.1.167. [DOI] [PubMed] [Google Scholar]
- 6.Heyman B. Regulation of antibody responses via antibodies, complement, and Fc receptors. Annu Rev Immunol. 2000;18:709–37. doi: 10.1146/annurev.immunol.18.1.709. [DOI] [PubMed] [Google Scholar]
- 7.Haczku A, Takeda K, Hamelmann E, et al. CD23 exhibits negative regulatory effects on allergic sensitization and airway hyperresponsiveness. Am J Resp Crit Care Med. 2000;161:952–60. doi: 10.1164/ajrccm.161.3.9905046. [DOI] [PubMed] [Google Scholar]
- 8.Nelson BH. Interleukin-2 signaling to the maintenance of self tolerance. Curr Dir Autoimmun. 2002;5:92–112. doi: 10.1159/000060549. [DOI] [PubMed] [Google Scholar]
- 9.Steele BW, Wu N-C, Whitcomb C. White blood cell and platelet counting performance by hematology analyzers: a critical evaluation. Lab Hematol. 2001;7:255–66. [Google Scholar]
- 10.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 11.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–8. [PubMed] [Google Scholar]
- 12.Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Meth Enzymol. 1990;186:407–13. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 13.Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–13. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 14.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 15.Kaaba SA, Al-Harbi SA. Abnormal lymphocyte subsets in Kuwaiti patients with type-1 insulin-dependent diabetes mellitus and their first-degree relatives. Immunol Lett. 1995;47:209–13. doi: 10.1016/0165-2478(95)00088-5. [DOI] [PubMed] [Google Scholar]
- 16.Kretowski A, Szelachowska M, Pietruczuk M, Kinalska I. CD23 antigen expression on B lymphocytes and soluble CD23 levels in peripheral blood of high-risk type I diabetes subjects. Scand J Immunol. 1999;49:78–81. doi: 10.1046/j.1365-3083.1999.00448.x. [DOI] [PubMed] [Google Scholar]
- 17.Mysliwiec J, Kretowski A, Kinalski M, Kinalska I. CD11a expression and soluble ICAM-1 levels in peripheral blood in high-risk and overt type I diabetes subjects. Immunol Lett. 1999;70:69–72. doi: 10.1016/s0165-2478(99)00133-9. [DOI] [PubMed] [Google Scholar]
- 18.Kretowski A, Maciej K, Ida K. The analysis of in vitro transforming growth factor-β1 (TGF-β1) production by peripheral blood in overt and pre-clinical type I diabetes mellitus. Immunol Lett. 2000;71:85–9. doi: 10.1016/s0165-2478(99)00174-1. [DOI] [PubMed] [Google Scholar]
- 19.Avanzini MA, Vitali L, d’Annunzio G, et al. Enhancement of soluble CD23 serum levels and cell-surface CD23-expression in subjects at increased risk of type I diabetes mellitus and in diabetic patients. Diabetes Med. 1998;15:320–6. doi: 10.1002/(SICI)1096-9136(199804)15:4<320::AID-DIA563>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 20.Von Andrian UH, Mackray CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–34. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi K, Honeyman MC, Harrison LC. Impaired yield, phenotype, and function of monocyte-derived dendritic cells in humans at risk for insulin-dependent diabetes. J Immunol. 1998;161:2629–35. [PubMed] [Google Scholar]
- 22.Kallmann BA, Lampeter EF, Hanifi-Moghaddam P, Hawa M, Leslie RD, Kolb H. Cytokine secretion patterns in twins discordant for Type I diabetes. Diabetologia. 1999;42:1080–5. doi: 10.1007/s001250051274. [DOI] [PubMed] [Google Scholar]
- 23.Karlsson MG, Lawesson SS, Ludvigsson J. Th1-like dominance in high-risk first-degree relatives of type I diabetic patients. Diabetologia. 2000;43:742–9. doi: 10.1007/s001250051372. [DOI] [PubMed] [Google Scholar]
- 24.Rouzaut A, Subirá ML, de Miguel C, et al. Co-expression of inducible nitric oxide synthase and arginases in different human monocyte subsets. Apoptosis regulated by endogenous NO. Biochim Biophys Acta. 1999;1451:319–33. doi: 10.1016/s0167-4889(99)00106-8. [DOI] [PubMed] [Google Scholar]
- 25.Katoh N, Kraft S, Weßendorf JHM, Bieber T. The high-affinity IgE receptor (Fc∈RI) blocks apoptosis in normal human monocytes. Lab Clin Invest. 2000;105:183–90. doi: 10.1172/JCI6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraft S, Novak N, Katoh N, Bieber T, Rupec RA. Aggregation of the high-affinity IgE receptor Fc∈RI on human monocytes and dendritic cells induces NF-κB activation. J Invest Dermatol. 2002;118:830–7. doi: 10.1046/j.1523-1747.2002.01757.x. [DOI] [PubMed] [Google Scholar]
- 27.Pedotti R, Mitchell D, Wedemeyer J, et al. An unexpected version of horror autotoxicus: anaphylactic shock to a self peptide. Nat Immunol. 2001;2:216–22. doi: 10.1038/85266. [DOI] [PubMed] [Google Scholar]
- 28.Kenny E, Mason D, Pombo A, Ramírez F. Phenotypic analysis of peripheral CD4+CD8+ T cells in the rat. Immunology. 2000;101:178–84. doi: 10.1046/j.1365-2567.2000.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiménez E, Sacedón R, Vicente A, Hernández-López C, Zapata AG, Varas A. Rat peripheral CD4+CD8+ lymphocytes are partially immunocompetent thymus-derived cells that undergo post-thymic maturation to become functionally mature CD4+ T lymphocytes. J Immunol. 2002;168:5005–13. doi: 10.4049/jimmunol.168.10.5005. [DOI] [PubMed] [Google Scholar]
- 30.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen non specific. J Immunol. 2000;164:183–90. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 31.Maekawa N, Kawabe T, Surgie K, et al. Induction of Fc∈RII/CD23 on PHA-activated human peripheral blood T lymphocytes and the association of Fyn tyrosine kinase with Fc∈RII/CD23. Res Immunol. 1992;143:422–5. doi: 10.1016/s0923-2494(05)80075-6. [DOI] [PubMed] [Google Scholar]
- 32.Nunez R, Matsui M, Yodoi J, Lynch RG. Identification of novel CD23 transcripts on human T and B lymphocytes and eosinophil cell line. Immunol Lett. 1995;44:169–74. doi: 10.1016/0165-2478(95)00210-v. [DOI] [PubMed] [Google Scholar]
- 33.Kawabe T, Maekawa N, Maeda Y, Hosoda M, Yodoi J. Induction of Fc epsilon RII/CD23 on phytohemagglutinin-activated human peripheral blood T lymphocytes. Enhancement by IL-2 and IL-4. J Immunol. 1991;147:548–53. [PubMed] [Google Scholar]
- 34.Corominas M, Mestre M, Bas J, Buendia E. Distinct modulation by interferon-gamma (IFN-γ) of CD23 expression on B and T lymphocytes of atopic subjects. Clin Exp Immunol. 1998;112:276–80. doi: 10.1046/j.1365-2249.1998.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species cell signaling and cell injury. Free Rad Biol Med. 2000;28:1456–62. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 36.Murata Y, Amao M, Yoneda J, Hamuro J. Intracellular thiol redox status of macrophages directs the Th1 skewing in thioredoxin transgenic mice during aging. Mol Immunol. 2001;38:747–57. doi: 10.1016/s0161-5890(01)00111-0. [DOI] [PubMed] [Google Scholar]
- 37.Hehner SP, Breitkreutz R, Shubinsky G, et al. Enhancement of T cell receptor signaling by a mild oxidative shift in the intracellular thiol pool. J Immunol. 2000;165:4319–28. doi: 10.4049/jimmunol.165.8.4319. [DOI] [PubMed] [Google Scholar]
- 38.Angelini G, Gardella S, Ardy M, et al. Antigen-presenting dendritic cells provide the reducing microenironment required for T lymphocyte activation. PNAS. 2002;99:1491–6. doi: 10.1073/pnas.022630299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gartner LA, Pfeifer MC, Albini C, Francis GL. Soluble interleukin 2 receptor levels in children with type I insulin-dependent diabetes mellitus. Ann Clin Lab Sci. 1995;25:44–51. [PubMed] [Google Scholar]
- 40.Erbagci AB, Tarakçioglu M, Coskun Y, Sivasli E, Namiduru ES. Mediators of inflammation in children with type I diabetes mellitus: cytokines in type I diabetic children. Clin Biochem. 2001;34:645–50. doi: 10.1016/s0009-9120(01)00275-2. [DOI] [PubMed] [Google Scholar]
- 41.Mohamed-Ali V, Armstrong L, Clarke D, Bolton CH, Pinkney JH. Evidence for the regulation of levels of plasma adhesion molecules by proinflammatory cytokines and their soluble receptors. J Int Med. 2001;250:415–21. doi: 10.1046/j.1365-2796.2001.00900.x. [DOI] [PubMed] [Google Scholar]
- 42.Targher G, Zenari L, Bertolini L, Muggeo M, Zoppini G. Elevated levels of interleukin-6 in young adults with type I diabetes without clinical evidence of microvascular and macrovascular complications. Diabetes Care. 2001;24:956–7. doi: 10.2337/diacare.24.5.956. [DOI] [PubMed] [Google Scholar]
- 43.Kallen K-J. The role of transsignaling via the agonistic soluble IL-6 receptor in human diseases. Biochim Biophys Acta. 2002;1592:323–43. doi: 10.1016/s0167-4889(02)00325-7. [DOI] [PubMed] [Google Scholar]
- 44.Lama G, Luongo I, Tirino G, et al. T-lymphocyte populations and cytokines in childhood nephrotic syndrome. Am J Kidney Dis. 2002;39:958–65. doi: 10.1053/ajkd.2002.32769. [DOI] [PubMed] [Google Scholar]