Abstract
Age is one of the main factors involved in the rapidity and the magnitude of CD4+ T cell repopulation in human immunodeficiency virus (HIV)-infected patients on highly active antiretroviral treatment (HAART). Improved thymic function has been suggested as the main factor associated with CD4+ T cell restoration after HAART. This work was undertaken to determine, among host factors, the predictor variable at baseline involved in the magnitude of short- and long-term recovery of CD4+ T cells after HAART. HIV-RNA levels and CD4+ T cell numbers were determined in 54 HIV-infected adults at baseline and at weeks 4, 12, 48 and 96 after HAART. T cell subpopulations were determined by flow cytometry, thymic volume by computed tomography, T cell receptor excision circle (TREC)-bearing cells by quantitative polymerase chian reaction (PCR) and interleukin (IL)-7 levels by enzyme linked immunosorbent assay at baseline. The phenotype of patients’ isolates was determined by infecting GHOST cells expressing CCR5 and CXCR4. The possible interference of phenotype with thymic function was also analysed. Baseline thymic volume was associated independently with the magnitude of short- and long-term recovery of CD4+ T cells after HAART, despite the patients’ viral phenotype. The measurement of thymic volume before therapy may predict the magnitude of T cell increase. This result could have important clinical implications not only in HIV-infected patients, but also in other scenarios of T cell depletion such as bone marrow transplantation and chemotherapy.
Keywords: CD4+, HIV, HAART, thymic function, TREC
INTRODUCTION
Several virological and host factors have been associated with CD4+ T cell recovery after highly active antiretroviral therapy (HAART). Among the virological factors, the magnitude of virological suppression has been correlated with increases in CD4+ T cell numbers [1,2]. Among host factors, age is one of the main players involved in the rapidity and the magnitude of CD4+ T cell repopulation in human immunodeficiency virus (HIV)-infected patients on HAART [3]. Moreover, naive T cell recovery has been associated with young individuals [4]. All such studies tried to explain that the magnitude of CD4+ T cell recovery is age related, and they suggested that improved thymic function could be associated with CD4+ T cell restoration after HAART. In fact, the role of the thymus after HAART is becoming accepted as one of the sources of CD4+ T cell recovery [5]. Hence, concomitant thymic volume increments, naive T cells and/or T cell receptor excision circle (TRECs)-bearing cells (the three most used thymic function-related markers) have been observed in children and adults under HAART [6,7]. Moreover, a poor CD4+ T cell recovery after HAART has been associated with impaired thymic T cell production [8]. We have reported recently that a larger thymic volume when starting HAART is associated with earlier CD4+ T cell repopulation [9]. In fact, among the most used thymic function-related markers, the baseline thymic volume has been reported to be the best predictor for the rapidity of CD4+ T cell recovery after HAART [10]. However, the association between these parameters and the magnitude of CD4+ T cell recovery after HAART has not yet been analysed.
On the other hand, the syncytium-inducing (SI) phenotype has been associated traditionally with a lower CD4+ T cell level and a rapid progression to AIDS [11], although nowadays this association is not clear. Hence, T-tropic strains might affect thymic function by inhibiting new T cell production. In relation to this, in vitro data have shown a high expression of the CXCR4 co-receptor in immature thymocytes [12] suggesting that the T-tropic phenotype might be associated with poorer CD4+ T cell recovery after HAART because T-tropic strains use this co-receptor preferentially. For this reason, it would be of special interest to study viral phenotype before treatment, predicting that the predominance of a T-tropic phenotype would be associated inversely with thymic function-related markers and with a poorer recovery of CD4+ T cell after HAART.
This work was undertaken to determine at baseline which variables among the host factors could predict the magnitude of short- and long-term CD4+ T cell recovery after HAART. We analysed T cell subsets, thymic function-related markers and other variables related to thymopoiesis, i.e. age and interleukin (IL)-7 levels. We also analysed the potential association between viral phenotype and the magnitude of CD4+ T cell gains and thymic function-related markers.
MATERIALS AND METHODS
Patients
In August 1998, the Viral Hepatitis and AIDS Study Group of Virgen del Rocio University Hospital began to study the role of the thymus in T cell repopulation in HIV-infected adults after HAART. The characteristics of this cohort have been described previously in other works [7,9,10,13]. Briefly, a total of 65 consecutive antiretroviral-naive HIV-infected patients starting HAART were included in an observational dynamic cohort. Up to March 2003, 54 patients were seen in at least two visits during follow-up (including baseline) and reported therapy compliance equal to or higher than 95%. All these patients agreed to participate in the study, providing written informed consent, and the Ethical Committee approved the study. HAART was defined as a combination of at least two nucleoside reverse transcriptase inhibitors plus a protease and/or a non-nucleoside reverse transcriptase inhibitor. Patients were evaluated at baseline and at weeks 4, 12, 48 and 96 after treatment. Each time-point was analysed for CD4+, CD8+ and CD3+ T cell subsets and HIV-1 RNA levels in fresh samples. Isolated peripheral blood mononuclear cells (PBMCs) were frozen in liquid nitrogen and serum samples were frozen at −20°C until further determinations. Thoracic computed tomography (CT) to measure thymic volume, T cell phenotype and TREC-bearing cell determinations were studied at baseline.
Thoracic computed tomography
Mediastinic CT was performed with a modified previously described method [14]. Briefly, coded samples were always measured by the same radiologist using a 3000 GE Sytec Scanner with 5 mm thick contiguous sections at 5 mm intervals. Thymic tissue was delimited carefully in all the slices between the first and the last in order to exclude mediastinic fat infiltration (a high density for soft tissue and a low density for surrounding fat). CT Sytec software (version 4·0, General Electrics Medical Systems, Milwaukee WI, USA) was used to analyse all the thymic areas along the slices to calculate thymic volume.
Flow cytometry
At each time-point, absolute numbers of CD4+, CD8+ and CD3+ T cells were determined in fresh samples by conventional flow cytometry. Percentages of naive CD4+ T cells (CD4+CD45RA+CD45RO–), memory CD4+ T cells (CD4+CD45RA–CD45RO+), naive CD8+ T cells (CD8+CD45RA+CD11alow) and memory CD8+ T cells (CD8+CD45RA–CD11ahigh) were determined using a frozen aliquot as described previously [15]. Naive and memory CD4+ and CD8+ T cell absolute numbers were calculated according to the CD4+ and CD8+ T cell counts obtained from fresh blood samples.
TREC-bearing cells: quantification in PBMCs
A modified polymerase chain reaction (PCR)-based method was used to quantify the δRec-ΨJα TREC number [16]. This method has been adapted to a real-time PCR using a LightCycler (Roche Molecular Biochemicals, Mannheim, Germany) to quantify the characteristic signal-joint sequences of the generated TREC and the β-globin gene [7]. TREC levels were measured in PBMC samples and given as TREC number per 106 PBMCs; in addition, the absolute count of TRECs/µl was calculated by multiplying the absolute T cell count by the proportion of TRECs in the CD3+ T cell subpopulation of PBMCs.
IL-7 quantification
Determination of IL-7 levels in serum samples was performed using a high sensitivity colourimetric enzyme-linked immunosorbent assay (ELISA) (QuantikineHS IL-7 immunoassay kit, R&D Systems, Minneapolis, MN, USA) according to the manufacturer's recommendations. This assay has a lower detection threshold of 0·1 pg/ml.
HIV-1 RNA quantification
Plasma HIV-1 RNA was measured by a quantitative PCR (HIV Monitor™ Test kit version 1·5, Roche Molecular Systems, Hoffman-La Roche, Basel, Switzerland) according to the manufacturer's instructions. This assay has a detection limit of 50 HIV-1 RNA copies/ml.
Hepatitis C virus (HCV) co-infection
Serum RNA HCV was measured by PCR (COBAS Amplicor, Roche Diagnostics, Barcelona, Spain).
Primary HIV-1 isolation
Patients’ PBMCs were co-cultured with phytohaemagglutinin (PHA, Boerhinger Ingelheim)-stimulated HIV–HCV-negative donor's PBMCs in equal proportion. Cells were cultured in RPMI-1640 media (Biochrom AG) supplemented with 20% fetal calf serum (FCS) (FCS, Biochrom AG), 2 µg/ml of IL-2 (R&D Systems), 20 µm glutamine, streptomycin and penicillin (Sigma Aldrich). Cultures were maintained for 3 weeks and supernatants were harvested three times a week and stored at −80°C. Cell-free supernatants with HIV-p24 antigen (ELISA, Zeptometrix, Belgium) above 500 pg/ml were used for further infection assays.
Determination of HIV-1 co-receptor usage
Co-receptor usage was determined using GHOST cell lines, a human osteosarcoma (HOS) cell line transfected with the human CD4 gene and one of the co-receptors, CCR5 or CXCR4. Cultures were maintained in Dulbecco's modified Eagle medium (DMEM; Biochrom AG) containing 10% FCS (Biochrom AG), streptomycin and penicillin (Sigma Aldrich). GHOST cells were plated in 48-well plates at 104 cells per well. Approximately 200 pg of HIV-p24 antigen of virus stocks from 30 patients were used to infect GHOST cells. Cultures were maintained for 7 days and supernatants were harvested every other day and tested for HIV-p24 antigen. We used the T-tropic NL4·3 strain and the M-tropic BaL strain as positive controls. HIV-p24 antigen levels above 20 pg/ml were considered positive. For statistical purposes, we used the ratio between the HIV-p24 antigen concentration obtained using GHOST-CD4-CXCR4 cells and the concentration obtained using GHOST-CD4-CCR5 cells at day 7 in order to transform a dichotomous variable into a continuous variable. A similar approach has been reported previously elsewhere [17,18].
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences software package (SPSS 11·0, Chicago, IL, USA). All continuous variables were expressed as median [interquartile (IQR) range] while categorical variables were expressed as number of cases (percentage). Pearson's correlation coefficient analysis (univariate analysis) was used to assess bivariate correlations between all the variables. All variables analysed that had a statistically significant association (P < 0·1) with changes in T cell counts at any point during follow-up versus baseline were included in a multivariate analysis using a multiple linear regression model with a stepwise procedure. Multivariate analysis was considered significant when P < 0·05.
RESULTS
Patients’ baseline characteristics
The patients’ baseline characteristics are summarized in Table 1. The study population was composed preferentially of young males with moderate immunosuppression (median 255·5 CD4+ T cells/µl) and with a high viral load. Almost half the patients were injecting drug users and HCV-co-infected.
Table 1.
Patients’ characteristics at baseline (n = 54)
| Risk group | |
| IDUa | 25 (46·3) |
| Homosexuala | 15 (27·8) |
| Heterosexuala | 14 (25·9) |
| Sex | |
| Malea | 42 (77·8) |
| HCV-co-infectiona | 26 (48·1) |
| Age (years)b | 37·0 [29·8–43·3] |
| Total CD4+ count (cells/µl)b | 255·5 [44·0–439·5] |
| Total CD8+ count (cells/µl)b | 756·0 [456·8–977·0] |
| Naive CD4+ count (cells/µl)b,c | 60·2 [7·90–140·5] |
| Naive CD8+ count (cells/µl)b,c | 45·1 [23·8–116·2] |
| Memory CD4+ count (cells/µl)b,c | 122·8 [41·8–181·0] |
| Memory CD8+ count (cells/µl)b,c | 248·5 [156·0–491·0] |
| IL-7 (pg/ml)b,c | 26·7 [19·5–41·8] |
| Viral load (log10copies/ml)b | 4·9 [4·4–5·2] |
| Thymic volume (cm3)b,d | 3·4 [1·8–6·2] |
| TREC-bearing cells (cells/µl)b,c | 0·74 [0·2–2·5] |
Number of subjects (percentage);
median (interquartile range);
availab1e in 42 patients;
availab1e in 49 patients. IDU, injecting drug user; HCV, hepatitis C virus; TREC, T cell excision circle.
Thymic volume is associated independently with the magnitude of CD4+ T cell recovery both short- and long-term after HAART
The Pearson test was used to correlate the changes in CD4+ T cells during follow-up with baseline parameters. Several variables correlated with the changes in CD4+ T cells, although only thymic volume correlated at each time-point throughout follow-up (Table 2). Variables showing an univariate association (P < 0·1) with changes in CD4+ T cells at weeks 4, 12, 48 and 96 were included in the multivariate analysis as potentially independently associated variables (age and HCV-co-infection were also included). Multiple linear regression using a stepwise procedure was performed considering changes in CD4+ T cell counts as dependent variables at weeks 4, 12, 48 and 96 versus baseline. Only the baseline thymic volume was associated independently with changes in CD4+ T cells at weeks 4, 12, 48 and 96 versus baseline (Table 3).
Table 2.
Univariate analysis between changes in total CD4+ T cells and different baseline parameters
| Week 4 (n = 49) | Week 12 (n = 50) | Week 48 (n = 33) | Week 96 (n = 27) | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | r | P | r | P | r | P | r | P |
| Age | −0·032 | 0·826 | −0·239 | 0·095 | 0·072 | 0·692 | −0·850 | 0·674 |
| Total CD4+ counts | 0·180 | 0·216 | 0·094 | 0·517 | −0·048 | 0·791 | −0·109 | 0·589 |
| Total CD8+ counts | −0·041 | 0·778 | −0·260 | 0·068 | −0·348 | 0·047 | −0·342 | 0·081 |
| Naive CD4+ T cells | 0·267 | 0·105 | 0·307 | 0·051 | 0·046 | 0·802 | 0·045 | 0·831 |
| Naive CD8+ T cells | 0·226 | 0·173 | 0·358 | 0·022 | −0·049 | 0·789 | 0·142 | 0·499 |
| Memory CD4+ T cells | 0·070 | 0·676 | −0·013 | 0·934 | −0·086 | 0·640 | −0·125 | 0·552 |
| Memory CD8+ T cells | −0·078 | 0·640 | −0·430 | 0·005 | −0·245 | 0·176 | −0·239 | 0·250 |
| IL-7 levels | −0·215 | 0·194 | −0·060 | 0·710 | −0·146 | 0·425 | −0·107 | 0·609 |
| Viral load | −0·083 | 0·585 | −0·247 | 0·094 | 0·019 | 0·921 | −0·301 | 0·144 |
| Thymic volume | 0·326 | 0·031 | 0·479 | 0·001 | 0·422 | 0·016 | 0·432 | 0·031 |
| TREC+ bearing-cells | 0·152 | 0·361 | 0·084 | 0·602 | −0·174 | 0·342 | 0·175 | 0·402 |
| X4/R5 ratio | −0·084 | 0·712 | −0·105 | 0·632 | 0·081 | 0·774 | 0·489 | 0·107 |
Significant values are underlined (P < 0·1). At weeks 4,12, 24, 48 and 96 a Pearson test was used. TREC, T cell excision circle.
Table 3.
Multivariate analysis between changes in total CD4+ T cells and different baseline parameters
| Week 4 (n = 49) | Week 12 (n = 50) | Week 48 (n = 33) | Week 96 (n = 27) | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | P | R2 | P | R2 | P | R2 | P | R2 |
| Age | 0·445 | – | 0·095 | – | 0·226 | – | 0·848 | – |
| Total CD8+ count | – | – | 0·078 | – | 0·079 | – | 0·256 | – |
| Naive CD4+ T cells | – | – | 0·996 | – | – | – | – | – |
| Naive CD8+ T cells | – | – | 0·519 | – | – | – | – | – |
| Memory CD8+ T cells | – | – | 0·025 | – | – | – | – | – |
| Thymic volume | 0·008 | 0·159 | <0·0001 | 0·332 | 0·016 | 0·150 | 0·031 | 0·151 |
| Viral load | – | – | 0·128 | – | – | – | – | – |
| HCV-co-infection | 0·624 | – | 0·359 | – | 0·351 | – | 0·863 | – |
Significant values are underlined. HCV, hepatitis C virus.
Association of viral phenotype with baseline parameters and the magnitude of CD4+ T cell recovery
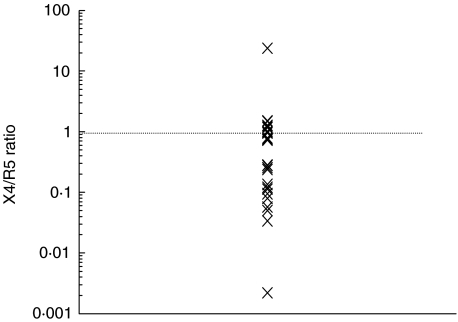
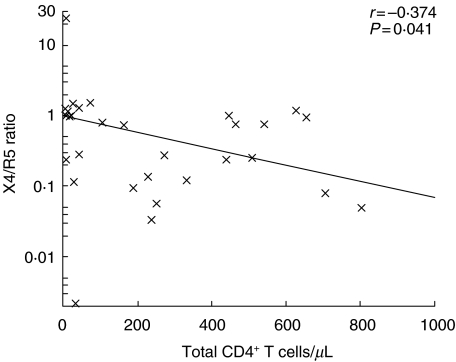
Co-receptor usage was determined in 30 isolates at baseline. The median X4/R5 ratio was 0·73 [0·12–1·05], indicating that most isolates can use both co-receptors with a tendency to use CCR5 preferentially. Only two isolates used one of the co-receptors almost exclusively (Fig. 1). Viral phenotype was associated inversely with baseline CD4+ T cells, i.e. total, naive and memory CD4+ T cells (P = 0·041, P = 0·013, P = 0·004, respectively; Fig. 2). Nevertheless, the X4/R5 ratio was not associated with either the baseline thymic function-related markers or the rest of the parameters at baseline. Viral phenotype was not associated with the magnitude of CD4+ T cell recovery at any week during follow-up (Table 2).
Fig. 1.
X4/R5 co-receptor ratio distribution of patients’ isolates. The median X4/R5 ratio was 0·73 [0·12–1·05]. Patients’ isolates used both co-receptors and preferentially R5; only two virus isolates used almost exclusively one co-receptor.
Fig. 2.
Correlation between X4/R5 ratio and total CD4+ T-cells at baseline. Spearman's test was used.
DISCUSSION
The results presented herein show for the first time that baseline thymic volume is associated independently with the magnitude of short- and long-term recovery of total CD4+ T cells in HIV-infected adults after HAART. Unexpectedly, the viral phenotype at baseline was not associated with either thymic function-related markers or the magnitude of CD4+ T cell count increases.
In many T cell depletion scenarios, such as intensive chemotherapy and haematopoietic stem cell transplantation, age has been found to be associated with CD4+ T cell recovery [19]. Among HIV-infected patients, younger subjects have also been associated with improved CD4+ cell recovery, in terms of rapidity and intensity [3]. Moreover, reconstitution of naive T cells in adults has been described to be dependent on age [4]. All these studies suggest that the greater the thymic function patients have at baseline, the better the immunological response they will obtain after HAART. In fact, restoration of CD4+ T cell numbers after HAART may be attributed partially to thymic function. Hence, increases in naive T cells, TREC-bearing cells, as well as increased thymic volume, after HAART support this affirmation [7]. Moreover, a small amount of thymic tissue after therapy has been associated with a poor CD4+ T cell recovery after suppression of HIV replication under HAART [8].
We have reported recently that baseline thymic volume is the main factor and the best thymic function-related marker to predict the rapidity of CD4+ T cell recovery after HAART [9,10]. Data presented herein support previous reported data among children [20]. Moreover, this study provides evidence, for the first time, that baseline thymic volume is associated independently with the magnitude of short-term (4 and 12 weeks) and long-term (1 and 2 years) recovery of CD4+ T cell numbers in adults after HAART. At week 12, memory baseline CD8+ T cell counts were associated independently with CD4+ T cell gains. However, only thymic volume was a predictor of the studied event. These data are very interesting, as several reports have established that the initial increase in CD4+ T cell numbers after HAART is due exclusively to redistribution from peripheral lymphoid organs [21–23]. In this work, we report a clear association between thymic volume and the short-term increase in CD4+ T cell numbers, suggesting active thymic output in the first weeks after treatment. According to these results, we may speculate that thymic function is not a late event after HAART but is an early mechanism, as is redistribution from peripheral lymphoid organs. This affirmation agrees with our previous work that described an early increment in thymic volume, naive T cell numbers and TREC-bearing cells after HAART [7,13,24], and also supports data reported by others [25].
Several in vitro and animal model studies have shown that different HIV-1 strains can productively infect and destroy thymocytes [12,26–29]. The wide distribution of CXCR4 on human thymocytes [12,18,30,31,32] suggests that a predominance of X4-tropic isolates could induce a greater impact on thymopoiesis, impairing CD4+ T cell recovery after HAART. Nevertheless, in this work viral phenotype was not found to be associated with the magnitude of CD4+ T cell repopulation after HAART. Because thymic volume is associated independently with CD4+ cell recovery, it would have been expected that the predominance of an X4-phenotype would have been associated inversely with thymic volume and, hence, with lower CD4+ T cell levels. Surprisingly, co-receptor usage, expressed as X4/R5 ratio, was not correlated with any thymic function-related marker at baseline. However, it was associated inversely with total CD4+ T cell levels supporting the biological value of the X4/R5 ratio for expressing viral phenotype according to previous data using SI strains [11]. The absence of correlation between viral phenotype, the magnitude of CD4+ T cell repopulation during follow-up and thymic function-related markers at baseline could be explained by the predominance of either a dual-tropic virus or a mixture of X4 and R5 viruses (Fig. 1). Perhaps the absence of correlation is due to either the narrow spectrum of viral strains in these patients or the low number of isolates available. On the other hand, recent reports have shown that both X4 and R5 isolates can infect thymocytes and cause the same cytopathic effect, supporting our results [33]. In fact, co-receptor use is not the only determinant of HIV pathogenesis in the thymus; different cytokine levels could also influence thymus impairment [33].
Baseline IL-7 levels have been related to increments in thymic volume after HAART [13]. Nevertheless, we found no correlation between baseline IL-7 levels and CD4+ T cell increments during follow-up. This could be explained because some patients whose thymic volume did not increase during follow-up may have had a bigger thymic volume at baseline and so undergone a substantial CD4+ cell increment after treatment. We also did not find a correlation between HCV co-infection and CD4+ T cell recovery, supporting previous studies [34,35], although a great controversy is growing regarding this topic [36,37].
Nowadays, the thymus has been proposed to be an important source of T cell recovery after severe immunosuppression events, as occurs in HIV infection. Data presented herein show for the first time in HIV-infected adults on HAART that the baseline thymic volume is associated independently with the magnitude of CD4+ cell recovery, irrespective of viral phenotype. These results also show that this thymic function is maintained both short- and long-term after antiretroviral therapy providing further evidence for the important role of the thymus after HAART.
This work has provided important clinical implications since thymic volume may predict the magnitude of CD4+ T cell recovery not only in HIV infection but also in other T cell depletion scenarios, i.e. bone marrow transplantation and chemotherapy. The role of the thymus might explain why young subjects respond better to chemotherapy, as younger patients should have a bigger thymus. Moreover, immunodeficiency after bone marrow transplantation might be explained in part by the absence of T cell maturation in a more involuted thymus. According to this hypothesis, it would be relevant to know the thymic volume before beginning these therapeutic interventions. New strategies to improve and preserve thymic function are under research and will have important implications for this treatment and other T cell depletion situations.
Acknowledgments
The authors wish to thank CRTS (Centro Regional de Transfusiones Sanguíneas), especially Dr Angel Ayala and Manuel Moyano for their continued support, Dr Aurelio Cayuela for his comments about the statistical methods and A. Gayoso, M. Olivera and M. del Mar Rodriguez for their technical support. This work was supported by research grants from Fondo para la Investigación y Prevención del SIDA en España (FIPSE 12304/02, integrated by Ministerio de Sanidad y Consumo, Abbott Laboratories, Boehringer Ingelgeim, Bristol Myers Squibb, GlaxoSmithKline, Merck Sharp and Dohme and Roche) and ‘Fondo de Investigaciones Sanitarias’ (FIS 00/0521), Red de Investigación en SIDA (RIS) by Ministerio de Sanidad y Consumo, Spain.
References
- 1.Connick E, Lederman MM, Kotzin BL, et al. Immune reconstitution in the first year of potent antiretroviral therapy and its relationship to virologic response. J Infect Dis. 2000;181:358–63. doi: 10.1086/315171. [DOI] [PubMed] [Google Scholar]
- 2.Wu H, Kuritzkes DR, McClernon DR, et al. Characterization of viral dynamics in human immunodeficiency virus type-1 infected patients treated with combination antiretroviral therapy. J Infect Dis. 1999;179:799–807. doi: 10.1086/314670. [DOI] [PubMed] [Google Scholar]
- 3.Viard JP, Mocroft A, Chiesi A, et al. Influence of age on CD4 cell recovery in human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy: evidence from the EuroSIDA study. J Infect Dis. 2001;183:1290–4. doi: 10.1086/319678. [DOI] [PubMed] [Google Scholar]
- 4.Cohen Stuart JW, Hamann D, Borleffs J, et al. Reconstitution of naive T cells during antiretroviral treatment of HIV-infected adults is dependent on age. AIDS. 2002;16:2263–6. doi: 10.1097/00002030-200211220-00005. [DOI] [PubMed] [Google Scholar]
- 5.Smith KY, Valdez H, Landay A, et al. Thymic size and lymphocyte restoration in patients with human immunodeficiency virus infection after 48 weeks of zidovudine, lamivudine, and ritonavir therapy. J Infect Dis. 2000;181:141–7. doi: 10.1086/315169. [DOI] [PubMed] [Google Scholar]
- 6.Vigano A, Vella S, Saresella M, et al. Early immune reconstitution after potent antiretroviral therapy in HIV-infected children correlates with the increase in thymus. AIDS. 2000;14:251–61. doi: 10.1097/00002030-200002180-00007. [DOI] [PubMed] [Google Scholar]
- 7.Franco JM, Rubio A, Martinez-Moya M, et al. T cell repopulation and thymic volume in HIV-1-infected adult patients after highly active antiretroviral therapy. Blood. 2002;99:3702–6. doi: 10.1182/blood.v99.10.3702. [DOI] [PubMed] [Google Scholar]
- 8.Texeira L, Valdez H, McCune J, et al. Poor CD4 T cell restoration after suppression of HIV-1 replication may reflect lower thymic function. AIDS. 2001;15:1749–56. doi: 10.1097/00002030-200109280-00002. [DOI] [PubMed] [Google Scholar]
- 9.De la Rosa R, Leal M, Rubio A, et al. Baseline thymic volume is a predictor for CD4+ T cell repopulation in adult HIV-infected patients under highly active antiretroviral therapy. Antivir Ther. 2002;7:159–63. [PubMed] [Google Scholar]
- 10.Ruiz-Mateos E, de la Rosa R, Soriano N, et al. Comparison of thymic function-related markers to predict early CD4+ T cell repopulation in adult HIV-infected patients on HAART. Antiviral Ther. 2003;8:289–94. [PubMed] [Google Scholar]
- 11.Koot M, Keet IP, Vos AH, et al. Prognostic value of HIV-1 biological phenotype for rate of CD4+ count cell depletion and progression to AIDS. Ann Intern Med. 1993;118:681–8. doi: 10.7326/0003-4819-118-9-199305010-00004. [DOI] [PubMed] [Google Scholar]
- 12.Zaitseva MB, Lee S, Rabin RL, et al. CXCR4 and CCR5 on human thymocytes: biological function and role in HIV-1 infection. J Immunol. 1998;161:3103–13. [PubMed] [Google Scholar]
- 13.Ruiz-Mateos E, de la Rosa R, Franco JM, et al. Endogenous interleukin-7 is associated with increased thymic volume in adult HIV-infected patients under HAART. AIDS. 2003;17:947–54. doi: 10.1097/00002030-200305020-00002. [DOI] [PubMed] [Google Scholar]
- 14.Choyke PL, Zeman RK, Gootenberg J, Greenberg JN, Hoffer F, Frank JA. Thymic atrophy and regrowth in response to chemotherapy: CT evaluation. Am J Roentgenol. 1987;149:269–72. doi: 10.2214/ajr.149.2.269. [DOI] [PubMed] [Google Scholar]
- 15.Franco JM, Leon-Leal JA, Leal M, et al. CD4+ and CD8+ T-lymphocytes regeneration after antiretroviral therapy in HIV-1 infected children and adult patients. Clin Exp Immunol. 2000;119:493–8. doi: 10.1046/j.1365-2249.2000.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during treatment of HIV infection. Nature. 1998;396:690–5. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 17.Simmons G, Wilkinson D, Reeves JD, et al. Primary, syncitium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as co-receptors for virus entry. J Virol. 1996;70:8355–60. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitt N, Chene L, Boutolleau D, et al. Positive regulation of CXCR4 expression and signaling by interleukin-7 in CD4+ mature thymocytes correlates with their capacity to favor human immunodeficiency X4 virus replication. J Virol. 2003;77:5784–93. doi: 10.1128/JVI.77.10.5784-5793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackall CL, Fleisher TA, Brown MR, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332:143–9. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 20.Clerici M, Saresella M, Trabattoni D, Ferrante P, Vanzulli A, Vigano A. Thymic volume predicts long-term immune reconstitution in HIV-infected children treated with highly active antiretroviral therapy. AIDS. 2002;16:2219–27. doi: 10.1097/00002030-200211080-00015. [DOI] [PubMed] [Google Scholar]
- 21.Pakker NG, Notermans DW, de Boer RJ, et al. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998;4:208–14. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 22.Bucy RP, Hockett RD, Derdeyn CA, et al. Initial increase in blood CD4+ lymphocytes after HIV antiretroviral therapy reflects redistribution from lymphoid tissues. J Clin Invest. 1999;103:1391–8. doi: 10.1172/JCI5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landay AL, Bettendorf D, Chan E, et al. Evidence of immune reconstitution in antiretroviral drug-experienced patients with advanced HIV disease. AIDS Res Hum Retrov. 2002;18:95–102. doi: 10.1089/08892220252779638. [DOI] [PubMed] [Google Scholar]
- 24.Rubio A, Martinez-Moya M, Leal M, et al. Changes in thymus volume in adult HIV-infected patients under HAART: correlation with the T cell repopulation. Clin Exp Immunol. 2002;1:121–6. doi: 10.1046/j.1365-2249.2002.01950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu H, Connick E, Kuritzkes DR, et al. Multiple CD4+ cell kinetic patterns and their relationships with baseline factors and virological responses in HIV type 1 patients receiving highly active antiretroviral therapy. AIDS Res Hum Retrov. 2001;17:1231–40. doi: 10.1089/088922201750461285. [DOI] [PubMed] [Google Scholar]
- 26.Bonyhadi ML, Rabin L, Salimi S, et al. HIV induces thymus depletion in vivo. Nature. 1993;363:690–5. doi: 10.1038/363728a0. [DOI] [PubMed] [Google Scholar]
- 27.Kaneshima H, Su L, Bonyhadi ML, Connor RI, Ho DD, McCune JM. Rapid-high, syncytium-inducing isolates of human immunodeficiency virus type 1 induce cytopathicity in the human thymus of the SCID-hu mouse. J Virol. 1994;68:8188–92. doi: 10.1128/jvi.68.12.8188-8192.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su L, Kaneshima H, Bonyhadi M, et al. HIV-1-induced thymocyte depletion is associated with indirect cytopathogenicity and infection of progenitor cells in vivo. Immunity. 1995;2:25–36. doi: 10.1016/1074-7613(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 29.Kollmann TR, Kim A, Pettoello-Mantovani M, et al. Divergent effects of chronic HIV-1 infection on human thymocyte maturation in SCID-hu mice. J Immunol. 1995;154:907–21. [PubMed] [Google Scholar]
- 30.Kitchen SG, Zack JA. CXCR4 expression during lymphopoiesis: implications for human immunodeficiency virus type 1 infection of the thymus. J Virol. 1997;71:6928–34. doi: 10.1128/jvi.71.9.6928-6934.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berkowitz RD, Beckerman K, Pk Schall TJ, McCune JM. CXCR4 and CCR5 expression delineates targets for HIV-1 disruption of T cell differentiation. J Immunol. 1998;161:3702–10. [PubMed] [Google Scholar]
- 32.Taylor JR, Kimbrell K, Scoggins R, Delaney M, Wu L, Camerini D. Expression and function of chemokine receptors on human thymocytes: implications for infection by human immunodeficiency virus type-1. J Virol. 2001;75:8752–60. doi: 10.1128/JVI.75.18.8752-8760.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pedroza-Martins L, Boscardin WJ, Anisman-Posner DJ, Schols D, Bryson YJ, Uittenbogaart CH. Impact of cytokines on replication in the thymus of primary human immunodeficiency virus type 1 isolates from infants. J Virol. 2002;76:6929–43. doi: 10.1128/JVI.76.14.6929-6943.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piroth L, Duong M, Quantin C, et al. Does hepatitis C virus co-infection accelerate clinical and immunological evolution of HIV-infected patients? AIDS. 1998;5:381–8. doi: 10.1097/00002030-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Sulkowski MS, Moore RD, Mehta SH, Chaisson RE, Thomas DL. Hepatitis C and progression of HIV disease. JAMA. 2002;288:199–206. doi: 10.1001/jama.288.2.199. [DOI] [PubMed] [Google Scholar]
- 36.Greub G, Ledergerber B, Battegay M, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356:1800–5. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 37.De Luca A, Bugarni R, Lepri AC et al. for the Italian Cohort Naive Antiretrovirals Study Group. Coinfection with hepatitis viruses and outcome of initial antiretroviral regimens in previously naive HIV-infected subjects. Arch Intern Med. 2002;162:2125–32. doi: 10.1001/archinte.162.18.2125. [DOI] [PubMed] [Google Scholar]